Abstract
Objective
To determine whether a pharmacist can effectively review repeat prescriptions through consultations with elderly patients in general practice.
Design
Randomised controlled trial of clinical medication review by a pharmacist against normal general practice review.
Setting
Four general practices.
Participants
1188 patients aged 65 or over who were receiving at least one repeat prescription and living in the community.
Intervention
Patients were invited to a consultation at which the pharmacist reviewed their medical conditions and current treatment.
Main outcome measures
Number of changes to repeat prescriptions over one year, drug costs, and use of healthcare services.
Results
590 (97%) patients in the intervention group were reviewed compared with 233 (44%) in the control group. Patients seen by the pharmacist were more likely to have changes made to their repeat prescriptions (mean number of changes per patient 2.2 v 1.9; difference=0.31, 95% confidence interval 0.06 to 0.57; P=0.02). Monthly drug costs rose in both groups over the year, but the rise was less in the intervention group (mean difference £4.72 per 28 days, −£7.04 to −£2.41); equivalent to £61 per patient a year. Intervention patients had a smaller rise in the number of drugs prescribed (0.2 v 0.4; mean difference −0.2, −0.4 to −0.1). There was no evidence that review of treatment by the pharmacist affected practice consultation rates, outpatient consultations, hospital admissions, or death rate.
Conclusions
A clinical pharmacist can conduct effective consultations with elderly patients in general practice to review their drugs. Such review results in significant changes in patients' drugs and saves more than the cost of the intervention without affecting the workload of general practitioners.
What is already known on this topic
Review of patients on long term drug treatment is important but is done inadequately
Evidence from the United States shows that pharmacists can improve patient care by reviewing drug treatment
What this study adds
Consultations with a clinical pharmacist are an effective method of reviewing the drug treatment of older patients
Review by a pharmacist results in more drug changes and lower prescribing costs than normal care plus a much higher review rate
Use of healthcare services by patients is not increased
Introduction
Over 80% of drugs prescribed by general practitioners in the United Kingdom are repeat prescriptions—that is, they are represcribed without a consultation between the doctor and the patient.1 Repeat prescribing is poorly managed in the United Kingdom.2 In 1994, the Audit Commission suggested that the review of long term treatment might be inadequate.3 Zermansky subsequently found that 72% of repeat prescriptions sampled in 50 practices had not been reviewed in the past 15 months.2 He concluded that this is potentially both wasteful and dangerous. Purves and Kennedy expressed concern about the variation in the quality of review between practices.4
The Royal College of Physicians and the recent National Service Framework for Older People emphasise the need for regular review of treatment for elderly patients.5,6 In view of the increasing workload of general practitioners, it has been proposed that pharmacists should review patients. Several North American trials have shown the benefits of pharmacists reviewing long term prescriptions in community practice.7–11 In the United Kingdom, two limited randomised controlled trials suggest that review of treatment by pharmacists identifies more drug related problems than normal care.12,13 We tested whether pharmacists can effectively review the conditions and treatments of elderly patients in consultation with the patient.
Participants and methods
Design
The study was a stratified randomised controlled trial and was approved by local research ethics committees. We calculated the sample size on the secondary outcome measure of cost of repeat drugs. This was because we expected the primary outcome, number of changes over 12 months, to show larger differences. The predicted difference in costs was £24 per patient a year, based on a previous study.14 We needed a sample size of 600 per group to give 80% power to detect a cost difference at the 5% significance level with a possible 15% loss to follow up.
Selection criteria and randomisation
We recruited general practices by randomly selecting them from a list of all practices in Leeds Health Authority with four or more partners, computerised repeat prescribing, no previous or current clinical pharmacist involvement, and prescribing costs close to average. We approached practices in random order until four had been recruited. One declined to participate, and we rejected another that was changing its computer system.
The participating practices provided complete lists of registered patients aged 65 and over who were receiving at least one drug on repeat prescription on 1 June 1999. We excluded patients in nursing or residential homes, those who had a terminal illness, and those who were in clinical trials. We wrote to eligible patients asking them to participate. Those who consented were randomised to an intervention group (clinical review by pharmacist) or control group (normal care) by computer generated random numbers. Randomisation was stratified by general practice (four levels), age (65-74 years v ⩾75 years), and number of drugs (⩽4 v ⩾5).
Intervention
The pharmacist (DRP) invited patients to his clinic when their next review was due. Patients with no review date were invited to attend when convenient. Immobile patients were visited at home. Non-attenders were invited once more by telephone.
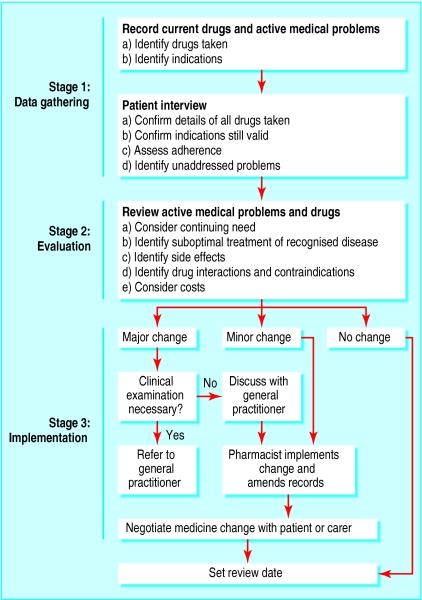
The intervention has been described previously and is summarised as an algorithm (fig 1).15 During the consultation with the patient, the pharmacist discussed each condition being treated and asked about relevant symptoms (such as swollen ankles and breathlessness in patients with heart failure). In conditions for which clinical or pathological monitoring was due, the pharmacist directed the patient to the practice nurse or doctor. The pharmacist did not physically examine the patient, although he noted signs that were obvious during the consultation, such as swollen ankles or rash. Patients with new clinical problems were referred to the doctor.
Clinical medication review
Clinical medication review is the process where a health professional reviews the patient, the illness, and the drug treatment during a consultation. It involves evaluating the therapeutic efficacy of each drug and the progress of the conditions being treated. Other issues, such as compliance, actual and potential adverse effects, interactions, and the patient's understanding of the condition and its treatment are considered when appropriate. The outcome of the review will be a decision about the continuation (or otherwise) of the treatment.
Figure 1.
Process for reviewing repeat prescriptions
Treatment recommendations were based on national, local, and (where available) practice guidelines. We agreed with each practice the level of intervention that the pharmacist could make without seeking prior approval.
Usual care
Patients in the control group continued to receive normal care from their general practitioner and primary healthcare staff. Patients were recalled for review of treatment by the general practitioner according to normal custom in the practice.
Outcome measures
The primary outcome measure was the number of changes to repeat prescriptions between baseline (June 1999) and the end of the 12 month study (June 2000). The secondary outcome measures were changes in number and cost of medicines and frequency of dose and effect on healthcare workload (general practitioner consultations, hospital outpatient attendances, and acute admissions).
Collection of data
As well as the age and sex of patients, we recorded number of repeat prescriptions, number of times doses were taken a day, and net ingredient cost of 28 days' supply (based on Drug Tariff and Monthly Index of Medical Specialties for December 1998) at baseline and the end of the study. The number of consultations within the practice, outpatient attendances, and acute admissions were recorded for the duration of the study. We recorded drop out due to death, leaving the practice, or going into a residential home. We also collected data for six months before the intervention to allow us to test whether the pharmacist's presence contaminated the control group.
Statistical analysis
Analysis was by intention to treat.16 We compared prescribing rates using standard two group comparisons. Regression analyses that adjusted for stratification factors did not qualitatively affect the conclusions and are not reported.
Results
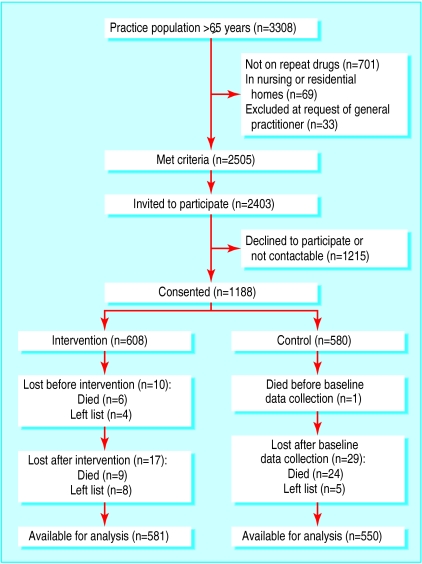
The four practices had a total list size of 28 202 (individual sizes 6342, 7647, 8759, and 5454) with 3308 patients aged 65 and over. In all, 2505 patients met our inclusion criteria, but 33 were excluded at their doctors' request. We contacted 2403 patients consecutively until the required number of participants was obtained;1188 consented. Figure 2 shows the progress of participants through the trial. There was even distribution of age, sex, practice, and number of drugs on repeat prescription between the intervention and control groups (table 1), and there were no differences in drug costs or number of doses (table 2) at baseline. Records on drugs were unavailable for 11 cases: seven had died (six in the intervention group) and four (all intervention group) had left the practices in the interval between consent and examining the records.
Figure 2.
Randomisation of patients and reasons for exclusion from final analysis
Table 1.
Stratification factors and demographic data for intervention and control groups at baseline
| Intervention* | Control | |
|---|---|---|
| No from each practice: | ||
| A | 147 | 124 |
| B | 182 | 175 |
| C | 148 | 144 |
| D | 131 | 137 |
| Total | 608 | 580 |
| Mean (SD age) | 74 (6.6) | 73 (6.4) |
| No (%) of women | 339 (56) | 325 (56) |
| No (interquartile range) of repeated drugs | 4 (2-7) | 4 (2-6) |
Four patients were subsequently found not to be receiving a repeat prescription.
Table 2.
Median (interquartile range) cost and dosage of repeat prescriptions at start of trial
| Factor | Intervention n=598 | Control n=579 | Total n=1177 |
|---|---|---|---|
| Repeats cost (£/month) | 20 (7-38) | 21 (7-39) | 20 (7-39) |
| No of dose times/day | 2 (1-3) | 2 (1-3) | 2 (1-3) |
Outcomes
By June 2000, 40 (3%) patients had died (15 intervention and 25 control group) and 17 (1%) had left the list (12 intervention and five control group). Records were unavailable for a further two patients (both intervention group), leaving 1131 patients with adequate data for inclusion in the principal analyses.
The mean number of changes per patient was 2.2 in the intervention group and 1.9 in the control group (difference=0.31, 95% confidence interval 0.06 to 0.57; P=0.02). Table 3 shows the numbers of patients who had at least one change to their treatment during the study. More patients in the control group than the intervention group started taking a new drug. There was no clear difference in the number of other changes.
Table 3.
Numbers (percentages) of patients whose repeat prescriptions were changed during the study. Some patients had more than one change
| Type of change | Intervention (n=581) | Control (n=550) | Total (n=1131) |
|---|---|---|---|
| New drug started | 265 (46) | 270 (49) | 535 (47) |
| Drug stopped | 239 (41) | 180 (33) | 419 (37) |
| Switched drug | 119 (20) | 93 (17) | 212 (19) |
| Dose changed | 98 (17) | 61 (11) | 159 (14) |
| Change to generic | 64 (11) | 37 (7) | 101 (9) |
| Formulation changed | 17 (3) | 12 (2) | 29 (3) |
| Frequency changed | 6 (1) | 0 | 6 (1) |
| Any of above | 438 (75) | 397 (72) | 835 (74) |
Table 4 shows the differences between baseline and follow up in the numbers, costs, and doses of repeat prescriptions. Numbers of drugs and cost rose in both groups, but for each the rise was significantly less in the intervention group. The number of daily doses did not differ significantly. There was no evidence of any adverse health outcome in the intervention group as measured by need for consultation with a general practitioner or hospital treatment (table 5). The number of deaths was 15 (2.5%) in the intervention group and 25 (4.3%) in the control group (odds ratio=0.56, 0.29 to 1.1).
Table 4.
Changes in treatment between the start and finish of study
| Intervention
|
Control
|
Difference between groups (95%CI) | P value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start (n=596) | Finish (n=576) | Change | Start (n=577) | Finish (n=549) | Change | ||||
| Mean No of repeat medicines | 4.8 | 5.0 | 0.2 | 4.6 | 5.0 | 0.4 | −0.2 (−0.4 to−0.1) | 0.01 | |
| Mean cost over 28 days (£) | 29.27 | 31.07 | 1.80 | 28.23 | 34.85 | 6.52 | −4.72 (−7.04 to −2.41) | 0.0001 | |
| Mean No of dose times/day | 2 | 1.9 | −0.1 | 2.1 | 1.9 | −0.2 | 0.1 (0.0 to 0.2) | 0.17 | |
t test.
Table 5.
Use of health services for 12 months from June 1999 to May 2000. Values are median (interquartile range) unless stated otherwise
| Service | Intervention (n=579) | Control (n=550) | P value |
|---|---|---|---|
| No of general practice consultations | 6 (3-10) | 6 (3-10) | 0.69* |
| No of outpatient appointments | 1 (03) | 1 (0-3) | 0.41* |
| No (%) admitted to hospital: | |||
| Never | 469 (81) | 458 (83) | |
| Once | 78 (13) | 55 (10) | 0.16† |
| More than once | 32 (6) | 37 (7) | |
Mann-Whitney test.
χ2 test.
In all, 590 (97%) intervention patients had a consultation with the pharmacist (one was seen twice). Of the 18 who were not seen, eight had died, four had moved, three declined, and three were not receiving repeat prescriptions. In the control group, 233 (44%) patients had a documented review with a doctor.
The pharmacist took an average of 20 minutes to conduct a review (excluding collection of research data). The gross cost of the pharmacist was £21 per hour, or £7 per patient reviewed. The average reduction in net cost of drugs per patient per 28 days was £4.72 (£2.41 to £7.04).
Discussion
We have shown that a trained pharmacist can conduct clinical medication reviews of elderly patients in the general practice setting. The pharmacist's review resulted in more changes to treatment than normal care and produced an important cost saving, even after the cost of the intervention was deducted.
Validity
We recruited half of contacted patients. There was concern that the participants might not be typical of the practices' eligible elderly populations. We have shown previously that the participants tended to be younger, male, and taking fewer drugs than non-participants.17 This suggests that our results may underestimate the effects of the review. Patients taking more drugs are more likely to benefit from the pharmacist's intervention, provided that they can be persuaded to attend a review. Attendance would be more likely in the context of care rather than a clinical trial.
The unit of randomisation was the patient. Thus practices contained both intervention and control patients. We collected data for the six months before the study started in response to concern that contamination could occur as a result of the pharmacist's presence in the practice. Comparison of these data with study data showed no evidence of contamination.
Reasons for difference between groups
The smaller increase in the mean number of repeat prescriptions in the intervention group was mainly due to these patients being more likely to have drugs stopped. Intervention patients had more changes to treatment in general, perhaps because the pharmacist did a more detailed review than the general practitioners. This effect could be important because patients' compliance has been shown to decrease with increasing number of drugs.18 Stopping unnecessary drugs may also reduce the risk of adverse effects and interactions.
Review of drug treatment by pharmacists could have increased general practice consultation rates if patients made appointments to confirm advice given by the pharmacist, to have tests done, or to have treatment recommendations implemented. Consultations did increase immediately after the review, but the total number in the year was not different from that in the control group. The increase in consultations was due to patients requiring tests (usually referred to the practice nurse) or to suspected worsening of an existing condition or a new condition (referral to the general practitioner). The extra workload was therefore appropriate and was balanced by a reduced workload in the subsequent months.
A potentially interesting outcome was the smaller number of deaths in the intervention group. We did not specify deaths as a secondary outcome, and the study was not powered to detect a difference in mortality. The non-significant difference may be due to the play of chance or to patients stopping inappropriate and harmful medicines, or perhaps because patients who had lost contact with their doctor were returned to surveillance.
We adopted a clinical patient centred approach rather than relying on technical appraisal of the drugs, as in some other studies.12,19 This resulted in more clinical interventions such as ensuring treatment was monitored, identifying new health problems, suggesting new interventions, and reinforcing compliance. Our study supports the concept of medication review suggested in the National Service Framework for Older People.6
The small scale of this trial, involving only four practices in one city and just one pharmacist, limits the generalisability of the results. Nevertheless, it shows that significant and clinically important results can be achieved by pharmacists reviewing patients and their treatment. A larger scale study with more practices and pharmacists is needed to clarify the practicality, costs, and benefits.
Acknowledgments
We thank Dr Dowson and partners, Dr Geraghty and partners, Dr Clements and partners, Dr Burkill and partners, and the patients for participating and D Buttress for help in recruiting patients and secretarial support. The views and opinions expressed do not necessarily reflect those of the funding body.
Footnotes
Funding: NHS Research and Development National Coordinating Centre for Health Technology Assessment.
Competing interests: None declared.
References
- 1.Harris CM, Dajda R. The scale of repeat prescribing. Br J Gen Pract. 1996;46:649–653. [PMC free article] [PubMed] [Google Scholar]
- 2.Zermansky AG. Who controls repeats? Br J Gen Pract. 1996;46:643–647. [PMC free article] [PubMed] [Google Scholar]
- 3.Audit Commission. A prescription for improvement. Towards more rational prescribing in general practice. London: HMSO; 1994. [Google Scholar]
- 4.Purves I, Kennedy J. The quality of general practice repeat prescribing. Newcastle: University of Newcastle upon Tyne; 1994. [Google Scholar]
- 5.Royal College of Physicians. Medication for older people. 2nd ed. London: RCP; 1997. [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health. Medicines and older people: implementing medicines related aspects of the National Service Framework for Older People. London: DoH; 2001. [Google Scholar]
- 7.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomised, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy IB, Keith MR, Coffey EL, Noyes A. Impact of pharmacist-operated general medicine chronic care refill clinics on practitioner time and quality of care. Ann Pharmacother. 1996;30:745–751. doi: 10.1177/106002809603000707. [DOI] [PubMed] [Google Scholar]
- 9.Borgsdorf LR, Miano JS, Knapp KK. Pharmacist-managed medication review in a managed care system. Am J Hosp Pharm. 1994;51:772–777. [PubMed] [Google Scholar]
- 10.Deady JE, Lepinski PW, Abramowitz PW. Measuring the ability of clinical pharmacists to effect drug therapy changes in a family practice clinic using prognostic indicators. Hospital Pharmacy. 1991;26:93–97. [PubMed] [Google Scholar]
- 11.Lobas NH, Lepinski PW, Abramowitz PW. Effects of pharmaceutical care on medication costs and quality of patient care in an ambulatory-care clinic. Am J Hosp Pharm. 1992;49:1681–1688. [PubMed] [Google Scholar]
- 12.Granas AG, Bates I. The effect of pharmaceutical review of repeat prescriptions in general practice. Int J Pharm Pract. 1999;7:264–275. [Google Scholar]
- 13.Mackie CA, Lawson DH, Campbell A, Maclaren AG, Waigh R. A randomised controlled trial of medication review in patients receiving polypharmacy in general practice. Pharm J. 1999;263:R7. [Google Scholar]
- 14.Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medication review and education programme in an elderly general practice. Br J Clin Pharmacol. 2000;50:172–175. doi: 10.1046/j.1365-2125.2000.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe CJ, Petty DR, Zermansky AG, Raynor DK. Development of a method for clinical medication review by a pharmacist in general practice. Pharm World Sci. 2000;22(4):121–126. doi: 10.1023/a:1008758823788. [DOI] [PubMed] [Google Scholar]
- 16.Piantadosi S. Clinical trials: a methodological perspective. New York: Wiley; 1997. [Google Scholar]
- 17.Petty DR, Zermansky AG, Raynor DK, Vail A, Lowe CJ, Freemantle N, et al. “No thank you”: Why elderly patients declined to participate in a research study. Pharm World Sci. 2001;23(1):22–27. doi: 10.1023/a:1011276924820. [DOI] [PubMed] [Google Scholar]
- 18.Murray MD, Birt JA, Manatunga AK. Medication compliance in elderly outpatients using twice-daily dosing and unit-of-use packaging. Ann Pharmacother. 1993;27:616–621. doi: 10.1177/106002809302700517. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein R, Hulme H, Willits J. Reviewing repeat prescribing—general practitioners and community pharmacists working together. Int J Pharm Pract. 1998;6:60–66. [Google Scholar]