Abstract
Background
Colorectal cancer liver metastases (CRLM) grow in distinct histological patterns that have been associated with outcome after surgical resection. We conducted a systematic review to evaluate the frequency of different CRLM growth patterns and their impact on prognosis.
Methods
We searched Embase and MEDLINE databases from inception to 1 December 2017 to identify studies that reported CRLM growth pattern histopathology, their frequencies, and/or data related to outcome.
Results
We included a total of 23 studies (2432 patients with CRLM) published between 1991 and 2017. There were variations in the terminology used to describe the growth patterns as well as in their histopathological definitions. A ‘desmoplastic’ pattern was most frequently considered, followed by ‘pushing’ and ‘replacement’ patterns. Data supported the presence of both intralesional and interlesional heterogeneity. There were no differences in growth pattern distribution stratified by chemotherapy. While heterogeneity of histopathology assessment precluded formal meta-analysis, the majority of articles found favourable outcomes for desmoplastic and unfavourable outcomes for replacement CRLM, independently of when the study was conducted.
Conclusions
The results suggest that CRLM growth patterns may have prognostic potential and that they may be considered for standardised routine histopathological reporting. Further understanding of the different growth patterns may provide important insights into the biological mechanisms that underlie metastatic growth in the liver.
Keywords: colorectal cancer, colorectal metastases, hepatic metastases, histopathology
Introduction
Colorectal cancer (CRC) is one of the most frequent malignancies worldwide.1 Multimodal stage-dependent therapy combining surgery and chemoradiotherapy achieves good long-term survival in patients without distant metastases.2 However, approximately half of all patients with CRC develop metastases during the course of their disease, at which point survival rates drop markedly.3 The liver is the organ in which CRC metastases are most frequently found.4 In contrast to most other cancer types, surgical resections of colorectal cancer liver metastases (CRLM) are routinely performed.5 Unfortunately, despite advances in surgical techniques and neoadjuvant chemotherapy, the management of CRLM remains a formidable challenge, as evidenced by the generally poor long-term survival rates.6
In primary CRC, the presence of different histological growth patterns is well known: Jass et al described an ‘infiltrating’ pattern, in which cancer cells invade diffusely into surrounding tissue, in contrast to a ‘pushing’ or ‘expanding’ growth pattern, in which the tumour border is clearly demarcated.7 This assessment of the tumour border has been well established in diagnostic pathology,8 and may serve for prognostic stratification, as primary CRCs with a predominantly ‘pushing’ border are associated with a more favourable outcome.9 10
With the increasing frequency of CRLM resections, the histomorphology of the metastatic front in liver metastases has become routinely accessible and several different CRLM growth patterns have been described: a ‘desmoplastic’ or ‘encapsulated’ pattern, in which the tumour cells are separated from the liver parenchyma by a rim of fibrotic stroma, is distinguished from a ‘pushing’ or ‘expansive’ pattern, in which the liver plates adjacent to the metastases are flattened, while intervening fibrotic tissue is absent. A further pattern is termed ‘invasive’ or ‘replacement’; in this case, the tumour cells infiltrate along the liver cell plates, replace hepatocytes and co-opt the sinusoidal stromal scaffold.11 12 In addition, overlaps of these patterns have been observed.13 14
Several studies have suggested that these distinct growth patterns are associated with differences in tumour recurrence and overall survival (OS).14–16 However, cohorts were of limited size, terminology and histological criteria were heterogeneous, and the morphology of the CRLM tumour border is not routinely reported in clinical practice.17 Systematic work by an international multidisciplinary team has recently resulted in the first consensus guidelines for scoring CRLM growth patterns.18 In the suggested guidelines, the authors define three main growth patterns, desmoplastic, replacement, and pushing. By applying this classification to a validation cohort of 374 patients, they find superior survival in the group with predominantly desmoplastic growth pattern, compared with the replacement type. Their work highlights that it is possible to systematise CRLM growth patterns in a reproducible and clinically valuable way.
From a tumour biology perspective, the modes of metastatic growth in the liver are of paramount interest. Recent studies have identified the local microenvironment as a major determinant of tumour aggressiveness in various cancer types including CRC.19–23 In each CRLM growth pattern, metastasised cancer cells interact in particular ways with different host cells such as hepatocytes, Ito cells, and endothelial cells, and are thus embedded in diverse biological microenvironments with hitherto unidentified impact on cancer cell behaviour. Hence, a deeper understanding of the different growth patterns holds potential for deciphering important mechanistic aspects of metastatic tumour progression.
We conducted this systematic review of the literature on CRLM growth patterns to systematically analyse variations in their nomenclature and to evaluate their reported frequencies as well as their impact on patient survival. Finally, we highlight recent functional evidence on the mechanistic underpinnings of this important clinicopathological phenomenon.
Methods
Literature search and selection criteria
We performed a systematic search for published studies on growth patterns in CRLM as of 1 December 2017 in Embase and MEDLINE databases. The search strings (online supplementary figures 1 and 2) were designed to find articles on liver metastases from adenocarcinomas of the colon or rectum that included a histological evaluation of the tumour border and assessed the growth pattern.
bmjgast-2018-000217supp001.pdf (907.6KB, pdf)
Additional searches were performed based on the lists of references in the eligible studies. Inclusion criteria for abstract review were defined as follows and judged independently by two reviewers (MG and CFM): (1) report on sporadic colon and/or rectum adenocarcinoma; (2) report on liver metastases; (3) histological assessment of the tumour border, either explicitly stated or implied (eg, by mentioning immunohistochemistry on CRLM sections); (4) species is human. Exclusion criteria were: (1) language other than English, Spanish, German or Swedish; (2) article is a case report; (3) article is a review.
After independent evaluation, MG and CFM discussed those articles for which there was disagreement on their inclusion. If no agreement was reached, BB was consulted.
This systematic review was not preregistered.
Data extraction
The full texts of potentially eligible articles were acquired by MG, assessed independently by and finally discussed between MG and CFM to reach agreement on their final inclusion. In the case of overlapping cohorts, the article with the largest sample size was included; if the exact same cohort was reported more than once (as identified by the number of patients, clinical institution, and dates of sample collection), the most recent publication was included. If CRLM growth patterns were assessed histologically, but not quantified, the corresponding author(s) were contacted by email and asked to share the following information: histopathological definition/growth pattern criteria, absolute frequencies, and available survival data. Requests were also made in cases of potential overlap of similar, but not identical, study cohorts (according to date, size, and reported data). If there was no reply within 1 week, an additional email was sent; in case of no response within a total of 2 weeks, the request was considered to have failed and the decision on inclusion was made jointly by CFM and MG; if no agreement could be reached, BB was consulted.
Estimates of internal study validity were recorded using a modified version of the Quality Assessment of Diagnostic Accuracy Studies criteria.24 Based on these data, Risk of Bias was assessed using an adapted version of the Cochrane Collaboration’s tool for assessing Risk of Bias25; ‘performance bias’ and ‘allocation bias’ were not assessed as the analysed studies were non-interventional.
Data handling
GraphPad Prism (V.6.0g) was used for data analysis. For word clustering, growth pattern terminology was extracted from all eligible studies and groups of terms were inferred by the descriptions of the histological patterns (eg, in a study where the term ‘sinusoidal’ was used to describe a pattern in which tumour cells replace hepatocytes, this was considered to represent the ‘replacement’ pattern). The absolute frequency of each term was calculated and the terms were displayed in a word clustering diagram with a proportional font size.
Results
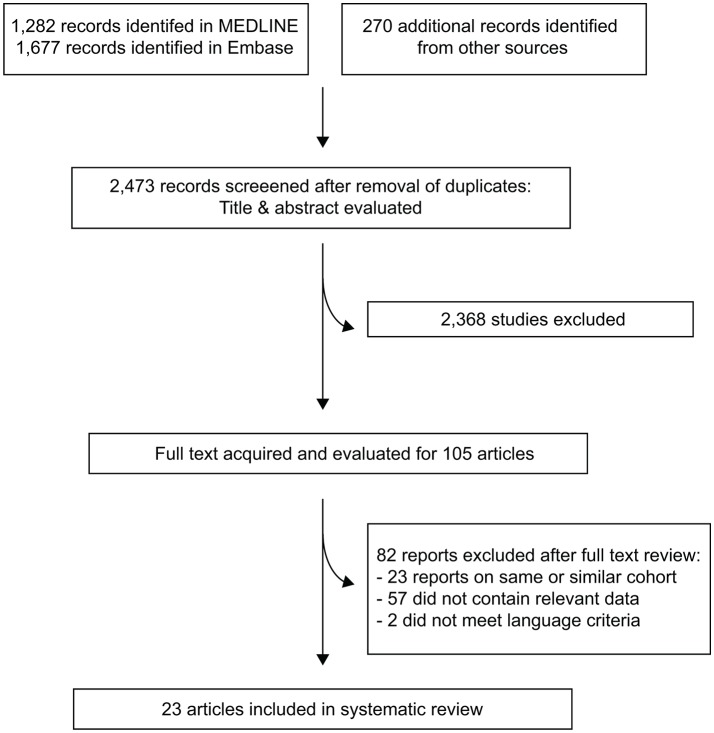
Figure 1 shows the study flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.26 The search strategy identified a total of 2473 articles, of which 2368 were excluded after abstract review. Full texts were acquired for 105 articles and after full text review, 23 studies reporting data on a total of 2432 patients were considered eligible.11–14 18 27–44 Table 1 presents the eligible studies included for systematic review and the clinicopathological characteristics of the cohorts; online supplementary figure 3 presents data on the risk of bias.
Figure 1.
Study flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. One study15was included specifically for outcome data, as it reported the outcome for a cohort published in a more recent article13; this study was excluded due to overlapping cohort from the main series. Another study27 included two independent patient cohorts that were reported separately; this study is counted as one item.
Table 1.
Overview of eligible studies included
| Study | Title | Publication year | Time period | Location | Male | Female | Primary in colon | Primary in rectum | Synchronous CRLM |
Metachronous CRLM |
Desmoplastic CRLM (%) | Pushing CRLM (%) | Replacement CRLM (%) |
Mixed CRLM (%) |
Capsule independent | Outcome assessed | Preoperative chemo |
| van Dam et al 18 | International consensus guidelines for scoring the histopathological growth patterns of liver metastasis | 2017 | 2000–2015 | Department of Surgical Oncology, Erasmus MC Cancer Institute (Rotterdam, The Netherlands) | 241 | 133 | 204 | 170 | 243 (defined as <1 year) | 131 | 49 | 3 | 47 | 1, excluded from survival analysis; >50% predominance was considered sufficient |
N | Y | ≥44% of patients |
| Frentzas et al,27 London cohort | Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases | 2016 | 2006–2012 | The Royal Marsden Hospital (RM), London, UK | 21 | 12 | 12 | 21 | NA | NA | 52.5 | 1.7 | 45.8 | NA | N | Y | All bev+chemo |
| Frentzas et al,27 Montréal cohort | Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases | 2016 | 2008–2014 | McGill University Health Centre (MUHC), Montréal, Canada | 35 | 24 | 39 | 20 | NA | NA | 51.6 | 3.1 | 45.3 | NA | N | Y | All bev+chemo |
| Siriwardana et al 28 | Biological and prognostic significance of the morphological types and vascular patterns in colorectal liver metastases (CRLM) looking beyond the tumor margin | 2016 | 1998–2008 | Royal Free Hospital, London, UK |
18 | 8 | 18 | 6 | 7 | 19 | 43.3 | NA | 43.3 | 10 | N | Y | N |
| Serrablo et al 29 | Impact of novel histopathological factors on the outcomes of liver surgery for colorectal cancer metastases | 2016 | 2004–2010 | NA, Spain and/or Italy | NA | NA | NA | NA | NA | NA | 17 | 52.3 | 46.3 | NA | Y | Y | Reported separately |
| Eefsen et al 13 | Microvessel density and endothelial cell proliferation levels in colorectal liver metastases from patients given neo-adjuvant cytotoxic chemotherapy and bevacizumab | 2015 (print 2016) |
2007–2011 | Rigshospitalet, Copenhagen, Denmark | 146 | 91 | 162 | 75 | 146 | 91 | 29.5 | 37.1 | 14.8 | 18.6 | N | N (for same cohort in ref 15) | Reported separately |
| Brunner et al 30 | Prognosis according to histochemical analysis of liver metastases removed at liver resection | 2014 | 2004–2010 | Regensburg, Germany | 118 | 83 | 119 | 82 | NA | NA | 37.8 | NA | NA | NA | N | Y | Reported separately |
| Pinheiro et al 31 | Tumor growth pattern as predictor of colorectal liver metastasis recurrence | 2014 | 2000–2009 | NA, likely Sao Paulo, Brazil | 50 | 41 | NA | NA | 43 | 48 | NA | 40.7 | 59.3 | NA | N | Y | Reported separately |
| Wiggans et al 32 | Extended pathology reporting of resection specimens of colorectal liver metastases: the significance of a tumour pseudocapsule | 2012 (print 2013) |
2010–2011 | Derriford Hospital, UK | 40 | 26 | NA | NA | 38 | 28 | 41.5 | NA | NA | NA | N | Y | Mixed |
| Nyström et al 33 | Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour | 2012 | 1998–2009 | Västerbotten County, Sweden |
25 | 23 | 29 | 18 | NA | NA | 45.8 | 52.1 | NA | NA | N | Y | Mixed |
| Van Den Eynden et al 14 | The histological growth pattern of colorectal cancer liver metastases has prognostic value | 2012 | 1997–2005 | Royal Hallamshire Hospital, Sheffield, UK | NA | NA | 104 | 91 | 101 | 98 | 34.6 | 15.6 | 27.8 | 17.6 | N | Y | NA |
| Rajaganeshan et al 34 | Biological characteristics and behaviour of putatively curatively resected colorectal liver metastases | 2007 (print 2008) |
1993–2004 | St James’s University Hospital, Leeds, UK |
60 | 49 | NA | NA | 19 | 90 | 34.8 | NA | NA | NA | N | Y | Untreated group reported in ref 46 |
| Stessels et al 35 | Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia | 2004 | NA | General Hospital Sint-Augustinus and of the University Hospital of Antwerp, Belgium |
NA | NA | NA | NA | NA | NA | 50 | 17.9 | 32.1 | NA | N | N | NA |
| Terayama et al 36 | Peritumoral rim enhancement of liver metastasis: hemodynamics observed on single-level dynamic CT during hepatic arteriography and histopathologic correlation | 2002 | NA | Kanazawa University School of Medicine, Japan | 24 | 5 | NA | NA | NA | NA | 10.5 | NA | NA | NA | N | N | NA |
| Yamaguchi et al 37 | Mode of infiltrative growth of colorectal liver metastases is a useful predictor of recurrence after hepatic resection | 2002 | 1981–1998 | Nagasaki University School of Medicine, Japan |
NA | NA | NA | NA | NA | NA | NA | 38.1 | 61.9 | NA | N | Y | NA |
| Vermeulen et al 12 | Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia | 2001 | NA | University Hospital Antwerp and AZ Sint-Augustinus, Belgium | NA | NA | NA | NA | NA | NA | 42.3 | 46.2 | 11.5 | NA | N | N | Excluded |
| Weber et al 38 | Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? | 2001 | 1988–1998 | Hôpital Universitaire de Hautepierre, Strasbourg, France | 132 | 89 | 142 | 76 | 90 | 131 | 28.5 | 34.8 | 65.2 | NA | Y | Y | NA |
| Lunevicius et al 39 | Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer | 2001 | 1983–1997 | Aichi Cancer Center, Japan |
NA | NA | NA | NA | NA | NA | 20.3 | NA | NA | NA | N | Y | NA |
| Okano et al 40 | Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma: clinicopathologic study of 152 first resection cases | 2000 | 1992–1996 | National Cancer Center Hospital, Tokyo, Japan | 104 | 48 | NA | NA | 46 | 106 | 61.1 | NA | NA | NA | N | Y | NA |
| Ambiru et al 41 | Hepatic resection for colorectal metastases: analysis of prognostic factors | 1999 | 1984–1997 | Chiba University School of Medicine, Japan | 104 | 62 | 109 | 53 | 71 | 91 | 27.9 | NA | NA | NA | N | Y | NA |
| Nagashima et al 11 | Histopathological prognostic factors influencing long-term prognosis after surgical resection for hepatic metastases from colorectal cancer | 1999 | 1981–1994 | Tokyo University Hospital, Japan | 48 | 11 | NA | NA | 24 | 35 | 80 | 91.5 | 8.5 | NA | Y | Y | NA |
| Terayama et al 42 | Histologic growth patterns of metastatic carcinomas of the liver | 1996 | NA | Kanazawa University School of Medicine, Japan |
6 | 7 | 13 | NA | NA | NA | 15.4 | 76.9 | 7.7 | NA | N | N | NA |
| Yamamoto et al 43 | Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer | 1995 | 1991–1992 | National Cancer Center Hospital, Tokyo, Japan | 26 | 14 | 27, multiple primaries | 12, multiple primaries | 8 | 32 | 70 | NA | NA | NA | N | N | NA |
| Morino et al 44 | Clinico-pathological features of liver metastases from colorectal cancer in relation to prognosis | 1991 | 1980–1986 | Kyoto University Hospital, Japan | 16 | 13 | 12 | 17 | 11 | 18 | 27.6 | 20.7 | 34.5 | 41.4 | Y | Y | NA |
Clinicopathological data were extracted as specified in the Methods section.
bev, bevacizumab; chemo, conventional cytotoxic chemotherapy; CRLM, colorectal cancer liver metastases; N, no; NA, not available; Y, yes.
Terminology of CRLM growth patterns
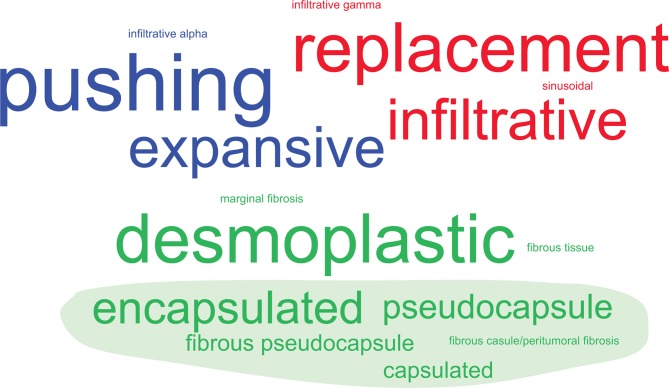
Although in general three major patterns were discernible across the studies, the terminology used to describe different growth patterns varied markedly (illustrated in figure 2). The most frequently used single term to describe the presence of fibrotic tissue separating tumour and liver cells was ‘desmoplastic’ (27% of all studies), but expressions referring to ‘capsule’ (such as ‘encapsulated’ or ‘pseudocapsule’) were cumulatively more frequent (54.5%). The most frequently used term to describe a pattern in which tumour cells infiltrate along the liver cell plates and replace the hepatocytes was ‘replacement’ (27.3%), followed by ‘infiltrative’ (22.7%). The third pattern, in which liver cell plates surrounding the tumour appear flattened (often referred to as ‘compressed’), while intervening fibrotic tissue is absent, was most frequently described as ‘pushing’ (31.8%), followed by ‘expansive’ (22.7%). Throughout the remainder of this article, we employ the most frequently used terms, ‘desmoplastic’, ‘replacement’, and ‘pushing’, adhering to the consensus guidelines.18
Figure 2.
Word cluster diagram of colorectal cancer liver metastases growth pattern terminology. Original terms for the histological growth patterns in the literature are shown in a font size proportional to their absolute frequency (n=22 studies, not considering the consensus guidelines18 as these already reflect a distillate from previous studies). These are grouped and coloured by similarity into ‘consensus terms’ (‘desmoplastic’, green; ‘pushing’, blue; and ‘replacement’, red). The filled green shape indicates terms related to ‘capsule’.
Classification schemes and histopathological criteria for growth pattern reporting
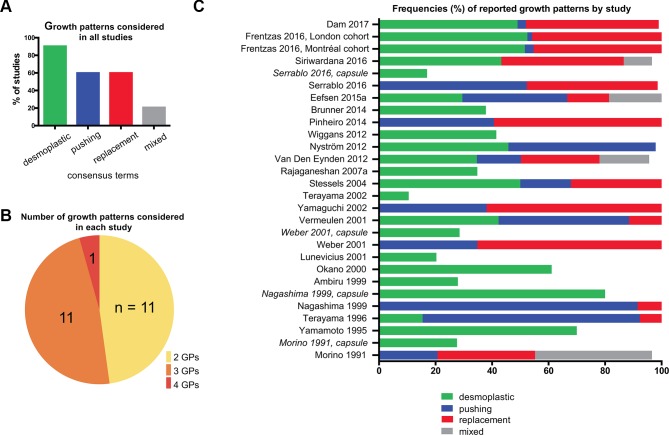
Not all studies considered all three major patterns. Overall, the desmoplastic pattern was considered most frequently (in 21 out of 23 studies), while the replacement and pushing patterns were described less often (both in 14 out of 23 studies; figure 3A). A total of 11 studies distinguished between two growth patterns, while 11 studies used a three-tier, and one study a four-tier classification system (figure 3B). The consensus paper specifically discusses two further rare growth patterns, ‘sinusoidal’ and ‘portal’,18 which are not systematically used in the literature and for the sake of practicability will not be considered further here.
Figure 3.
Classification systems and reported frequencies of colorectal cancer liver metastases (CRLM) growth patterns. (A) Overall reported frequencies of CRLM growth patterns. Heterogeneous expressions used to describe growth patterns between studies were grouped by similarity with respect to their individual terms and associated descriptions into ‘consensus terms’ (related to figure 2). (B) Number of growth patterns considered in each study. Note that some studies regarded the presence/absence of a fibrotic capsule as a parameter independent of the histological growth patterns—that is, ‘desmoplastic’ metastases could be simultaneously classified as ‘pushing’ or ‘replacement’. ‘Mixed’ patterns are not included, as they do not define a specific category. (C) Percentage frequencies of reported growth patterns by study, according to consensus terms. For studies (in italics) in which a capsule was assessed independently, its frequency is represented in a separate bar. Note: In some studies, single metastases could not be classified by the reporting authors, in which case the bars do not add up to 100%. Although Terayama et al 42 considered a total of four growth patterns the description of ‘sinusoidal’ was considered to closely represent the ‘replacement’ pattern in this specific study and counted as such. GP, growth pattern.
The exact definitions of the histopathological criteria varied substantially (online supplementary table 1). For example, describing the desmoplastic pattern, some definitions required the metastases to be ‘completely surrounded by fibrous tissue’38 or to be of a certain thickness that varied between studies,29 32 while other definitions were less specific and based on the presence of a ‘fibrotic capsule’.30 42 44
Four studies reported the presence or absence of a fibrotic capsule as a feature separate from either the replacement or pushing growth patterns (table 1). In general, a heterogeneous picture of histopathological definitions and classification schemes became evident.
Reported frequencies of CRLM growth patterns
Reported unweighted median frequency for the desmoplastic pattern was 41.7% (range: 10.5%–80%), for the pushing pattern 37.1% (range: 1.7%–91.5%), and for the replacement pattern 43.3% (range: 7.7%– 65.2%) (figure 3C).
Reported frequencies of CRLM growth patterns in relation to preoperative chemotherapy
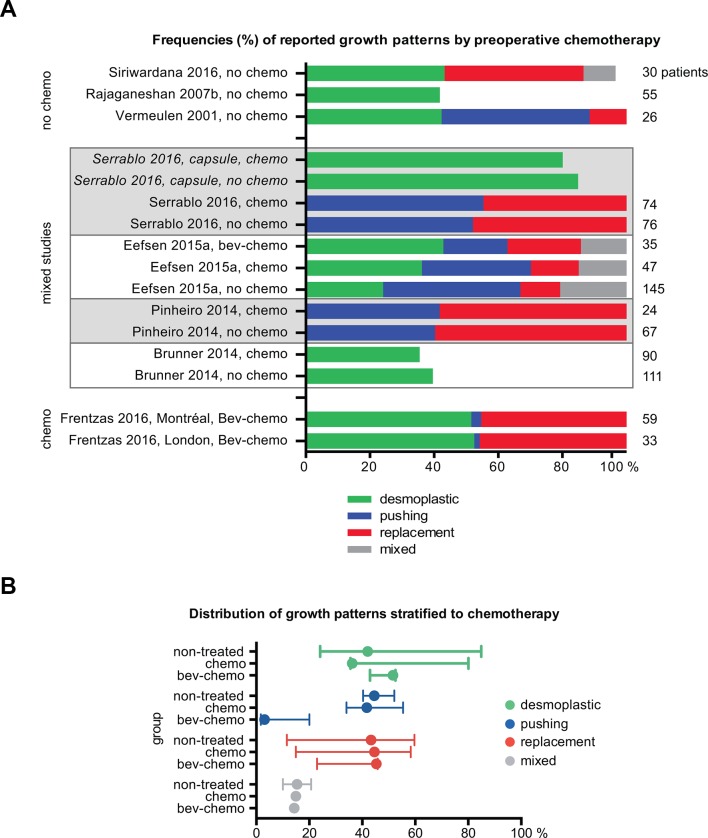
Following current treatment routines, most patients with CRLM will undergo preoperative chemotherapy,45 which may impact directly on the growth pattern phenotypes or select for those types possibly resistant to chemotherapy. Thus, we assessed whether studies explicitly reported data on chemonaïve patients. For articles published in the 1990s, systemic preoperative chemotherapy was unlikely, but given regional and historic differences in treatment regimens that may not have been reported in detail, we included only those studies with explicit statements. We identified two studies in which only patients who had not received preoperative chemotherapy were included.12 28 In a third study (not included in the eligible studies due to overlap with a more recent report), data for patients without preoperative chemotherapy were published separately.46 In addition, four studies reported growth pattern frequencies for chemonaïve and chemotherapy-treated patients separately.13 29–31 In one study with two cohorts,27 all patients had received cytotoxic therapy in combination with the anti-vascular endothelial growth factor antibody bevacizumab, while in another study, the cohort receiving bevacizumab in addition to chemotherapy was analysed individually.13 Figure 4A shows the distribution of growth patterns stratified according to the administration of preoperative chemotherapy. Although there was substantial variation between studies with respect to histopathological analysis, which precluded quantitative synthesis, there were no obvious differences in overall growth pattern distribution across treatment groups (figure 4B). However, a decrease in the frequency of the pushing pattern was observed in patients treated with both bevacizumab and chemotherapy, likely due to differences in histologic classification27 as discussed below.
Figure 4.
Reported frequencies stratified according to preoperative chemotherapy. (A) Percentage frequencies of reported growth patterns by study, grouped according to treatment: no preoperative treatment, cytotoxic chemotherapy, or cytotoxic chemotherapy plus bevacizumab. For studies (in italics) in which the capsule was assessed independently, its frequency is represented in a separate bar. Studies that reported both treated and untreated patients are grouped and indicated with grey or white background. The total number of patients in each study cohort is given to the right of each bar. (B) Reported frequencies for the three treatment groups; lines indicate minimum and maximum, filled circle indicates median (frequency in %). Note: The mixed pattern was reported in one study only for chemo and bev-chemo groups. bev, bevacizumab; chemo, conventional cytotoxic chemotherapy.
Intralesional heterogeneity of CRLM growth patterns
The fact that some authors assessed desmoplasia/encapsulation independently of the other growth patterns indicated heterogeneity within single metastases. This is also reflected in the recent consensus guidelines, in which a cut-off >50% of the tumour-liver interface is considered defining the predominant growth pattern.18 Therefore, next we assessed how frequently heterogeneous patterns were reported. We found that in addition to the four studies in which the desmoplastic pattern was a separate parameter, a ‘mixed’ pattern was reported independently of the three main patterns in a further three studies,14 15 44 and independently of the desmoplastic and replacement patterns in a fourth.28 Frequencies for a mixed pattern were 18.6%15 and 17.6%14 in two larger cohorts and 10%28 and 41.4%44 in two smaller ones. Intralesional heterogeneity was also reflected indirectly in some studies that did not quantify this feature, stating that heterogeneity was ‘uncommon’,31 or that ‘usually’ or ‘in most of the cases’, only one growth pattern was present.12 33
Similar to the consensus guidelines, a recent study addressed intralesional heterogeneity by scoring the percentage of each pattern for every individual lesion.27 Of note, the authors refined their own previously developed criteria,12 as they observed that in some cases in which tumour cells seemed to directly replace hepatocytes, flattening of the surrounding liver plates—a characteristic of the pushing type—was also apparent.27
Interlesional heterogeneity of CRLM growth patterns
Approximately half of all patients with CRLM present with more than one hepatic lesion at the time of diagnosis.45 47 Hence, an important question was whether interlesional heterogeneity was present and how it was assessed. Various approaches were used: three studies included a statement that multiple lesions showed similar pathological features,12 40 43 while in others, quantitative differences in growth patterns between multiple lesions were not commonly reported. In studies with statements on multiple lesions, the largest metastasis (or the lesion with the largest tumour-liver interface) was most frequently used for growth pattern assessment.12 33 40 43 Given the lack of quantitative reporting, interlesional heterogeneity could not be analysed further for review purposes. However, one additional paper (not included in the eligible studies due to likely cohort overlap) provided a systematic analysis of interlesional heterogeneity in chemonaïve patients with multiple CRLM (n=24).48 Applying a three-tier system for desmoplastic, pushing, and replacement patterns, and also considering mixed cases, the authors found that in 20 patients with a uniform growth pattern in a single metastasis, 12 had a single growth pattern in all metastases, while eight had CRLM of more than one type. Overall, the authors concluded that the data suggested ‘… that the growth pattern of liver metastases is not a random phenomenon'.48 Two studies reported data on repeated hepatic resections. Of note, the majority of recurring CRLMs showed the same growth pattern as in the first resection (n=5 and n=21, respectively).30 33
Prognostic potential of growth pattern assessment
Among the eligible studies, a total of 17 investigated the prognostic impact of CRLM growth patterns (online supplementary table 2). One additional study15 reported recurrence risk specifically for a cohort included in a more recent study.13 One study reported two cohorts separately,27 yielding a total of 19 different patient cohorts for analysis of outcome. The most commonly reported metrics were overall survival, disease-free survival, and disease recurrence.
However, reported outcome data across studies were not applicable to pooled quantitative analysis due to substantial heterogeneity in terminology, definitions of histopathological criteria, classification systems (two or three-tiered), independent assessment of the capsule, and different outcome metrics.
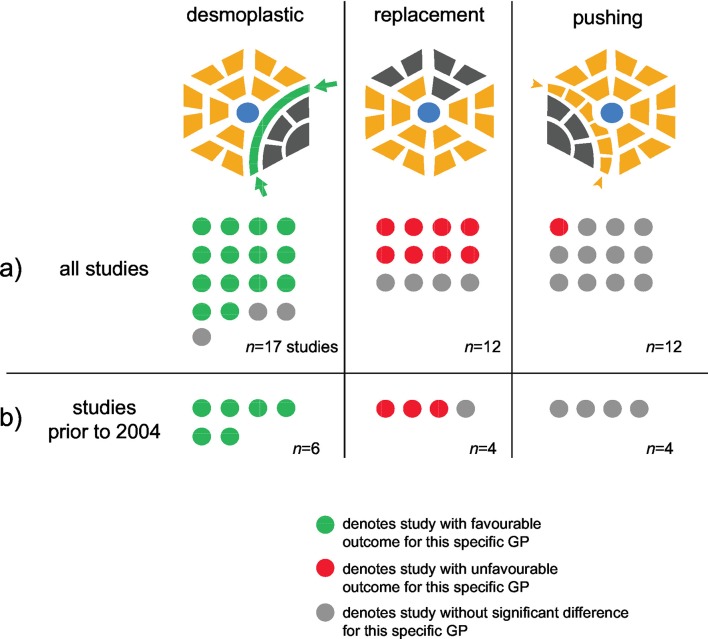
Nevertheless, descriptive analysis (figure 5A) showed that for 14 out of 17 cohorts (82.4%) in which the CRLM growth pattern was assessed, a statistically significant favourable outcome was reported for patients with desmoplastic CRLM. In eight out of 12 cohorts (66.7%) in which the pattern was assessed, a significantly unfavourable outcome for patients with a predominantly replacement-type CRLM was found. No study reported the opposite effects, that is, neither inferior outcome for desmoplastic pattern nor beneficial outcome for replacement pattern CRLM was found in any of the cohorts. Two studies found no significant differences, and one out of 12 studies (8.3%) that considered this pattern showed unfavourable survival for patients with pushing-type CRLM.
Figure 5.
Impact of main growth patterns on prognosis. First column: The desmoplastic pattern is characterised by a rim of fibrotic stroma (green) that separates tumour cells from the surrounding hepatocytes. Second column: In the replacement pattern, tumour cells (grey) invade along the liver cell plates, replacing the hepatocytes (yellow). Third column: In the pushing pattern, the liver plates adjacent to the metastases appear flattened (arrowheads); blue: central veins. (A) Dot plot of all studies reporting outcome for every growth pattern; one dot corresponds to one study; (B) as in (A), but only considering studies published prior to Food and Drug Administration’s (FDA) approval of bevacizumab (indicated by thick line in online supplementary table 2). Note that some studies found effects for more than one pattern.
It has recently been shown that survival of patients with desmoplastic CRLM compared with the replacement type was favourable only in patients receiving the anti-angiogenic antibody bevacizumab, while there was no difference in the cohort that did not receive the drug.27 When focusing on articles published before the approval of bevacizumab by the US Food and Drug Administration (FDA) in 2004,49 50 we found that all six studies that considered the desmoplastic growth pattern reported a significantly better outcome for patients with this pattern, while three out of four studies that considered the replacement-type growth pattern found a significantly poorer survival associated with replacement pattern CRLM (figure 5B); two of these studies found both effects.
Discussion
In the earliest systematic description of CRLM growth patterns identified in this systematic analysis of the literature, Morino et al classified the tumour border of CRLM inspired by previous observations in hepatocellular carcinoma51; the authors distinguished between ‘sinusoidal’ (largely corresponding ‘replacement’, but also used as a term to describe a very rare pattern for CRLM in the consensus guidelines18) and ‘expansive’ (corresponding to ‘pushing’). They assessed ‘capsule formation’ as an independent feature.44 This early description established the morphological framework, and modifications of the initial terminology have been followed in all studies included in this systematic review.
The rationale behind the need for routine reporting of CRLM growth patterns is at least twofold: on one hand, the patterns may be useful for prognostication and may aid treatment decisions27; on the other hand, it is likely that understanding these distinct patterns of metastatic progression will provide important insights into the biological mechanisms that support tumour growth in the liver. To this end, Frentzas et al showed that microvessel co-option, besides providing vascular supply, renders metastases of the replacement type resistant to anti-angiogenic therapy.27 Furthermore, the authors suggested an alternative approach for treating replacement-type metastases by suppressing cancer cell motility, which they achieved experimentally by knocking down the actin-related protein 2/3 complex. It is worth noting that several studies published before FDA approval of anti-angiogenic therapy found a favourable prognosis for patients with desmoplastic-type CRLM, suggesting that there are important mechanistic insights beyond differences in angiogenesis to be gained from the different growth patterns (figure 5B).
The most significant limitation of this systematic review is intrinsic to the studies analysed and highlights a major challenge of the field: Pronounced study heterogeneity—most importantly in classification systems and histopathological definitions as summarised in online supplementary table 1—precluded quantitative synthesis. Although formal meta-analysis was not possible, a high number of studies that assessed outcome concluded that the desmoplastic pattern is associated with a favourable prognosis. This was independent of the era in which the patient cohorts were treated and thus the data indicate that specific treatment regimens cannot sufficiently explain the positive prognostic effect of the desmoplastic pattern. In addition, more than one quarter of all studies found that the replacement pattern was associated with unfavourable prognosis, suggesting that distinguishing between the desmoplastic and replacement patterns and understanding the underlying mechanisms likely is of highest impact for developing prognostic tools and treatment strategies.
bmjgast-2018-000217supp002.docx (149.1KB, docx)
As highlighted by our results, comparable data are essential for future quantification, and following common terminologies as well as growth pattern definitions is warranted. We suggest, in line with the recent consensus guidelines,18 the most frequently used terms, ‘desmoplastic’, ‘replacement’, and ‘pushing’. We hope that this review will contribute to routine pathological reporting of the histological growth patterns in CRLM.
Acknowledgments
MG acknowledges grants from the Swedish Cancer Society and the Ruth and Richard Julin Foundation. The authors are grateful to Gun Brit Knutssøn and Susanne Gustafsson at Karolinska Institutet University Library for their help with the literature search and to Rune Toftgård for comments on the manuscript. The authors thank Tobias Grönlund for help with the growth pattern illustrations and Sarah Lang for editing the manuscript.
Footnotes
Contributors: CFM and MG designed the study, extracted and analysed the data and wrote the manuscript. BB contributed to the conceptualisation of the study, analysed the data upon consultation by CFM and MG and commented on the manuscript.
Funding: This study was funded by the Swedish Cancer Society (Cancerfonden 2014/1376) and the Ruth and Richard Julin Foundation (2016juli46405 and 2015juli44263).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data generated during and/or analysed for the current study are available from the corresponding author (MG) upon reasonable request.
References
- 1. Siegel RL, Miller KD, Jemal A, et al. . CA Cancer J Clin 2016;2016:7–30. [DOI] [PubMed] [Google Scholar]
- 2. O’Connell JB, Maggard MA, Cy K. Staging Colon Cancer Survival Rates With the New American Joint Committee on Cancer. JNCI J Natl Cancer Inst 2004;96:1420–5. [DOI] [PubMed] [Google Scholar]
- 3. Wong SL, Mangu PB, Choti MA, et al. . American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493–508. 10.1200/JCO.2009.23.4450 [DOI] [PubMed] [Google Scholar]
- 4. Riihimäki M, Hemminki A, Sundquist J, et al. . Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765 10.1038/srep29765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nordlinger B, Van Cutsem E, Rougier P, et al. . Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 2007;43:2037–45. 10.1016/j.ejca.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 6. Adam R, de Gramont A, Figueras J, et al. . Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015;41:729–41. 10.1016/j.ctrv.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 7. Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet 1987;1:1303–6. 10.1016/S0140-6736(87)90552-6 [DOI] [PubMed] [Google Scholar]
- 8. Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. . American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000;88:1739–57. [DOI] [PubMed] [Google Scholar]
- 9. Karamitopoulou E, Zlobec I, Koelzer VH, et al. . Tumour border configuration in colorectal cancer: proposal for an alternative scoring system based on the percentage of infiltrating margin. Histopathology 2015;67:464–73. 10.1111/his.12665 [DOI] [PubMed] [Google Scholar]
- 10. Zlobec I, Baker K, Minoo P, et al. . Tumor border configuration added to TNM staging better stratifies stage II colorectal cancer patients into prognostic subgroups. Cancer 2009;115:4021–9. 10.1002/cncr.24450 [DOI] [PubMed] [Google Scholar]
- 11. Nagashima I, Oka T, Hamada C, et al. . Histopathological prognostic factors influencing long-term prognosis after surgical resection for hepatic metastases from colorectal cancer. Am J Gastroenterol 1999;94:739–43. 10.1111/j.1572-0241.1999.00945.x [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen PB, Colpaert C, Salgado R, et al. . Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 2001;195:336–42. 10.1002/path.966 [DOI] [PubMed] [Google Scholar]
- 13. Eefsen RL, Engelholm L, Willemoe GL, et al. . Microvessel density and endothelial cell proliferation levels in colorectal liver metastases from patients given neo-adjuvant cytotoxic chemotherapy and bevacizumab. Int J Cancer 2016;138:1777–84. 10.1002/ijc.29904 [DOI] [PubMed] [Google Scholar]
- 14. Van den Eynden GG, Bird NC, Majeed AW, et al. . The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis 2012;29:541–9. 10.1007/s10585-012-9469-1 [DOI] [PubMed] [Google Scholar]
- 15. Eefsen RL, Vermeulen PB, Christensen IJ, et al. . Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis 2015;32:369–81. 10.1007/s10585-015-9715-4 [DOI] [PubMed] [Google Scholar]
- 16. Nielsen K, Rolff HC, Eefsen RL, et al. . The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol 2014;27:1641–8. 10.1038/modpathol.2014.4 [DOI] [PubMed] [Google Scholar]
- 17. The Royal College of Pathologists. Reporting proforma for liver resection - colorectal cancer metastasis (Appendix C5). https://www.rcpath.org/resourceLibrary/liver-resection-colorectal-cancer-metastasis.html (accessed 8 Jun 2017).
- 18. van Dam PJ, van der Stok EP, Teuwen LA, et al. . International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 2017;117:1427 10.1038/bjc.2017.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhim AD, Oberstein PE, Thomas DH, et al. . Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerling M, Büller NV, Kirn LM, et al. . Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat Commun 2016;7:12 10.1038/ncomms12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mezheyeuski A, Bradic Lindh M, Guren TK, et al. . Survival-associated heterogeneity of marker-defined perivascular cells in colorectal cancer. Oncotarget 2016;7:41 10.18632/oncotarget.9632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isella C, Terrasi A, Bellomo SE, et al. . Stromal contribution to the colorectal cancer transcriptome. Nat Genet 2015;47:312–9. 10.1038/ng.3224 [DOI] [PubMed] [Google Scholar]
- 23. Cunha SI, Bocci M, Lövrot J, et al. . Endothelial ALK1 Is a therapeutic target to block metastatic dissemination of breast cancer. Cancer Res 2015;75:2445–56. 10.1158/0008-5472.CAN-14-3706 [DOI] [PubMed] [Google Scholar]
- 24. Whiting P, Rutjes AW, Reitsma JB, et al. . The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]; Table 8.5.a: The Cochrane Collaboration tool for assessing risk of bias. 2011. http://handbook.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm (accessed 16 Jun 2017).
- 26. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frentzas S, Simoneau E, Bridgeman VL, et al. . Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 2016;22:1294–302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siriwardana PN, Luong TV, Watkins J, et al. . Biological and Prognostic Significance of the Morphological Types and Vascular Patterns in Colorectal Liver Metastases (CRLM): Looking Beyond the Tumor Margin. Medicine 2016;95:e2924 10.1097/MD.0000000000002924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serrablo A, Paliogiannis P, Pulighe F, et al. . Impact of novel histopathological factors on the outcomes of liver surgery for colorectal cancer metastases. Eur J Surg Oncol 2016;42:1268–77. 10.1016/j.ejso.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 30. Brunner SM, Kesselring R, Rubner C, et al. . Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg 2014;101:1681–91. 10.1002/bjs.9627 [DOI] [PubMed] [Google Scholar]
- 31. Pinheiro RS, Herman P, Lupinacci RM, et al. . Tumor growth pattern as predictor of colorectal liver metastasis recurrence. Am J Surg 2014;207:493–8. 10.1016/j.amjsurg.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 32. Wiggans MG, Shahtahmassebi G, Malcolm P, et al. . Extended pathology reporting of resection specimens of colorectal liver metastases: the significance of a tumour pseudocapsule. HPB 2013;15:687–94. 10.1111/hpb.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nyström H, Naredi P, Berglund A, et al. . Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour. Anticancer Res 2012;32:5183–91. [PubMed] [Google Scholar]
- 34. Rajaganeshan R, Jayne DG, Malik HZ, et al. . Biological characteristics and behaviour of putatively curatively resected colorectal liver metastases. Eur J Surg Oncol 2008;34:439–44. 10.1016/j.ejso.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 35. Stessels F, Van den Eynden G, Van der Auwera I, et al. . Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 2004;90:1429–36. 10.1038/sj.bjc.6601727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terayama N, Matsui O, Ueda K, et al. . Peritumoral rim enhancement of liver metastasis: hemodynamics observed on single-level dynamic CT during hepatic arteriography and histopathologic correlation. J Comput Assist Tomogr 2002;26:975–80. 10.1097/00004728-200211000-00021 [DOI] [PubMed] [Google Scholar]
- 37. Yamaguchi J, Komuta K, Matsuzaki S, et al. . Mode of infiltrative growth of colorectal liver metastases is a useful predictor of recurrence after hepatic resection. World J Surg 2002;26:1122–5. 10.1007/s00268-002-6267-y [DOI] [PubMed] [Google Scholar]
- 38. Weber JC, Nakano H, Bachellier P, et al. . Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? Am J Surg 2001;182:81–8. 10.1016/S0002-9610(01)00656-0 [DOI] [PubMed] [Google Scholar]
- 39. Lunevicius R, Nakanishi H, Ito S, et al. . Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer. J Cancer Res Clin Oncol 2001;127:193–9. 10.1007/s004320000199 [DOI] [PubMed] [Google Scholar]
- 40. Okano K, Yamamoto J, Kosuge T, et al. . Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma. Cancer 2000;89:267–75. [DOI] [PubMed] [Google Scholar]
- 41. Ambiru S, Miyazaki M, Isono T, et al. . Hepatic resection for colorectal metastases. Diseases of the Colon & Rectum 1999;42:632–9. 10.1007/BF02234142 [DOI] [PubMed] [Google Scholar]
- 42. Terayama N, Terada T, Nakanuma Y. Histologic growth patterns of metastatic carcinomas of the liver. Jpn J Clin Oncol 1996;26:24–9. 10.1093/oxfordjournals.jjco.a023174 [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto J, Sugihara K, Kosuge T, et al. . Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg 1995;221:74–8. 10.1097/00000658-199501000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morino T, Tanaka J, Tobe T. Clinico-pathological features of liver metastases from colorectal cancer in relation to prognosis. Nihon Geka Hokan 1991;60:154–64. [PubMed] [Google Scholar]
- 45. Nordlinger B, Sorbye H, Glimelius B, et al. . Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–15. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 46. Rajaganeshan R, Prasad R, Guillou PJ, et al. . The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer 2007;96:1112–7. 10.1038/sj.bjc.6603677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. . Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 48. Eefsen RL, Van den Eynden GG, Høyer-Hansen G, et al. . Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol 2012;2012:1–12. 10.1155/2012/907971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hurwitz H, Fehrenbacher L, Novotny W, et al. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 50. Ferrara N, Hillan KJ, Gerber HP, et al. . Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004;3:391–400. 10.1038/nrd1381 [DOI] [PubMed] [Google Scholar]
- 51. Nakashima T, Kojiro M, Kawano Y, et al. . Histologic growth pattern of hepatocellular carcinoma: relationship to orcein (hepatitis B surface antigen)-positive cells in cancer tissue. Hum Pathol 1982;13:563–8. 10.1016/S0046-8177(82)80272-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2018-000217supp001.pdf (907.6KB, pdf)
bmjgast-2018-000217supp002.docx (149.1KB, docx)