Abstract
Introduction
Given that polypharmacy and potentially inappropriate prescribing are common in elderly orthopaedic patients, pharmacist interventions to improve medication practices among this population are important. However, past studies have reported mixed results regarding the effectiveness of pharmacist-led interventions in inpatient elderly care. Furthermore, few randomised controlled trials have evaluated patient-relevant outcomes as a primary endpoint. Therefore, we will evaluate whether a pharmacist-led intervention could reduce readmission of hospitalised elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing.
Methods and analysis
This is an ongoing single-centre, prospective, non-blinded, randomised controlled trial designed to evaluate the superiority of a pharmacist-led intervention for hospitalised elderly patients compared with usual care. The trial will include newly admitted orthopaedic patients 70 years of age and older with polypharmacy or at least one potentially inappropriate prescription, as identified by the screening tool of older people’s prescriptions (STOPP) criteria. Usual care includes medication reconciliation, patient education and monitoring, as well as providing information about discharge medications. Pharmacist interventions, in addition to usual care, include advising the patient’s physician to stop unnecessary or inappropriate medications and start necessary medications. The primary outcome is the 1-year readmission rate. Secondary outcomes are the proportion of patients who undergo emergency department visits and the occurrences of all-cause death, a new fracture, myocardial infarction and ischaemic stroke. The study started in November 2017, and up to approximately 220 patients will be enrolled.
Ethics and dissemination
The protocol was approved by the Medical Ethics Committee of the National Hospital Organization Tochigi Medical Center (No. 29–22). The trial was registered at the University Hospital Medical Information Network (UMIN) clinical registry. The results of this trial will be submitted for publication in a peer-reviewed journal.
Trial registration number
UMIN000029404.
Keywords: clinical pharmacology, geriatric medicine
Strengths and limitations of this study.
This randomised controlled trial will evaluate the effectiveness of pharmacist interventions for hospitalised orthopaedic elderly patients, using patient-relevant outcomes as the primary outcomes.
This is a single-centre study with a small sample size and short-term follow-up.
Orthopaedic patients, who are admitted electively or discharged within less than 7 days after admission, will be excluded.
Orthopaedic patients, who are prescribed fewer than five medications and are taking no potentially inappropriate medications at admission, will be excluded.
Introduction
In recent decades, as the population has aged, polypharmacy and multimorbidities have become more complicated problems among elderly patients.1–3 Polypharmacy in elderly patients is associated with inappropriate prescribing4 and adverse events, such as adverse drug events and death.5 Because adverse drug events are a primary cause of preventable hospital admissions among elderly patients,6 strategies to prevent drug-related events has been proposed in recent decades.7–9 These strategies include deprescribing for polypharmacy9 and reducing potentially inappropriate prescribing and potential prescription omissions.7 8
Polypharmacy and potentially inappropriate prescribing among elderly patients are particularly common in acute care settings compared with primary care settings.10–12 Therefore, it is important to improve the appropriateness of medications used during hospitalisation. In fact, the American College of Emergency Physicians Geriatric Emergency Department Guidelines recommend a multidisciplinary team intervention for all elderly patients who present to the emergency department and are prescribed more than five medications or at least one potentially inappropriate medication, regardless of the presenting complaint.13 Given that physicians are often unaware of adverse drug events,14 15 the role of hospital pharmacists in improving polypharmacy and potentially inappropriate prescribing in hospitalised elderly patients is important. Nonetheless, past studies have reported mixed results regarding the effectiveness of a pharmacist-led intervention in improving the appropriateness of medications in inpatient elderly care. Although pharmacist intervention can improve the appropriateness of medications in hospitalised elderly patients,16 the conclusions of past systematic reviews and meta-analyses have been inconsistent regarding whether patient-relevant outcomes, such as mortality and readmission, were improved by these interventions.17–20 One recent meta-analysis that included seven randomised controlled trials that evaluated the effectiveness of a pharmacist-led intervention in inpatient elderly care also reported little impact of pharmacist interventions on readmission rates.21 However, most trials included in this meta-analysis were considered to have a high risk of bias. Furthermore, only two of the seven randomised controlled trials included in the meta-analysis evaluated patient-relevant outcomes as primary endpoints.22 23 In one of those two trials, a comprehensive pharmacist intervention for hospitalised elderly patients with polypharmacy led to a significant reduction in hospital visits.23 Therefore, it is still too early to conclude that pharmacist-led interventions for hospitalised elderly patients do not improve patient-relevant outcomes. Furthermore, most studies have targeted internal medicine patients, while few studies have ever investigated the effectiveness of pharmacist interventions for elderly patients hospitalised in an orthopaedic ward.21 The prevalence of polypharmacy and potentially inappropriate prescribing is particularly high in elderly orthopaedic patients, and these practices often continue after recovery from a fracture.24 25 Furthermore, polypharmacy is associated with an increased risk of fall and fracture.5 26 Therefore, pharmacist interventions for improving the appropriateness of medications in hospitalised elderly orthopaedic patients may be associated with better patient outcomes compared with other settings. Thus, we will conduct a randomised controlled trial to evaluate whether a pharmacist-led intervention reduces readmission in hospitalised elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing.
Objectives
Primary objective
Our primary objective is to determine whether pharmacist intervention for elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing at admission reduces 1-year readmission rates compared with usual care. Based on a past study,23 we selected a readmission time frame of 1 year for the primary objective.
Secondary objectives
The key secondary objectives are to determine whether pharmacist intervention for elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing at admission reduces patient-relevant outcomes, such as all-cause death, myocardial infarction, ischaemic stroke and any fractures, compared with usual care. Other secondary objectives are to determine whether pharmacist intervention for elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing at admission reduces the total number of medications, potentially inappropriate prescribing and potential prescription omissions.
Literature search and review
We performed a literature search and review of pharmacist interventions in elderly hospitalised orthopaedic patients. We used the terms ‘pharmacist’, ‘polypharmacy’, ‘medication review’ and ‘inappropriate prescribing’ alone and in combination to search the PubMed and Google Scholar databases until 5 August 2017 without limits for the year when the articles were published. We restricted our review to full-text articles published in English or Japanese. We also identified references from the relevant articles. We primarily selected randomised controlled trials, systematic reviews and meta-analyses. We found a recent systematic review regarding the effectiveness of pharmacist-led intervention on patient outcomes in elderly hospitalised patients.21 Based on this systematic review, we designed this trial.
Methods and analysis
Trial design
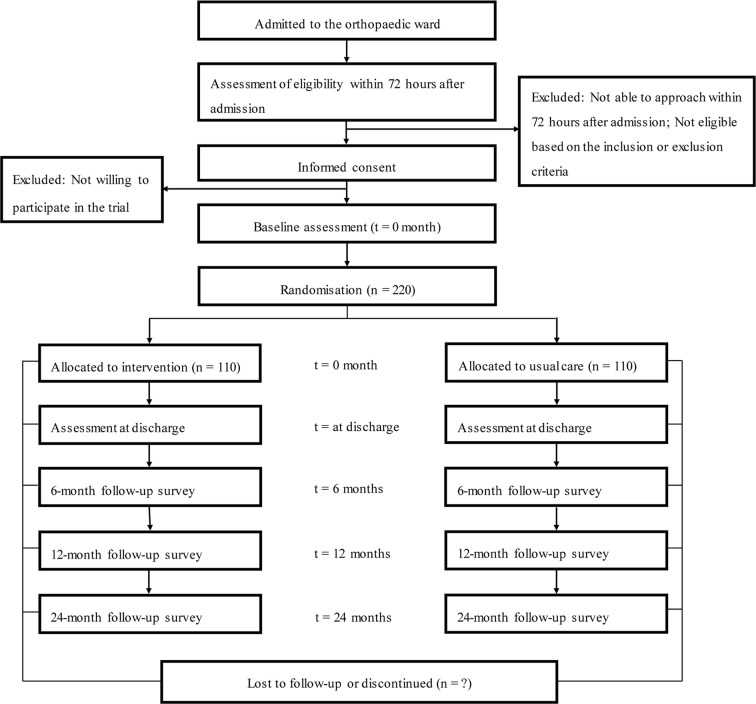
This study is a single-centre, prospective, non-blinded, randomised, controlled, superiority trial with two parallel groups. All participants who provide consent for participation and fulfil the inclusion criteria will be randomly assigned to the pharmacist intervention group or the usual care group with a 1:1 allocation. The study will be conducted in accordance with the Declaration of Helsinki. Standard Protocol Items: Recommendations for Interventional Trials checklist27 was followed in designing the study protocol (online supplementary appendix). Figure 1 summarises the design of the trial, and each of the trial aspects is described in detail below.
Figure 1.
Flow diagram of the participant.
bmjopen-2018-021924supp001.pdf (176.6KB, pdf)
Study setting
This study will be conducted in the orthopaedic ward at the National Hospital Organization Tochigi Medical Center. Our hospital is a 350-bed acute care community hospital and is one of five main hospitals that serve approximately 0.5 million individuals in Utsunomiya in the Tochigi prefecture in Japan.
Eligibility criteria
Eligible patients are those who meet all the following inclusion criteria and who do not have any listed exclusion criteria. Based on the American College of Emergency Physicians Geriatric Emergency Department Guidelines,11 the number of medications taken or the presence of potentially inappropriate prescribing at admission will be used as the inclusion criteria. However, the minimum number of medications for inclusion will be five, based on a past study showing that taking five or more medications was a useful parameter for estimating medication-related adverse effects related to frailty, disability and mortality among men aged 70 years and older.28 As-needed medications will be not be considered in the medication count.
Inclusion criteria
Age 70 years and older.
Polypharmacy (defined as five or more medications) or at least one potentially inappropriate prescription (as defined by the 2015 STOPP criteria8) on admission.
Exclusion criteria
Elective admission.
Inability to contact patient within 72 hours after their admission.
Expected hospital stay duration of <1 week.
Study duration, enrolment and number of sites
The study will be conducted at a single hospital in Japan. The planned sample size is approximately 220 patients. This study began after November 2017. The planned follow-up duration for each patient will be 2 years after the randomisation. Our investigation period is projected to be 3 years. However, unless we can recruit the planned number of patients within 3 years after beginning this study, we will extend the investigation duration to achieve the planned number of patients.
Screening and registration
All elderly patients, who are hospitalised in an orthopaedic ward in our hospital, will be screened for eligibility for the trial by one of three pharmacists (KS, ST or MK) every weekday morning. Patients, who are hospitalised on weekends, will be screened on the following Monday morning. If the screened patients are not eligible, we will document the reason for ineligibility for the trial and the number of ineligible patients. All patients, who fulfil the inclusion criteria and have no exclusion criteria, will be registered by one of three pharmacists in the central data centre at the National Hospital Organization Tochigi Medical Center. Unless written informed consent is provided by the patients, we will document the reasons why the patients did not provide consent to participate in the trial and document the number of patients who declined to participate in the trial.
Randomisation and allocation concealment
All patients who provide consent for participation and who fulfil the inclusion criteria will be randomised. Randomisation will be requested by one of three pharmacists (KS, ST or MK) to the independent randomisation centre at the National Hospital Organization Tochigi Medical Center via webmail. Participants will be randomly assigned to either the pharmacist intervention group or the usual care group. Randomisation will be performed as block randomisation with a 1:1 allocation. The computer-generated random allocation sequence will be provided by an independent staff pharmacist who is not involved in the treatment of patients or with the assessment of patient outcomes. The randomisation will not be stratified. The block sizes will be concealed until the primary outcome is analysed. Throughout the study, the randomisation list will also be concealed until the end of the study.
Blinding
Due to the nature of the intervention, neither the participants nor the clinical pharmacists can be blinded to the allocation. Patients will be informed of the group to which they have been randomly allocated. Assessments regarding the outcomes will be conducted by an assessor who knows the treatment allocation. The analysis regarding the primary outcome will be conducted by independent investigators who are blinded to the treatment allocation and are not involved in the assessment of patient outcomes.
Pharmacist intervention group
Before starting the study, three study pharmacists (KS, ST and MK) were trained during a 3-month period from May 2017 to July 2017. To standardise the intervention by these pharmacists, approximately 16 sessions (1 hour per session) regarding medication use in elderly patients based on the screening tool of older people’s prescriptions (STOPP) and screening tool to alert to right treatment (START) criteria8 were provided by one internal medicine physician (JK). Therefore, these pharmacists will perform the interventions by following the 2015 STOPP/START criteria. However, the use of these criteria for the pharmacist intervention will not be mandatory because some criteria have uncertain applicability to Japanese patients. For example, according to the 2015 START criteria, statin therapy is recommended for patients with a history of cerebral vascular disease unless the patient’s status is end of life or the patient is aged >85 years. However, the effectiveness of statin therapy for patients with ischaemic stroke without dyslipidaemia has not been clearly demonstrated in Japan.29 One of these trained pharmacists (KS, ST or MK) will treat the participants from admission to discharge at the following three stages.
Intervention at admission
A comprehensive list of current medications will be compiled within 72 hours after admission. A drug review will be performed, and advice regarding the following factors will be provided to one of five orthopaedic physicians who care for patients: (1) deprescribing inappropriate or unnecessary medications, (2) starting effective or necessary medications and (3) modifying medication dosages. However, the final decision to adhere to the advice provided by pharmacists will be determined by the orthopaedic physician in charge. Pharmacists will document whether the orthopaedic physicians follow their advice. If the orthopaedic physicians accept the advice but defer action to the primary care physicians, pharmacists will send the discharge summary including their advice to the primary care physicians.
Intervention during hospitalisation
During the hospital stay, patients will be educated about the harms and benefits of their medications. Pharmacists will also provide information about the rationale for medication use and therapeutic goals. Patients will be monitored after starting or stopping medications.
Intervention at discharge
Information about discharge medications (eg, rationale for changes and monitoring needs for newly started or stopped medications) will be summarised in a written document by the pharmacists. Patients will receive discharge counselling with this summary. The summary will also be sent to the primary care physicians and community pharmacists.
Usual care group
Usual care typically includes the same elements as those received by the intervention group but is less extensive. In the usual care group, a comprehensive list of current medications will be compiled by the pharmacists (KS, ST or MK) within 72 hours after admission. Patients will be monitored and educated about newly started medications by their physician and will receive discharge counselling. However, unlike in the intervention group, advice from pharmacists about deprescribing and starting medications will not be provided to the patient’s physician, except in cases of apparent harmful effects of medications that are judged to be symptomatic by pharmacists. Furthermore, pharmacists will neither prepare the summary about discharge medications nor send it to the primary care physicians and community pharmacists. However, at the discretion of the pharmacist providing advice about medications for the physicians, the summary about discharge medications will be prepared. These procedures are the standard practice for pharmacists in most Japanese hospitals.30
Data collection
One of the pharmacists (ST, KS or MK) will collect the demographic and baseline medical information from the patients and/or their caregivers at admission and summarise this information on a patient registration form. Participants will be followed and assessed for 2 years after study entry (table 1). One of the pharmacists (ST, KS or MK) will assess outcomes at discharge. We will survey the participants or their caregivers regarding information about primary and secondary outcomes by sending letters at 6 months, 12 months and 24 months after randomisation. If the participants do not respond to the survey appropriately, we will contact them or their caregivers by telephone to minimise the effect of missing data on study outcomes. Furthermore, to collect more accurate data, we will also use data from electronic medical records of our hospital if the participants are admitted or visit our hospital regularly during the study period.
Table 1.
Time schedule of participant enrolment, interventions and assessments
| Timepoint* | Study period | |||||
| Enrolment | Allocation | Postallocation | ||||
| -t1 | 0 | t1 | t2 | t3 | t4 | |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Allocation | X | |||||
| Interventions | ||||||
| Pharmacist intervention |

|
|||||
| Usual care (control) |

|
|||||
| Assessments | ||||||
| No of medications | X | X | X | X | X | |
| No of PIP† | X | X | X | X | X | |
| No of PPO† | X | X | X | X | X | |
| Adverse drug events | X | |||||
| Discharge destination | X | |||||
| Duration of hospital stay | X | |||||
| All-cause death | X | X | X | X | ||
| Readmission‡ | X | X | X | |||
| ED visit | X | X | X | |||
| Myocardial infarction | X | X | X | X | ||
| Ischaemic stroke | X | X | X | X | ||
| Fracture | X | X | X | X | ||
*-t1, within 72 hours after admission; t1, at discharge; t2, 6 months after randomisation; t3, 12 months after randomisation; t4, 24 months after randomisation.
†PIP and PPO are defined based on the 2015 STOPP/START criteria.
‡Includes all-cause hospitalisation regardless of the cause of hospitalisation.
ED, emergency department; PIP, potentially inappropriate prescribing; PPO, potential prescribing omission.
Outcomes
Primary outcome
The primary outcome is the readmission rate within 1 year after randomisation. The readmission rate is defined as the proportion of participants who are rehospitalised regardless of the cause of hospitalisation (all-cause readmission). Patients who visit an emergency department but are not hospitalised will not be counted. We will evaluate the difference in the readmission rate within 1 year after randomisation between the two treatment groups.
Secondary outcomes
The secondary outcomes are readmission rates within 6 and 24 months after randomisation. We will evaluate the differences in the readmission rates at 6 and 24 months between the two treatment groups. The other secondary outcomes are provided below. These outcomes will be evaluated at discharge and at 6 months, 12 months and 24 months after randomisation. We will evaluate the differences between the two treatment groups regarding these outcomes at discharge, 6 months, 12 months and 24 months.
Statistical analysis
Sample size calculation
We estimated that a sample of 200 patients would provide the study with a power of at least 80% to show a relative risk reduction of 33% for the primary outcome in the intervention group compared with the usual care group (at a two-sided alpha level of 0.05), assuming that the proportion of patients who are readmitted within 1 year is 60% in the usual care group (based on a previous study).23 Assuming that the drop-out rate is 10%, we would need to enrol approximately 220 patients.
Statistical analysis
The baseline characteristics of the study population will be summarised using descriptive statistics. The intervention group will be compared against the usual group for all primary and secondary outcomes (table 2). We will use a χ2 test for binary outcomes and Student’s t-test for continuous outcomes. We will calculate the relative risk and number needed to treat with corresponding 95% CIs to compare dichotomous variables, and the difference in the means will be used for an additional analysis of continuous variables. For all tests, we will use two-sided p values with an alpha <0.05 for the level of significance.
Table 2.
Variables, measures and analysis methods
| Variable/outcome | Hypothesis | Measured outcomes | Methods of analysis |
| Primary | |||
| Readmission* at 12 months | Improvement occurred | Readmission rate % (binary) | χ2 test |
| Secondary | |||
| No of medications at discharge and at 6, 12 and 24 months | Decline occurred | Total no of medications (continuous) | Student’s t-test |
| PIP† at discharge and at 6, 12 and 24 months | Decline occurred | Total no of PIP (continuous) | Student’s t-test |
| Improvement occurred | Proportion of patients who take any PIP % (binary) | χ2 test | |
| PPO† at discharge and at 6, 12 and 24 months | Decline occurred | Total no of PPO (continuous) | Student’s t-test |
| Improvement occurred | Proportion of patients who take any PPO % (binary) | χ2 test | |
| Readmission* at 6 and 24 months | Improvement occurred | Readmission rate % (binary) | χ2 test |
| ED visit at 6, 12 and 24 months | Improvement occurred | Proportion of patients who visit ED % (binary) | χ2 test |
| All-cause death at 6, 12 and 24 months | Improvement occurred | All-cause mortality % (binary) | χ2 test |
| Acute myocardial infarction at 6, 12 and 24 months | Improvement occurred | Proportion of patients whom acute myocardial infarction occurred % (binary) | χ2 test |
| Acute ischaemic stroke at 6, 12 and 24 months | Improvement occurred | Proportion of patients whom acute ischaemic stroke occurred % (binary) | χ2 test |
| Any fractures at 6, 12 and 24 months | Improvement occurred | Proportion of patients whom any fractures occurred % (binary) | χ2 test |
*Includes all-cause hospitalisation regardless of the cause of hospitalisation.
†PIP and PPO are defined based on the 2015 STOPP/START criteria.
ED, emergency department; PIP, potentially inappropriate prescribing; PPO, potential prescribing omission.
Analyses for all outcomes will include all patients who have undergone randomisation and have provided valid informed consent (intention-to-treat population). Regarding the procedure for missing data, we will exclude the data from participants who are lost to follow-up or whose outcomes are missing. These analyses will be performed using IBM SPSS Statistics Base V.21.0 (IBM) or Excel statistical software package V.2.11 (Bellcurve for Excel; Social Survey Research Information, Tokyo, Japan). All analyses will be conducted by investigators who are blinded to the study group allocations.
Data management
The trial data of the study participants will be transmitted to and stored in the research database at National Hospital Organization Tochigi Medical Center. This data will not include the participants’ identifying information. Instead, individual participants and research data will be identified by unique study identification numbers. At the end of the study, the data will be locked. The data will be stored for at least 5 years after study completion. Access to the stored data will be limited to investigators. The data will be stored using codes assigned by the investigators and kept on password-protected computers.
Monitoring
Data monitoring
The risk associated with participation in this study is low, because our aim is to improve the quality of medications in patients. According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects (as of March 2015), our intervention corresponds with a non-invasive procedure. Therefore, we will not need a data monitoring committee. However, an independent staff pharmacist who is not involved with the trial intervention will monitor the data periodically to ensure safety.
Adverse events
In our study, an adverse event will be defined as any undesirable medical occurrence in a participant without regard to the possibility of a causal relationship. Data on adverse events will be collected after the participants have provided consent and enrolled in the study. If a participant experiences an adverse event after the informed consent document is signed and the participant has not yet started to receive the study intervention, the event will be reported as not being related to the study intervention. All adverse events that occur after entry into the study and for 2 years after randomisation will be recorded. A serious adverse event for this study is any undesirable medical occurrence that is believed by the investigators to be causally related to the study intervention and results in any of the following: a life-threatening condition (ie, immediate risk of death) or severe or permanent disability.
Auditing
According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects (as of March 2015), our intervention corresponds with a non-invasive procedure. Furthermore, past studies investigating the effectiveness of a pharmacist intervention have reported few adverse events.16–23 Therefore, we will not need auditing.
Ethics and dissemination
This study protocol was approved by the Medical Ethics Committee of the National Hospital Organization Tochigi Medical Center (Tochigi, Japan). They judged the study design, ethics and safety. Substantial amendments to the study protocol must be approved by the Medical Ethics Committee of the National Hospital Organization Tochigi Medical Center. The trial was registered at the University Hospital Medical Information Network clinical registry on 3 October 2017. We will obtain informed consent from the trial participants or their authorised surrogates according to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects (as of March 2015). One of three pharmacists (ST, KS or MK) will introduce the trial to patients and discuss the trial with all patients using the information sheets about the nature, purpose, and possible risks and benefits of the trial, which was approved by the Medical Ethics Committee of the National Hospital Organization Tochigi Medical Center. Then, the pharmacists will obtain written informed consent from patients willing to participate in the trial. To assure confidentiality, trial participants will be allocated a unique trial identification number throughout the trial. A manuscript with the results of this study will be published in a peer-reviewed journal.
Patient involvement
No patients were involved in determining the research question or outcome measures nor were any patients involved in developing plans to design or implement the study. No patients were involved in evaluating the burden of the intervention. There are no plans to disseminate the results of this research to study participants or the relevant patient community.
Discussion
Given that polypharmacy and potentially inappropriate prescribing among elderly patients are common in acute care settings,10 it is important to improve the appropriateness of medications during hospitalisation. Therefore, the role of hospital pharmacists in improving polypharmacy and potentially inappropriate prescribing in hospitalised elderly patients is important. Nonetheless, there are conflicting results regarding the effectiveness with which pharmacist interventions in elderly inpatient care can improve polypharmacy and potentially inappropriate prescribing to affect patient-relevant outcomes.17–21 Given that few past randomised controlled trials have evaluated a patient-relevant outcome as a primary endpoint,22 23 it is important to conduct a randomised controlled trial to evaluate whether a pharmacist-led intervention improves patient-relevant outcomes, such as readmission and death, in hospitalised elderly orthopaedic patients with polypharmacy or potentially inappropriate prescribing.
There are several limitations to this study. First, the non-blinded study design may overestimate the effectiveness of pharmacist intervention.31 However, due to the nature of the intervention, it is difficult for both participants and clinical pharmacists to be blinded to the allocation. Second, this study is a single-centre trial. Although most past randomised controlled trials were also single-centre trials,21 23 32–35 the external validity of this study is limited. Therefore, an additional randomised controlled trial may be needed. Third, we will exclude elderly orthopaedic patients who are admitted electively or who are taking less than five prescribed medications or have no potentially inappropriate prescriptions. Furthermore, elderly patients admitted to other specialty wards, such as internal medicine or general surgery, will also be excluded. Therefore, it is unclear whether the findings of this trial will be applicable to elderly patients who are admitted electively or to other wards besides the orthopaedic ward. Fourth, medication reconciliation is included in the usual care group in this study. The possible beneficial effect of medical reconciliation for hospitalised patients36 may mitigate the effectiveness of the pharmacist intervention in this study. Finally, we will not assess the cost-effectiveness of the intervention.
Although these limitations are important, this study is one of a few randomised controlled trials to investigate the effectiveness of a pharmacist-led intervention and use a patient-relevant outcome as the primary outcome for hospitalised elderly patients. Given that the burdens of polypharmacy and multimorbidities among elderly patients have increased in recent years, this trial will provide important information on improving the acute care of elderly patients with polypharmacy or potentially inappropriate prescribing.
Supplementary Material
Footnotes
Contributors: KS and JK conceived the project. JK performed the literature search and review. KS, JK, ST and MK designed the study. KS and JK wrote the draft of the protocol for the study. All authors contributed equally to writing the original protocol for this study. KS is the chief investigator of this study. JK wrote the draft of this manuscript. All authors provided final approval for submission of this manuscript for publication consideration.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: This study was approved by the Medical Ethics Committee of the National Hospital Organization Tochigi Medical Center (No. 29–22).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162:2269–76. 10.1001/archinte.162.20.2269 [DOI] [PubMed] [Google Scholar]
- 2. Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002;287:337–44. 10.1001/jama.287.3.337 [DOI] [PubMed] [Google Scholar]
- 3. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases. JAMA 2005;294:716–24. 10.1001/jama.294.6.716 [DOI] [PubMed] [Google Scholar]
- 4. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc 2006;54:1516–23. 10.1111/j.1532-5415.2006.00889.x [DOI] [PubMed] [Google Scholar]
- 5. Fried TR, O’Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014;62:2261–72. 10.1111/jgs.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002;24:46–54. 10.1023/A:1015570104121 [DOI] [PubMed] [Google Scholar]
- 7. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–46. [DOI] [PubMed] [Google Scholar]
- 8. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015;175:827–34. 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 10. Gallagher P, Lang PO, Cherubini A, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 2011;67:1175–88. 10.1007/s00228-011-1061-0 [DOI] [PubMed] [Google Scholar]
- 11. Tommelein E, Mehuys E, Petrovic M, et al. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol 2015;71:1415–27. 10.1007/s00228-015-1954-4 [DOI] [PubMed] [Google Scholar]
- 12. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One 2012;7:e43617 10.1371/journal.pone.0043617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The American College of Emergency Physicians, the American Geriatrics Society, Emergency Nurses Association and the Society for Academic Emergency Medicine. Geriatric emergency department guidelines. Ann Emerg Med 2014;63:e7–e25. [DOI] [PubMed] [Google Scholar]
- 14. Hohl CM, Zed PJ, Brubacher JR, et al. Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann Emerg Med 2010;55:493–502. 10.1016/j.annemergmed.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 15. Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003;348:1556–64. 10.1056/NEJMsa020703 [DOI] [PubMed] [Google Scholar]
- 16. Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists' interventions in secondary care. Age Ageing 2016;45:201–9. 10.1093/ageing/afv190 [DOI] [PubMed] [Google Scholar]
- 17. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med 2006;166:955–64. 10.1001/archinte.166.9.955 [DOI] [PubMed] [Google Scholar]
- 18. Holland R, Desborough J, Goodyer L, et al. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol 2008;65:303–16. 10.1111/j.1365-2125.2007.03071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koshman SL, Charrois TL, Simpson SH, et al. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med 2008;168:687–94. 10.1001/archinte.168.7.687 [DOI] [PubMed] [Google Scholar]
- 20. Renaudin P, Boyer L, Esteve MA, et al. Do pharmacist-led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta-analysis. Br J Clin Pharmacol 2016;82:1660–73. 10.1111/bcp.13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas R, Huntley AL, Mann M, et al. Pharmacist-led interventions to reduce unplanned admissions for older people: a systematic review and meta-analysis of randomised controlled trials. Age Ageing 2014;43:174–87. 10.1093/ageing/aft169 [DOI] [PubMed] [Google Scholar]
- 22. Nazareth I, Burton A, Shulman S, et al. A pharmacy discharge plan for hospitalized elderly patients--a randomized controlled trial. Age Ageing 2001;30:33–40. 10.1093/ageing/30.1.33 [DOI] [PubMed] [Google Scholar]
- 23. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009;169:894–900. 10.1001/archinternmed.2009.71 [DOI] [PubMed] [Google Scholar]
- 24. McMahon CG, Cahir CA, Kenny RA, et al. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing 2014;43:44–50. 10.1093/ageing/aft114 [DOI] [PubMed] [Google Scholar]
- 25. Kragh A, Elmståhl S, Atroshi I. Older adults' medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc 2011;59:863–8. 10.1111/j.1532-5415.2011.03372.x [DOI] [PubMed] [Google Scholar]
- 26. Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010;89:295–9. 10.1097/MD.0b013e3181f15efc [DOI] [PubMed] [Google Scholar]
- 27. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–95. 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 29. Hosomi N, Nagai Y, Kohriyama T, et al. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine 2015;2:1071–8. 10.1016/j.ebiom.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakagawa S, Kume N. Pharmacy Practice in Japan. Can J Hosp Pharm 2017;70:232–42. 10.4212/cjhp.v70i3.1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601–5. 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lisby M, Thomsen A, Nielsen LP, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol 2010;106:422–7. 10.1111/j.1742-7843.2009.00511.x [DOI] [PubMed] [Google Scholar]
- 33. McMullin ST, Hennenfent JA, Ritchie DJ, et al. A prospective, randomized trial to assess the cost impact of pharmacist-initiated interventions. Arch Intern Med 1999;159:2306–9. 10.1001/archinte.159.19.2306 [DOI] [PubMed] [Google Scholar]
- 34. Spinewine A, Swine C, Dhillon S, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc 2007;55:658–65. 10.1111/j.1532-5415.2007.01132.x [DOI] [PubMed] [Google Scholar]
- 35. Lipton HL, Bird JA. The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial. Gerontologist 1994;34:307–15. 10.1093/geront/34.3.307 [DOI] [PubMed] [Google Scholar]
- 36. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6:e010003 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-021924supp001.pdf (176.6KB, pdf)