Abstract
Cytochrome P450 enzymes from the CYP2C subfamily play a prominent role in the metabolic clearance of many drugs. CYP2C enzymes have also been implicated in the metabolism of arachidonic acid to vasoactive epoxyeicosatrienoic acids. CYP2C8, CYP2C9, and CYP2C19 are expressed in the adult liver at significant levels; however, the expression of CYP2C enzymes in extrahepatic tissues such as the brain is less well characterized. Form-specific antibodies to CYP2C9 and CYP2C19 were prepared by affinity purification of antibodies raised to unique peptides. CYP2C9 and CYP2C19 were located in microsomal fractions of all five human brain regions examined, namely the frontal cortex, hippocampus, basal ganglia, amygdala, and cerebellum. Both CYP2C9 and CYP2C19 were detected predominantly within the neuronal soma but with expression extending down axons and dendrites in certain regions. Finally, a comparison of cortex samples from alcoholics and age-matched controls suggested that CYP2C9 expression was increased in alcoholics.
Introduction
Cytochrome P450 (P450) enzymes play a dominant role in the metabolic clearance of drugs and other environmental chemicals. However, it is increasingly apparent that P450s are expressed differentially in extrahepatic tissues in a region-specific fashion that may influence the tissue-specific clearance of drugs. In particular, P450s are expressed in the brain in a highly cell-specific fashion. Localized metabolism of drugs in the brain may have significant implications for the efficacy and side-effect profile of neuroactive medicines. Given the role of CYP2C forms in the metabolism of a number of drugs affecting the central nervous system (Guengerich, 2005), it is of significant interest to characterize their expression in the brain. Moreover, CYP2C enzymes in animals have been proposed to contribute to the regulation of cerebral blood flow via generation of epoxyeicosatrienoic acid metabolites from arachidonic acid (Alkayed et al., 1996; Iliff et al., 2007). Although a few studies have been undertaken to detect CYP2C transcript expression in the human brain (McFayden et al., 1998; Klose et al., 1999; Dauchy et al., 2008; Dutheil et al., 2009), the detection of CYP2C proteins has been limited by the paucity of human brain tissue and form-specific antibodies. This study aimed to characterize the expression of CYP2C9 and CYP2C19 proteins in discrete regions of the human brain.
Materials and Methods
Polyvinylidene fluoride and BioTrace NT nitrocellulose membranes were obtained from Pall Corporation (East Hills, NY). Alexa Fluor 680– and Alexa Fluor 488–labeled goat anti-rabbit IgG antibodies were purchased from Invitrogen (Carlsbad, CA) and IRDye 800-labeled donkey anti-mouse IgG antibody was obtained from Rockland Immunochemicals (Gilbertsville, PA). Mouse anti–human-α-tubulin monoclonal primary antibody was purchased from Sigma (St. Louis, MO). Fluorescence mounting medium (DAKO, Glostrup, Denmark) was used to maintain fluorophore stability.
All work described here was done under protocols approved by the University of Queensland Molecular Biosciences Animal Ethics Committee and the University of Queensland Human Ethics Committee. CYP2C9- and CYP2C19-specific antibodies were raised in rabbit, against two unique His-tagged 13–amino acid sequences for each P450 in regions predicted to be immunogenic: LMKMEKEKHNQPC-HHHHHH and GGNFKKSKYFC-HHHHHH (CYP2C9) and LIKMEKEKQNQQC-HHHHHH and GGNFKKSNYFC-HHHHHH (CYP2C19), respectively (bold type indicates residues that differ from the corresponding peptide in other CYP2C forms). The peptides were conjugated to keyhole limpet hemocyanin then used to immunize two 12-week-old New Zealand white rabbits (150 μg initially using Freund’s complete adjuvant with three subsequent boosters of 75 μg each fortnightly thereafter in Freund’s incomplete adjuvant). A third antibody preparation was raised against recombinant CYP2C18 protein, expressed in Escherichia coli with a C-terminal hexa-His tag, and purified by Ni2+ chelate chromatography as previously described (Cuttle et al., 2000; Shukla et al., 2005). Purified CYP2C18 was used to immunize another pair of rabbits as above but with 100 μg initially followed by three subsequent boosters of 50 μg each fortnightly thereafter. Prior to injection of antigen, 20 ml of preimmune serum was collected from each animal to check background immunogenicity. Animals were exsanguinated 11 weeks after initial immunization, whole blood was collected, IgG fractions were prepared, and antibodies were affinity purified as previously described (Booth Depaz et al., 2013) using bacterial membrane preparations containing recombinant CYP2C enzymes. Isolated antibodies raised against CYP2C9, CYP2C19, or CYP2C18 were tested for specificity to all four recombinant human CYP2C proteins (CYP2C9, CYP2C19, CYP2C8, and CYP2C18) expressed in bacterial membrane fractions (Supplemental Table 1) (Cuttle et al., 2000; Shukla et al., 2005).
Frozen human brain samples were obtained from the New South Wales Tissue Resource Centre. Five brain regions [frontal cortex (Brodmann area 9), anterior hippocampus, basal ganglia, amygdala, and cerebellum] from three different alcohol– and illicit drug–free patient samples (a total of 15 tissue samples) were provided as dissected sections from the right hemisphere. The histologic appearance was reported as normal at both the macroscopic and microscopic levels. Patient histories are given in Supplemental Table 2. Samples used to compare the expression of P450s in the alcoholic and nonalcoholic brain were obtained from 12 different male subjects matched for age (Supplemental Table 3). Brain microsomes were prepared as previously described (Booth Depaz et al., 2013) from approximately 500 mg frozen brain tissue.
Either 10 µg (CYP2C18 antibody) or 20 μg protein (CYP2C9 and CYP2C19 antibodies) was subjected to immunoblotting as previously described (Booth Depaz et al., 2013). β-actin immunoreactivity detected with a mouse anti−β-actin monoclonal primary antibody (used at a dilution of 1:20,000; Sigma) was used as a loading control. Immunoreactive protein was detected with an Odyssey near-infrared imaging system (LI-COR, Lincoln, NE) using Alexa Fluor 680–labeled goat anti-rabbit IgG (1:5000; Invitrogen) and IRDye 800-labeled donkey anti-mouse IgG (1:20,000; Rockland Immunochemicals) secondary antibodies. For quantitation of the alcoholic frontal cortex immunoblots, individual sample optical densities (ODs) were corrected against β-actin immunoreactivity (OD of target protein/OD β-actin) and the corrected measurements were used for statistical analysis. Data were subjected to t test analysis with a 95% confidence interval. Fluorescent immunohistochemistry of paraffin-embedded sections was done as previously described using an Alexa Fluor 488–labeled goat anti-rabbit IgG secondary antibody (Booth Depaz et al., 2013).
Results and Discussion
The affinity-purified CYP2C9 and CYP2C19 peptide antibodies specifically detected their respective target proteins; no cross-reactivity against the other known human CYP2C P450s was observed in immunoblot analyses under the conditions used (Supplemental Fig. 1). By contrast, the antibody raised against recombinant CYP2C18 protein detected all four CYP2C forms tested in this study and is referred to hereafter as the “all-2C” antibody (Supplemental Fig. 1). CYP2C9 and CYP2C19 were both expressed in the microsomal (110,000 × g pellet) fractions of the cortex, hippocampus, amygdala, basal ganglia, and cerebellum of the human brain (Fig. 1, A and B). The all-2C antibody detected CYP2C in microsomal fractions of all five brain regions as well as liver microsomes (Fig. 1C).
Fig. 1.
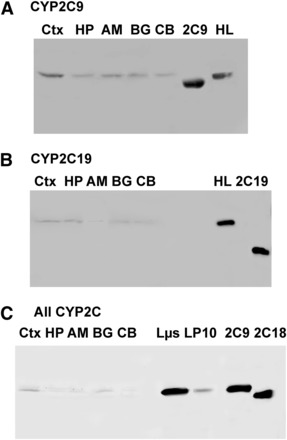
CYP2C protein expression in human brain microsomes. (A–C) Affinity-purified polyclonal antibodies raised against CYP2C9, CYP2C19, and recombinant CYP2C18 (“All CYP2C”) were used to assess CYP2C9 (A), CYP2C19 (B), and total CYP2C expression (C) in the cortex, hippocampus, basal ganglia, amygdala, and cerebellum. For CYP2C9 and CYP2C19 blots, 20 μg protein pooled from the three samples described in Supplemental Table 2 were loaded in each lane. For the blot incubated with the all-2C antibody, 10 μg protein was loaded from one representative brain sample. Recombinant proteins used as standards in (A) and (B) contained truncated N-terminal peptide sequences compared with the full-length, native proteins in liver and brain fractions. Recombinant CYP2C9 and CYP2C18 in (C) were expressed from full-length constructs (Cuttle et al., 2000; Shukla et al., 2005). 2C9, recombinant CYP2C9; 2C18, recombinant CYP2C18; 2C19, recombinant CYP2C19; AM, amygdala; BG, basal ganglia; CB, cerebellum; CTX, cortex; HL, human liver microsome; HP, hippocampus; Lµs, human liver pellet microsomal fraction from centrifugation at 110,000 × g; LP10, human liver pellet fraction from centrifugation at 110,000 × g.
CYP2C9 and CYP2C19 were expressed predominantly in the somatic region of neuronal cells, with expression frequently extending to the axonal hillock (Figs. 2 and 3), and further down axons and dendrites in some brain regions. Both CYP2C9 and CYP2C19 were detected from layer 2 through layer 6 of the frontal cortex, although CYP2C9 was detected to a lesser extent in layer 2 (Fig. 2A). CYP2C9 and CYP2C19 were both located in the hippocampus, where expression of both P450s appeared to be highest in the CA1 and CA3 regions (Fig. 2B). CYP2C9 and CYP2C19 expression was predominantly localized in the soma of neurons in the amygdala, but both somatic and axonal expression was observed in the basal ganglia. Both forms were localized to the pyramidal cells and the granular cell layer of the cerebellar cortex (Fig. 3).
Fig. 2.
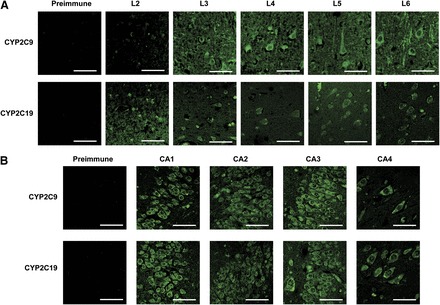
(A and B) Immunohistochemical detection of CYP2C9 and CYP2C19 expression in the human frontal cortex (A) and hippocampus (B). Negative controls incubated with preimmune sera for CYP2C9 and CYP2C19 were performed with layers 5 and 6 (A) and CA4 (B), respectively, and were representative of other layers. (A) CYP2C9 and CYP2C19 proteins were detected in layer 2 through layer 6 of the frontal cortex, predominantly in the soma of neuronal cells. Expression of CYP2C9 was also observed in neuronal axons and dendrites, predominantly in layers 5 and 6. (B) CYP2C9 and CYP2C19 were both located throughout the hippocampus; however, expression appeared to be highest in the CA1 region in both cases. In addition, CYP2C9 was also highly expressed in the CA3 region. Bar, 50 μm.
Fig. 3.
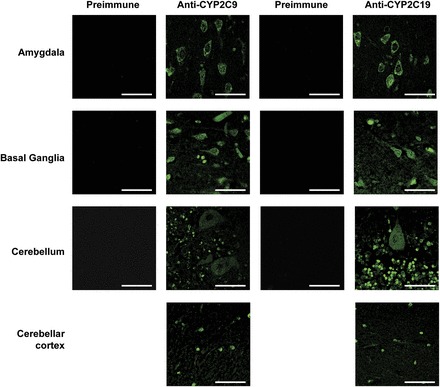
Immunohistochemical detection of CYP2C9 and CYP2C19 expression in the human amygdala, basal ganglia, and cerebellum. Negative controls incubated with preimmune sera for CYP2C9 and CYP2C19 were performed with the molecular layer of the cerebellum but were representative of other areas. Both P450s were expressed in the amygdala, basal ganglia, and cerebellum. In the amygdala, expression was predominantly localized in the soma of neurons, whereas both somatic and axonal expression of both CYP2C9 and CYP2C19 was observed in the basal ganglia. Expression of both CYP2C9 and CYP2C19 was localized to the pyramidal cells and the granular cell layer of the cerebellar cortex. Bar, 50 μm.
To the best of our knowledge, this is the first study to characterize the expression of individual CYP2C forms at the protein level in the human brain; however, several groups have reported CYP2C mRNA in the brain. McFadyen et al. (1998) detected CYP2C mRNA in the frontal and temporal cortex, basal ganglia, midbrain, and cerebellum but not in the pons or medulla, by PCR using primers designed to amplify all CYP2C forms, subsequently identifying the product as CYP2C8. Klose et al. (1999) found both CYP2C8 and CYP2C18 but not CYP2C9 or CYP2C19 mRNA in the whole brain. Dauchy et al. (2008, 2009) also found CYP2C8 in the cortex and microvessels, and both CYP2C8 and CYP2C18 in a cerebral microvascular endothelial cell line. Dutheil et al. (2009) found sufficient CYP2C8 expression to quantify in the total human brain, whereas CYP2C9 was detected but not quantifiable, and neither CYP2C18 nor CYP2C19 was detected. In addition, CYP2C9 was observed in several different types of brain tumor (Knüpfer et al., 1999). Finally, data from vast transcriptomic studies reported in the GeneCards database (http://www.genecards.org/, accessed in November 2014) suggest that all CYP2C transcripts might be expressed, to some extent, in several human brain regions; however, the specificity and tissue source for those data are unclear, and thus may not be directly comparable to those obtained in our study and those cited above.
There is also evidence that specific CYP2C forms are expressed in the rodent brain, including the cortex and hippocampus (Alkayed et al., 1996; Luo et al., 1998; Riedl et al., 2000; Iliff et al., 2007). CYP2C11 expressed in astrocytes and perivascular neurons was proposed to be an arachidonic acid epoxygenase involved in the regulation of cerebral blood flow in rats (Alkayed et al., 1996; Iliff et al., 2007). Human CYP2C family enzymes may play a similar role in regulating cerebral blood flow (Gervasini et al., 2004); CYP2C8 and CYP2C9 were shown to have arachidonic acid epoxygenase activity (Rifkind et al., 1995) and CYP2C9 has been suggested to regulate blood flow in skeletal muscle during exercise (Hillig et al., 2003).
The fact that CYP2C9 and CYP2C19 protein were clearly detected in specific cell types across several brain regions in this study contrasts with the limited detection of mRNA for these two forms in previous studies. Although the affinity-purified antibodies appeared specific for the cognate antigens when tested on immunoblots, we cannot exclude the possibility that the antibodies are detecting an altered CYP2C antigen in the immunohistochemical analyses. However, a BLAST search failed to find any proteins other than CYP2C enzymes with the relevant linear epitopes, so any non-CYP2C proteins detected would represent conformational mimics, which is an unlikely, but not impossible, prospect. Alternatively, the P450 proteins, or at least peptide antigens derived therefrom, may be more resistant to degradation during the postmortem delay compared with mRNA. Significantly, both CYP2C18 and CYP2C19 were expressed in the brain at the mRNA level in mice transgenic for these two genes (Löfgren et al., 2008). Moreover, in the liver, P450 protein expression correlates with mRNA transcript levels for only some P450s (Ohtsuki et al., 2012).
CYP2C9 expression was higher in the microsomal fractions of the frontal cortex of brains from individuals with a history of alcohol abuse than in age- and sex-matched controls (Fig. 4A; P < 0.05, 95% confidence interval). By contrast, no difference was observed in the expression of CYP2C19 in the microsomal fractions between brains from alcoholics and controls (Fig. 4B). CYP2C9 expression does not appear to be regulated by alcohol exposure in liver (Guengerich, 2005); however, other reports have shown that the regulation of other P450s differs both quantitatively and qualitatively between the liver and brain (Hesse et al., 2004), so this statistically significant association, albeit of small effect size, merits further exploration.
Fig. 4.
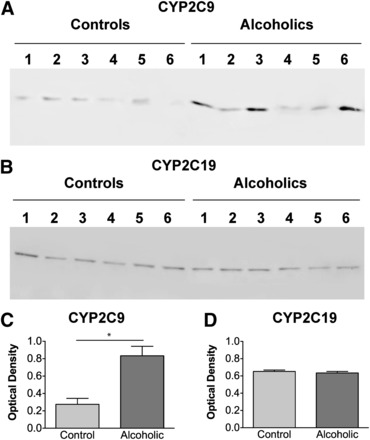
(A and B) Immunoblot of microsomal fractions (pellets from centrifugation at 110,000 × g) of the frontal cortex of six individual control and six individual alcoholic case-matched human brain samples using antibodies detecting CYP2C9 (A) and CYP2C19 (B). An equivalent amount (20 μg) of total protein was loaded in each lane. (C) Individual sample densities were corrected against beta-actin and the corrected data were subjected to a t test with a 95% confidence interval. Data are expressed as mean ± S.E.M. The asterisk indicates that CYP2C9 expression in samples from alcoholics was significantly elevated over expression in controls (n = 6; P < 0.05, 95% confidence interval). No significant differences were seen in CYP2C19 expression between samples from alcoholics and controls.
Acknowledgments
The authors thank the donors and their next of kin as well as the neuropathologists from the New South Wales Tissue Resource Centre for providing the tissue samples used in this study. The authors also thank Dr. P. Beaune (Rene Descartes University) and Dr. F.J. Gonzalez (National Institutes of Health) for donation of the CYP2C18 and CYP2C8 cDNAs, as well as Dr. Peter Dodd for helpful comments.
Abbreviations
- OD
optical density
- P450
cytochrome P450
Authorship Contributions
Participated in research design: Booth Depaz, Wilce, Gillam.
Conducted experiments: Booth Depaz, Toselli.
Contributed new reagents or analytic tools: Wilce.
Performed data analysis: Booth Depaz, Toselli, Gillam.
Wrote or contributed to the writing of the manuscript: Booth Depaz, Toselli, Gillam.
Footnotes
This research was supported by the Australian National Health and Medical Research Council (NHMRC) [Project Grant 210215]. The New South Wales Tissue Resource Centre and Australian Brain Donor Program are supported by the University of Sydney, the NHMRC [Grant 401551], the Schizophrenia Research Institute, the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [R24 Grant AA12404], and the New South Wales Department of Health.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. (1996) Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol 271:H1541–H1546. [DOI] [PubMed] [Google Scholar]
- Booth Depaz IM, Toselli F, Wilce PA, Gillam EM. (2013) Differential expression of human cytochrome P450 enzymes from the CYP3A subfamily in the brains of alcoholic subjects and drug-free controls. Drug Metab Dispos 41:1187–1194. [DOI] [PubMed] [Google Scholar]
- Cuttle L, Munns AJ, Hogg NA, Scott JR, Hooper WD, Dickinson RG, Gillam EMJ. (2000) Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab Dispos 28:945–950. [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Declèves X. (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 107:1518–1528. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud P-O, Weaver RJ, Weksler B, Romero I-A, Scherrmann J-M, De Waziers I, Decleves X. (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 77:897–909. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottée L, Bièche I, Ingelman-Sundberg M, Flinois JP, de Waziers I, et al. (2009) Xenobiotic-metabolizing enzymes and transporters in the normal human brain: regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab Dispos 37:1528–1538. [DOI] [PubMed] [Google Scholar]
- Gervasini G, Carrillo JA, Benitez J. (2004) Potential role of cerebral cytochrome P450 in clinical pharmacokinetics: modulation by endogenous compounds. Clin Pharmacokinet 43:693–706. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (2005) Human cytochrome P450 enzymes, in Cytochrome P450 (Ortiz de Montellano PR. ed) pp 377–530, Kluwer Academic/Plenum Publishing, New York. [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. (2004) Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. (2003) Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol 546:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Close LN, Selden NR, Alkayed NJ. (2007) A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92:653–658. [DOI] [PubMed] [Google Scholar]
- Klose TS, Blaisdell JA, Goldstein JA. (1999) Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol 13:289–295. [DOI] [PubMed] [Google Scholar]
- Knüpfer H, Knüpfer MM, Hotfilder M, Preiss R. (1999) P450-expression in brain tumors. Oncol Res 11:523–528. [PubMed] [Google Scholar]
- Löfgren S, Baldwin RM, Hiratsuka M, Lindqvist A, Carlberg A, Sim SC, Schülke M, Snait M, Edenro A, Fransson-Steen R, et al. (2008) Generation of mice transgenic for human CYP2C18 and CYP2C19: characterization of the sexually dimorphic gene and enzyme expression. Drug Metab Dispos 36:955–962. [DOI] [PubMed] [Google Scholar]
- Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA. (1998) Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch Biochem Biophys 357:45–57. [DOI] [PubMed] [Google Scholar]
- McFadyen MC, Melvin WT, Murray GI. (1998) Regional distribution of individual forms of cytochrome P450 mRNA in normal adult human brain. Biochem Pharmacol 55:825–830. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, et al. (2012) Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab Dispos 40:83–92. [DOI] [PubMed] [Google Scholar]
- Riedl AG, Watts PM, Douek DC, Edwards RJ, Boobis AR, Rose S, Jenner P. (2000) Expression and distribution of CYP2C enzymes in rat basal ganglia. Synapse 38:392–402. [DOI] [PubMed] [Google Scholar]
- Rifkind AB, Lee C, Chang TKH, Waxman DJ. (1995) Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys 320:380–389. [DOI] [PubMed] [Google Scholar]
- Shukla A, Gillam EM, Mitchell DJ, Bernhardt PV. (2005) Direct electrochemistry of enzymes from the cytochrome P450 2C family. Electrochem Commun 7:437–442. [Google Scholar]


