Abstract
Alopecia areata (AA) is a common hair loss disorder worldwide with characteristic exclamation mark hairs. Although AA is self-limited, it can last for several months or even years in some patients. Currently, there is no US Food and Drug Administration-approved treatment for AA. Many off-label treatments are available but with limited efficacy. Through a better understanding of molecular biology, many targeted therapies have emerged as new alternatives for various autoimmune diseases. Various janus kinase (JAK) and signal transducer and activator of transcription (STAT) proteins form signaling pathways, which transmit extracellular cytokine signals to the nucleus and induce DNA transcriptions. By inhibiting JAK, T-cell-mediated inflammatory responses are suppressed. Increasing evidence suggests that JAK inhibitors (JAKis) are effective in the treatment of many autoimmune diseases, including AA. Among these, several studies on tofacitinib, ruxolitinib, and baricitinib in AA had been published, demonstrating promising outcomes of these agents. Unlike oral formulations, efficacy of topical forms of tofacitinib and ruxolitinib reported in these studies is still unsatisfactory and requires improvement. This review aims to summarize evidence of the efficacy and safety of JAKis in the treatment of AA.
Keywords: baricitinib, JAK, JAK inhibitors, JAK-STAT pathway, ruxolitinib, tofacitinib
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by a nonscarring patch or patches of hair loss with characteristic exclamation mark hairs. AA is a relatively common disease with a worldwide prevalence of 0.1%–0.2%.1 It is found to be associated with atopic dermatitis,2 vitiligo, systemic lupus erythematosus, and autoimmune thyroid diseases.3,4 Other than autoimmune factors, AA is thought to be driven by genetic predisposition. There have been several studies suggesting genetic background with familial incidence of 7%–18% depending on the type of AA.5,6 Evidence, of its prevalence in the population of about 2%, concordance in twins, a Gaussian distribution of severity, a 10-fold increased risk of first-degree relatives of affected individuals, and the aggregation of affected individuals in families with no clear Mendelian pattern of inheritance, suggests that AA fits a complex or multifactorial genetic pattern.7 Furthermore, AA is found to be associated with several human leukocyte antigens, such as DQ3, DR4, DR11, and DQ7.8 Currently, there is no United State Food and Drug Administration (FDA)-approved treatment for AA. Many off-label treatments are available but with limited efficacy. Thus, there is still room for new alternatives for the treatment of AA. Increasing evidence suggests that JAK inhibitors (JAKis) are effective in the treatment of AA. This review aims to summarize evidence on the efficacy and safety of JAKis, in the hope of improving the understanding and treatment of AA, and to suggest future directions in which JAKis may be promising candidates for the treatment of other hair loss disorders.
Pathogenesis of AA
Hair follicles are immune-privileged sites with complex and intricate structures to maintain their immunity against the body immune system. The key features of an immune-privileged site are low major histocompatibility complex (MHC) class I and II expression and well-suppressed natural killer (NK) cells.9 Disruption of the system, namely upregulation of MHC class I or UL16-binding protein 3 (ULBP3) molecules10 or defect in NK cell inhibition or containment, results in loss of immune privilege and ultimately causes AA.11 ULBP3 was identified as an important factor in AA pathogenesis by various genome-wide association studies. Its overexpression leads to the attack of cytotoxic cluster of differentiation 8-positive (CD8+) NK group 2D-positive (NKG2D+) T cells to the hair follicles.12,13 Hair follicles that lose their immune privilege during anagen phase become the target of CD8+ T cells and NKG2D+ cells.14 This hypothesis is supported by the findings of CD8+ T cells and NKG2D+ cells around the peribulbar area of the affected hair follicles.11,15 Concurrently, marked interferon (IFN)-γ response and upregulation of several γ-chain (γc) cytokines, including interleukin (IL)-2, IL-7, IL-15, and IL-21, and IFN-γ elements, promote activation and survival of IFN-γ-producing CD8+ NKG2D+ T cells and contribute to immune privilege collapse of hair follicles.10 These events ultimately lead to hair follicle dystrophy and accelerate hair follicles into catagen phase.16
JAK-STAT signaling pathway and its role in AA
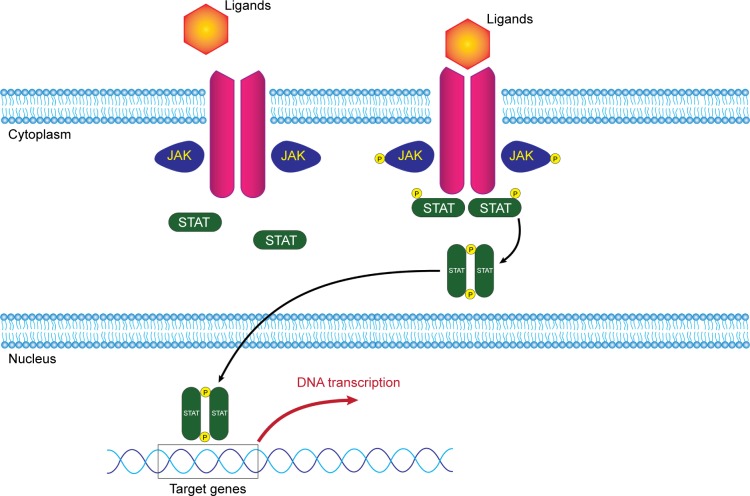
JAK-STAT signaling pathway consists mainly of three components: receptor, janus kinase (JAK), and signal transducer and activator of transcription (STAT) (Figure 1). The receptor, on the cell surface, binds to specific ligands, such as IFNs, ILs, and various other cytokines and hormones. JAK is a member of tyrosine kinase family, which consists of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). JAK, after receiving signal by the ligands, phosphorylates its own tyrosine component to activate its kinase function, which in turn phosphorylates STAT component. Phosphorylation of the STAT component dimerizes and activates STATs. The activated STATs then will, in turn, translocate to the DNA in the nucleus and promote transcription of a specific region of the DNA, leading to gene expression (Figure 1). This fundamental process of gene expression mediates cellular processes through activation of cytokines. Function of the JAK-STAT signaling pathway was first discovered as a pathway for IFN signaling.17–19 Subsequently, a large number of cytokines, particularly γc cytokines, have been found to activate the JAK-STAT pathway, leading to a myriad of gene expression.20 JAK-STAT pathway is vital in maintaining innate and adaptive immunity. Any defect in JAK component results in certain hematologic or immune-related diseases, such as myeloproliferative neoplasms or severe combined immunodeficiency.21,22
Figure 1.
Illustration of JAK-STAT signaling pathway.
Notes: Specific ligands bind to their corresponding receptors and activate JAK component. JAK phosphorylates its own tyrosine component to activate its kinase function which in turn phosphorylates STAT component. Activated STATs translocate to promote transcription of DNA in the nucleus.
Abbreviations: JAK, janus kinase; STAT, signal transducer and activator of transcription.
Given a crucial role that JAK-STAT pathway plays in mediating the CD8+ NKG2D+T cell reaction, which is a component of AA pathogenesis, JAKis seem to be an appealing option for the treatment of AA. Moreover, inhibition of this pathway results in promotion of hair growth cycle, which increases effectiveness of hair loss treatment.10 Figure 2 demonstrates the interaction between JAK-STAT pathway, in pathogenesis of AA, and JAKis. Further information regarding the relationship between JAK-STAT pathway and hair growth cycle, as well as JAKis and AA, is discussed in the next sections.
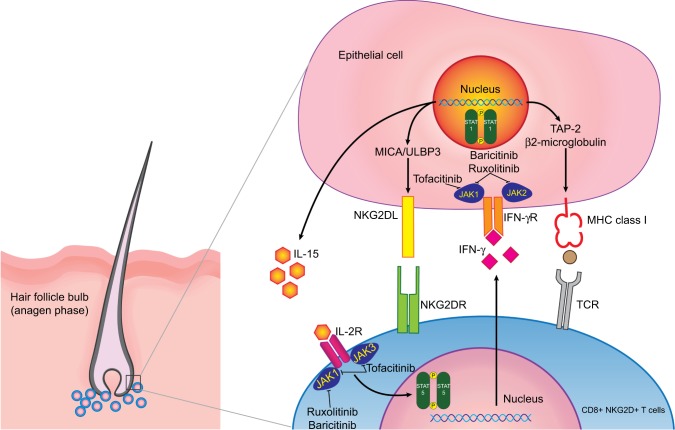
Figure 2.
Interaction between follicular epithelial cells and CD8+ NKG2D+T cells.
Notes: CD8+ NKG2D+T cells form immune synapses with follicular epithelial cells and in turn upregulate MHC class I expression through JAK1 and JAK2. Concurrently, NKG2D ligands (NKG2DL), such as MICA and ULBP-3, are also upregulated through JAK1 and JAK3. Activated CD8+ NKG2D+T cells release IFN-γ that binds to its receptor on follicular epithelial cells, causing transition into catagen phase. This also causes follicular epithelial cells to promote the production of IL-15 through JAK1 and JAK2. IL-15 in turn binds to its receptor on CD8+ T cells and induces JAK1- and JAK3-mediated IFN-γ production and ultimately completes the feedback loop. Tofacitinib mainly inhibits JAK1 and JAK3, while ruxolitinib predominantly inhibits JAK1 and JAK2. Lastly, baricitinib selectively inhibits JAK1 and JAK2. These inhibitions interfere with the feedback loop and alleviate AA.
Abbreviations: IFN, interferon; IL, interleukin; JAK, janus kinase; MHC, major histocompatibility complex; NK, natural killer; STAT, signal transducer and activator of transcription; TAP-2, transporter associated with antigen processing-2; TCR, T-cell receptor.
JAK and hair growth cycle
In terms of hair growth, key genes in the JAK-STAT pathway including Stat5A/B, Stat3, Jak1, Jak3, and Socs2/3 were highly expressed in catagen and telogen phases but suppressed in early anagen phase.23 IL-6 and oncostatin M (OSM), which signal via JAK-STAT pathway, have been shown to play a role in hair growth regulation. Overexpression of IL-6 in keratinocytes in mice results in hair growth retardation.24 IL-6 is also found to be more prominent in balding dermal papilla compared with nonbalding dermal papilla. The same study also showed that injection of recombinant IL-6 into anagen skin can induce premature onset catagen phase.25 Finally, IL-6 and OSM were found to inhibit hair shaft elongation in the human organ culture model.25,26 Anagen extension and hair regrowth were found in mice receiving tofacitinib, a JAKi. The study also proved that, after inhibiting JAK-STAT pathway, vascular endothelial growth factor is upregulated, resulting in angiogenesis. This suggests the role of JAK in hair growth.27 Harel et al showed that inhibiting JAK-STAT pathway promotes hair growth by stimulating the activation and/or proliferation of hair follicle stem cells and other unknown mechanisms.23 It was also shown that suppression of JAK signaling activates an antiquiescence signal during telogen phase and accelerates reentry into anagen phase in mice. However, no study was able to establish the same effect on human hair follicles.
JAKis and AA
Over the past few years, various JAKis have been reported to have promising efficacy in various autoimmune disorders, such as rheumatoid arthritis28 and psoriasis,29 and myeloproliferative disorders, such as myelofibrosis or polycythemia vera.30 In the same manner, AA was also found to be responsive to JAKi treatment. Several studies had helped bring light to the mechanism of JAKis in stimulating hair growth in AA. Overexpression of JAK3 and, to a lesser extent, JAK1 and JAK2 was observed in skin biopsy specimens of patients with AA.31 In terms of hair growth in AA, a two-step mechanism needs to be fulfilled.32 First, T-cell-mediated immune response on the hair follicle must be terminated. Xing et al demonstrated that the involvement of γc cytokine and receptor family members in AA and JAKis blocked the downstream signal of such cytokines.10 JAKis also disrupt the production of inflammatory T helper (Th) 17 cells and Th1 and Th2 differentiation (Figure 2).33 Second, anagen phase must be reinstated. Restoration of anagen phase of the hair follicle by JAK inhibition has been discussed previously in this article (see JAK and hair growth cycle). Currently, there are three medications that have been reported in various trials for the treatment of AA. Each of which is reviewed in this article.
Tofacitinib
Tofacitinib (CP-690,550, formerly tasocitinib) is the first of the JAKi family. Its chemical formula is C16H20N6O (Figure 3).34 It selectively inhibits JAK1- and JAK3-dependent STAT activation over JAK2, with minimal effects on TYK2 pathway.35 Tofacitinib blocks STAT phosphorylation induced by IFN-γ, IL-2, IL4, IL-7, IL-15, and IL-21, which clearly affects the signaling pathway downstream of JAK1- and JAK3-dependent γc receptors in both mice and humans. IL-12 signaling, which depends on JAK2 and TYK2, is blocked for STAT1 activation but only mildly suppressed for STAT4.36 Additionally, anti-inflammatory effects of tofacitinib have also been described in some studies.27,33,36
Figure 3.
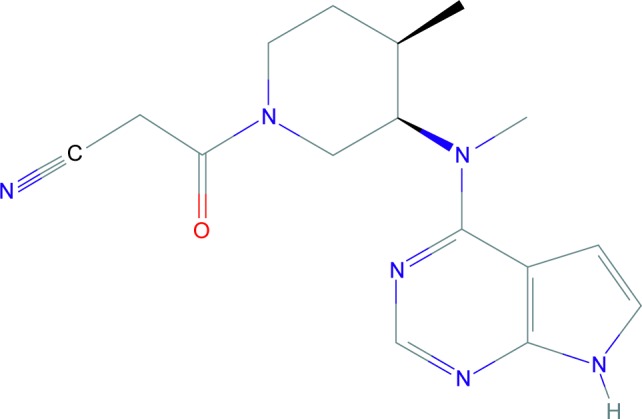
Tofacitinib.
Efficacy of tofacitinib in AA was first reported by Craiglow and King in 2014.37 A 25-year-old male patient with psoriasis and, coincidentally, alopecia universalis (AU) was treated with oral tofacitinib, showing improvement in both psoriasis and AU. Full regrowth of hair at all body sites was observed after 8 months of therapy with 15 mg per day of oral tofacitinib. Since then, several clinical studies on adolescent and adult patients have been published (Table 1).37–58 These cases were mostly diagnosed with AU and some with AA. Most of the cases were also unresponsive to their previous treatments, including various regimens of corticosteroid, cyclosporine, and/or methotrexate. In a 38-year-old male with AU and nail dystrophy associated with AA, total hair regrowth and normalization of nails were observed after 10 months of treatment with oral tofacitinib 5 mg twice daily.43 A case report of a 40-year-old woman with moderate-to-severe AA demonstrated almost complete regrowth of hair after 4 months of treatment with oral tofacitinib 5 mg twice daily. The same study also found that initial elevation of CXCL10 (an IFN-induced chemokine), IFN, and cytotoxic T lymphocyte signatures was decreased after 4 weeks of treatment. However, cessation of tofacitinib resulted in near-complete hair loss.44
Table 1.
Characteristics of clinical studies of tofacitinib in the treatment of AA
| Authors | Year | Study design | Patients | Indication | JAK inhibitor (dose) | Outcome | Side effects | |
|---|---|---|---|---|---|---|---|---|
| 1 | Craiglow and King37 | 2014 | Case report | 1 | Alopecia universalis and plaque-type psoriasis | Oral tofacitinib (15 mg daily) | • Patient experienced full regrowth in 8 months | – |
| 2 | Kennedy Crispin et al38 | 2016 | Open-label, single-arm trial | 66 | Alopecia areata and variants | Oral tofacitinib (5 mg BID) | • 64% of patients responded to treatment • 32% of patients had SALT reduced ≥50% in 3 months • All patients experienced hair loss in 8.5 weeks after cessation |
• Grade I and II infections (17): eg, URI (11), UTI (2), zoster (1) • Headache (5), abdominal pain (5), acne (5), diarrhea (4), fatigue (4), hot flashes (3), pruritus (2), folliculitis (2), numbness (2), cough (1), nausea (1), amenorrhea (1), dry eyes (1), weight gain (1), AST/ ALT elevation (1) |
| 3 | Liu et al39 | 2017 | Retrospective study | 90 | Alopecia areata and variants | Oral tofacitinib (5, >5 mg BID with and without prednisone) | • 20% (13) of patients were complete responders (>90% reduction in SALT), median 15 months • 38.4% (25) of patients were intermediate responders (51%–90% reduction in SALT), median 14 months • 18.5% (12) of patient were moderate responders (6%–50% reduction in SALT), median 11 months • 23.1% (15) of patients were nonresponders (<5% reduction in SALT), median 7 months |
• Grade I and II infections (35): eg, URI (26), UTI (3), zoster (2) • AST/ALT elevation (1), transient • Leukopenia (1), transient • Increased TG (6) • Increased LDL (15) |
| 4 | Gupta et al40 | 2016 | Case series | 2 | Alopecia universalis | Oral tofacitinib (5 mg BID) | • Hair growth was observed in 1 and 3 months • Both patients had full regrowth in 8 months |
• Viral infection and fatigue (1) |
| 5 | Dhayalan et al41 | 2016 | Case series | 3 | Alopecia universalis and nail dystrophy associated with alopecia areata | Oral tofacitinib (5 mg BID) | • All patients experienced remission of nail change within 5–6 months • Two patients experienced hair growth |
– |
| 6 | Anzengruber et al42 | 2016 | Case report | 1 | Alopecia universalis | Oral tofacitinib (5 mg BID) | • Patient had terminal hair growth after 3 months but returned to baseline in 1 month | – |
| 7 | Ferreira et al43 | 2016 | Case report | 1 | Alopecia universalis and nail dystrophy associated with alopecia areata | Oral tofacitinib (5 mg BID) | • Patient had total hair regrowth and normalization of nails in 10 months | – |
| 8 | Jabbari et al44 | 2016 | Case report | 1 | Alopecia universalis | Oral tofacitinib (5 mg BID) | • Patient had almost complete regrowth in 4 months • Patient had near-complete hair loss after cessation • CXCL10, IFN-γ in tissue and ALADIN score decreased at 4 weeks |
– |
| 9 | Mrowietz et al45 | 2017 | Case report | 1 | Alopecia universalis, plaque-type psoriasis and psoriatic arthritis | Oral tofacitinib (10, 15 mg daily) | • Patient had full regrowth in 6 months • Psoriatic arthritis also resolved • Patient developed new psoriatic plaque refractory to tofacitinib |
• Herpes zoster (1) |
| 10 | Liu et al46 | 2018 | Open-label, pilot, single- arm trial | 10 | Alopecia areata | Topical 2% tofacitinib (BID) | • One patient had excellent regrowth • Two patients had partial regrowth • Seven patients showed no regrowth |
• Scalp irritation (4) (40%) • Folliculitis (1) (10%) • Minimal elevation of total cholesterol (4) (40%) |
| 11 | Craiglow et al47 | 2017 | Retrospective study | 13 | Alopecia areata and variants | Oral tofacitinib (10, 15 mg daily) | • Nine patients experienced significant hair regrowth • Median % SALT changed was 93% (mean 61%), mean duration was 6.5 months |
• Headache (3) • URI (4) • Transient elevation of liver transaminase (4) |
| 12 | Ibrahim et al48 | 2017 | Retrospective study | 13 | Alopecia areata and variants | Oral tofacitinib (10, 15, 20 mg daily) | • Seven patients achieved regrowth ≥50%, mean duration 4.2 months • Two patients experienced hair loss back to baseline after 2 weeks of discontinuation |
• Morbilliform eruption and peripheral edema leads to medication withdrawal (1) • Lipid and liver abnormalities, resolved with dose reduction (2) |
| 13 | Park et al49 | 2017 | Retrospective study | 32 | Alopecia areata and variants | Oral tofacitinib (various doses) | • Six patients had 5%–50% regrowth, median duration was 7 months • Nine patients had 50%–90% regrowth, median duration was 10 months • Nine patients had >90% regrowth, median duration was 10 months • Eight patients showed no response |
– |
| 14 | Bayart et al50 | 2017 | Case series | 6 | Alopecia areata and variants | • Four patients: Topical 2% tofacitinib (BID) • Two patients: Topical 1%, 2% ruxolitinib (BID) |
• One patient had 20% regrowth of eyebrows • One patient had 95% regrowth • One patient had 80% regrowth after 1 year • One patient had no response (only data from tofacitinib-treated patients) |
• Transient elevation of liver transaminase (1) • Transient leukopenia (1) (only data from tofacitinib-treated patients) |
| 15 | Castelo- Soccio51 | 2017 | Case series | 8 | Alopecia universalis | Oral tofacitinib (5 mg BID) | • All patients experienced >50% regrowth in scalp hair by 5 months • All patients experienced significant improvement in SALT • Two patients experienced improvement in nail pitting |
– |
| 16 | Scheinberg et al52 | 2017 | Case series | 4 | Alopecia universalis (Two patients failed topical tofacitinib before this trial) | Oral tofacitinib (5, 10 mg daily) | • One patient had progressive hair growth after 9 months • One patient had some regrowth after 6 weeks • One patient had hair regrowth after 7 months • One patient had full regrowth after 6 months |
– |
| 17 | Kim and Kim53 | 2017 | Case report | 1 | Alopecia universalis | Oral tofacitinib (5 mg BID) | • Patient had full regrowth in 32 weeks | – |
| 18 | Strazzulla et al54 | 2017 | Case report | 1 | Alopecia universalis | Oral tofacitinib (unspecified dose) | • Patient had near-complete regrowth in 10 months | – |
| 19 | Erduran et al55 | 2017 | Case report | 1 | Alopecia universalis | Oral tofacitinib (10, 15 mg daily) | • Patient had full regrowth in 6 months | – |
| 20 | Salman et al56 | 2017 | Case report | 1 | Alopecia universalis and plaque-type psoriasis | Oral tofacitinib (5 mg BID) | • Patient had no hair regrowth despite almost total clearance of psoriasis | – |
| 21 | Jabbari et al57 | 2018 | Open-label, single-arm trial | 12 | Alopecia areata and variants | Oral tofacitinib (10, 15, 20 mg daily) | • Eight patients showed $50% regrowth from baseline • Three patients showed, 50% regrowth from baseline • One patient showed no regrowth • Reduction in ALADIN score in responders • No significant change in ALADIN score in nonresponder |
• Grade I and II infection (12): eg, URI (11), conjunctivitis (1) • Loose stool (3), constipation (1), bloating (1), mild acne (3), weight gain (2), hypertensive urgency (1), dizziness (1), headache (1), neuropathic pain (1), urinary retention (1), vaginal spotting (1) • Blood on urinalysis (4), asymptomatic bacteriuria (2), transaminitis (1) |
| 22 | Patel et al58 | 2018 | Case series | 2 | Alopecia universalis | Oral tofacitinib (5, 10 mg daily) | • One patient had 85% reduction in SALT score • One patient had 90% reduction in SALT score |
– |
Abbreviations: AA, alopecia areata; ALADIN, Alopecia Areata Disease Activity Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, bis in die (twice daily); CXCL10, C-X-C motif chemokine ligand 10; γ, interferon γ; JAK, janus kinase; LDL, low-density lipoprotein; SALT, Severity of Alopecia Tool; TG, triglyceride; URI, upper respiratory tract infection; UTI, urinary tract infection.
Several retrospective studies have also been published. In the largest study conducted by Liu et al,39 efficacy of oral tofacitinib 5 mg or more twice daily as monotherapy or as combination therapy with prednisone was evaluated in 90 patients with AA and its variants. Patients aged 18–70 years old were evaluated for Severity of Alopecia Tool (SALT) score at baseline and after various treatment durations (4–12 months). Sixty-five patients were identified as potential responders (patients whose current episode of alopecia totalis [AT] or AU was 10 years or less or patients with AA). Of the potential responders, 13 patients (20%) were complete responders having >90% reduction in SALT. Twenty-five (38.4%) patients were intermediate responders (51%–90% reduction in SALT), 12 (18.5%) were moderate responders (6%–50% reduction in SALT), and 15 (23.1%) were nonresponders (≤5% reduction in SALT).
In an open-label, single-arm study, 66 patients with AA who had >50% hair loss, AT, or AU were given oral tofacitinib 5 mg twice daily for 3 months. Outcome was evaluated from regrowth of scalp hair assessed by SALT, duration of hair growth after completion of therapy, and disease transcriptome. Of 66 treated subjects, 32% of patients had ≥50% reduction in SALT. AA and ophiasis subtypes were more responsive than AT or AU. Shorter duration and histologic peribulbar inflammation of pretreated scalp biopsies were associated with improvement in SALT.38 Recently, in 2018, another open-label, single-arm study on 12 patients with moderate-to-severe AA, AT, or AU had been published. Each patient was given oral tofacitinib for 10 mg daily for 1 month and the dosage was increased gradually to 15 mg daily and 20 mg daily if the patient did not achieve at least ≥50% regrowth of hair from baseline. A full course of treatment was defined as having $50% regrowth of hair from baseline for 6–12 months while taking tofacitinib. Patients were then required to stop tofacitinib after completing a full course treatment and reevaluating after 6 months of tofacitinib cessation. Eleven of the 12 patients completed the full course treatment with minimal adverse events. Eight patients experienced ≥50% regrowth of hair from baseline, while three experienced ≤50% regrowth of hair and one experienced no regrowth.57
An open-label, single-arm trial and a case series investigated the safety and efficacy of topical tofacitinib in patients with AA.46 In the clinical study, 10 patients with AA were treated with 2% tofacitinib ointment twice daily. Regrowth of scalp hair was assessed by using SALT; one achieved excellent regrowth, two had partial regrowth, and seven had no regrowth.
In terms of safety, reported side effects include only mild symptoms, grade I and II infections, as well as transient elevation of liver transaminase, and cholesterol level (Table 1).38,39,47,48,50,57 Most of these symptoms or abnormalities were transient and reversible either spontaneously or with discontinuation of tofacitinib. One retrospective study by Ibrahim et al reported a patient having morbilliform eruption and peripheral edema leading to cessation of oral tofacitinib.48 Information on long-term safety of tofacitinib was indirectly extrapolated from clinical trials in rheumatoid arthritis. Tuberculosis was observed in 10 mg dosage groups (incidence rate 0.5 events/100 patient-years) but not in 5 mg dosage groups (95% CI 0.1–0.9). Rate of opportunistic infection other than tuberculosis was low and limited to herpes zoster without visceral involvement or death.59 Lung cancer and breast cancer were the most common malignancies that occurred during tofacitinib treatment. The overall rate of occurrence of malignancies, excluding nonmelanoma skin cancer, was 0.939 events/100 patient-years (95% CI 0.737–1.198).60 However, all this information of adverse events must be taken into account that the studied population could be affected by rheumatoid arthritis and its treatment.
Ruxolitinib
Ruxolitinib (INC424 or INCB018424), a JAKi with chemical structure of C17H18N6 (Figure 4), 61 is FDA-approved for the treatment of myelofibrosis.62,63 It selectively inhibits JAK1 and JAK2 and, to some extent, TYK2.64 Other than the effects of JAK-STAT pathway inhibition, ruxolitinib has been shown to have anti-inflammatory effects, which are thought to be due to interruption of the IL-17 signaling axis.65 Concurrently, ruxolitinib has been demonstrated to reduce cytokine-induced phosphorylation of STAT364,66 and levels of circulating inflammatory cytokines such as tumor necrosis factor-α and IL-6 in mice.64 Eyelash growth was observed in a patient with hypereosinophilic syndrome after having taken ruxolitinib, but the responsible mechanism was not elucidated.67
Figure 4.
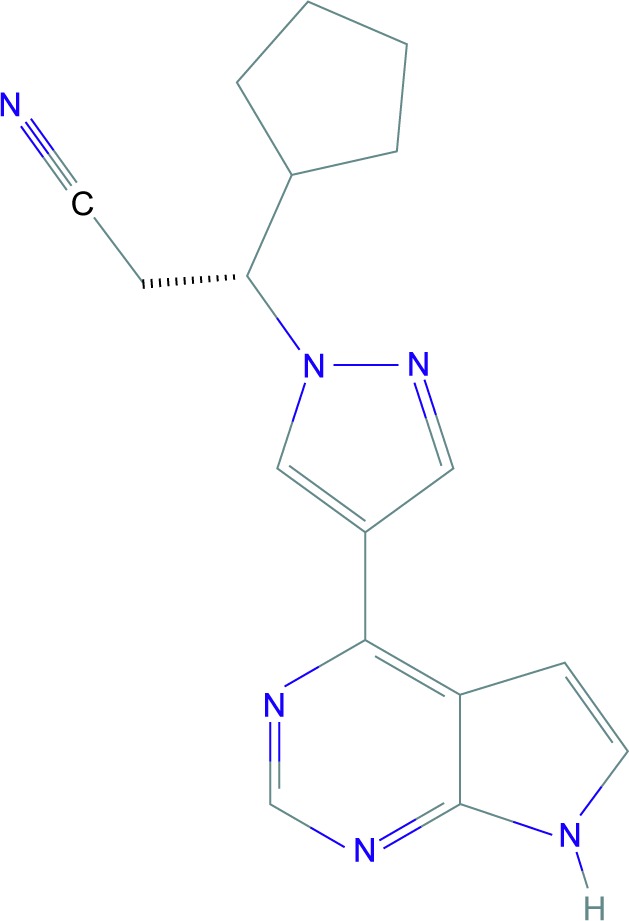
Ruxolitinib.
A number of case reports and one open-label, single-arm trial of ruxolitinib have been published (Table 2).10,50,68–73 Concerning oral ruxolitinib, Xing et al, apart from establishing the effects of JAKis in alopecic skin through in vivo study, had reported three cases of AA treated with 20 mg of oral ruxolitinib twice daily.10 All three patients experienced near-complete regrowth within 3–5 months. Pieri et al later reported a near-complete hair growth after 15 mg of oral ruxolitinib twice daily was administered to a patient with AU and essential thrombocythemia.68
Table 2.
Characteristics of clinical studies of ruxolitinib and baricitinib in the treatment of AA
| Authors | Year | Study design | Patients | Indication | JAK inhibitor (dose) | Outcome | Side effects | |
|---|---|---|---|---|---|---|---|---|
| 1 | Xing et al10 | 2014 | Case series | 3 | Alopecia areata | Oral ruxolitinib (20 mg BID) | • All patients experienced near- complete regrowth within 3–5 months | – |
| 2 | Pieri et al68 | 2015 | Case report | 1 | Alopecia universalis and essential thrombocythemia | Oral ruxolitinib (15 mg BID) | • Patient had near- complete regrowth within 10 months | – |
| 3 | Craiglow et al69 | 2016 | Case report | 1 | Alopecia universalis | Topical 0.6% ruxolitinib (BID) | • Patient had near- complete regrowth of eyebrows and 10% regrowth of scalp hair in 12 weeks | • Leukopenia (1) |
| 4 | Mackay- Wiggan et al70 | 2016 | Open-label, single-arm trial | 12 | Alopecia areata | Oral ruxolitinib (20 mg BID) | • Nine patients had regrowth ≥50% • Nine responders experienced reduction of hair loss from baseline by 92% |
• Bacterial skin infection (3) • URI (9) • UTI (1) • Mild pneumonia (1) • Postoperative conjunctival hemorrhage (1) • Mild GI symptoms (1) • Lower hemoglobin: resolved with dose reduction (1) |
| 5 | Bayart et al50 | 2017 | Case series | 6 | Alopecia areata and variants | • Two patients: topical 1%, 2% ruxolitinib (BID) • Four patients: topical 2% tofacitinib (BID) |
• (only data from ruxolitinib- treated patients) One patient showed no response • One patient experienced 75% regrowth of only upper eyelashes; no regrowth of eyebrows |
– (only data from ruxolitinib- treated patients) |
| 6 | Vandiver et al71 | 2017 | Case series | 2 | Alopecia areata | Oral ruxolitinib (10–30 mg daily) | • One patient had complete regrowth in 8 months •One patient had near- complete regrowth in 6 months |
• Five-pound weight gain (1) • Bloating and bruising (1) |
| 7 | Deeb and Beach72 | 2017 | Case report | 1 | Alopecia areata | Topical 0.6% ruxolitinib (BID) | • Patient showed no improvement | – |
| 8 | Ramot and Zlotogorski73 | 2018 | Case report | 1 | Alopecia universalis | Oral ruxolitinib (20 mg BID) | • Patient had full regrowth of beard and 50% regrowth of scalp hair | – |
| 9 | Jabbari et al77 | 2015 | Case report | 1 | Alopecia areata and CANDLE syndrome | Oral baricitinib (7 mg morning and 4 mg evening) | • Patient had full regrowth in 9 months | – |
Abbreviations: AA, alopecia areata; BID, bis in die (twice daily); CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; GI, gastrointestinal; JAK, janus kinase; URI, upper respiratory tract infection; UTI, urinary tract infection.
In an open-label, single-arm trial for oral ruxolitinib, 12 patients with moderate-to-severe AA were given 20 mg of oral ruxolitinib twice daily for 3–6 months. The proportion of patients with 50% or greater hair regrowth from baseline was the primary endpoint. Nine patients (75%) had significant hair growth (mean improvement of 92%).70
Despite promising outcomes in a mouse model reported by Xing et al, unfavorable efficacy of the topical form of ruxolitinib had been demonstrated in three case reports.10 Craiglow et al reported a case of AU treated with 0.6% ruxolitinib cream.69 The patient had near-complete regrowth of eyebrows, but only 10% growth of scalp hair after 12 weeks. Bayart et al revealed two cases of AU treated with 1% and 2% ruxolitinib in liposomal base.50 No response was observed in the patient treated with 2% ruxolitinib, whereas the other receiving 1% ruxolitinib experienced regrowth of only upper eyelashes but not the eyebrows. Similarly, Deeb and Beach reported no improvement in the patient with AA who underwent the treatment with 0.6% ruxolitinib cream.72
As for the safety issue of ruxolitinib, all clinical studies on AA patients have reported only mild symptoms and grade I or II infection (Table 2).69–71 Data from studies on ruxolitinib in myelofibrosis patients show hematologic adverse events mainly dose-related anemia, thrombocytopenia, and neutropenia. These conditions can be explained by the fact that ruxolitinib inhibits JAK2-STAT signaling in normal hematopoiesis. Common nonhematologic adverse events included nonsevere bruising, dizziness, and headache.74 Ruxolitinib proved to be noncarcinogenic in the 6-month Tg.rasH2 transgenic mouse model and in a 2-year carcinogenicity study in the rat. Furthermore, there has yet to be any report on ruxolitinib-related malignancy in the literature.66
Baricitinib
Baricitinib (LY3009104 or INCB028050) is a potent selective JAK1 and JAK2 inhibitor with a recognizable degree of JAK3 and various kinase inhibition. Its chemical structure is C16H17N7O2S (Figure 5).75 In cell-based assays relevant to autoimmune diseases, baricitinib has been shown to inhibit JAK signaling and function initiated by IL-6 and IL-23.76 Anti-inflammatory effects have also been observed in a mouse model with reduced CD8 infiltration and reduced MHC class I and class II expression when compared with vehicle control-treated mice.77
Figure 5.
Baricitinib.
There has only been one case report concerning the efficacy of baricitinib in AA (Table 2).77 In 2015, Jabbari et al reported a patient with chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome (CANDLE syndrome) and AA who was treated with oral baricitinib 7 mg in the morning and 4 mg in the evening.77 Complete regrowth of scalp hair was observed after 9 months of treatment.
With regard to safety, Jabbari et al did not report any adverse events following baricitinib use in AA.77 A study on baricitinib in healthy volunteers showed no serious adverse events. Reported adverse events were neutropenia and reduced reticulocyte count.78 Baricitinib was considered to be noncarcinogenic based on the study of carcinogenicity assessment of baricitinib in mice.79
Future directions
Despite exceptional results in mice,10 researchers are still unable to reproduce similar efficacy of the topical preparation of tofacitinib and ruxolitinib in humans. Bayart et al demonstrated that a patient, who initially did not respond to the treatment, attained 95% hair growth after switching from tofacitinib in VersaBase® cream formulation to liposomal base formulation.50 This suggests that there is still room for improvement for topical JAKis especially in terms of pharmacokinetics and phar-macodynamics. In fact, researchers have already begun investigating into nanotechnology in the hope of better drug delivery, which might greatly enhance topical JAKi efficacy.80
Second-generation JAKis, equipped with a better selectivity of JAK receptor, are currently being studied in RA. Filgotinib (GLPG0634/GS-6034) and ABT-494, selective inhibitors of JAK1, and decernotinib (VX-509), a selective inhibitor of JAK3, have shown promising efficacy in Phase II studies of rheumatoid arthritis.81–83 WYE-151650, a selective inhibitor of JAK3, has been shown to have exceptional efficacy in collagen-induced arthritis in mice.84 These second-generation JAKis might be applicable in AA with similar efficacy and less toxicity and side effects. Future studies are needed to shed more light on the safety and efficacy of these new members of the JAKi family.
As previously mentioned, JAKis have many explained and unexplained roles in hair growth cycle regulation in addition to their anti-inflammatory effects. Several studies regarding their mechanism on hair growth cycle are ongoing. The implementation of JAKis in hair loss disorders, including scarring and nonscarring alopecia, other than AA may also be possible and should be investigated. In the near future, JAKis may become one of the effective treatment options for various hair loss disorders.
Conclusion
In recent years, safety and efficacy of various JAKis have been demonstrated in previous trials of RA, myelofibrosis, and various autoimmune and hematologic diseases. Currently, there is yet to be an FDA-approved JAKi for dermatologic indication. However, based on greater knowledge in the pathogenesis of AA and molecular biochemistry, JAKis are emerging as a promising treatment for AA. In this group, tofacitinib, ruxolitinib, and baricitinib have been studied in AA and its variants with varying outcomes. These studies demonstrated exceptional efficacy of oral tofacitinib and ruxolitinib in severe AA or refractory AA but unfavorable efficacy for topical preparation. Many case reports also showed promising results of oral tofacitinib and ruxolitinib in recalcitrant cases. Furthermore, side effects of tofacitinib and ruxolitinib demonstrated in AA cases were mostly transient and nonsevere. These data suggest that JAKis could be a great addition to the dermatologist’s armament for tackling AA and a possible alternative in cases unresponsive to standard treatments. However, current evidence is based on case reports and uncontrolled trials. Well-controlled prospective studies are needed to determine long-term efficacy, safety, and cost-effectiveness, as well as to elucidate undisclosed mechanisms responsible for hair growth. As for baricitinib, available data are too sparse to conclude its efficacy and safety in AA. Further studies are needed for better understanding of baricitinib. Although JAKis are effective in various diseases, they, so far, have not been shown to provide long-term efficacy after stopping treatment in these diseases. Thus, JAKis might be more suitable for diseases with short duration or that are self-limited. Finally, from current evidence, JAKis are considered breakthrough treatment for AA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–89. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Huang KP, Mullangi S, Guo Y, Qureshi AA, Autoimmune QAA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789–794. doi: 10.1001/jamadermatol.2013.3049. [DOI] [PubMed] [Google Scholar]
- 3.Chu SY, Chen YJ, Tseng WC, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65(5):949–956. doi: 10.1016/j.jaad.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Lee NR, Kim BK, Yoon NY, Lee SY, Ahn SY, Lee WS. Differences in comorbidity profiles between early-onset and late-onset alopecia areata patients: a retrospective study of 871 Korean patients. Ann Dermatol. 2014;26(6):722–726. doi: 10.5021/ad.2014.26.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–297. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 6.de Weert J, Temmerman L, Kint A. Alopecia areata: a clinical study. Dermatologica. 1984;168(5):224–229. doi: 10.1159/000249708. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80(2):316–328. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombe BW, Price VH, Khoury EL, Garovoy MR, Lou CD. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5 Pt 1):757–764. [PubMed] [Google Scholar]
- 9.Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8(2):188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 10.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 12.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. doi: 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc. 2013;16(1):S25–S27. doi: 10.1038/jidsymp.2013.7. [DOI] [PubMed] [Google Scholar]
- 15.Sperling LC, Lupton GP. Histopathology of non-scarring alopecia. J Cutan Pathol. 1995;22(2):97–114. doi: 10.1111/j.1600-0560.1995.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Ito N, Saathoff M, Bettermann A, Takigawa M, Paus R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br J Dermatol. 2005;152(4):623–631. doi: 10.1111/j.1365-2133.2005.06453.x. [DOI] [PubMed] [Google Scholar]
- 17.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 18.Darnell JE. STATs and gene regulation. Science. 1997;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 20.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;(109 Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 21.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 22.Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377(6544):65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 23.Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1(9):e1500973. doi: 10.1126/sciadv.1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turksen K, Kupper T, Degenstein L, Williams I, Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992;89(11):5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132(1):43–49. doi: 10.1038/jid.2011.274. [DOI] [PubMed] [Google Scholar]
- 26.Yu M, Kissling S, Freyschmidt-Paul P, Hoffmann R, Shapiro J, Mcelwee KJ. Interleukin-6 cytokine family member oncostatin M is a hair-follicle-expressed factor with hair growth inhibitory properties. Exp Dermatol. 2008;17(1):12–19. doi: 10.1111/j.1600-0625.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 27.Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309(9):729–738. doi: 10.1007/s00403-017-1777-5. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon S. Tofacitinib: a review in rheumatoid arthritis. Drugs. 2017;77(18):1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CM, Tung TH, Wang SH, Chi CC. Efficacy and safety of tofacitinib for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2018;32(3):355–362. doi: 10.1111/jdv.14695. [DOI] [PubMed] [Google Scholar]
- 30.Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017;130(2):115–125. doi: 10.1182/blood-2017-04-742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves de Medeiros AK, Speeckaert R, Desmet E, van Gele M, de Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11(10):e0164080. doi: 10.1371/journal.pone.0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divito SJ, Kupper TS. Inhibiting Janus kinases to treat alopecia areata. Nat Med. 2014;20(9):989–990. doi: 10.1038/nm.3685. [DOI] [PubMed] [Google Scholar]
- 33.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Biotechnology Information PubChem Compond Database: CID=9926791. [Accessed April 3, 2018]. [updated 2018 Mar 31] cited 2018 Apr 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/9926791.
- 35.Meyer DM, Jesson MI, Li X, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm. 2010;7:41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134(12):2988–2990. doi: 10.1038/jid.2014.260. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi: 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi: 10.1016/j.jaad.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Gupta AK, Carviel JL, Abramovits W. Efficacy of tofacitinib in treatment of alopecia universalis in two patients. J Eur Acad Dermatol Venereol. 2016;30(8):1373–1378. doi: 10.1111/jdv.13598. [DOI] [PubMed] [Google Scholar]
- 41.Dhayalan A, King BA. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152(4):492–493. doi: 10.1001/jamadermatol.2015.3772. [DOI] [PubMed] [Google Scholar]
- 42.Anzengruber F, Maul JT, Kamarachev J, Trüeb RM, French LE, Navarini AA. Transient efficacy of tofacitinib in alopecia areata universalis. Case Rep Dermatol. 2016;8(1):102–106. doi: 10.1159/000445182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira SB, Scheinberg M, Steiner D, Steiner T, Bedin GL, Ferreira RB. Remarkable improvement of nail changes in alopecia areata universalis with 10 months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8(3):262–266. doi: 10.1159/000450848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25(8):642–643. doi: 10.1111/exd.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mrowietz U, Gerdes S, Gläser R, Schröder O. Successful treatment of refractory alopecia areata universalis and psoriatic arthritis, but not of plaque psoriasis with tofacitinib in a young woman. Acta Derm Venereol. 2017;97(2):283–284. doi: 10.2340/00015555-2491. [DOI] [PubMed] [Google Scholar]
- 46.Liu LY, Craiglow BG, King BA. Tofacitinib 2% ointment, a topical Janus kinase inhibitor, for the treatment of alopecia areata: A pilot study of 10 patients. J Am Acad Dermatol. 2018;78(2):403–404. doi: 10.1016/j.jaad.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 47.Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76(1):29–32. doi: 10.1016/j.jaad.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim O, Bayart CB, Hogan S, Piliang M, Bergfeld WF. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153(6):600–602. doi: 10.1001/jamadermatol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park HS, Kim MW, Lee JS, et al. Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: A case series. J Am Acad Dermatol. 2017;77(5):978–980. doi: 10.1016/j.jaad.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 50.Bayart CB, Deniro KL, Brichta L, Craiglow BG, Sidbury R. Topical janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol. 2017;77(1):167–170. doi: 10.1016/j.jaad.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Castelo-Soccio L. Experience with oral tofacitinib in 8 adolescent patients with alopecia universalis. J Am Acad Dermatol. 2017;76(4):754–755. doi: 10.1016/j.jaad.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 52.Scheinberg M, de Lucena Couto Ocea RA, Cruz BA, Ferreira SB. Brazilian experience of the treatment of alopecia universalis with the novel antirheumatic therapy tofacitinib: a case series. Rheumatol Ther. 2017;4(2):503–508. doi: 10.1007/s40744-017-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim BY, Kim HS. Successful hair regrowth in a Korean patient with alopecia universalis following tofacitinib treatment. Singapore Med J. 2017;58(5):279–280. doi: 10.11622/smedj.2017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strazzulla LC, Avila L, Lo Sicco K, Shapiro J. Image Gallery: Treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176(6):e125. doi: 10.1111/bjd.15483. [DOI] [PubMed] [Google Scholar]
- 55.Erduran F, Adışen E, Aksakal AB. Excellent response to tofacitinib treatment in a patient with alopecia universalis. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26(2):47–49. doi: 10.15570/actaapa.2017.15. [DOI] [PubMed] [Google Scholar]
- 56.Salman A, Sarac G, Ergun T. Alopecia universalis unresponsive to treatment with tofacinitib: report of a case with a brief review of the literature. Dermatol Online J. 2017;23(7):13030/qt224878kb. [PubMed] [Google Scholar]
- 57.Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138(7):1539–1545. doi: 10.1016/j.jid.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel NU, Oussedik E, Grammenos A, Pichardo-Geisinger R. A case report highlighting the effective treatment of alopecia universalis with tofacitinib in an adolescent and adult patient. J Cutan Med Surg. 2018;22(4):439–442. doi: 10.1177/1203475418760512. [DOI] [PubMed] [Google Scholar]
- 59.XELJANZ®/XELJANZ XR® (tofacitinib) tablets, film coated, extended release [pescribing information] New York: Pfizer Laboratories Div Pfizer Inc; 2012. [Accessed April 3, 2018]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=959. [Google Scholar]
- 60.Curtis JR, Lee EB, Kaplan IV, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–841. doi: 10.1136/annrheumdis-2014-205847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Center for Biotechnology Information PubChem Compound Database CID=25126798 [updated 2018 Mar 31 cited 2018 Apr 3] 2018. [Accessed April 3, 2018]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/25126798.
- 62.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 64.Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67(4):658–664. doi: 10.1016/j.jaad.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 66.Jakafi® (ruxolitinib) tablets [prescribing information] Delaware: Incyte Corporation; 2011. [Accessed April 3, 2018]. Available from: http://www.jakafi.com/pdf/prescribing-information.pdf. [Google Scholar]
- 67.Song J, Song A, Palmares T, Song M, Song H. Ruxolitinib found to cause eyelash growth: a case report. J Med Case Rep. 2017;11(1):189. doi: 10.1186/s13256-017-1304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90(1):82–83. doi: 10.1002/ajh.23871. [DOI] [PubMed] [Google Scholar]
- 69.Craiglow BG, Tavares D, King BA. Topical ruxolitinib for the treatment of alopecia universalis. JAMA Dermatol. 2016;152(4):490–491. doi: 10.1001/jamadermatol.2015.4445. [DOI] [PubMed] [Google Scholar]
- 70.Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi: 10.1172/jci.insight.89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandiver A, Girardi N, Alhariri J, Garza LA. Two cases of alopecia areata treated with ruxolitinib: a discussion of ideal dosing and laboratory monitoring. Int J Dermatol. 2017;56(8):833–835. doi: 10.1111/ijd.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deeb M, Beach RA. A case of topical ruxolitinib treatment failure in alopecia areata. J Cutan Med Surg. 2017;21(6):562–563. doi: 10.1177/1203475417716363. [DOI] [PubMed] [Google Scholar]
- 73.Ramot Y, Zlotogorski A. Complete regrowth of beard hair with rux-olitinib in an alopecia universalis patient. Skin Appendage Disord. 2018;4(2):122–124. doi: 10.1159/000479722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galli S, Mclornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014;13(7):967–976. doi: 10.1517/14740338.2014.916273. [DOI] [PubMed] [Google Scholar]
- 75.National Center for Biotechnology Information NationalCenter for Biotechnology Information. [Accessed April 3, 2018]. PubChem Compound Database; CID=44205240 [updated 2018 Mar 31 cited 2018 Apr 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/44205240.
- 76.Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical char-acterization of INCB028050. J Immunol. 2010;184(9):5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 77.Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2(4):351–355. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi JG, Chen X, Mcgee RF, et al. The pharmacokinetics, pharmacody-namics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644–1654. doi: 10.1177/0091270010389469. [DOI] [PubMed] [Google Scholar]
- 79.Carfagna M, Cannady E, Ryan T, et al. Carcinogenicity assessment of baricitinib in Tg.rasH2 mice and Sprague-Dawley (Crl:CD) rats. Regul Toxicol Pharmacol. 2018;92:458–471. doi: 10.1016/j.yrtph.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 80.Boca S, Berce C, Jurj A, et al. Ruxolitinib-conjugated gold nanoparticles for topical administration: An alternative for treating alopecia? Med Hypotheses. 2017;109:42–45. doi: 10.1016/j.mehy.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 81.Vanhoutte F, Mazur M, Voloshyn O, et al. Efficacy, safety, pharmacoki-netics, and pharmacodynamics of filgotinib, a selective JAK-1 inhibitor, after short-term treatment of rheumatoid arthritis: results of two random-ized phase IIa trials. Arthritis Rheumatol. 2017;69(10):1949–1959. doi: 10.1002/art.40186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–2866. doi: 10.1002/art.39808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Genovese MC, van Vollenhoven RF, Pacheco-Tena C, Zhang Y, Kinnman N. VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):46–55. doi: 10.1002/art.39473. [DOI] [PubMed] [Google Scholar]
- 84.Lin TH, Hegen M, Quadros E, et al. Selective functional inhibition of JAK-3 is sufficient for efficacy in collagen-induced arthritis in mice. Arthritis Rheum. 2010;62(8):2283–2293. doi: 10.1002/art.27536. [DOI] [PubMed] [Google Scholar]