Abstract
Disorders of adrenal steroidogenesis comprise autosomal recessive conditions affecting steroidogenic enzymes of the adrenal cortex. Those are located within the 3 major branches of the steroidogenic machinery involved in the production of mineralocorticoids, glucocorticoids, and androgens. This mini review describes the principles of adrenal steroidogenesis, including the newly appreciated 11-oxygenated androgen pathway. This is followed by a description of pathophysiology, biochemistry, and clinical implications of steroidogenic disorders, including mutations affecting cholesterol import and steroid synthesis, the latter comprising both mutations affecting steroidogenic enzymes and co-factors required for efficient catalysis. A good understanding of adrenal steroidogenic pathways and their regulation is crucial as the basis for sound management of these disorders, which in the majority present in early childhood.
Keywords: Steroidogenesis, Congenital adrenal hyperplasia, Androgen excess
Introduction
The first case of a patient with a steroidogenic disorder, likely congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency, was published more than 150 years ago [1, 2]: a phenotypical male with ambiguous genitalia, female internal anatomy, and large adrenal glands on autopsy. It took another century until the biochemistry of this condition became clear and treatment was available [3]. Only after further advances in molecular biology and protein biochemistry, the molecular basis of CAH was unravelled from the 1980s onwards. Since then, our understanding of the physiology and pathophysiology of steroid hormones has vastly improved [4]. Although we now have a nearly complete understanding of the underlying nature of steroidogenic disorders, diagnosis and management continue to pose challenges and require expert guidance. Novel diagnostic approaches promise to improve rapid diagnosis and treatment monitoring. In particular, modified release glucocorticoid hormone formulations are promising new therapies to mimic the diurnal endogenous secretion pattern of cortisol to provide more physiologic replacement.
Overview of Adrenal Steroidogenesis
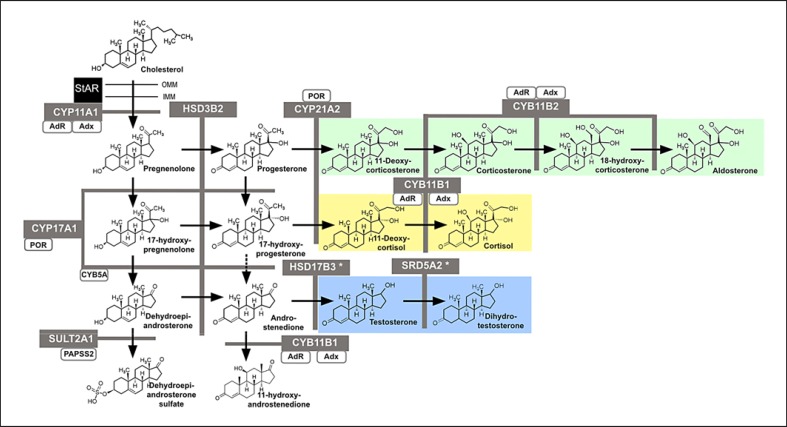
Steroidogenesis in the adrenal cortex describes a series of consecutive enzymatic reactions converting a precursor hormone into intermediates on their way to active steroid hormones for 3 major steroid classes: mineralocorticoids (MCs), glucocorticoids (GCs), and precursors of active androgens (and ultimately, oestrogens) (Fig. 1). The principal enzymes involved are either cytochrome P450 enzymes (CYPs) or hydroxysteroid dehydrogenases (HSDs). Their expression in distinct layers of the adrenal cortex determines the zonal specific steroid output and provides functional separation of 3 layers: the MC-producing zona glomerulosa as the outer layer, the GC-producing zona fasciculata in the middle, and the zona reticularis generating androgen precursors as the innermost layer, adjacent to the adrenal medulla.
Fig. 1.
Schematic representation of human adrenal steroidogenesis. Steroidogenic enzymes are depicted in grey boxes supporting catalytic activities indicated by the black arrow. Co-factors for steroidogenic enzymes are represented by white boxes. The three main steroidogenic pathways are coloured as green for the mineralocorticoid, yellow for the glucocorticoid, and blue for the androgen pathway. An asterisk marks enzymes that are predominantly expressed in extra-adrenal tissues. StAR, steroidogenic acute regulatory protein; CYP11A1, cholesterol side-chain cleavage enzyme; CYP17A1, 17α-hydroxylase/17,20 lyase; CYB5A, cytochrome b5; SULT2A1, DHEA sulfotransferase; POR, P450 oxidoreductase; CYP21A2, 21-hydroxylase; HSD3B2, 3β-hydroxysteroid dehydrogenase; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; HSD17B3, 17β-hydroxysteroid dehydrogenase type 3; SRD5A2, 5α-reductase type 2; Adx, adrenodoxin; AdR, adrenodoxin reductase.
As a simplified approach to explain adrenal steroidogenesis, in particular when explaining it to patients and lay people, it helps to compare the adrenal steroid machinery to a highway system with 3 main junctions (see Fig. 1): precursors are travelling to their distinct destinations, taking either the MC, GC, or sex steroid junction. Traffic flow is controlled via ACTH on junction 2 and 3, and via the renin-angiotensin-aldosterone system (RAAS) on junction 1. A block at any junction (i.e., a distinct enzyme deficiency) causes congestion on the motorway, and the precursor traffic will accumulate before the block and try to find its way out through the other junctions that are open.
Cholesterol is the principal substrate for all steroid hormones. It can be synthesized de novo from acetate [5] but is mostly supplied from dietary cholesterol transported to the cell via LDL and HDL [6]. The side-chain cleavage enzyme (P450scc or CYP11A1) is located at the inner mitochondrial membrane and responsible for the first catalytic step, generating pregnenolone. The exact mechanisms underlying mitochondrial cholesterol import are complex and despite intensive research efforts not understood in all detail yet [7]. There are excellent reviews on the extensive work that has been done on cholesterol import and trafficking [4, 7]; this topic is beyond the scope of this article.
The steroidogenic acute regulatory protein (StAR) is a key player shifting cholesterol from the outer to the inner mitochondrial membrane, where CYP11A1 resides. CYP11A1 exhibits 3 consecutive reactions modifying the cholesterol molecule: (1) 20α-hydroxylation, (2) 22R-hydroxylation, and (3) carbon side-chain cleavage of C20-C22 yielding pregnenolone [8, 9]. As a mitochondrial type 1 CYP enzyme, it requires electron provision from NADPH facilitated by adrenodoxin (Adx) and adrenodoxin reductase (AdR) [10]. CYP11A1 is expressed in all 3 layers of the adrenal cortex [11] and its function to generate pregnenolone is the rate-limiting step of all steroid hormone biosynthesis. The downstream conversion and “fate” of its product depends on the zonal-specific presence and activity of a distinct combination of steroidogenic enzymes.
MC Production: Zona Glomerulosa
The enzyme 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) generates progesterone from pregnenolone in the zona glomerulosa. It has 2 catalytic activities: (1) with its dehydrogenase activity it oxidizes the hydroxyl group at carbon 3 to a keto group and (2) subsequently, with its Δ4/Δ5 isomerase activity, HSD3B2 changes the carbon-carbon double bond from the position between C5/6 (“Δ5”) to C4/5 (“Δ4”) [12, 13]. The microsomal (type 2) cytochrome P450 enzyme 21-hydroxylase (CYP21A2) then converts progesterone via hydroxylation on carbon atom 21 to the potent MC precursor 11-deoxycorticosterone (DOC). At this stage, the “fate” of the precursors is determined as they have entered the MC pathway (“junction 1”).
Further activation of DOC to aldosterone is performed in 3 subsequent steps by the mitochondrial CYP enzyme 11B2 (CYP11B2), or also “aldosterone synthase”: (1) 11β-hydroxylation of DOC yielding corticosterone, (2) 18-hydroxylation of corticosterone to form 18-hydroxycorticosterone, and finally (3) the 18-methyl oxidation of corticosterone generating aldosterone [14]. Of note, CYP11B1, which is not expressed in the zona glomerulosa, is also able to perform the first two catalytic reactions of CYP11B2: however, the 18-methyl oxidation generating aldosterone is unique to the type 2 isozyme [15].
GC Production: Zona Fasciculata
Both zona fasciculata and glomerulosa express high amounts of HSD3B2 [11]. The microsomal CYP enzyme 17α-hydroxylase (CYP17A1) is highly expressed in the zona fasciculata and reticularis in postadrenarchal children, but absent in the glomerulosa [11], and competes with HSD3B2 for substrates. CYP17A1 - the gatekeeper towards GC and sex steroid biosynthesis - has 2 catalytic activities [16, 17]. First, with its 17α-hydroxylase activity CYP17A1, it introduces a hydroxyl group on carbon position 17 of the pregnenolone or progesterone molecule to form 17α-hydroxypregnenolone (17OHPreg) and 17α-hydroxyprogesterone (17OHP), respectively. Second, with its 17,20 lyase activity CYP17A1, it cleaves 2 carbon atoms off position 17 from the hydroxylated compounds, yielding dehydroepiandrosterone (DHEA) and androstenedione. Both catalytic activities require electron transfer from NADPH, which is facilitated by the redox enzyme P450 oxidoreductase (POR), which provides electrons to all microsomal (type 2) CYPs involved in steroidogenesis. CYP17A1 17,20 lyase activity, the sex steroid-producing step, needs more electrons and further interaction with the small haemoprotein cytochrome b5, which is only expressed in the zona reticularis, facilitating DHEA generation ([18, 19], see below).
The combination of HSD3B2 and CYP17A1 activities generates 17OHP as the principal GC precursor entering the GC pathway (“junction 2”). 17OHP is converted to 11-desoxycortisol, the central GC precursor, by the enzyme CYP21A2. Finally, the microsomal CYP enzyme 11β-hydroxylase (CYP11B1) converts 11-desoxycortisol in one single oxidative reaction to the major active GC cortisol.
Androgen Precursor Production: Zona Reticularis
The lack of HSD3B2 with strong expression of CYP17A1 and both ample presence of the co-factors POR and cytochrome b5 (CYB5A) enables the zona reticularis to produce robust amounts of the androgen precursor DHEA [20]. It is important to note that in normal physiology CYP17A1 17,20 lyase activity has a 100-fold higher substrate preference for 17OH-Preg than for 17OHP [21], which means that under physiologic conditions most androgen synthesis in the adrenal goes through DHEA in the “Δ5 pathway.” The mechanisms by which CYB5A facilitates the 17,20 lyase activity of CYP17A1 are not entirely understood; it has been suggested that CYB5A enhances allosteric interaction between the POR and CYP17A1 proteins, with only indirect involvement in electron transfer [21]. Recent work, however, indicates direct binding between CYB5A and CYP enzymes [22].
Adrenal Precursors of the Classic Androgen Pathway
Cortisol and aldosterone leave the adrenal cortex as “end products” with high affinities to their respective receptors. By contrast, DHEA requires further conversion to active sex steroids in the gonads and peripheral target tissues of androgen action [23]. The conversion of DHEA to androstenedione is catalyzed by HSD3B2, and although the zona reticularis expresses very little of this enzyme, 50% of circulating plasma androstenedione is of adrenal origin [24]. It was proposed that the overlapping area between the zona fasciculata and reticularis, where both CYP17A1/POR/CYB5A and HSD3B2 are expressed, is responsible to generate adrenal androstenedione [25]. Ovarian HSD3B2 may also contribute to androstenedione generation in premenopausal women; however, the HSD3B type 1 enzyme expressed in many nonsteroidogenic tissues (i.e., liver, skin, adipose, kidney, and others) also generates androstenedione from DHEA [26, 27]. Further extra-adrenal downstream conversion towards potent, androgen receptor-activating androgens is performed by 17β-hydroxysteroid dehydrogenases (HSD17Bs) yielding testosterone. Testosterone generation is also catalyzed in small proportions in the adrenal cortex by the enzyme 17β-hydroxysteroid dehydrogenase type 5 (AKR1C3) [28]. Finally, peripheral 5α-reduction by 5α- reductases (SRD5s) yield 5α-dihydrotestosterone (DHT), the most potent androgen [4, 29].
The majority of DHEA leaves the adrenal in form of its sulfate ester DHEAS, which is by far the most abundant steroid hormone in the postadrenarchal circulation in higher primate species [30]. The conversion of DHEA to DHEAS is catalyzed in the zona reticularis by the enzyme DHEA sulfotransferase (SULT2A1) [11]. DHEA sulfation is energetically a highly expensive reaction, which requires activated sulfate in the form of 3′-phosphoadenosine 5′-phosphosulfate (PAPS), generated and delivered to SULT2A1 by PAPS synthase 2 (PAPSS2) [31].
Adrenal Precursors of the 11-Oxygenated Androgen Pathway
Recent work has highlighted the significance of the conversion of androstenedione to 11β-hydroxyandrostenedione by adrenal CYP11B1, which occurs in significant quantities [32, 33]. This metabolite was previously thought to be a “waste product” of adrenal androgen production [34, 35, 36]. However, 11β-hydroxylation of androstenedione, catalyzed by CYP11B1, forms 11β-hydroxyandrostenedione, a precursor to an alternative pathway to active 11-oxygenated androgens [37, 38] (Fig. 1). These are secreted by the adrenal in huge quantities, even superseding the amounts of adrenally derived androstenedione, both at baseline and after ACTH stimulation [32, 34]. Further conversion via 11-keto-androstenedione generates 11-keto-testosterone (11-keto-T) and further downstream 11-keto-dihydrotestosterone (11-keto-DHT) [34, 39]. Importantly, the in vitro androgen receptor-transactivating activity of 11-keto-T and 11-keto-DHT is similar to that of testosterone and DHT [32, 39, 40, 41]. The implications of this additional androgen class are wide-ranging: recent studies have shown that they play a key role in androgen excess states, representing the majority of circulating androgens in polycystic ovary syndrome [42]. In addition, 11-oxygenated androgens are found to be increased in CAH due to CYP21A2 deficiency [43, 44, 45, 46].
Monogenic Disorders of Adrenal Steroidogenesis
Disorders Affecting Cholesterol Import and Metabolism
StAR Deficiency - Congenital Lipoid Adrenal Hyperplasia
A key player in shifting cholesterol from the outer to the inner mitochondrial membrane is StAR [47], and mutations in the STAR gene result in a disorder with disruption of all steroidogenesis, congenital lipoid adrenal hyperplasia (CLAH) [48]. In its most severe form, affected babies cannot produce significant amounts of any steroid and present with salt-wasting adrenal crisis in the neonatal period. They exhibit high ACTH levels, increased plasma renin activity, and grossly enlarged adrenal glands, which contain excessive amounts of cholesterol and its derivatives [49]. The complex physiology of StAR was elucidated by studying patients with disease-causing mutations and led to a “two-hit” disease model for congenital lipoid hyperplasia in StAR deficiency [48]: most (but not all) steroidogenesis is StAR dependent, and the loss of StAR, the first hit, results in compensatory activation of the ACTH axis and de novo cholesterol biosynthesis, leading to accumulation of cholesterol in the cell. The destruction of the cell's capacity to generate steroids due to toxic effects of accumulating cholesterol molecules and its metabolites then follows as the second hit. Some of the unusual features observed in patients with StAR mutation are better explained with the following model. (1) 46,XY babies present with female external genitalia but wolffian internal structures: androgen-producing testicular Leydig cells are destroyed during the first trimester when gonadal steroidogenesis peaks. Sertoli cells remain intact and adequate levels of anti-müllerian hormone inhibit the development of female internal structures. (2) As placental steroidogenesis is not dependent on StAR and the placenta is able to produce sufficient amounts of progesterone to maintain pregnancy, babies come to term with no significant antenatal complications. (3) Fetal adrenal androgen production is also compromised, leading to low maternal estriol (E3) levels, which can be detected antenatally in a maternal urine sample [50]. (4) Salt and water balance is controlled antenatally by the placenta, but postnatally overt MC deficiency emerges within 2–3 weeks, the delay is best being explained by progressively emerging cellular damage and some remaining StAR-independent MC biosynthesis. (5) Lastly, adolescent 46,XX females were reported to exhibit low sex steroid production that is sometimes sufficient to initiate the development of secondary sexual characteristics and pubertal progression [51, 52]: as the ovary is steroidogenically quiescent until puberty, it is “protected” from cellular damage until steroidogenesis starts. Progressive cellular damage in the luteal phase of the menstrual cycle due to accumulating cholesterol results in anovulatory cycles; however, (unopposed) oestrogen production can be sufficient for breast development and cyclical vaginal bleeding.
Milder clinical phenotypes have been described as “nonclassic” forms of CLAH, where residual activity of the mutant StAR protein was evident (up to 25–30% of wild-type activity): patients presented with adrenal insufficiency after infancy, male external genitalia in 46,XY individuals, mild hypergonadotrophic hypogonadism, and mild MC deficiency [53, 54, 55].
Overall, StAR deficiency is rare in most populations. To date, just about 80 genetic alterations of the StAR gene associated with lipoid CAH have been reported; half of them are missense mutations (www.hgmd.cf.ac.uk). StAR deficiency is relatively common in East Asia due to the p.Q258X founder mutation [56, 57]. Founder effects contribute to a higher disease frequency in Arab [48, 58] and Swiss [59] populations.
Children with CLAH need steroid replacement therapy with GCs, MCs, and sodium during infancy and may require sex steroid replacement later on.
P450 Cytochrome Side-Chain Cleavage (CYP11A1) Deficiency
It was thought that foetuses lacking the CYP11A1 enzyme could not survive pregnancy as placental steroidogenesis and, hence, the progesterone production maintaining pregnancy depends on this enzyme. However, cases with mutations in the CYP11A1 gene have been reported in individuals, which were clinically and biochemically identical to CLAH [50, 60, 61, 62, 63]. The fact that affected foetuses survive pregnancy could be explained by prolonged progesterone production from the corpus luteum, sufficient to maintain the nourishing characteristics of the endometrium. However miscarriages and preterm delivery have been reported in pedigrees with CYP11A1 deficiency carrying mutations predicted to severely affect enzyme function [50, 63].
Nonclassic CYP11A1 deficiency has been described in nearly a dozen cases where residual enzymatic activity was maintained in in vitro assays [64, 65, 66, 67]. Good correlation between clinical phenotype and residual enzyme activity has been reported [67]. Similar to nonclassic StAR deficiency, patients can present with late-onset adrenal insufficiency manifesting in early to mid-childhood with variable degrees of sex steroid deficiency [66]. Also, isolated adrenal insufficiency with normal sex steroid production has been reported in single cases [66, 67].
The main difference between StAR and CYP11A1 deficiency is the size of the adrenals, as there is no cholesterol accumulation in CYP11A1 deficiency, and thus the adrenals (and gonads) remain small. However, the only way to differentiate them diagnostically is via genetic testing [60].
Management of CYP11A1 deficiency is similar to CLAH due to STAR mutations, with replacement of GC, MC, and sex steroids, as required.
Monogenic Disorders due to Mutations in Adrenal Steroidogenic Enzymes
The focus of this section is on the variants of CAH, a group of autosomal recessive diseases caused by inactivating mutations in genes encoding enzymes or co-factors involved in cortisol biosynthesis. Depending on the exact location of the steroidogenic block, there can be excess or deficiency of MCs and adrenal androgen synthesis, respectively. Steroidogenic enzymes implicated in the pathophysiology of CAH include 21-hydroxylase (CYP21A2), 11β-hydroxylase (CYP11B1), 17α-hydroxylase/17,20 lyase (CYP17A1), and 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2). Mutations in POR, an electron donor enzyme serving CYP21A2 and CYP17A1, cause the most recently identified variant of CAH, which will be discussed in the section Monogenic Disorders Affecting Co-Factor Enzymes of Steroidogenesis.
For each condition, we mention a brief outline on management focusing on hormonal replacement therapy. Patients with disorders of sex development (DSD) require holistic care within an experienced multi-disciplinary service, where also the issue of genital surgery needs to be addressed individually in accord with evidence-based guidelines on the management of DSD. To discuss the complexity of this issue is beyond the scope of this mini review and we would refer to excellent reviews and consensus guidelines recently published [68, 69].
21-Hydroxylase (CYP21A2) Deficiency
21-Hydroxylase deficiency is by far the most common form of CAH, accounting for more than 95% of cases. The incidence of the classical form of 21-hydroxylase deficiency is reported to be in the region of 1 in 9,800–16,000 live births, but this varies greatly according to ethnicity and location [70, 71]. Heterozygous carriers are found in most populations with a prevalence of 1 in 50.
CYP21A2 is a key enzyme required for both MC and GC synthesis, but not for production of adrenal androgens (Fig. 1). Defective 21-hydroxylation results in deficient GC and MC synthesis and leads to accumulation of precursor steroids prior to the enzymatic block, most notably 17OHP, which is used as the marker steroid for diagnosis and neonatal screening [72]. Following the lack of negative feedback from reduced cortisol, increased amounts of ACTH continue to stimulate the adrenal cortex. However, ACTH stimulation can only achieve upregulation of adrenal androgen output and, after long-lasting stimulation, adrenal hyperplasia, which gave the condition its name. Due to the accumulation of 17OHP, there is also increased generation of androstenedione directly from 17OHP. This conversion does not contribute much to androgen synthesis under physiological conditions, as CYP17A1 has a strong substrate preference for 17Preg rather than 17OHP (Fig. 1). The hallmark combination of raised 17OHP with elevated adrenal androgens establishes the diagnosis, which can be confirmed with genetic testing [71].
CYP21A2 and its nonfunctional but highly homologous pseudogene (CYP21A1P) are located close together in the HLA class III region on the short arm of chromosome 6 [73]. Approximately 95% of disease-causing mutations in 21-hydroxylase deficiency are variants, point mutations, or deletions caused by meiotic recombination events between CYP21A2 and CYP21A1P [70, 74]. Approximately 1% of inactivating mutations in CYP21A2 arise de novo. Most CAH patients (65–75%) are found to have compound heterozygous mutations with the clinical phenotype determined by the more productive allele [70]. Complete inactivating mutations result in the salt-losing phenotype, whereas 1–2% of 21-hydroxylase function is sufficient to manifest with the simple virilizing phenotype [70, 75].
Depending on the degree of loss-of-function caused by a distinct mutation, either, only GC or both GC and MC deficiency are present. In childhood, the classical forms of 21-hydroxylase deficiency are accordingly differentiated into “salt-wasting” CAH (75%; GC + MC deficiency) and “simple virilizing” (25%; GC deficiency only) [71]. In the absence of neonatal screening, the salt-wasting form presents with life-threatening adrenal “salt-losing” crisis within the first 2 weeks of life and ambiguous genitalia in affected 46,XX babies from birth (46,XX DSD). Deficiency of cortisol affects the growth subsequent function of the adrenal medulla. Reduced production of epinephrine and metanephrine exacerbates the potential for hypoglycaemia during adrenal crisis [76].
The simple virilizing form presents with symptoms of androgen excess in isolation, i.e., 46,XX DSD from birth [3]; 46,XY individuals affected by the simple virilizing form may present with premature pubic/axillary hair growth or precocious pseudo-puberty in early childhood and rapid skeletal growth with advanced bone age [3, 77].
Treatment requires GC and, if deficient, MC replacement, during infancy also additional sodium supplementation [72]. GC dosing has to be increased during times of physical stress/illness (stress dose cover). As with other virilizing forms of CAH, the aim is not only to replace for GC deficiency and prevent adrenal crisis but also to “switch off” adrenal androgen production by reducing ACTH drive. Inadequate dosing results in increased risk of adrenal crisis and inadequate androgen suppression, the former being a leading cause of mortality in CAH [78] and the latter leading to rapid premature skeletal growth and reduced height potential in affected children [79]. Therefore, GC doses required may be high, usually 10–15 mg/m2/day of hydrocortisone. However, attempts to completely normalize 17OHP levels frequently result in overtreatment. Patients run the risk of overtreatment with developing Cushingoid side effects impacting on growth and metabolic health. In the long term, patients have an increased risk of cardiovascular disease and metabolic syndrome [80]. Fertility problems are frequent in both sexes, related to both under- and overtreatment of GCs. Women are affected by menstrual irregularities and chronic anovulation; and many male patients have compromised fertility due to testicular adrenal rest tissue with compromised spermatogenesis and frequent hypothalamic-pituitary-gonadal dysfunction [81, 82, 83].
Though patients with simple virilizing CAH do not present with clinically overt MC deficiency, subclinically deficient MC production has been found in all forms, not exclusively the salt-wasting phenotype [84, 85, 86]. A plasma aldosterone to renin ratio can be used to evaluate the degree of MC insufficiency [72]. Newborns and infants are relatively MC resistant as they have immature renal tubules with a limited capacity to reabsorb sodium, hence the requirement for additional salt supplementation during this time [72].
During childhood, the GC most widely used and recommended is short-acting hydrocortisone (= cortisol); this is also the GC of choice in adulthood. However, multiple hydrocortisone doses are not sufficient to control androgen excess, sometimes management of adult patients requires long-acting synthetic GCs such as prednisolone or, in some cases, dexamethasone.
Monitoring GC treatment in CAH can be challenging. The current recommended indicators for treatment efficacy are measurement of 17OHP, androstenedione, and testosterone in children coupled with height velocity and (after 2 years of age) annual bone age assessment [72]. Again, the overall goal is not to aim for 17OHP to normalize as this would results in overtreatment with GCs. It is very difficult to physiologically replace GCs orally in a circadian manner and most patients show androgen excess with upregulation of both classic and alternative androgen pathways [87]; a recent study has shown that whilst classic androgen synthesis appeared controlled, the alternative androgen pathways can still produce in excess on standard GC therapy in CAH [45]. The challenges with treatment monitoring may be greater during puberty because of activation of the hypothalamic-pituitary-gonadal axis, altered adrenal enzymatic expression leading to increased androgen production, physiological rapid growth, and psychological sequelae conferred with chronic disease. After puberty, the emphasis for treatment shifts to prevention of long-term adverse effects [88].
11β-Hydroxylase (CYP11B1) Deficiency
11β-hydroxylase deficiency is the second most common cause of CAH, accounting for 2–5% of reported cases of European ancestry, with an overall incidence of 1 in 100,000–200,000 live births. It is caused by inactivating mutations in the CYP11B1 gene. Due to founder mutations, the condition is more common in Israel among Jewish immigrants from Morocco [89]. The CYP11B1 gene is located on the long arm of chromosome 8 and in very close proximity to the highly homologous CYP11B2 (aldosterone synthase) gene [70]. Recombination events have been reported where the CYP11B1 gene is under control of the CYP11B2 promoter, which responds to angiotensin II and not ACTH, resulting clinically in classic CYP11B1 deficiency [90]. More than 100 mutations have been reported to date in the CYP11B1 gene (www.hgmd.cf.ac.uk). There is no mutational hotspot, and mutations have been found along the whole gene, most being missense mutations significantly reducing enzymatic activity [91].
Most cases of 11β-hydroxylase deficiency present with a classical phenotype with the hallmark features of androgen excess and hypertension [92]. CYP11B1 catalyzes the conversion of 11-deoxycortisol to cortisol and of 11-deoxycorticosterone to corticosterone in the zona fasciculata (see Fig. 1). Reduced cortisol concentrations in CYP11B1 deficiency lead to HPA axis activation. Under the influence of ACTH, concentrations of the MC receptor agonist corticosterone increase markedly, leading to hypertension, suppression of the RAAS and low aldosterone concentration despite the ability of the zona glomerulosa to produce aldosterone. Hypertension might not become apparent during the neonatal period due to the renal MC resistance, and some newborns may even present with salt loss [93]. Androgen excess can be severe, presenting with 46,XX DSD from birth and precocious pseudo-puberty in males.
Non-classic forms of CYP11B1 deficiency are rare and have a similar phenotype to non-classic CYP21A2 deficiency, exhibiting signs of androgen excess like precocious pseudo-puberty or symptoms suggestive of polycystic ovary syndrome in post-pubertal females [91, 94, 95, 96]. Arterial hypertension is not commonly found early in the non-classic form, but can develop later in life, making it a diagnosis not to be missed, as health implications can be severe [96].
Diagnostically, elevated serum corticosterone, 11-deoxycorticosterone and 11-deoxycortisol levels, or their urinary metabolites (tetrahydrocorticosterone, tetrahydro-11-deoxycorticosterone, tetrahydro-11-deoxycortisol) are strong biochemical markers for this condition [92, 96, 97] (Table 1). As 17OHP is accumulating prior to the more pronounced increase in 11-deoxycortisol, some babies in newborn screening programs are wrongly diagnosed as suffering from CYP21A2 deficiency [98].
Table 1.
Overview of monogenic disorders of adrenal steroidogenesis with associated effects on steroidogenic pathways and their specific key analytic steroid abnormalities detected with either serum or urinary steroid profiling useful in the diagnosis for each condition
| Name | Gene | Effect on MC synthesis | Effect on GC synthesis | Effect on sex steroid synthesis | Key analytic steroid abnormalities | Other |
|---|---|---|---|---|---|---|
| Congenital lipoid adrenal hyperplasia | StAR |
Classic: hyperreninemic, hypokalaemic hypotension/neonatal salt-wasting crisis Nonclassic: variable degrees/normal |
Classic: neonatal adrenal insufficiency Non-classic: late-onset adrenal insufficiency |
Classic: female external genitalia in 46,XY; spontaneous ovarian sex steroid production in 46,XX reported Nonclassic: variable degrees of 46,XY DSD |
Absence/reduction of all steroid classes, including precursors | Large adrenals with accumulating cholesterol and cholesterol metabolites Also affects gonadal androgen production |
| P450 side-chain cleavage deficiency | CYP11A1 | As above | As above | As above | As above | Small, hypoplastic adrenals Also affects gonadal androgen production |
| CAH due to 21-hydroxylase deficiency | CYP21A2 |
Classic: hyperreninemic, hypokalaemic hypotension/neonatal salt-wasting crisis Nonclassic: variable degrees/normal |
Classic: neonatal adrenal insufficiency Nonclassic: various degrees of (partial) adrenal insufficiency; normal |
Classic: prenatal androgen excess (46,XX DSD); maybe the only symptom from birth (“simple virilizers”) Nonclassic: various degrees of adrenal androgen excess after birth |
Serum: elevated 17OHP and 21-deoxycortisol Urine: elevated metabolites of 17OHP (17OH-pregnanolone [17HP], pregnanetriolone [PT]) and 21-deoxycortisol (pregnanetriolone [P'TONE]) |
Enlarged adrenals |
| CAH due to 11-hydroxylase deficiency | CYP11B1 |
Classic: low renin hypertension with normal aldosterone levels Nonclassic: mild/absent hypertension |
Classic: adrenal insufficiency Nonclassic: various degrees of partial adrenal insufficiency; normal |
Classic: prenatal androgen excess (46,XX DSD) Nonclassic: various degrees of androgen excess after birth |
Serum: elevated 11-deoxycortisol [S] and 11-deoxycorticosterone [DOC] Urine: elevated metabolites of S (tetrahydro-11-deoxycortisol [THS]) and DOC (tetrahydrocorticosterone [THDOC]) |
|
| CAH due to 17-hydroxylase deficiency | CYP17A1 | Low-renin, hypokalaemic hypertension; can be absent in partial defects | Various degrees of adrenal insufficiency, often rarely adrenal crisis (cross-reactivity of MC precursors on GC receptor) | 46,XY DSD from birth; absence of secondary sexual characteristics in both sexes |
Serum: elevated corticosterone [B] and 11-deoxycorticosterone [DOC]; low androgens Urine: increased ratio of MC over GC metabolites to assess 17-hydroxylase activity; increased ratio of androgens over GC metabolites to assess 17,20 lyase activity |
Also affects gonadal androgen production |
| CAH due to 3ß-hydroxysteroid-dehydrogenase deficiency | HSD3B2 |
Classic: hyperreninemic, hypokalaemic hypotension/neonatal salt-wasting crisis Nonclassic: variable degrees/normal |
Classic: neonatal adrenal insufficiency Nonclassic: various degrees of (partial) adrenal insufficiency; normal |
Classic: DSD in both sexes Nonclassic: premature adrenarche, precocious pseudopuberty, irregular menstrual cycles |
Serum: increased (stimulated) ratio of Δ4 (progesterone, 17OHP, androstenedione) over 5 steroids (pregnenolone, 17Preg, DHEA) Urine: elevated ratio of DHEA over GC metabolites and 5-pregnenetriol (5PT) over GC metabolites |
Also affects gonadal androgen production |
| CAH due to P450 oxidoreductase deficiency | POR | Some elevation of MC metabolites but overt arterial hypertension has not been reported | Various degrees of GC deficiency in 85% of patients | DSD in both sexes from birth in 75% of patients; delayed puberty in both sexes of various degrees |
Serum: unspecific mild elevation of 17OHP; increased pregnenolone, progesterone, 17OHP Urine: combined impairment of diagnostic ratios for CYP17A1 and CYP21A2; distinct accumulation of pregnenolone metabolites (pregnanediol [PD]) |
Also affects gonadal androgen production; affects bone development: skeletal malformations of the Antley-Bixler phenotype spectrum |
| Cytochrome b5 deficiency | CYB5A | None | None | 46,XY DSD; absence of puberty in 46,XX |
Serum: isolated sex steroid deficiency Urine: normal ratio of MC over GC metabolites reflecting normal 17-hydroxylase activity; increased ratio of androgen over GC metabolites reflective of 17,20 lyase activity |
Raised methaemoglobin levels; also affects gonadal androgen production |
| Apparent DHEA sulfotransferase deficiency | PAPSS2 | None | None | Premature adrenarche and polycystic ovary syndrome (hyperandrogenemic oligoanovulation) |
Serum: very low/undetectable DHEAS and sulfated steroid compounds, with high DHEA and downstream androgens (testosterone, androstenedione) Urine: high androgen metabolites and precursors |
Also causes skeletal abnormalities: brachyolmia type 4 |
MC, mineralocorticoid; GC, glucocorticoid; CAH, congenital adrenal hyperplasia; DSD, disorders of sex development.
Treatment requires GC replacement in doses sufficient to ameliorate hypothalamic-pituitary-adrenal axis feedback to control androgen excess and hypertension, which may also require treatment with MC receptor antagonists.
17α-Hydroxylase/17,20 Lyase (CYP17A1) Deficiency
CAH due to CYP17A1 deficiency is rare, accounting for 1% of CAH cases. CYP17A1 deficiency affects both GC and androgen production, which channels steroidogenesis to the MCs causing excess production (see Fig. 1). CYP17A1 deficiency is caused by inactivating mutations in the CYP17A1 gene located on the long arm of chromosome 10. Inactivating mutations have been located along the whole length of the gene, without any distinct hot spot, although N-terminal mutations are found more frequently. To date, just over 100 mutations have been reported, 75% of which are missense mutations (www.hgmd.cf.ac.uk). There are some frequently occurring mutations which are ethnic background-specific, in particular a small 4bp insertion in exon 8 in Dutch Friedlaenders, a 3 amino acid in-frame deletion of exon 8 in South East Asia, the p.F53/54del mutation, and, within the Brazil ian population, the missense mutations p.W406R and p.R362C [99].
Classically, 46,XX patients affected by CYP17A1 deficiency present at early pubertal age, due to lack of pubertal development including primary amenorrhea, with marked hypergonadotrophic hypogonadism. Partial adrenal insufficiency might become evident with synacthen testing, and low renin hypertension is usually manifest. In contrast, 46,XY patients present with DSD, having undervirilized external genital from birth. The fact that ‘asymptomatic’ 46,XX patients rarely present with adrenal crisis despite being profoundly GC deficient is best explained due to the affinity of MC precursors (in particular DOC) towards the GC receptor. The accumulation of DOC, enhanced by the activation of the hypothalamic-pituitary-adrenal axis with some regulatory effect on the MC pathway, results in suppression of the RAAS and aldosterone production with biochemical findings of hypernatremia, hypokalemia, hyporeninemia, and arterial hypertension.
Milder forms with some residual enzyme activity (partial defects) have been reported; whilst those always disrupt 17,20 lyase activity, the impact on the enzyme's 17α-hydroxylase activity can be milder. Such cases, sometimes labelled as “isolated 17,20 lyase deficiency,” are extremely rare, and patients present with isolated androgen deficiency [100]. However, their capacity to produce sufficient amounts of GCs is attenuated, indicating some degree of 17α-hydroxylase impairment [100, 101, 102].
The two key pillars of treatment are GC substitution and anti-hypertensive management [99]: although GC replacement is less essential (due to DOC accumulation binding to the GC receptor), GC will lower DOC levels and augment blood pressure and potassium levels. Ideally, partial substitution with GC would allow DOC to reduce but not quite normalize to allow protection from adrenal crisis. In addition, side effects from GC therapy are reduced. With this approach, additional anti-hypertensive therapy is required, as blood pressure would not normalize completely, and MC receptor antagonists would be the first-line therapy. In puberty, the careful introduction of oestrogen will be necessary in 46,XX females; equally, gonadal testosterone production alone is not sufficient to bring an 46,XY boy through puberty, and induction with exogenous testosterone is required.
HSD3B2 Deficiency
HSD3B2 deficiency is a rare form of CAH, presenting in early infancy [103, 104]. HSD3B2 is at a critical branch point in steroidogenesis, gating entrance to all three steroid pathways (Fig. 1). The two isoforms of 3β-hydroxysteroid dehydrogenase are encoded by genes located in close proximity to each other on the short arm of chromosome 1 (HSD3B1 and HSD3B2); HSD3B2 is expressed in adrenals and gonads, whilst HSD3B1 is expressed in multiple peripheral tissues. More than 60 mutations of the HSD3B2 gene have been described (www.hgmd.cf.ac.uk), and good genotype-phenotype correlations exists with regards to MC deficiency, where severe loss-of-function mutations predict neonatal salt-wasting. There are no reported mutations in the human HSD3B1 gene to date.
Classically, affected patients present in infancy with salt-wasting adrenal crisis and high-renin hypotension, similar to CYP21A2 deficiency. However, both sexes can present with DSD: in 46,XY babies, deficiency of adrenal and gonadal androgens result in undermasculinization. The observation that 46,XX babies can present with virilized genitalia is explained by the compensatory action of the HSD3B type 1 isoform expressed in placenta and peripheral tissues: HSD3B1 facilitates downstream conversion of accumulating adrenal DHEA to androstenedione in the periphery, causing androgen excess. Apparently, this is sufficient to virilize an 46,XX baby, but would not compensate for the loss of gonadal HSD3B2 activity in 46,XY babies.
The presence of the HSD3B1 isoform can make this diagnosis difficult: one would expect that 17OHP levels are low; however, HSD3B1 peripherally converts 17α- hydroxypregnenolone (17OHPreg), which accumulates before the block, into 17OHP, which can be increased in newborns with HSD3B2 deficiency. The overall steroid precursor constellation in this condition is a predominance of Δ5 steroids (i.e., pregnenolone, 17Preg, and DHEA) over Δ4 steroids (progesterone, 17OHP, and androstenedione); this ratio is further exaggerated after ACTH stimulation (Table 1). Urinary steroid profiling is similarly accurate and less invasive to establish the diagnosis [97] (Table 1).
There is broad phenotypic variation, and presentation can vary from severe salt-wasting forms, to non-salt-wasters with variable degrees of virilization. This may include late-onset forms manifesting with hirsutism and irregular periods resembling a polycystic ovary syndrome phenotype in patients with confirmed pathogenic mutations. In addition, a clinical and biochemical phenotype exists, where androgen excess is present with predominance of Δ5 steroids, where no genetic abnormalities in the HSD3B2 gene were detected [105, 106]. The molecular basis of this condition, often referred to as “functional HSD3B2 deficiency,” is currently unknown.
Monogenic Disorders Affecting Co-Factor Enzymes of Steroidogenesis
POR Deficiency
POR is required as a crucial electron donor enzyme for all microsomal (type 2) CYP enzymes, including steroidogenic enzymes CYP21A2, CYP17A1, and, to a lesser degree, CYP19A1 (P450 aromatase). The discovery of the genetic cause in 2004 [107, 108] explained the pathophysiology of what was described before as “apparent combined 17α-hydroxylase and 21-hydroxylase deficiency,” reported in 1985 [109]. The exact incidence is unknown, but POR deficiency is a rare CAH variant, with approximately 75 mutations in 140 individuals reported to date (www.hgmd.cf.ac.uk) [110]. The POR gene is located on the long arm of chromosome 7. There are 2 mutations which are frequently found in distinct ethnic groups: the p.A287P mutation is most common in White Caucasians, whereas the p.R457H mutation is most commonly found in individuals of Asian ancestry.
The majority of affected patients will have some degree of cortisol deficiency, with 90% requiring at least stress-related GC replacement. Therefore, all affected patients should undergo cosyntropin stimulation testing, regardless of presence or absence of DSD [111]. Approximately 50% of those who require GC treatment will need permanent hydrocortisone replacement, with the other 50% requiring replacement during periods of stress. 75% of patients will have some degree of DSD, and this has been reported in both sexes: 46,XY babies are often undermasculinized and 46,XX babies are born virilized without progression of virilization postnatally. This intriguing finding is explained by the presence of an alternative pathway to 5α-DHT synthesis only active during fetal life [108], elements of which were first described in the foetal gonad of the tammar wallaby pouch young [112]. Postnatally, activity of the pathway ceases, so there is sex steroid deficiency in both sexes. In puberty, both sexes can present with delayed development of sexual characteristics, in particular females often develop significant hypergonadotropic hypogonadism and severe ovarian cysts prone to torsion, which can be difficult to manage [113, 114]. In addition, expectant mothers carrying affected foetuses can experience virilization during pregnancy, which characteristically resolves postpartum [107, 108, 111, 113, 115]; this is explained by the production of 5α-reduced androgens via the alternative pathway by the foetus, that cannot be aromatized by the placenta and is transferred to the maternal circulation.
POR deficiency is not only a steroidogenic but also a multi-system disorder, as it affects all microsomal CYP enzymes that require POR for proper function (Fig. 2). The most striking clinical findings in the majority of affected POR-deficient patients are skeletal abnormalities, which have been described as part of the Antley-Bixler syndrome phenotype. Typical malformations include large joint synostosis such as radio-humeral synostosis, congenital bowing of the femurs, and hand and foot malformations such as long palms, camptodactyly, and rocker bottom feet. Craniofacial anomalies are frequent, such as craniosynostosis with midface hypoplasia, most commonly involving the coronal and lambdoid sutures, which can in severe forms be complicated by hydrocephalus requiring surgical intervention [111]. The number and severity of associated malformation features form the basis of a scoring system proposed to classify the severity of Antley-Bixler syndrome phenotype [111]. It is thought that the molecular basis of the malformation phenotype is the disruption of the activity CYP enzymes involved in sterol synthesis and retinoic acid metabolism, both of which have been shown to cause a skeletal malformation phenotype in murine knockout models [116].
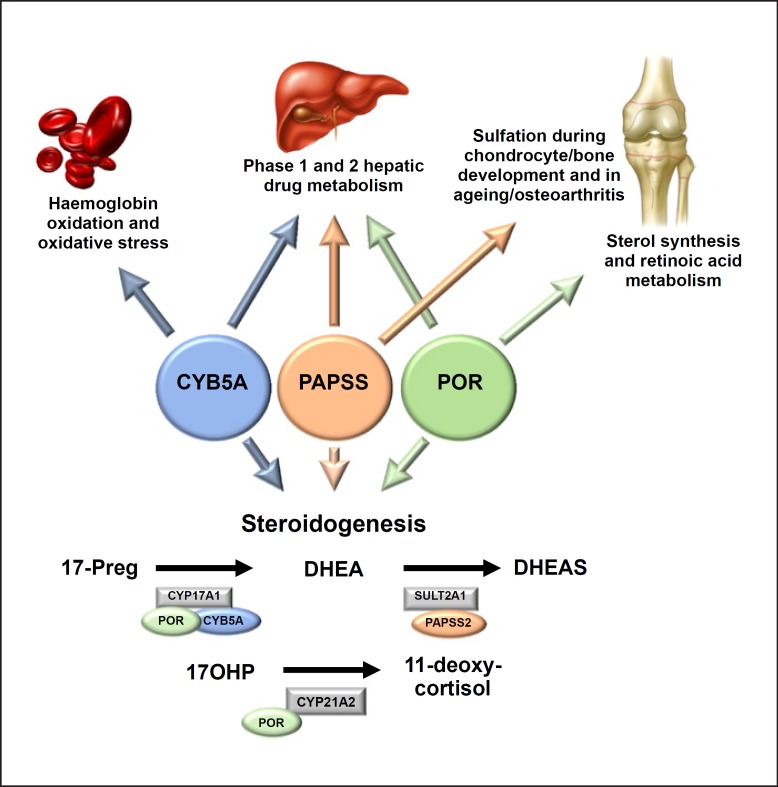
Fig. 2.
Schematic diagram illustrating the known implications of co-factors CYB5A, PAPSS2, and POR beyond steroid hormone biosynthesis on haemoglobin, hepatic, and chondrocyte metabolism. CYB5A, cytochrome b5; PAPSS2, PAPS synthase 2; POR, P450 oxidoreductase.
In addition, loss of POR function affects a number of key CYP enzymes involved in hepatic phase 1 metabolism, including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4, which metabolize about 80% of xenobiot ics and drugs used in clinical practice [117]. In addition, CYP3A4 metabolizes oestrogens and GCs, which is important for treatment considerations in patients with POR deficiency as the metabolic clearance of exogenous hormone replacement is expected to be reduced, thus potentially exposing patients to higher GC and oestrogen levels than intended. So far, various studies have assessed the impact of certain POR mutations on POR-dependent hepatic cytochromes in vitro (see [118, 119] for a comprehensive overview), with one detailed in vivo phenotyping study confirming altered drug detoxification for a variety of substances [120].
A number of studies have looked at genotype-phenotype correlations [111, 113, 115]. Generally, phenotypic expression of a distinct genotype can be variable, but mutations causing 46,XX DSD usually present with normal genital phenotype in 46,XY individuals and vice versa; similarly, the severity of the skeletal malformation phenotype is increased in carriers of major loss-of-function mutations [110, 111]. With severe phenotypes, there have been reported cases of stillbirth and early neonatal death [121].
Diagnosis of POR deficiency can usually be readily established by urinary steroid profiling by gas chromatography-mass spectrometry, which reveals the hallmark feature of combined impairment of CYP21A2 and CYP17A1 activities [97, 111] and a characteristic accumulation of pregnenolone metabolites [97] (Table 1). Molecular genetic testing will then confirm the diagnosis. Prenatal testing is possible using maternal urinary steroid profiling from 12 weeks onwards [121] or through molecular genetic techniques once the POR pathogenic variant has been ascertained for a given family [110].
Hormonal replacement therapy consists of GC replacement, mostly only required as stress dose cover, depending on the degree of adrenal insufficiency. Overall, GC doses are lower as adrenal androgen suppression is not necessary. In addition, treatment goals should holistically aim to address other associated features as well. Genital abnormalities may require surgical intervention, and this should be addressed within a holistic and experienced DSD service. Males may require testosterone replacement during puberty; similarly, females may require oestrogen replacement during this time, ideally as oestrogen patch to avoid hepatic first-pass metabolism (see above). Skeletal malformations may require physical and occupational therapy or surgical intervention.
Cytochrome b5 (CYB5A) Deficiency
CYB5A deficiency is a very rare monogenic disorder, with only 5 cases reported so far [122, 123, 124]. In addition to conveying 17,20 lyase activity of CYP17A1 (Fig. 1), CYB5A also plays key roles in haemoglobin synthesis and hepatic phase 1 drug metabolism (Fig. 2). The first reported case of CYB5A deficiency was a patient with severe methaemoglobinaemia presenting with fatal cyanosis at birth [122, 125]. The baby, who carried a severe splice-site mutation, was also found to have ambiguous genitalia; however, detailed endocrine investigations were not performed [125]. Only recently, 4 children from 2 different pedigrees with CYB5A deficiency have been reported: all affected children did not have any clinical cyanosis, but insufficient masculinization in 46,XY individuals (46,XY DSD) and lack of pubertal development in the only affected 46,XX individual [123, 126]. Methaemoglobin levels were found to be mildly raised without clinical evidence of cyanosis. Affected children were homozygous for a novel nonsense mutation (p.W27X), likely to abolish protein activity [123], and a homozygous missense mutation, p.H44L, which retains minimal protein function on 17,20 lyase activity in vitro [126].
Biochemical investigations, in particular urinary steroid profiling, revealed an exclusive impairment of the 17,20 lyase activity of CYP17A1 with intact 17α-hydroxylase activity. This reflects normal GC and MC production with isolated androgen deficiency, in essence true isolated 17,20 lyase deficiency [126]. Some cases of CYP17A1 and POR deficiency have also been described as isolated 17,20 lyase deficiency; however, biochemistry reveals impaired 17α-hydroxylase activity in both instances [102, 127, 128]. Three affected individuals from a Swiss pedigree with isolated 17,20 lyase deficiency reported in the 1970s [129] have been shown to have mutations in the AKR1C2 and AKR1C4 genes [130]. Both enzymes are thought to be involved in the proposed alternative “backdoor” pathway to DHT, exemplified by POR deficiency and described in the respective above section of this review. CYP17A1 17,20 lyase activity supported by CYB5A is required in the early part of that pathway. These cases suggest the need for the alternative pathway to be active for normal male sexual differentiation [130].
PAPSS2 Deficiency
DHEA is the principal adrenal androgen precursor synthesized in the adrenal zona reticularis in post-adrenarche children and in the adrenal fetal zone prior to birth (see Fig. 1). Previous assumptions regarded the interconversion of DHEA and its sulfate ester DHEAS as a well-balanced equilibrium, in which DHEAS serves a storage pool for the (re)generation of active androgens. This equilibrium was thought to be maintained by 2 counteracting enzymes, the DHEA sulfotransferase SULT2A1, responsible for sulfate conjugation of the DHEA molecule, and steroid sulfatase, STS, cleaving the sulfate group off the DHEAS molecule to make it accessible for downstream activation towards active androgens. However, in a number of in vivo studies, we have previously shown that the inactivating step catalyzed by SULT2A1 is the predominant direction in normal human physiology, whereas STS activity makes only minor contributions to androgen activation [131, 132, 133].
The seminal case of apparent DHEA sulfotransferase activity due to PAPSS2 deficiency, a 6-year-old girl, first presented with signs of hyperandrogenism (premature adrenarche and later on hirsutism and irregular menstrual cycles) [134]. Strikingly, her biochemical assessment revealed undetectable serum DHEAS while circulating androstenedione and testosterone levels were increased. A defect in the DHEA sulfotransferase enzyme SULT2A1 was hypothesized, but no mutations were found. All sulfotransferases depend on the provision of the universal sulfate donor PAPS, which is generated by the human iso-enzymes PAPS synthase 1 and 2. Genetic analysis of both PAPSS1 and PAPSS2 in our patient revealed compound heterozygous mutations in PAPSS2, disrupting DHEA sulfotransferase activity [134].
PAPSS2 deficiency had previously been described in a large Pakistani kindred presenting with spondyloepimetaphyseal dysplasia (SEMD)/brachyolmia type 4 (MIM #603005) [135, 136]; interestingly, in our patient only subtle vertebral abnormalities could be detected radiologically but not clinically, suggesting that the residual enzymatic activity of the only partially activating mutation was largely sufficient to support sulfation processes required in chondrocyte and bone development [134]. Over recent years, a number of PAPSS2 mutations have been reported in patients with SEMD/brachyolmia, with only very limited data on endocrine function [137, 138, 139]. Steroid metabolome profiling following an oral DHEA challenge, performed in a family with 2 brothers affected by PAPSS2 deficiency, revealed that also the mother, a heterozygous carrier of a severe loss-of-function mutation, had impaired DHEA sulfation with clinical and biochemical evidence of androgen excess [140].
Although the underlying defect is rare, the elucidation of PAPSS2 deficiency has provided fundamental insights into the mechanisms controlling the equilibrium between androgen activation and inactivation. It highlights the crucial role of DHEA sulfation as a gatekeeper to human androgen synthesis: disruption of DHEA sulfation drives unconjugated DHEA molecules towards active androgen synthesis, causing androgen excess. To what extent PAPSS2 contributes to common phenotypic androgen excess remains to be established. In-depth phenotyping studies with targeted sequencing are lacking. Population-based GWAS studies describing only minor effects of more common variants in PAPSS2, SULT2A1, and STS [141, 142]. Like POR deficiency and CYB5A deficiency, PAPSS2 deficiency results in a multi-system disorder and in addition to impaired DHEA sulfation it impacts on chondrocyte and bone development and is likely to impact on hepatic phase 2 drug and xenobiotic metabolism (Fig. 2).
Outlook and Future Directions
Novel GC Replacement Strategies - Modified Release Formulations
Treatment for CAH with steroid hormone replacement was not available until the 1950s. Since this time, there has been little development in the way we conduct steroid hormone replacement therapy, particularly in the field of paediatrics. Coming to adulthood, a prospective study on the health status of CAH patients in the UK (CaHASE) showed alarming results: the majority of patients have poorly controlled androgen levels with nonphysiological GC replacement regimes affecting fertility and quality of life [80], which could be improved with better treatment options [143]. Only in recent years, there have been encouraging trials to explore new GC formulations for replacement therapy and to suppressing parts of the hypothalamic-pituitary-adrenal axis. Oral GC replacement in its current form is adequate at ameliorating cortisol deficiency, but fails to account for the normal physiological diurnal rhythm of cortisol production, fails to adequately suppress ACTH production via negative feedback mechanisms, and fails to suppress production of androgens without risking the side effects of gross overtreatment [144]. Learning from advances in the field of diabetes, trials with continuous subcutaneous infusions of hydrocortisone have been performed in patients with adrenal insufficiency [145, 146, 147]. Though the outcomes were generally favourable, with findings of lower mean ACTH levels and more physiological variation in cortisol levels, this is a costly strategy which requires intensive patient education; improvement in quality of life measures were inconsistent and side effects such as insertion site reactions were common. Methods to deliver oral GCs more physiologically are also in development. Plenadren® (Shire Pharmaceuticals Ltd.) is a dual-release hydrocortisone, consisting of an extended-release core surrounded by an immediate-release coating. It is licensed for once daily dosing in adults and was designed in the hope to improve treatment compliance as well as achieve more physiological GC replacement. Trials have shown sustained improvement in quality of life, reduction in central adiposity and improvements in lipid profile in adult patients with adrenal insufficiency [148]. Peak concentrations of cortisol are reached in the late morning and nadir lower in the late evening than a conventional regime [149]. However, as it is taken after awakening, Plenadren cannot mimic the physiological early morning cortisol peak and therefore is unlikely to achieve adequate androgen suppression required for treatment of 21-hydroxylase deficiency.
Chronocort® (Diurnal Ltd.) is another modified release form of hydrocortisone that has a delayed release and then sustained absorption profile. In contrast to Plenadren, it does not start to release cortisol immediately but only after a lag time of several hours after intake. It has been designed for twice daily dosing so that the larger evening dose has its peak effect in the early morning hours and smaller morning dose peaks in the afternoon/evening and provides GC cover for the day, in order to achieve a more physiological cortisol profile [149, 150]. Small early clinical trials have demonstrated cortisol profiles similar to physiologic cortisol secretion, a decrease in hydrocortisone dose equivalent to achieve lower androstenedione and 17-OHP levels compared to standard GC treatment. It also increased lean body mass, morning HOMA-IR, and osteocalcin [151, 152]. The trial did however report a slightly decreased bone mineral density (despite increased osteocalcin), some sleep disturbances and there was no reported difference in quality of life measure. Larger and more in-depth studies are required (and indeed currently ongoing) to fully assess the potential benefits, efficacy and long-term effect of this medication.
Other Approaches to Control CAH-Related Androgen Excess
Two recent proof-of-concept studies have shown novel approaches to decreasing androgen excess in CAH. The first involved the use of the CYP17A1 inhibitor abiraterone, originally designed for prostate cancer, where it profoundly decreases serum testosterone and improves survival in men with this condition. A small trial has been undertaken in women with CAH secondary to 21-hydroxylase deficiency, who were administered abiraterone acetate alongside physiological doses of hydrocortisone. This trial showed a significant reduction in androgen production (both androstenedione and testosterone), with no major side effects reported [153]. Thus, abiraterone treatment might facilitate reduction in the GC dose required as androgen control with combination therapy is improved. However, this does not address the effects of a persistent early morning rise in ACTH observed with standard GC treatment regimens and would be contraindicated in anyone requiring fertility due to effects on gonadal function.
Another recent novel approach has targeted the hyperactivity of the HPA axis in CAH. To reduce the ACTH-driven increase in androgen production in CAH, a recent study has administered a corticotropin-releasing hormone receptor antagonist. This initial proof-of-concept study demonstrated a reduction in ACTH and 17OHP, but no consistent trend with androstenedione or testosterone after a single dose of a CRF1 receptor antagonist [154]. Further studies will be required to fully demonstrate the efficacy of this treatment strategy.
Learning More from the Few - The Use of Registries
All disorders of steroidogenesis are rare diseases. As with any condition, a better understanding and improving patient care require experience from large patient cohorts. International registries allowing anonymized and secure data exchange and hence an accumulation of knowledge have been developed over the past decade and have been shown to improve our understanding of rare diseases, such as DSD [155, 156]. The international registry on CAH (www.i-cah.org) seeks to connect clinical and research centres on all 5 continents by a “virtual research environment” aiming to improve the understanding of CAH and ultimately aiming to provide better management for affected patients as a result. Another important advance is the initiation of the European Reference Networks (ERNs) for rare disease, which has seen the creation of EndoERN (https://endo-ern.eu), with an adrenal-specific subgroup working towards an international standard of care for patients with rare adrenal monogenic disorders. With the possibility to get affected patients themselves involved as well, these initiatives clearly set the tune for the future approach to rare disease management by creating a platform of exchange for evidence-based multidisciplinary care.
Disclosure Statement
The authors have nothing to declare.
Acknowledgment
Financial support was provided by the Medical Research Council UK (Programme Grant G0900567, to W.A.), the National Institute of Health Research (NIHR) UK (Lecturer Fellowship to J.I.); and Birmingham Children's Hospital Foundation (Springboard Fellowship, to E.S.B.)
References
- 1.De Creccio L. Sopra un caso di apparence virili in una donna. Il Morgagni. 1865 Jul;1:151–189. [Google Scholar]
- 2.Delle Piane L, Rinaudo PF, Miller WL. 150 years of congenital adrenal hyperplasia: translation and commentary of De Crecchio's classic paper from 1865. Endocrinology. 2015 Apr;156((4)):1210–7. doi: 10.1210/en.2014-1879. [DOI] [PubMed] [Google Scholar]
- 3.El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017 Nov;390((10108)):2194–210. doi: 10.1016/S0140-6736(17)31431-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011 Feb;32((1)):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason JI, Rainey WE. Steroidogenesis in the human fetal adrenal: a role for cholesterol synthesized de novo. J Clin Endocrinol Metab. 1987 Jan;64((1)):140–7. doi: 10.1210/jcem-64-1-140. [DOI] [PubMed] [Google Scholar]
- 6.Gwynne JT, Strauss JF., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3((3)):299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 7.Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017 Jan;165(Pt A):18–37. doi: 10.1016/j.jsbmb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Shikita M, Hall PF. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. II. Subunit structure. J Biol Chem. 1973 Aug;248((16)):5605–9. [PubMed] [Google Scholar]
- 9.Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci USA. 1986 Dec;83((23)):8962–6. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura T, Suzuki K. Enzymatic reduction of non-heme iron protein (adrenodoxin) by reduced nicotinamide adenine dinucleotide phosphate. Biochem Biophys Res Commun. 1965 Aug;20((4)):373–9. doi: 10.1016/0006-291x(65)90585-1. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000 Dec;53((6)):739–47. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomas JL, Myers RP, Strickler RC. Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5----4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem. 1989 Aug;33((2)):209–17. doi: 10.1016/0022-4731(89)90296-3. [DOI] [PubMed] [Google Scholar]
- 13.Lachance Y, Luu-The V, Labrie C, Simard J, Dumont M, de Launoit Y, et al. Characterization of human 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase gene and its expression in mammalian cells. J Biol Chem. 1990 Nov;265((33)):20469–75. [PubMed] [Google Scholar]
- 14.Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, et al. Role of steroid 11 beta-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci USA. 1992 Feb;89((4)):1458–62. doi: 10.1073/pnas.89.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulatero P, Curnow KM, Aupetit-Faisant B, Foekling M, Gomez-Sanchez C, Veglio F, et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab. 1998 Nov;83((11)):3996–4001. doi: 10.1210/jcem.83.11.5237. [DOI] [PubMed] [Google Scholar]
- 16.Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986 Dec;234((4781)):1258–61. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 17.Chung BC, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shively JE, et al. Cytochrome P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA. 1987 Jan;84((2)):407–11. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995 Mar;317((2)):343–7. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 19.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995 Jun;308((Pt 3)):901–8. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller WL. Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol. 2002 Dec;198((1-2)):7–14. doi: 10.1016/s0303-7207(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 21.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998 Feb;273((6)):3158–65. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 22.Bart AG, Scott EE. Structural and functional effects of cytochromeb5interactions with human cytochrome P450 enzymes. J Biol Chem. 2017 Dec;292((51)):20818–33. doi: 10.1074/jbc.RA117.000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003 Apr;24((2)):152–82. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 24.Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974 Dec;39((6)):1020–4. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Xing Y, Hui XG, Kurotaki Y, Ono K, Cohen T, et al. Human adrenal cells that express both 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production. J Steroid Biochem Mol Biol. 2011 Feb;123((3-5)):122–6. doi: 10.1016/j.jsbmb.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999 Sep;341((14)):1013–20. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]
- 27.Arlt W, Haas J, Callies F, Reincke M, Hübler D, Oettel M, et al. Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. J Clin Endocrinol Metab. 1999 Jun;84((6)):2170–6. doi: 10.1210/jcem.84.6.5789. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009 Jun;94((6)):2192–8. doi: 10.1210/jc.2008-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong AN, Yang L, Ma M, Tao D, Bjornsson TD. Molecular cloning of the alcohol/hydroxysteroid form (hSTa) of sulfotransferase from human liver. Biochem Biophys Res Commun. 1992 Aug;187((1)):448–54. doi: 10.1016/s0006-291x(05)81514-1. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein RM, Sterner KN, Wildman DE. Adrenal androgen production in catarrhine primates and the evolution of adrenarche. Am J Phys Anthropol. 2012 Mar;147((3)):389–400. doi: 10.1002/ajpa.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev. 2015 Oct;36((5)):526–63. doi: 10.1210/er.2015-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013 Mar;98((3)):1182–8. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol. 2018 Apr;456:4–26. doi: 10.1016/j.mce.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pretorius E, Arlt W, Storbeck KH. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 2017 Feb;441:76–85. doi: 10.1016/j.mce.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Byrnes WW, Shipley EG. Guinea pig copulatory reflex in response to adrenal steroids and similar compounds. Endocrinology. 1955 Jul;57((1)):5–9. doi: 10.1210/endo-57-1-5. [DOI] [PubMed] [Google Scholar]
- 36.Dorfman RI. In vivo metabolism of neutral steroid hormones. J Clin Endocrinol Metab. 1954 Mar;14((3)):318–25. doi: 10.1210/jcem-14-3-318. [DOI] [PubMed] [Google Scholar]
- 37.Schloms L, Storbeck KH, Swart P, Gelderblom WC, Swart AC. The influence of Aspalathus linearis (Rooibos) and dihydrochalcones on adrenal steroidogenesis: quantification of steroid intermediates and end products in H295R cells. J Steroid Biochem Mol Biol. 2012 Feb;128((3-5)):128–38. doi: 10.1016/j.jsbmb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, et al. 11β-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione. J Steroid Biochem Mol Biol. 2013 Nov;138:132–42. doi: 10.1016/j.jsbmb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013 Sep;377((1-2)):135–46. doi: 10.1016/j.mce.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, et al. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008 Apr;149((4)):1786–92. doi: 10.1210/en.2007-1015. [DOI] [PubMed] [Google Scholar]
- 41.Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-Ketodihydrotestosterone in Castration Resistant Prostate Cancer: Potent Androgens Which Can No Longer Be Ignored. PLoS One. 2016 Jul;11((7)):e0159867. doi: 10.1371/journal.pone.0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, et al. 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017 Mar;102((3)):840–8. doi: 10.1210/jc.2016-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turcu AF, Auchus RJ. Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2015 Jun;44((2)):275–96. doi: 10.1016/j.ecl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, et al. 11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2017 Aug;102((8)):2701–10. doi: 10.1210/jc.2016-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones CM, Mallappa A, Reisch N, Nikolaou N, Krone N, Hughes BA, et al. Modified-Release and Conventional Glucocorticoids and Diurnal Androgen Excretion in Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2017 Jun;102((6)):1797–806. doi: 10.1210/jc.2016-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2018 Apr;178:221–8. doi: 10.1016/j.jsbmb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994 Nov;269((45)):28314–22. [PubMed] [Google Scholar]
- 48.Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL, International Congenital Lipoid Adrenal Hyperplasia Consortium The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996 Dec;335((25)):1870–8. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- 49.Sandison AT. A form of lipoidosis of the adrenal cortex in an infant. Arch Dis Child. 1955 Dec;30((154)):538–41. doi: 10.1136/adc.30.154.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, et al. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008 Mar;93((3)):696–702. doi: 10.1210/jc.2007-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, et al. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997 Mar;99((6)):1265–71. doi: 10.1172/JCI119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997 May;82((5)):1511–5. doi: 10.1210/jcem.82.5.3962. [DOI] [PubMed] [Google Scholar]
- 53.Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, et al. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006 Dec;91((12)):4781–5. doi: 10.1210/jc.2006-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009 Oct;94((10)):3865–71. doi: 10.1210/jc.2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahakitrungruang T, Soccio RE, Lang-Muritano M, Walker JM, Achermann JC, Miller WL. Clinical, genetic, and functional characterization of four patients carrying partial loss-of-function mutations in the steroidogenic acute regulatory protein (StAR) J Clin Endocrinol Metab. 2010 Jul;95((7)):3352–9. doi: 10.1210/jc.2010-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo HW, Kim GH. Molecular and clinical characterization of Korean patients with congenital lipoid adrenal hyperplasia. J Pediatr Endocrinol Metab. 1998 Nov-Dec;11((6)):707–11. doi: 10.1515/jpem.1998.11.6.707. [DOI] [PubMed] [Google Scholar]
- 57.Nakae J, Tajima T, Sugawara T, Arakane F, Hanaki K, Hotsubo T, et al. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum Mol Genet. 1997 Apr;6((4)):571–6. doi: 10.1093/hmg/6.4.571. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Baker BY, Abduljabbar MA, Miller WL. A genetic isolate of congenital lipoid adrenal hyperplasia with atypical clinical findings. J Clin Endocrinol Metab. 2005 Feb;90((2)):835–40. doi: 10.1210/jc.2004-1323. [DOI] [PubMed] [Google Scholar]
- 59.Flück CE, Maret A, Mallet D, Portrat-Doyen S, Achermann JC, Leheup B, et al. A novel mutation L260P of the steroidogenic acute regulatory protein gene in three unrelated patients of Swiss ancestry with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2005 Sep;90((9)):5304–8. doi: 10.1210/jc.2005-0874. [DOI] [PubMed] [Google Scholar]
- 60.Gucev ZS, Tee MK, Chitayat D, Wherrett DK, Miller WL. Distinguishing deficiencies in the steroidogenic acute regulatory protein and the cholesterol side chain cleavage enzyme causing neonatal adrenal failure. J Pediatr. 2013 Apr;162((4)):819–22. doi: 10.1016/j.jpeds.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (p450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab. 2001 Aug;86((8)):3820–5. doi: 10.1210/jcem.86.8.7748. [DOI] [PubMed] [Google Scholar]
- 62.Katsumata N, Ohtake M, Hojo T, Ogawa E, Hara T, Sato N, et al. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J Clin Endocrinol Metab. 2002 Aug;87((8)):3808–13. doi: 10.1210/jcem.87.8.8763. [DOI] [PubMed] [Google Scholar]
- 63.Hiort O, Holterhus PM, Werner R, Marschke C, Hoppe U, Partsch CJ, et al. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J Clin Endocrinol Metab. 2005 Jan;90((1)):538–41. doi: 10.1210/jc.2004-1059. [DOI] [PubMed] [Google Scholar]
- 64.Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009 Mar;94((3)):936–9. doi: 10.1210/jc.2008-1118. [DOI] [PubMed] [Google Scholar]
- 65.Sahakitrungruang T, Tee MK, Blackett PR, Miller WL. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2011 Mar;96((3)):792–8. doi: 10.1210/jc.2010-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tee MK, Abramsohn M, Loewenthal N, Harris M, Siwach S, Kaplinsky A, et al. Varied clinical presentations of seven patients with mutations in CYP11A1 encoding the cholesterol side-chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2013 Feb;98((2)):713–20. doi: 10.1210/jc.2012-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, et al. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1) J Clin Endocrinol Metab. 2011 Nov;96((11)):E1798–806. doi: 10.1210/jc.2011-1277. [DOI] [PubMed] [Google Scholar]
- 68.Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, et al. Global DSD Update Consortium Global Disorders of Sex Development Update since 2006: Perceptions, Approach and Care. Horm Res Paediatr. 2016;85((3)):158–80. doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015) Clin Endocrinol (Oxf) 2016 May;84((5)):771–88. doi: 10.1111/cen.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009 Apr;23((2)):181–92. doi: 10.1016/j.beem.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, et al. 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia. 1993 [Google Scholar]
- 72.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Endocrine Society Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010 Sep;95((9)):4133–60. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci USA. 1986 Jul;83((14)):5111–5. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000 Jun;21((3)):245–91. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]
- 75.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003 Aug;349((8)):776–88. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 76.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000 Nov;343((19)):1362–8. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 77.Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011 Aug;165((2)):189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- 78.Falhammar H, Frisén L, Norrby C, Hirschberg AL, Almqvist C, Nordenskjöld A, et al. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014 Dec;99((12)):E2715–21. doi: 10.1210/jc.2014-2957. [DOI] [PubMed] [Google Scholar]
- 79.Hargitai G, Sólyom J, Battelino T, Lebl J, Pribilincová Z, Hauspie R, et al. MEWPE-CAH Study Group Growth patterns and final height in congenital adrenal hyperplasia due to classical 21-hydroxylase deficiency. Results of a multicenter study. Horm Res. 2001;55((4)):161–71. doi: 10.1159/000049990. [DOI] [PubMed] [Google Scholar]
- 80.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010 Nov;95((11)):5110–21. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claahsen-van der Grinten HL, Otten BJ, Takahashi S, Meuleman EJ, Hulsbergen-van de Kaa C, Sweep FC, et al. Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J Clin Endocrinol Metab. 2007 Feb;92((2)):612–5. doi: 10.1210/jc.2006-1311. [DOI] [PubMed] [Google Scholar]
- 82.Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, et al. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007 Sep;92((9)):3674–80. doi: 10.1210/jc.2007-0337. [DOI] [PubMed] [Google Scholar]
- 83.Engels M, Gehrmann K, Falhammar H, Webb EA, Nordenström A, Sweep FC, et al. dsd-LIFE group Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018 Mar;178((3)):285–94. doi: 10.1530/EJE-17-0862. [DOI] [PubMed] [Google Scholar]
- 84.Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007 Jan;92((1)):137–42. doi: 10.1210/jc.2006-0964. [DOI] [PubMed] [Google Scholar]
- 85.Frisch H, Battelino T, Schober E, Baumgartner-Parzer S, Nowotny P, Vierhapper H. Salt wasting in simple virilizing congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2001 Nov-Dec;14((9)):1649–55. doi: 10.1515/jpem.2001.14.9.1649. [DOI] [PubMed] [Google Scholar]
- 86.Fiet J, Gueux B, Raux-DeMay MC, Kuttenn F, Vexiau P, Brerault JL, et al. Increased plasma 21-deoxycorticosterone (21-DB) levels in late-onset adrenal 21-hydroxylase deficiency suggest a mild defect of the mineralocorticoid pathway. J Clin Endocrinol Metab. 1989 Mar;68((3)):542–7. doi: 10.1210/jcem-68-3-542. [DOI] [PubMed] [Google Scholar]
- 87.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012 Mar;97((3)):E367–75. doi: 10.1210/jc.2011-1997. [DOI] [PubMed] [Google Scholar]
- 88.Webb EA, Krone N. Current and novel approaches to children and young people with congenital adrenal hyperplasia and adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2015 Jun;29((3)):449–68. doi: 10.1016/j.beem.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Rösler A, Leiberman E, Cohen T. High frequency of congenital adrenal hyperplasia (classic 11 beta-hydroxylase deficiency) among Jews from Morocco. Am J Med Genet. 1992 Apr;42((6)):827–34. doi: 10.1002/ajmg.1320420617. [DOI] [PubMed] [Google Scholar]
- 90.Hampf M, Dao NT, Hoan NT, Bernhardt R. Unequal crossing-over between aldosterone synthase and 11beta-hydroxylase genes causes congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001 Sep;86((9)):4445–52. doi: 10.1210/jcem.86.9.7820. [DOI] [PubMed] [Google Scholar]
- 91.Parajes S, Loidi L, Reisch N, Dhir V, Rose IT, Hampel R, et al. Functional consequences of seven novel mutations in the CYP11B1 gene: four mutations associated with nonclassic and three mutations causing classic 11{beta}-hydroxylase deficiency. J Clin Endocrinol Metab. 2010 Feb;95((2)):779–88. doi: 10.1210/jc.2009-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White PC, Curnow KM, Pascoe L. Disorders of steroid 11 beta-hydroxylase isozymes. Endocr Rev. 1994 Aug;15((4)):421–38. doi: 10.1210/edrv-15-4-421. [DOI] [PubMed] [Google Scholar]
- 93.Holcombe JH, Keenan BS, Nichols BL, Kirkland RT, Clayton GW. Neonatal salt loss in the hypertensive form of congenital adrenal hyperplasia. Pediatrics. 1980 Apr;65((4)):777–81. [PubMed] [Google Scholar]
- 94.Joehrer K, Geley S, Strasser-Wozak EM, Azziz R, Wollmann HA, Schmitt K, et al. CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11 beta-hydroxylase deficiency. Hum Mol Genet. 1997 Oct;6((11)):1829–34. doi: 10.1093/hmg/6.11.1829. [DOI] [PubMed] [Google Scholar]
- 95.Peters CJ, Nugent T, Perry LA, Davies K, Morel Y, Drake WM, et al. Cosegregation of a novel homozygous CYP11B1 mutation with the phenotype of non-classical congenital adrenal hyperplasia in a consanguineous family. Horm Res. 2007;67((4)):189–93. doi: 10.1159/000097244. [DOI] [PubMed] [Google Scholar]
- 96.Reisch N, Högler W, Parajes S, Rose IT, Dhir V, Götzinger J, et al. A diagnosis not to be missed: nonclassic steroid 11β-hydroxylase deficiency presenting with premature adrenarche and hirsutism. J Clin Endocrinol Metab. 2013 Oct;98((10)):E1620–5. doi: 10.1210/jc.2013-1306. [DOI] [PubMed] [Google Scholar]
- 97.Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J Steroid Biochem Mol Biol. 2010 Aug;121((3-5)):496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peter M, Janzen N, Sander S, Korsch E, Riepe FG, Sander J. A case of 11beta-hydroxylase deficiency detected in a newborn screening program by second-tier LC-MS/MS. Horm Res. 2008;69((4)):253–6. doi: 10.1159/000113027. [DOI] [PubMed] [Google Scholar]
- 99.Auchus RJ. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol. 2017 Jan;165(Pt A):71–8. doi: 10.1016/j.jsbmb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997 Oct;17((2)):201–5. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- 101.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003 Dec;278((49)):48563–9. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 102.Idkowiak J, Randell T, Dhir V, Patel P. A missense mutation in the human cytochrome b5 gene causes 46, XY disorder of sex development due to true isolated 17, 20 lyase deficiency. The Journal of …. 2011 doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bongiovanni AM. The adrenogenital syndrome with deficiency of 3 beta-hydroxysteroid dehydrogenase. J Clin Invest. 1962 Nov;41((11)):2086–92. doi: 10.1172/JCI104666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rhéaume E, Simard J, Morel Y, Mebarki F, Zachmann M, Forest MG, et al. Congenital adrenal hyperplasia due to point mutations in the type II 3 beta-hydroxysteroid dehydrogenase gene. Nat Genet. 1992 Jul;1((4)):239–45. doi: 10.1038/ng0792-239. [DOI] [PubMed] [Google Scholar]
- 105.Mermejo LM, Elias LL, Marui S, Moreira AC, Mendonca BB, de Castro M. Refining hormonal diagnosis of type II 3beta-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J Clin Endocrinol Metab. 2005 Mar;90((3)):1287–93. doi: 10.1210/jc.2004-1552. [DOI] [PubMed] [Google Scholar]
- 106.Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, et al. Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 2002 Jun;87((6)):2611–22. doi: 10.1210/jcem.87.6.8615. [DOI] [PubMed] [Google Scholar]
- 107.Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004 Mar;36((3)):228–30. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 108.Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004 Jun;363((9427)):2128–35. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- 109.Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases. A new variant of congenital adrenal hyperplasia. N Engl J Med. 1985 Nov;313((19)):1182–91. doi: 10.1056/NEJM198511073131903. [DOI] [PubMed] [Google Scholar]
- 110.Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al. Cytochrome: Oxidoreductase Deficiency; 1993. p. p. 450. [Google Scholar]
- 111.Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012 Feb;97((2)):E257–67. doi: 10.1210/jc.2011-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, et al. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003 Feb;144((2)):575–80. doi: 10.1210/en.2002-220721. [DOI] [PubMed] [Google Scholar]
- 113.Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, et al. Cytochrome P450 Oxidoreductase Deficiency: Identification and Characterization of Biallelic Mutations and Genotype-Phenotype Correlations in 35 Japanese Patients. J Clin Endocrinol Metab. 2009 Mar;3:94–1731. doi: 10.1210/jc.2008-2816. [DOI] [PubMed] [Google Scholar]
- 114.Idkowiak J, O'Riordan S, Reisch N, Malunowicz EM, Collins F, Kerstens MN, et al. Pubertal presentation in seven patients with congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2011 Mar;96((3)):E453–62. doi: 10.1210/jc.2010-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005 May;76((5)):729–49. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ribes V, Otto DM, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, et al. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol. 2007 Mar;303((1)):66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 117.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002 Oct;360((9340)):1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 118.Pandey AV, Sproll P. Pharmacogenomics of human P450 oxidoreductase. Front Pharmacol. 2014 May;5:103. doi: 10.3389/fphar.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burkhard FZ, Parween S, Udhane SS, Flück CE, Pandey AV. P450 Oxidoreductase deficiency: analysis of mutations and polymorphisms. J Steroid Biochem Mol Biol. 2017 Jan;165((Pt A)):38–50. doi: 10.1016/j.jsbmb.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010 Dec;163((6)):919–24. doi: 10.1530/EJE-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reisch N, Idkowiak J, Hughes BA, Ivison HE, Abdul-Rahman OA, Hendon LG, et al. Prenatal diagnosis of congenital adrenal hyperplasia caused by P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2013 Mar;98((3)):E528–36. doi: 10.1210/jc.2012-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med. 1986 Mar;314((12)):757–61. doi: 10.1056/NEJM198603203141206. [DOI] [PubMed] [Google Scholar]
- 123.Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010 Mar;95((3)):994–9. doi: 10.1210/jc.2008-1745. [DOI] [PubMed] [Google Scholar]
- 124.Idkowiak J, Malunowicz EM, Dhir V, Reisch N, Szarras-Czapnik M, Holmes DM, et al. Concomitant mutations in the P450 oxidoreductase and androgen receptor genes presenting with 46,XY disordered sex development and androgenization at adrenarche. J Clin Endocrinol Metab. 2010 Jul;95((7)):3418–27. doi: 10.1210/jc.2010-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giordano SJ, Kaftory A, Steggles AW. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Hum Genet. 1994 May;93((5)):568–70. doi: 10.1007/BF00202825. [DOI] [PubMed] [Google Scholar]
- 126.Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, et al. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012 Mar;97((3)):E465–75. doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geller D, Auchus R, Miller W. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- 128.Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, et al. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab. 2008 Sep;93((9)):3584–8. doi: 10.1210/jc.2008-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zachmann M, Völlmin JA, Hamilton W, Prader A. Steroid 17,20-desmolase deficiency: a new cause of male pseudohermaphroditism. Clin Endocrinol (Oxf) 1972 Oct;1((4)):369–85. doi: 10.1111/j.1365-2265.1972.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 130.Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011 Aug;89((2)):201–18. doi: 10.1016/j.ajhg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arlt W, Hammer F, Sanning P, Butcher SK, Lord JM, Allolio B, et al. Dissociation of serum dehydroepiandrosterone and dehydroepiandrosterone sulfate in septic shock. J Clin Endocrinol Metab. 2006 Jul;91((7)):2548–54. doi: 10.1210/jc.2005-2258. [DOI] [PubMed] [Google Scholar]
- 132.Hammer F, Subtil S, Lux P, Maser-Gluth C, Stewart PM, Allolio B, et al. No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J Clin Endocrinol Metab. 2005 Jun;90((6)):3600–5. doi: 10.1210/jc.2004-2386. [DOI] [PubMed] [Google Scholar]
- 133.Idkowiak J, Taylor AE, Subtil S, O'Neil DM, Vijzelaar R, Dias RP, et al. Steroid Sulfatase Deficiency and Androgen Activation Before and After Puberty. J Clin Endocrinol Metab. 2016 Jun;101((6)):2545–53. doi: 10.1210/jc.2015-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, et al. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009 May;360((22)):2310–8. doi: 10.1056/NEJMoa0810489. [DOI] [PubMed] [Google Scholar]
- 135.Faiyaz ul Haque M, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, et al. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat Genet. 1998 Oct;20((2)):157–62. doi: 10.1038/2458. [DOI] [PubMed] [Google Scholar]
- 136.Ahmad W, Brancolini V, ul Faiyaz MF, Lam H, ul Haque S, Haider M, et al. A locus for autosomal recessive hypodontia with associated dental anomalies maps to chromosome 16q12.1. Am J Hum Genet. 1998 Apr;62((4)):987–91. doi: 10.1086/301799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tüysüz B, Yılmaz S, Gül E, Kolb L, Bilguvar K, Evliyaoğlu O, et al. Spondyloepimetaphyseal dysplasia Pakistani type: expansion of the phenotype. Am J Med Genet A. 2013 Jun;161A((6)):1300–8. doi: 10.1002/ajmg.a.35906. [DOI] [PubMed] [Google Scholar]
- 138.Miyake N, Elcioglu NH, Iida A, Isguven P, Dai J, Murakami N, et al. PAPSS2 mutations cause autosomal recessive brachyolmia. J Med Genet. 2012 Aug;49((8)):533–8. doi: 10.1136/jmedgenet-2012-101039. [DOI] [PubMed] [Google Scholar]
- 139.Iida A, Simsek-Kiper PÖ, Mizumoto S, Hoshino T, Elcioglu N, Horemuzova E, et al. Clinical and radiographic features of the autosomal recessive form of brachyolmia caused by PAPSS2 mutations. Hum Mutat. 2013 Oct;34((10)):1381–6. doi: 10.1002/humu.22377. [DOI] [PubMed] [Google Scholar]
- 140.Oostdijk W, Idkowiak J, Mueller JW, House PJ, Taylor AE, O'Reilly MW, et al. PAPSS2 deficiency causes androgen excess via impaired DHEA sulfation—in vitro and in vivo studies in a family harboring two novel PAPSS2 mutations. J Clin Endocrinol Metab. 2015 Apr;100((4)):E672–80. doi: 10.1210/jc.2014-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Louwers YV, de Jong FH, van Herwaarden NA, Stolk L, Fauser BC, Uitterlinden AG, et al. Variants in SULT2A1 affect the DHEA sulphate to DHEA ratio in patients with polycystic ovary syndrome but not the hyperandrogenic phenotype. J Clin Endocrinol Metab. 2013 Sep;98((9)):3848–55. doi: 10.1210/jc.2013-1976. [DOI] [PubMed] [Google Scholar]
- 142.Haring R, Wallaschofski H, Teumer A, Kroemer H, Taylor AE, Shackleton CH, et al. A SULT2A1 genetic variant identified by GWAS as associated with low serum DHEAS does not impact on the actual DHEA/DHEAS ratio. J Mol Endocrinol. 2012 Dec;50((1)):73–7. doi: 10.1530/JME-12-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krone N, Rose IT, Willis DS, Hodson J, Wild SH, Doherty EJ, et al. United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) Genotype-phenotype correlation in 153 adult patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency: analysis of the United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) cohort. J Clin Endocrinol Metab. 2013 Feb;98((2)):E346–54. doi: 10.1210/jc.2012-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Debono M, Mallappa A, Gounden V, Nella AA, Harrison RF, Crutchfield CA, et al. Hormonal circadian rhythms in patients with congenital adrenal hyperplasia: identifying optimal monitoring times and novel disease biomarkers. Eur J Endocrinol. 2015 Dec;173((6)):727–37. doi: 10.1530/EJE-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009 Sep;94((9)):3477–80. doi: 10.1210/jc.2009-0630. [DOI] [PubMed] [Google Scholar]
- 146.Nella AA, Mallappa A, Perritt AF, Gounden V, Kumar P, Sinaii N, et al. A Phase 2 Study of Continuous Subcutaneous Hydrocortisone Infusion in Adults With Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2016 Dec;101((12)):4690–8. doi: 10.1210/jc.2016-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oksnes M, Björnsdottir S, Isaksson M, Methlie P, Carlsen S, Nilsen RM, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of addison's disease: a randomized clinical trial. J Clin Endocrinol Metab. 2014 May;99((5)):1665–74. doi: 10.1210/jc.2013-4253. [DOI] [PubMed] [Google Scholar]
- 148.Johannsson G, Nilsson AG, Bergthorsdottir R, Burman P, Dahlqvist P, Ekman B, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012 Feb;97((2)):473–81. doi: 10.1210/jc.2011-1926. [DOI] [PubMed] [Google Scholar]
- 149.Porter J, Blair J, Ross RJ. Is physiological glucocorticoid replacement important in children? Arch Dis Child. 2017 Feb;102((2)):199–205. doi: 10.1136/archdischild-2015-309538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009 May;94((5)):1548–54. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2010 Apr;72((4)):441–7. doi: 10.1111/j.1365-2265.2009.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mallappa A, Sinaii N, Kumar P, Whitaker MJ, Daley LA, Digweed D, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015 Mar;100((3)):1137–45. doi: 10.1210/jc.2014-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Auchus RJ, Buschur EO, Chang AY, Hammer GD, Ramm C, Madrigal D, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014 Aug;99((8)):2763–70. doi: 10.1210/jc.2014-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Turcu AF, Spencer-Segal JL, Farber RH, Luo R, Grigoriadis DE, Ramm CA, et al. Single-Dose Study of a Corticotropin-Releasing Factor Receptor-1 Antagonist in Women With 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2016 Mar;101((3)):1174–80. doi: 10.1210/jc.2015-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ahmed SF, Rodie M. Investigation and initial management of ambiguous genitalia. Best Pract Res Clin Endocrinol Metab. 2010 Apr;24((2)):197–218. doi: 10.1016/j.beem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 156.Ahmed SF, Bryce J, Hiort O. International networks for supporting research and clinical care in the field of disorders of sex development. Endocr Dev. 2014;27:284–92. doi: 10.1159/000363676. [DOI] [PubMed] [Google Scholar]