Abstract
Purpose
The immune system is considered to be affected by aging, which is linked to various immune pathogeneses. The purpose of this study was to determine age-associated changes in immune function of healthy dogs (beagles), specifically those of naive and memory T lymphocytes, based on cytokine synthesis.
Patients and methods
Blood samples were obtained from 44 healthy beagles that were divided into three age-groups: young (<4 years), middle-aged (4–8 years), and older dogs (>8 years). Subpopulations of T lymphocytes were determined by flow cytometry. Transcriptional (mRNA) levels of cytokines were determined for primary-cultured leukocytes using quantitative real-time polymerase chain reaction.
Results
There were negative correlations between dogs’ ages and the number of peripheral blood mononuclear cells, T cells, and B cells. In particular, the number of naive CD4+ CD45RA+ T cells and CD8+ CD45RA+ T cells significantly decreased with age. The mRNA levels for interleukin (IL)-2, IL-2Rα, and interferon-gamma were significantly higher in young or middle-aged dogs (P < 0.05), whereas IL-4 mRNA expression was not significantly different over the different age-groups. IL-2Rγ mRNA expression tended to decrease with age.
Conclusion
Decreases of naive CD4+ and naive CD8+ T cells may be related to age-related immunosenescence in dogs. With regard to cytokine production, leukocyte IL-4 and IL-10 mRNA levels did not change with age, whereas IL-2, IL-2Rα, and IL-2Rγ mRNA levels decreased with age. These altered cytokine mRNA expression patterns may contribute to decreased naive T-cell function(s) with aging.
Keywords: aging, leukocyte subpopulation, cytokine, dog, naive T cell, IL-2R
Introduction
The occurrence of age-related disorders, such as tumors in dogs1 and renal inflammation in cats,2 increases with extended life span. The immune system is considered to be affected by aging, which is linked to various immune pathogeneses.3,4 The following age-related immunosenescent changes have been reported for dogs: reduced proliferative responses of peripheral blood mononuclear cells (PBMCs);5–7 reduced major histocompatibility complex (MHC) class II expression by peripheral blood lymphocytes;8 reduced numbers of peripheral blood T and B cells;9,10 reduced numbers of CD4+ T cells;1,11 and reduced CD4/8 T-cell ratios.8,12–14 Moreover, compared with young dogs, aged dogs have reduced numbers of circulating naive T cells and increased numbers of memory T cells.15 Naive T cells are important to mount an immune response to various newly encountered antigens; naive CD4+ and CD8+ T cells, in particular, are important for defenses against externally derived (bacteria, viruses) and internally derived (neoplastic cells) antigenic challenges, respectively.
To assess the basic immune functions of T cells, it is useful to determine their cytokine synthesis capability. CD4+ T cells are divided into two distinct subsets based on their cytokine production patterns: type 1 (Th1) and type 2 (Th2). Th1 cytokines, such as interleukin (IL)-2 and interferon (IFN)-γ, are required for the generation of cytotoxic T cells and the activation of natural killer cells. IL-2 produced by activated T cells promotes the proliferation, differentiation, and survival of mature T and B cells.16
We investigated the mRNA expressions of Th1 and Th2 cytokines and the IL-2 receptor (IL-2R) in peripheral blood leukocytes of dogs (beagles) as a function of age. Our purpose was to determine if there were age-associated changes in immune functions in dogs, specifically those of naive and memory T lymphocytes, based on cytokine synthesis.
Materials and methods
Animals
Normal healthy beagles (n = 44) were used for this study. All dogs had been referred to the veterinary teaching hospitals of Kitasato University during 2007–2008. Dogs were divided into three age-groups, as in a previous report:17 young (1–3 years old; n = 18); middle-aged (4–7 years old; n = 13); and older dogs (8–12 years old; n = 13). A body-condition score (BCS) was assessed and expressed on a five-point scale: 1, thin; 2, lean; 3, optimal; 4, obese, 5, gross, as described by Laflamme.18 This study followed the Guidelines for Institutional Laboratory Animal Care and Use of the School of Veterinary Medicine of Kitasato University.
Biochemical and hormonal analysis of blood
Peripheral blood was collected from a cervical vein into sodium fluoride-coated tubes without an anticoagulant. To obtain serum, a sample was centrifuged at 1500 g for 10 minutes. Biochemical analyses of these samples used an AU400 Clinical Chemistry Analyzer (Olympus, Tokyo, Japan).
Classification of canine peripheral blood mononuclear cells
Peripheral blood from a cervical vein was collected into EDTA tubes. PBMCs were isolated by gradient centrifugation at 750 g for 60 minutes using Lymphoprep (Axis-Shield, Oslo, Norway; specific gravity = 1.077 ± 0.001). Cells were washed twice with phosphate-buffered saline (PBS). Lymphocytes were labeled with CD molecules by incubating cells with anti-mouse immunoglobulin (Ig)G antibodies (CD4, CD8, CD21, MHC, CD45R, and CD14; Serotec, Kidlington, UK; VMRD, Pullman, WA; Beckman Coulter, Fullerton, CA) for 60 minutes at 4°C. The cells were then washed and incubated with anti-mouse IgG antibodies labeled with phycoerythrin or fluorescein isothiocyanate for 30 minutes at 4°C. Cells were washed with PBS, and CD antigen expressions were analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). The CellQuest software package (BD, Franklin Lakes, NJ) was used for data acquisition and analysis. Absolute counts for lymphocyte subsets were determined by: (lymphocyte absolute counts × percentage of fluorescent positive cells within lymphogate)/100.
Primary culture of PBMCs
Peripheral blood from a cervical vein was collected into heparin tubes, and PBMCs were prepared using Lymphoprep. These cells were cultured at a density of 5 × 106 cells/mL in Roswell Park Memorial Institute 1640 culture medium supplemented with 10% fetal calf serum at 37°C in 5% CO2 for 12 hours with or without stimulation of PBMC. After culture, canine cells were harvested by centrifugation and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA).
RNA purification
RNA purification, reverse transcription, and mRNA expression were examined according to the manufacturer’s instructions. Briefly, after the addition of 0.2 mL of chloroform and thorough mixing, an RNA solution in TRIzol was incubated on ice for 5 minutes. Then, the sample was centrifuged at 7500 g for 15 minutes at 4°C. After the addition of 0.2 mL of isopropanol to the separated aqueous layer, the sample was incubated on ice for 15 minutes. After centrifugation at 7500 g for 15 minutes at 4°C, the supernatant was removed, 70% ethanol was added, and it was dried. Finally, total RNA was dissolved in 20 μL of nuclease-free water. RNA concentration was determined with a Nano Drop ND-1000 Spectrophotometer (LMS, Tokyo, Japan). cDNA synthesis used 1 μg of RNA, adjusted to 10 μL with nuclease-free water, which was reverse-transcribed with oligo dT primers. After incubation at 68°C for 5 minutes, the sample was added to a mixed liquor containing 5× first-strand buffer (4 μL), 10 mM dNTP mix (1 μL), 25 mM MgCl2 solution (2 μL), 0.1 M DTT (2 μL), and Superscript II RNaseH Reverse Transcriptase (0.7 μL) (Invitrogen, Carlsbad, CA). This sample was first incubated at 42°C for 50 minutes and then at 68°C for 15 minutes.
Real-time PCR analysis
Quantification of mRNA used real-time polymerase chain reaction (PCR) analysis. The sequences for canine IL-2, IFN-γ, IL-4, IL-10, IL-2 receptors (IL-2Rα and IL-2Rγ), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were obtained from GenBank. Our designed primers were; IFN-γ: 5′-CCAGATGTATCGGAGCGGTGG TTATCGCCTTGCGCTGGACC-3′, IL-2: 5′-GCATCGCACTGACGCTTGTATTGCTCCATCTGTTGCTCT-GTT-3′, IL-2Rγ 5′-CAAGAGCATCTGCAAAACCAGCTTGCTTGAAGCTCTTCGT-3′, IL-2Rγ: 5′-CCCCATGTTACACCCTAAAGCCTGAAAGGTTTCCGGGCCCTCACATTG-3′, IL-4: 5′-ATGGGTCTCACCTCCCAACTG TCAATGCCTGTAGTATTTCTTC-3′, IL-10: 5′-TACCTGGGTTGCCAAGCCCT TTCACAGAGAAGCTCAGTAAAT-3′, GAPDH: 5′-GGGGCCATCCACAGTCTTCTGCCAAAAGGGTCATCATCTC-3′. Mixed solutions of primers and SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA) used a 7700 Sequence Detector (PE Applied Biosystems) with denaturing at 50°C for 30 minutes and 95°C for 10 minutes, followed by 45 cycles at 95°C for 15 seconds and 60°C for 1 minute. The target gene expression was normalized against endogenous GAPDH giving a VCT value and calibrated with the VCT value from the sample with lowest expression giving a VVCT. Relative expression values were transfrmed from logarithmic to linear form putting VVCT into the equation 2−ΔΔCT.
Statistical analysis
Results are given as mean ± standard error. Relationships between dogs’ age and peripheral blood leukocyte subpopulations were assessed using Spearman’s rank-order correlation coefficients. Statistical analysis using the group comparisons was done by Student’s t-test or one-way analysis of variance using GraphPad Prism software (GraphPad, San Diego, CA). P-values of <0.05 were considered significant.
Results
For this study, blood samples were obtained from healthy beagles of various ages. These were divided into three groups based on age: young (1–3 years old; n = 18); middle-aged (4–7 years old; n = 13); and older dogs (8–12 years old; n = 13). Their BCSs ranged from 2.5 to 4.0. There was no significant correlation between age and BCS (Table 1). There were no significant differences in white blood cell and red blood cell counts, except for mean corpuscular volume: middle-aged dogs had greater mean corpuscular volume values than dogs in the other age-groups (P < 0.05; Table 1).
Table 1.
Clinical characteristics of dogs
| Variable | Young (18) | Middle-aged (13) | Aged (13) |
|---|---|---|---|
| BCS | 3.13 ± 0.09 | 2.96 ± 0.09 | 3.17 ± 0.17 |
| Age (years) | 1.72 ± 0.14a | 5.02 ± 0.23b | 10.24 ± 0.52c |
| WBC (×102/mL) | 139.39 ± 10.24 | 129.69 ± 12.16 | 113.23 ± 7.94 |
| RBC (×104/mL) | 690.00 ± 30.20 | 705.15 ± 34.71 | 637.54 ± 12.66 |
| Hb (g/dL) | 15.94 ± 0.70 | 16.61 ± 0.84 | 14.92 ± 0.31 |
| Ht (%) | 45.17 ± 1.97 | 47.82 ± 2.35 | 42.05 ± 0.76 |
| MCV (fl) | 65.56 ± 0.63a | 67.84 ± 0.62b | 66.02 ± 0.58a |
| MCH (pg) | 23.12 ± 0.25 | 23.55 ± 0.29 | 23.42 ± 0.30 |
| MCHC (g/dL) | 35.28 ± 0.17 | 34.70 ± 0.19 | 35.48 ± 0.25 |
| PLT (×104/mL) | 21.22 ± 2.45 | 16.69 ± 2.42 | 19.08 ± 1.92 |
Notes: Values are represented as mean ± standard deviation; different letters, a, b, c indicate statistically significant differences between groups (P < 0.05).
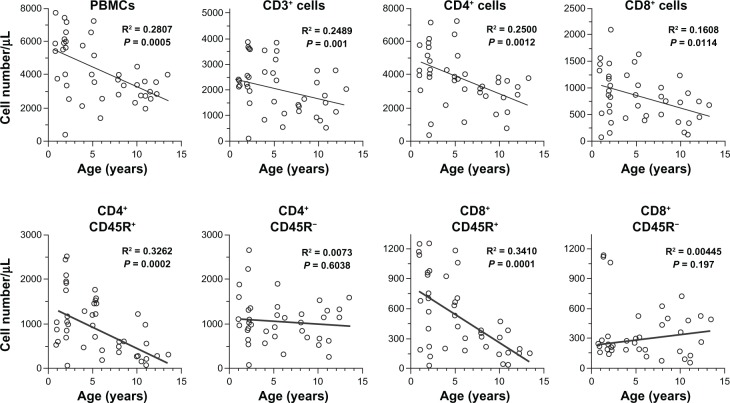
To determine if there were alterations with age in peripheral blood leukocyte subpopulations in dogs, lymphocyte subsets were evaluated using a flow cytometric immunophenotyping method. Representative subpopulations are shown in the scatter diagrams (Figure 1). There were significant negative correlations between dogs’ ages and the number of PBMCs, CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD21+ B cells. Notably, the number of CD4+ CD45R+ T cells (naive helper T cells) and CD8+ CD45R+ T cells (naive killer T cells) decreased significantly with age. The numbers of CD45R− T cells (memory T cells) did not change with age for either CD4+ or CD8+ T cells.
Figure 1.
Scatter diagrams for age-related changes in peripheral blood leukocyte subpopulation in beagles.
Notes: Numbers of peripheral blood mononuclear cells (PBMCs), CD3+ T cells, CD4+ T cells, CD8+ T cells and CD45R+/− cells, CD4+ or CD8+ T cells versus age in individual dogs are shown by open circles (n = 44). All of these variables, except for CD4+ CD45R− T cells and CD8+ CD45− T cells, were significantly negatively correlated with dogs’ age (P < 0.05).
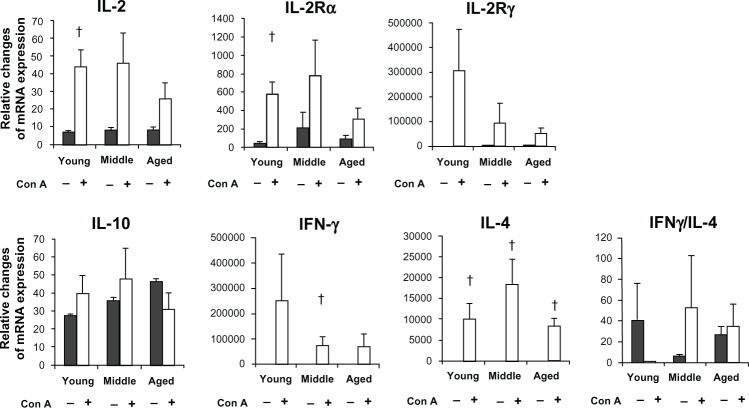
The mRNA levels for various cytokines in cultured PBMCs were analyzed using quantitative real-time PCR (Figure 2). Leukocytes were cultured for 12 hours under optimized conditions. mRNA levels for IL-2 and IL-2Rα were significantly increased only in the young and middle-aged groups, respectively. IL-2Rγ mRNA expression tended to decline with age. By comparison, IL-4 and IL-10 mRNA expressions did not change with age.
Figure 2.
Age-related changes in the transcriptional (mRNA) levels for IL-2, IL-2Rα, IL-2Rγ, IL-10, IFNγ, IL-4, and IFNγ/IL-4. mRNA levels were determined 12 hours after stimulation of PBMCs with Con A.
Notes: The control mRNA levels (gray bars) and mRNA levels induced by con-A stimulation (open bars) in PBMCs are shown in each age-group. IL-2, IL-2Rα, and IFNγ mRNA levels were significantly increased only in young or middle-aged dogs (P < 0.05). IL-4 mRNA expression was significantly increased in all age-groups after Con-A stimulation (P < 0.05).
Abbreviations: IL, interleukin; PBMCs, peripheral blood mononuclear cells; IFN, interferon.
Discussion
In the present study, no changes were found in the clinical conditions and the biochemical examinations of healthy beagles in different age-groups. However, for peripheral blood leukocyte subpopulations, there were significant negative correlations between the numbers of PBMCs, CD4+ T cells, CD8+ T cells, CD4+ CD45R+ T cells (possibly naive cells), CD8+ CD45R+ T cells, CD21+ B cells and dogs’ ages. In contrast, there were no significant correlations between the numbers of CD4+ CD45R− T cells (possibly memory cells) and CD8+ CD45R− T cells and dogs’ ages. Thus, the subpopulations that were reduced in older healthy dogs were characteristically naive T cells. These results are in agreement with human studies.19,20 Thus, the decrease in naive T cells may contribute to reduced T-cell responses to newly encountered antigens in aged dogs, as is observed in humans.21
In this study, the IL-2 and IFN-γ mRNA levels in Con A–stimulated PBMCs were significantly increased in cells from young and middle-aged dogs, respectively. In rats, naive T cells are the best IL-2 producers.22 IFN-γ is also primarily produced by naive but not memory T cells.23 Therefore, the age-related reductions of IL-2 and IFN-γ mRNA expressions may be due to the reduced numbers of naive T cells in older dogs. In contrast, IL-4 and IL-10 mRNA expressions appeared not to change with age. Memory T cells can produce significant amounts of IL-4.23 Thus, it is possible that memory T cells, which did not change with age, may be primarily related to age-independent IL-4 mRNA expression.
Age-associated alterations in IL-2R mRNA expression will require further investigation. In this study, IL-2Rγ mRNA expression tended to be downregulated with dogs’ age. IL-2Rγ–related cytokines appear to be essential for naive cell survival through the maintenance of antiapoptotic pathways.24,25 These changes in IL-2Rγ mRNA patterns may also contribute to the decreases in naive T-cell functions observed with aging. In addition, IL-2Rα mRNA expression decreased in an age-dependent manner. IL-2Rα can effectively increase the formation of ternary complexes.26 Because IL-2Rα transmits much stronger proliferative and differentiation signals, age-related decreases in these IL-2R mRNA expressions may play an essential role in the decline of naive T cells.
The decline in immune function in older animals may be related to an increased incidence of certain diseases. However, a direct association between a decline in immune function and an increased susceptibility to diseases has not been reported. From the results of this study, the age-related decreases in naive T cells and IL-2R mRNA expression may contribute to the decline in immune function. Further studies will be necessary to determine whether these results are directly linked to clinical problems.
Conclusion
In summary, we analyzed the relationship between peripheral blood leukocyte subpopulations and their cytokine synthesis and age in healthy dogs. First, the numbers of naive T cells decreased with age, which may contribute to the reduced cytokine production by these cells. Second, the IL-2Rα and IL-2Rγ mRNA expression levels in PBMCs decreased with age. These reduced mRNA expressions may contribute to the decline in naive T cells. Reduced numbers of naive T cells and IL-2R mRNA expression could affect each other and be related to immunosenescence associated with aging.
Acknowledgments
We thank Drs Hori Yasutomo and Okano Shozo for their help with animal care.
Footnotes
Disclosure
This work was supported by the Japan Pet Nutrition Society. The authors declare no conflicts of interest in this work.
References
- 1.Watabe A, Fukumoto S, Komatsu T, Endo Y, Kadosawa T. Alterations of lymphocyte subpopulations in healthy dogs with aging and in dogs with cancer. Vet Immunol Immunopathol. 2011;42:189–200. doi: 10.1016/j.vetimm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Lawler DF, Evans RH, Chase K, et al. The aging feline kidney: a model mortality antagonist? J Feline Med Surg. 2006;8:363–371. doi: 10.1016/j.jfms.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finch EC. Longevity, Senescence and the Genome. Chicago: University of Chicago Press; 1994. p. 922. [Google Scholar]
- 4.Rose MR. Evolutionary Biology of Ageing. New York: Oxford University Press; 1991. [Google Scholar]
- 5.Greeley EH, Kealy RD, Ballam JM, Lawler DF, Segre M. The influence of age on the canine immune system. Vet Immunol Immunopathol. 1996;55:1–10. doi: 10.1016/s0165-2427(96)05563-8. [DOI] [PubMed] [Google Scholar]
- 6.Greeley EH, Ballam JM, Harrison JM, Kealy RD, Lawler DF, Segre M. The influence of age and gender on the immune system: a longitudinal study in Labrador retriever dogs. Vet Immunol Immunopathol. 2001;82:57–71. doi: 10.1016/s0165-2427(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 7.Greeley EH, Spitznagel E, Lawler DF, Kealy RD, Segre M. Modulation of canine immunosenescence by life-long caloric restriction. Vet Immunol Immunopathol. 2006;111:287–299. doi: 10.1016/j.vetimm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 8.HogenEsch H, Thompson S, Dunham A, Ceddia M, Hayek M. Effect of age on immune parameters and the immune response of dogs to vaccines: a cross-sectional study. Vet Immunol Immunopathol. 2004;97:77–85. doi: 10.1016/j.vetimm.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Strasser A, Teltscher A, May B, Sanders C, Niedermüller H. Age-associated changes in the immune system of German shepherd dogs. J Vet Med A Physiol Pathol Clin Med. 2000;47:181–192. doi: 10.1046/j.1439-0442.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 10.Blount DG, Pritchard DI, Heaton PR. Age-related alterations to immune parameters in Labrador retriever dogs. Vet Immunol Immunopathol. 2005;108:399–407. doi: 10.1016/j.vetimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Meydani SN, Hayek MG, Wu D, et al. Vitamin E and immune response in aged dogs. In: Reinhart GA, Carey DP, editors. Recent Advances in Canine and Feline Nutrition. Vol. 3. Wilmington: Orange Frazer Press; 2000. pp. 295–303. [Google Scholar]
- 12.Heaton PR, Blount DG, Devlin P, et al. Assessing age-related changes in peripheral blood leukocyte phenotypes in Labrador retriever dogs using flow cytometry. J Nutr. 2002;132:1655S–1657S. doi: 10.1093/jn/132.6.1655S. [DOI] [PubMed] [Google Scholar]
- 13.Heaton PR, Blount DG, Mann SJ, et al. Assessing age-related changes in peripheral blood leukocyte phenotypes in domestic shorthaired cats using flow cytometry. J Nutr. 2002;132:1607S–1609S. doi: 10.1093/jn/132.6.1607S. [DOI] [PubMed] [Google Scholar]
- 14.Faldyna M, Levá L, Knötigová P, Toman M. Lymphocyte subsets in peripheral blood of dogs – a flow cytometric study. Vet Immunol Immunopathol. 2001;82:23–37. doi: 10.1016/s0165-2427(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 15.Reis AB, Carneiro CM, Carvalho MG, et al. Establishment of a microplate assay for flow cytometric assessment and it is use for the evaluation of age-related phenotypic changes in canine whole blood leukocytes. Vet Immunol Immunopathol. 2005;103:173–185. doi: 10.1016/j.vetimm.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 17.Tapp PD, Siwak CT, Estrada J, et al. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in ageing. Learn Mem. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10–15. [Google Scholar]
- 19.Todo-Bom A, Mota-Pinto A, Alves V, Santos-Rosa M. Aging and asthma – changes in CD45RA, CD29 and CD95 T cells subsets. Allergol Immunopathol (Madr) 2012;40:14–19. doi: 10.1016/j.aller.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Provinciali M, Moresi R, Donnini A, Lisa RM. Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontology. 2009;55:314–321. doi: 10.1159/000199451. [DOI] [PubMed] [Google Scholar]
- 21.Stulnig T, Maczek C, Böck G, Majdic O, Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from ‘healthy’ young and aged subjects. Int Arch Allergy Immunol. 1995;108:205–210. doi: 10.1159/000237155. [DOI] [PubMed] [Google Scholar]
- 22.Hedlund G, Dohlsten M, Ericsson PO, Sjögren HO. Rapid response to Con A by CD4+ CD45R-rat memory lymphocytes as compared to CD4+ CD45R+ lymphocytes. Cell Immunol. 1989;119:317–326. doi: 10.1016/0008-8749(89)90247-5. [DOI] [PubMed] [Google Scholar]
- 23.Sasama J, Vyas B, Vukmanovic-Stejic M, Kemeny DM. Effect of IL-4, IFN-gamma and IL-12 on cytokine production from human CD45RA and CD45RO CD4 T cell precursors. Int Arch Allergy Immunol. 1998;117:255–262. doi: 10.1159/000024020. [DOI] [PubMed] [Google Scholar]
- 24.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 25.Vivien L, Benoist C, Mathis D. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol. 2001;13:763–768. doi: 10.1093/intimm/13.6.763. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]