Abstract
Background
Community Care of North Carolina (CCNC) initiated an innovative medical home program in the 1990s to improve primary care in Medicaid-insured populations. CCNC has been successful in improving asthma, diabetes, and cardiovascular outcomes but has not been evaluated in the context of cancer care. We explored whether CCNC enrollment was associated with guideline-concordant follow-up care among breast cancer survivors.
Methods
Using state cancer registry records matched to Medicaid claims, we identified women 18 to 64 years old who were diagnosed with stage 0, I, II, or unstaged breast cancer from 2003 to 2007 and tracked their monthly CCNC enrollment. Using published American Society for Clinical Oncology guidelines to define our outcomes, we employed multivariate logistic regressions to examine, as a function of CCNC enrollment, receipt of mammogram and at least 2 physical examinations/ history-taking visits within observational windows consistent with the guidelines.
Results
Of the 840 women, approximately half were enrolled into the CCNC for some time during the study period. Between 40% and 85% received follow-up mammogram in accordance with guidelines, with significant variation by CCNC status, and 95% of women received at least 2 physical examinations/history-taking visits. In multivariate models, increasing months of CCNC enrollment was significantly positively associated with receipt of follow-up mammogram but not with physical examinations/history-taking visits.
Conclusions
Results suggest that CCNC enrollment is associated with guideline-concordant follow-up care for Medicaid-insured survivors. Given the growing population of cancer survivors and increased emphasis on primary care medical homes, future studies should explore what factors are associated with medical home participation and whether similar findings are observed with extended follow-up.
Keywords: medical home, breast cancer, survivorship, Medicaid, primary care
Cancer survivors are a population with complex and dynamic health needs.1–3 After an intensive cancer therapeutic period, many survivors subsequently “fall through the cracks” in the health care system, failing to receive appropriate follow-up surveillance and supportive care coordination.4 The quality of survivorship care varies widely, despite the existence of cancer surveillance and management guidelines for most common cancers.3–6 One challenge in managing cancer survivors is the lack of clarity regarding which provider—the oncologist or the primary care physician—is responsible for ensuring appropriate survivorship care.7
The role of primary care practitioners (PCPs) in caring for the growing cancer survivor population is becoming increasingly important.1,8,9 Most cancer survivors will transition, at some point, from care largely provided by the oncologists to care largely provided by the PCPs.7 Because cancer surveillance remains important and many survivors have persistent late effects associated with cancer therapy,10 PCPs must be familiar with and capable of providing survivorship care in concordance with clinical guidelines. However, 21%–50% of PCPs do not feel sufficiently knowledgeable about the cancer surveillance guidelines,2,11 and 48% of PCPs express challenges promoting cancer-related risk reduction.2 In addition, PCPs should be fully informed of the oncology care their patients received, which requires good communication between the PCPs and oncology care teams.12 Unfortunately, only 20%–30% of survivors feel that their PCPs and oncologists communicate well about their treatment.11,13
Despite these challenges, PCPs are well positioned to manage complex survivorship care needs and are capable of providing high-quality survivorship care that monitors for symptoms of recurrence and assures timely oncology referrals.2,7,5 One study randomly assigned breast cancer survivors to follow-up with either their PCPs or oncologists and found that PCPs detected serious recurrence events at the same rate as the oncologists and that both groups had similar health-related quality of life scores and levels of anxiety.7 Importantly, patients followed by PCPs tend to receive more appropriate noncancer preventive care than those seen by oncologists alone.1,6,9 Not surprisingly, general health promotion is often overlooked by oncologists because the primary interest of oncologists is monitoring for cancer recurrence, when in fact, many adult cancer survivors are at risk for and will die from cardiovascular disease, stroke, or diabetes complications rather than recurrent cancer.
The medical home is an evolving concept of care led by a medical provider with the capacity to direct and coordinate the provision of comprehensive, high-quality, accessible, community-based care.14,15 In NC and elsewhere, medical homes have been shown to improve disease management, ensure receipt of general preventive services, reduce the risks of medication contraindications, reduce health disparities, and create efficiency gains.16,17 Moreover, medical homes may be useful in improving cancer survivorship care, given that the management of cancer survivors requires coordination of multiple medications, providers, and procedures to ensure optimal cancer follow-up and management of noncancer health care needs.18–21
Community Care of North Carolina (CCNC) is an innovative medical home program initiated in the 1990s to enhance primary care case management in vulnerable populations insured by Medicaid. Medicaid patients whose providers are members of one of the CCNC networks throughout the state are enrolled into a CCNC medical home, and their providers and the network receive per member per month payments for care coordination.16 Medicaid beneficiaries who are not enrolled in a CCNC medical homes receive care through a more generic primary care case management program or through fee for service.16 The medical homes model in NC, or elsewhere, has not been studied empirically in the context of improving cancer care, although many have proposed using medical home shared-care concepts to coordinate cancer survivorship care.22,18,20 Capitalizing on NC’s use of medical homes for its Medicaid population, the purpose of our research was to examine whether adherence to cancer survivorship guidelines as set forth by the American Society for Clinical Oncology (ASCO)5 was higher among CCNC enrollees. We focused our efforts on breast cancer because it is one of the most commonly diagnosed malignancies in women23 and has good prognosis when diagnosed early. As a result, we identified a large number of breast cancer survivors in our Medicaid population of interest.
METHODS
Data Sources
Our dataset was created using NC Central Cancer Registry and Medicaid claims to identify women diagnosed with incident stage 0, I, II, or unstaged breast cancer in the years 2003–2007, using a previously published protocol.24 The combined dataset consisted of patient demographics, tumor information, claims for emergency, inpatient, and outpatient services, and prescription drugs paid for by Medicaid.
Study Population
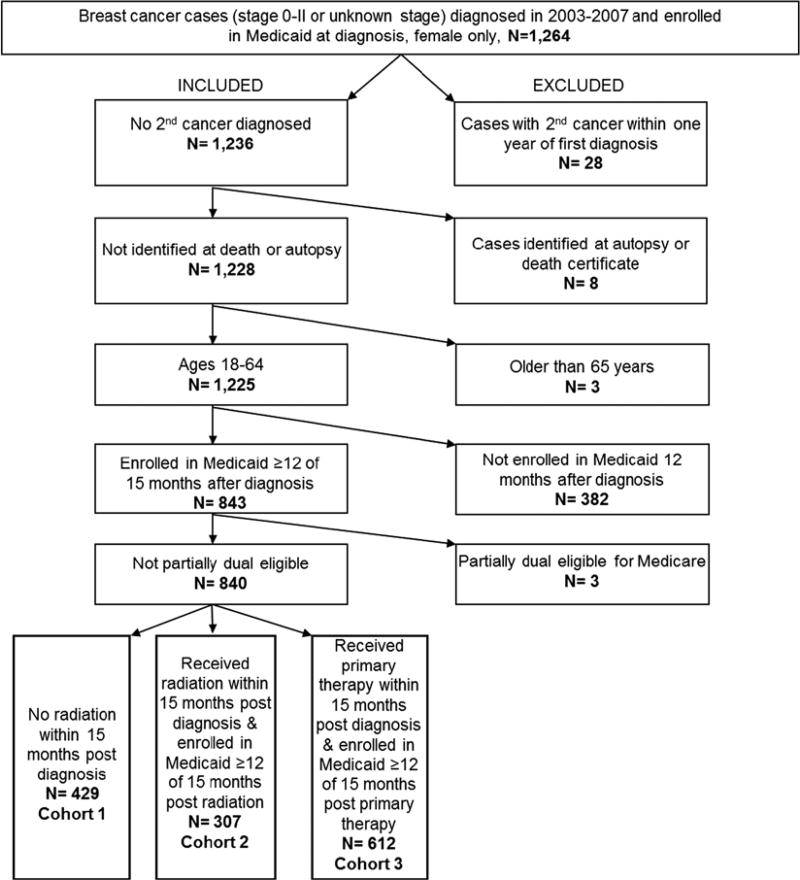
We restricted our analyses to women who were 18 to 64 years old during our study period. Women aged 65 and older at the time of diagnosis were excluded because our focus was on younger, Medicaid-insured, breast cancer patients, a relatively understudied population that may have less comorbidity and different patterns of health care use when compared with older women. Eligible women were fully enrolled in Medicaid (excluding partial duals) at least 1 month before their index breast cancer diagnosis and at least 12 of 15 months postdiagnosis (to balance the need to capture complete claims for cancer treatment with the recognition that Medicaid enrollment can sometimes be transient). We excluded cases diagnosed at autopsy or death certificate25 and cases dying of any cause in the same month as diagnosis. We also excluded a very small number of cases with an additional primary cancer diagnosis within 1 year of the index diagnosis (n = 28) because the vast majority of early-stage breast cancer patients will not experience a second cancer within this time frame, and those who do are very different in terms of their health and health care utilization.25 We were not able to determine whether sample participants experienced breast cancer recurrence because of the fact that the NC Central Cancer Registry does not capture same-breast recurrences, and there is no standardized, validated approach for identifying same-breast recurrences in claims data, because of the lack of specificity in coding around laterality.26 Finally, we required at least 12 of 15 months of Medicaid enrollment during each time window, we sought to observe the outcome of interest. We explored how these decisions affected our sample characteristics and outcomes in sensitivity analyses. Inclusion/exclusion criteria are summarized in Figure 1.
FIGURE 1.
Inclusion and exclusion criteria and cohort selection.
Dependent Variables
Dependent variables were inspired by ASCO guidelines,5 which state that (1) first follow-up mammogram should be received 1 year after the initial diagnostic mammogram among women not receiving radiation therapy (RT); (2) first post-RT mammogram should be received 6 months to 1 year after RT completion among women receiving RT; and (3) history-taking/physical examination should be received every 3–6 months for the first 3 years after primary therapy completion. In accordance with these guidelines, we developed 3 sets of quality of care measures examining: receipt of first follow-up mammogram in the 15-month period postdiagnosis for women not receiving RT (cohort 1); receipt of first follow-up mammogram in the 15-month period after RT completion (as reflected by the last claim date associated with a primary RT cycle) for women receiving RT (cohort 2); and receipt of at least 2 visits with history-taking/physical examination in the 15-month period after primary treatment completion among women who received primary treatment within 15 months of diagnosis (cohort 3). We defined the latter observational window by identifying the dates of all treatment-related procedural codes in the claims postdiagnosis. We then identified the last treatment (surgery, RT, or chemotherapy) received before a 90-day gap in any therapy. We then defined the last claim date associated with that treatment as the date of completion of primary therapy.27 Although ASCO guidelines recommend that follow-up mammography and physical examinations should be received within 12 months, we extended our time window from 12 to 15 months to allow possible scheduling delays and late claims filing. Table 1 summarizes our patient cohort definitions, variable measurement windows, and modeling approach.
TABLE 1.
Summary of Statistical Models
| Model | Cohort | Cohort Size |
Outcome | Key Independent Variables | Control Variables | Analysis |
|---|---|---|---|---|---|---|
| 1 | Women who did not receive radiation and were enrolled in Medicaid ≥ 12 of 15 mo after diagnosis | 429 | Follow-up mammogram within 15 mo of diagnosis | Months enrolled in Medical home | Comorbidities; stage of disease; hormone receptor status; age at diagnosis; race; rural/urban residence; BCCCP participation; aged/blind/disabled; fully dual eligible; surgery type; chemotherapy; diagnosis year | Logit |
| 2 | Women who received radiation and were enrolled in Medicaid ≥ 12 of 15 mo after radiation completion | 307 | Follow-up mammogram within 15 mo of radiation completion | Months enrolled in Medical home | Same as model 1 | Logit |
| 3a | Women who were enrolled in Medicaid ≥ 12 of 15 mo after primary therapy completion | 612 | At least 2 physical exams within 15 mo of primary therapy completion | Months enrolled in Medical home | Same as model 1; radiation therapy | Logit |
| 3b | Women who had at least 2 physical exams and were enrolled in Medicaid ≥ 12 of 15 mo after primary therapy completion | 581 | Count of physical exams within 15 mo of primary therapy completion | Months enrolled in Medical home | Same as model 3a | OLS |
BCCCP indicates Breast and Cervical Cancer Control Program; OLS, ordinary least squares.
Procedural codes used to identify services can be found online (Table, Supplemental Digital Content 1, http://links.lww.com/MLR/A436). Mammograms received within 30 days of diagnosis were excluded as these likely reflect diagnostic work up rather than surveillance. Differentiating mammography intent any further is not straightforward in insurance claims data (eg, distinguishing symptom-driven mammography vs. general surveillance mammography vs. diagnostic mammography).28
Independent Variables
Patients were identified as being in the CCNC program in any given month when a management fee was paid to both the PCP and the CCNC network in the corresponding month (Table, Supplemental Digital Content 1, http://links.lww.com/MLR/A436). For each of the analyses described above and in Table 1, months of CCNC enrollment was measured in the 15-month period postdiagnosis (cohort 1); 15-month period post-RT completion (cohort 2); and 15-month period after primary therapy completion (cohort 3). In the final models, we included the number of months of medical home enrollment and its quadratic form.
Control Variables
On the basis of the previous research, we included 3 categories of control variables that are associated with health care services utilization among breast cancer patients—sociodemographic, clinical (overall and tumor-related), and cancer therapeutic characteristics.29–31 Patient sociodemographic measures included race/ethnicity, age at diagnosis, rural/urban residence at diagnosis,32 reason for Medicaid eligibility, and diagnosis year. For Medicaid eligibility categorization, we included indicators for full dual enrollment in Medicare, enrollment in the Breast and Cervical Cancer Control Program (BCCCP), and being classified as aged/ blind/disabled for Medicaid eligibility for any of the months corresponding to the observational window of each analysis (Table 1).
Clinical variables measured included cancer stage at diagnosis, hormone receptor positivity at diagnosis, and presence of comorbid conditions. Stage was derived from the American Joint Committee on Cancer grouping; Surveillance, Epidemiology and End Results summary stage or Tumor, Node, and Metastasis was used when the American Joint Committee on Cancer was not available. Hormone receptor positivity was coded as positive or elevated estrogen, or positive or elevated progesterone receptor status. Presence of comorbid conditions was determined using the breast cancer–specific National Cancer Institute Combined Index33 modified to use any available claims from the index diagnosis month through 15 months postdiagnosis.
Therapeutic characteristics of interest that defined our patient cohorts and observational time windows and that may also influence subsequent health services utilization included receipt of breast cancer surgery [no surgery, breast conserving surgery (BCS) only, mastectomy only, or both BCS and mastectomy], RT, and chemotherapy. We assume that these measures are characteristics of the women and their tumors rather than endogenous choices about therapeutic options. Breast cancer treatments were identified using relevant diagnostic and procedural codes from Medicaid claims (Table, Supplemental Digital Content 1, http://links.lww.com/MLR/A436).
Statistical Analysis
Continuous variables were summarized by their means and SDs and compared with CCNC status using t tests. The χ2 statistic was used to test overall associations for each categorical covariate by CCNC status. We used multivariate logistic regression to examine, as a function of CCNC status, receipt of mammogram within 15 months of diagnosis among women who did not receive RT (cohort 1); receipt of mammogram within 15 months of RT completion among women who received RT (cohort 2); and receipt of at least 2 physical examinations/history-taking visits within 15 months of primary therapy completion (cohort 3). Of those who received at least 2 visits, we estimated using Ordinary Least Squares regression the total number of visits as a function of CCNC enrollment (cohort 3) (Table 1).
We examined functional form of age, comorbidity, and year of diagnosis using z statistics and −2 Log Pseudolikelihood and ultimately used the continuous form of age, categorical specifications of comorbidity, and categorical specifications of year of diagnosis. On the basis of feedback received from an expert advisory committee (described in detail below), we included an interaction term for BCCCP-by-urban residence to reflect potentially stronger relationships between the BCCCP program and survivorship care quality in urban areas (where BCCCP is better resourced and more accessible to women in need).
We report average marginal effects on predicted probabilities because odds ratios are often misinterpreted and misunderstood, particularly when interaction terms are used.34 In our analysis, a marginal effect for any given explanatory variable can be interpreted as the average difference in the predicted probability of the outcome based on changing values of that explanatory variable, relative to other covariates, across all observations in the estimation sample.35 SEs for marginal effects were estimated using the Delta method,34 from which we report 95% confidence intervals using the “margins” command in STATA 12.0 (StataCorp, College Station, TX).
Expert Advisory Committee
To enable us to better interpret our research findings, to reflect meaningfully upon the limitations of our data, and to plan for future community and CCNC research engagement, we assembled an expert advisory committee early in the research process. The 10-member committee included oncologists, PCPs, a breast cancer survivor, an advocate, a nurse navigator, Medicaid and uninsured case/social workers, and CCNC and other statewide organizational leaders.
RESULTS
A total of 840 women were included in our overall sample, of which 429 were included in cohort 1, 307 were included in cohort 2, and 612 were included in Cohort 3 analyses (Fig. 1). Table 2 depicts bivariate descriptive statistics, by medical home status, in cohorts 1, 2, and 3. Overall, average age at cancer diagnosis was 50.3 years, 44% of women were non-Hispanic white, 43% were non-Hispanic black, and almost two thirds lived in an urban area. Approximately half were involved in a CCNC medical home at any point during our study period, with significant variation in CCNC enrollment by patient sample characteristics (Table 2). Comparing our final overall analytic sample (which required at least 12 of 15 mo Medicaid enrollment postdiagnosis) with the complete sample of women enrolled in Medicaid for any amount of time, CCNC participation for any period of time was higher in our final overall analytic sample (51%) when compared with the complete sample (30%).
TABLE 2.
Descriptive Summary of Patterns of Care for Breast Cancer Survivors
| n (%) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cohort 1 No RT† (n = 429) | Cohort 2 RT† (n = 307) | Cohort 3 Primary Therapy (n = 612) | ||||
|
|
|
|
||||
| Patient Characteristics | Ever in MH [210 (49.0)] |
Never in MH [219 (51)] |
Ever in MH [168 (54.7)] |
Never in MH [139 (45.3)] |
Ever in MH [334 (54.6)] |
Never in MH [278 (45.4)] |
| Mean no. months in MH (SD) | 10.9 (4.2) | 11.3 (4.4) | 11.4 (4.4) | |||
| Clinical characteristics | ||||||
| Comorbidity index | ||||||
| Score 0 | 60.0 (126) | 58.0 (127) | 58.3 (98) | 56.1 (78) | 58.7 (196) | 55.0 (153) |
| Score > 0 <1 | 31.4 (66) | 34.2 (75) | 36.3 (61) | 35.3 (49) | 33.5 (112) | 36.7 (102) |
| Score ≥ 1 <2 | 6.7 (14) | 5.9 (13) | 2.4 (4) | 6.5 (9)* | 5.1 (17) | 6.1 (17) |
| Score ≥ 2 | 1.9 (4) | 1.8 (4) | 3.0 (5) | 2.2 (3) | 2.7 (9) | 2.2 (6) |
| Tumor stage | ||||||
| Stage 0 | 28.6 (60) | 27.4 (60) | 22.6 (38) | 25.2 (35) | 25.7 (86) | 26.6 (74) |
| Stage 1 | 34.8 (73) | 34.7 (76) | 40.5 (68) | 46.8 (65) | 39.2 (131) | 40.3 (112) |
| Stage 2 | 23.3 (49) | 28.3 (62) | 28.0 (47) | 20.9 (29) | 25.1 (84) | 25.6 (71) |
| Stage unknown | 13.3 (28) | 9.6 (21) | 8.9 (15) | 7.2 (10) | 9.9 (33) | 7.5 (21) |
| Hormone receptor positive | 40.5 (85) | 37.0 (81) | 48.8 (82) | 46.0 (64) | 44.0 (147) | 43.2 (120) |
| Sociodemographic | ||||||
| Age mean (SD) | 48.1 (9.8) | 52.6 (9.1)*** | 48.9 (8.9) | 53.6 (9.0)*** | 48.4 (9.5) | 53.5 (8.8)*** |
| Race | ||||||
| White | 40.0 (84) | 47.5 (104) | 34.5 (58) | 48.2 (67)* | 37.7 (126) | 50.0 (139)*** |
| Black | 43.8 (92) | 41.1 (90) | 55.4 (93) | 36.0 (50)*** | 49.1 (164) | 38.1 (106)*** |
| Hispanic | 4.8 (10) | 1.8 (4)* | 3.6 (6) | 2.2 (3) | 4.8 (16) | 1.4 (4)** |
| Other | 4.3 (9) | 0.5 (1)*** | 2.4 (4) | 2.2 (3) | 2.4 (8) | 1.1 (3) |
| Multiple | 7.1 (15) | 9.1 (20) | 4.2 (7) | 11.5 (16)** | 6.0 (20) | 9.4 (26) |
| Urban residence | 61.9 (130) | 58.9 (129) | 63.7 (107) | 64.0 (89) | 62.9 (210) | 58.3 (162) |
| ABD classification | 58.1 (122) | 68.9 (151)** | 67.3 (113) | 75.5 (105) | 64.7 (216) | 76.3 (212)** |
| BCCCP participation | 3.3 (7) | 14.6 (32)*** | 7.1 (12) | 9.4 (13) | 5.1 (17) | 11.9 (33)*** |
| Fully dual eligible | 17.6 (37) | 36.5 (80)*** | 16.1 (27) | 47.5 (66)*** | 16.5 (55) | 47.5 (132)*** |
| Year of diagnosis | ||||||
| 2003 | 18.1 (38) | 20.5 (45) | 13.7 (23) | 23.0 (32)** | 18.3 (61) | 19.8 (55) |
| 2004 | 14.3 (30) | 15.5 (34) | 16.1 (27) | 25.2 (35)** | 15.6 (52) | 21.6 (60)* |
| 2005 | 21.9 (46) | 21.5 (47) | 23.2 (39) | 18.7 (26) | 22.8 (76) | 22.7 (63) |
| 2006 | 21.0 (44) | 19.6 (43) | 28.6 (48) | 18.0 (25)** | 23.4 (78) | 18.0 (50) |
| 2007 | 24.8 (52) | 22.8 (50) | 18.5 (31) | 15.1 (21) | 20.1 (67) | 18.0 (50) |
| Therapeutic characteristics | ||||||
| Surgery type | ||||||
| Both BCS and mastectomy | 35.2 (74) | 27.4 (60)* | 50.6 (85) | 52.5 (73) | 46.1 (154) | 45.7 (127) |
| No surgery | 19.0 (40) | 34.3 (75)*** | 15.5 (26) | 15.8 (22) | 10.5 (35) | 14.0 (39) |
| Breast conserving surgery only | 9.0 (19) | 10.5 (23) | 27.4 (46) | 25.9 (36) | 18.6 (62) | 20.5 (57) |
| Mastectomy only | 36.7 (77) | 27.9 (61)** | 6.6 (11) | 5.8 (8) | 24.9 (83) | 19.8 (55) |
| Chemotherapy | 41.0 (86) | 39.3 (86) | 61.3 (103) | 53.2 (74) | 51.8 (173) | 47.8 (133) |
| Receipt of recommended follow-up care | ||||||
| MM within 15 mo of diagnosis | 50.0 (105) | 40.2 (88)** | — | — | — | — |
| MM within 15 mo of RT | — | — | 85.1 (143) | 77.0 (107)* | — | — |
| Mean days to first MM | 281 (116) | 298 (81) | 157 (91) | 174 (94)* | — | — |
| Two physical exams within 15 mo of primary therapy | — | — | — | — | 96.7 (323) | 92.8 (258)** |
| Mean no. physical exams within 15 mo of primary therapy | — | — | — | — | 14.3 (8.3) | 15.6 (9.5)* |
Statistical significance of t tests and χ2 indicated by ***P < 0.01, **P < 0.05, *P < 0.1.
Receipt of radiation within 15 mo of diagnosis.
ABD indicates aged/blind/disabled; BCCCP, Breast and Cervical Cancer Control Program; BCS, breast conserving surgery; MH, medical home; MM, mammography; RT, radiation therapy.
Among women not receiving RT (cohort 1), 45% received follow-up mammogram within 15 months postdiagnosis, and mean time to first follow-up mammogram was 289 days postdiagnosis. Among women receiving RT (cohort 2), 81% received follow-up mammogram within 15 months post-RT completion, and mean time to first follow-up mammogram was 165 days post-RT completion. Approximately 52% of women in the RT group received a follow-up mammogram before the recommended 6 months post-RT completion.
Table 3 depicts average marginal effects indicating differences in the probability of receipt of follow-up mammogram based on multivariate logistic regressions. In cohort 1, number of months of CCNC enrollment was significantly positively associated with receipt of follow-up mammogram within 15 months postdiagnosis (P = 0.021). In other words, each additional month of CCNC enrollment corresponds to a 1.2% point increase in the probability of receiving a follow-up mammogram within the observational time period. In cohort 2, CCNC participation was significantly positively associated with receipt of follow-up mammogram within 15 months post-RT completion (P = 0.031). In other words, each additional month of CCNC enrollment corresponds to a 1.4% point increase in the probability of receiving a follow-up mammogram within the observational time period. The results for physical examination/history-taking visits (cohort 3) are presented in Table 4. We found no statistically significant differences in this outcome by CCNC status.
TABLE 3.
Average Marginal Effects on Follow-up Mammography for Breast Cancer Survivors From Multivariate Logistic Regression
| Cohort 1 No RT† | Cohort 2 RT† | |||
|---|---|---|---|---|
|
|
|
|||
| Marginal Effect on MM Within 15 mo of Diagnosis |
95% CI | Marginal Effect on MM Within 15 mo of RT |
95% CI | |
| Months in medical home | 0.012 | 0.002, 0.023** | 0.014 | 0.001, 0.027** |
| Clinical characteristics | ||||
| Comorbidity index | ||||
| Score 0 (reference category) | ||||
| Score > 0 <1 | −0.014 | −0.112, 0.084 | −0.014 | −0.104, 0.076 |
| Score ≥ 1 <2 | −0.026 | −0.208, 0.156 | −0.026 | −0.192, 0.140 |
| Score ≥2 | −0.090 | −0.391, 0.210 | −0.244 | −0.451, −0.038** |
| Tumor stage | ||||
| Stage 0 (reference category) | ||||
| Stage 1 | −0.002 | −0.118, 0.113 | 0.124 | −0.002, 0.249* |
| Stage 2 | −0.182 | −0.307, −0.056*** | 0.141 | −0.005, 0.288* |
| Stage unknown | −0.038 | −0.207, 0.131 | 0.057 | −0.096, 0.210 |
| Hormone receptor positive | 0.015 | −0.092, 0.123 | 0.084 | −0.005, 0.172* |
| Sociodemographic | ||||
| Age | 0.006 | 0.000, 0.011** | 0.003 | −0.002, 0.009 |
| Race | ||||
| White (reference category) | ||||
| Black | 0.007 | −0.092, 0.105 | −0.034 | −0.123, 0.056 |
| Hispanic | −0.024 | −0.244, 0.196 | −0.068 | −0.323, 0.186 |
| Other | 0.027 | −0.234, 0.287 | −0.109 | −0.309, 0.091 |
| Multiple | 0.055 | −0.125, 0.236 | −0.105 | −0.240, 0.030 |
| Urban residence | −0.034 | −0.130, 0.062 | −0.048 | −0.126, 0.030 |
| ABD classification | −0.080 | −0.200, 0.039 | −0.063 | −0.175, 0.049 |
| BCCCP participation | 0.151 | −0.026, 0.328* | −0.036 | −0.180, 0.109 |
| Fully dual eligible | 0.042 | −0.077, 0.160 | 0.129 | 0.023, 0.235** |
| Year of diagnosis | ||||
| 2003 (reference category) | ||||
| 2004 | 0.049 | −0.111, 0.208 | −0.090 | −0.216, 0.035 |
| 2005 | −0.015 | −0.161, 0.132 | −0.004 | −0.118, 0.109 |
| 2006 | 0.058 | −0.099, 0.214 | −0.062 | −0.173, 0.049 |
| 2007 | 0.068 | −0.084, 0.220 | −0.104 | −0.274, 0.066 |
| Therapeutic characteristics | ||||
| Surgery type | ||||
| Both BCS and mastectomy (reference category) | ||||
| No surgery | −0.234 | −0.362, −0.106*** | −0.116 | −0.222, −0.010** |
| Breast conserving surgery only | −0.059 | −0.225, 0.106 | 0.101 | −0.033, 0.236 |
| Mastectomy only | 0.027 | −0.089, 0.143 | −0.261 | −0.386, −0.137*** |
| Chemotherapy | 0.097 | −0.003, 0.197* | 0.004 | −0.116, 0.124 |
| Observations | 429 | 307 | ||
Interaction term BCCCP × urban included.
P < 0.01,
P < 0.05,
P < 0.1.
Receipt of radiation within 15 mo of diagnosis.
ABD indicates aged/blind/disabled; BCCCP, Breast and Cervical Cancer Control Program; BCS, breast conserving surgery; CI, confidence interval; MM, mammography; RT, radiation therapy.
TABLE 4.
Average Marginal Effects on Physical Exams for Breast Cancer Survivors After Primary Therapy† From 2-part Model
| Cohort 3a | Cohort 3b | |||
|---|---|---|---|---|
|
|
|
|||
| Marginal Effect on Receipt of 2 Physical Exams‡ |
95% CI | Marginal Effect on No. Physical Exams‡ |
95% CI | |
| Months in medical home | 0.001 | −0.007, 0.008 | −0.066 | −0.197, 0.065 |
| Clinical characteristics | ||||
| Comorbidity index | ||||
| Score 0 (reference category) | ||||
| Score >0 <1 | −0.021 | −0.056, 0.015 | 3.907 | 2.419, 5.396*** |
| Score ≥1§ | 0.045 | −0.038, 0.128 | 6.874 | 4.298, 9.450*** |
| Tumor stage | ||||
| Stage 0 (reference category) | ||||
| Stage 1 | −0.040 | −0.086, 0.006* | 0.043 | −1.745, 1.832 |
| Stage 2 | −0.039 | −0.092, 0.014 | −1.705 | −3.776, 0.366 |
| Stage unknown | −0.013 | −0.088, 0.061 | −2.060 | −4.901, 0.782 |
| Hormone receptor positive | 0.052 | 0.014, 0.090*** | 0.221 | −1.357, 1.800 |
| Sociodemographic | ||||
| Age | −0.004 | −0.006, −0.001*** | −0.058 | −0.149, 0.033 |
| Race | ||||
| White (reference category) | ||||
| Black | −0.034 | −0.072, 0.004* | −2.702 | −4.163, −1.240*** |
| Hispanic | −0.052 | −0.126, 0.022 | −1.127 | −5.110, 2.856 |
| Other | −0.118 | −0.225, −0.011** | −3.254 | −8.576, 2.069 |
| Multiple | −0.049 | −0.110, 0.011 | −1.534 | −4.224, 1.156 |
| Urban residence | −0.047 | −0.078, −0.016*** | −0.843 | −2.255, 0.569 |
| ABD classification | 0.027 | −0.028, 0.081 | 2.625 | 0.743, 4.507*** |
| BCCCP participation | 0.016 | −0.032, 0.064 | 2.371 | −0.205, 4.948* |
| Fully dual eligible | 0.029 | −0.012, 0.071 | −0.191 | −1.948, 1.565 |
| Year of diagnosis | ||||
| 2003 (reference category) | ||||
| 2004 | −0.011 | −0.048, 0.025 | −0.187 | −2.452, 2.078 |
| 2005 | −0.066 | −0.117, −0.015** | −0.318 | −2.620, 1.984 |
| 2006 | −0.028 | −0.071, 0.015 | −1.797 | −4.109, 0.515 |
| 2007 | −0.078 | −0.138, −0.017** | −1.765 | −4.289, 0.759 |
| Therapeutic characteristics | ||||
| Surgery type | ||||
| Both BCS and mastectomy (reference category) | ||||
| No surgery | −0.040 | −0.095, 0.014 | −0.823 | −3.206, 1.560 |
| Breast conserving surgery only | 0.027 | −0.024, 0.079 | 0.205 | −1.778, 2.188 |
| Mastectomy only | 0.045 | −0.006, 0.096* | −0.324 | −2.209, 1.561 |
| Chemotherapy | 0.067 | 0.022, 0.113*** | 4.689 | 3.132, 6.247*** |
| Radiation | 0.056 | 0.016, 0.095*** | 1.301 | −0.237, 2.839* |
| Observations | 612 | 581 | ||
Interaction term BCCCP × urban included.
P < 0.01,
P < 0.05,
P < 0.1.
Receipt of primary therapy within 15 mo of diagnosis.
Physical exams received within 15 mo of completion of primary therapy.
Comorbidity categories were combined due to small cell sizes.
ABD indicates aged/blind/disabled; BCCCP, Breast and Cervical Cancer Control Program; BCS, breast conserving surgery; CI, confidence interval.
CONCLUSIONS
This study examined the relationship between CCNC enrollment and adherence to ASCO surveillance and follow-up guidelines in a population of low-income women with breast cancer. Our findings indicate that CCNC enrollment is associated with increased probability of receiving timely follow-up mammography. More broadly, results of this study suggest that primary care medical homes may be associated with improved follow-up care among Medicaid-insured breast cancer survivors. To our knowledge, this is the first study to demonstrate the potential benefits of primary care medical homes for cancer survivors. Although our analyses preclude us from commenting definitively on causal relationships, these findings point to the need for more research to identify how primary care medical homes can be helpful in the context of cancer survivorship care quality.
In this study, a large number of women failed to receive guideline-concordant follow-up mammogram within recommended time windows, a concerning finding that is consistent with other studies.25,36 Another potential indicator of poor access to care and quality of care was the fact that 12%–27% of women did not receive any surgery (depending on the cohort examined). Many of the women who did not undergo surgery had missing stage information. In multivariate analyses, receiving no surgery was associated with lower probability of guideline-recommended follow-up mammogram, further suggesting poorer quality of survivorship care in these women. In addition, between 31% and 51% of women received both BCS and mastectomy, which may indicate insufficient initial surgical procedures. In contrast, there is some overlap in billing codes used to identify surgical biopsies (a diagnostic procedure) and breast conserving surgery (a therapeutic procedure); it is therefore possible that some women classified as receiving BCS followed by mastectomy, in fact, received diagnostic surgical biopsies as opposed to therapeutic BCS.
As with any observational study, limitations exist. First, because we used claims data linked to cancer registry data, we did not have access to patients’ medical records. Thus, we lacked potentially important contextual information such as quality of patient-provider communication and detailed information about laterality, which would reflect whether a radical bilateral mastectomy had been received previously. Unfortunately, identifying bilateral mastectomies from claims data is prone to measurement error.37 Moreover, we only observed procedures corresponding to claims paid by Medicaid, therefore, some procedures may be underascertained. Nevertheless, using these data, we were able to document overall patterns of care by CCNC status for an important vulnerable subpopulation of women, and we have no reason to think that such measurement issues would be differential by CCNC status, biasing our results in any particular direction. Second, our NC-specific findings may not be generalizable to other states. Nonetheless, the CCNC program has been heralded nationally as innovative and effective in enhancing care coordination; therefore, because NC is a national model in this respect and has one of the largest Medicaid programs in the country, providing additional data on the potential benefits of CCNC, as we have done here, is important. Finally, because of a limited sample size, we do not control for selection into the CCNC program and therefore cannot rule out that the factors that drive women to CCNC providers over other Medicaid providers are responsible for the relationships observed. It is also possible that areas with CCNC network providers have other health care resources such as survivor support groups that influence follow-up care. Such unmeasured factors were not available in our data and may confound the relationship between CCNC enrollment and receipt of high-quality survivorship care.
Strengths of this study include its novelty in examining patterns of cancer survivorship care within primary care medical homes. We had a unique opportunity to capitalize on the existing CCNC program that has been shown to improve non-cancer care coordination. To our knowledge, this study is the first to document that shared-care models like CCNC are associated with higher quality cancer survivorship care. Another strength is our approach to outcome measurement, which reflects published, evidence-based guidelines for breast cancer surveillance and follow-up.5 Finally, bringing an expert advisory committee into our research process allowed us to (a) better understand and interpret findings from data analyses; (b) reflect upon care processes in community settings; and (c) prepare for community involvement in future research and implementation efforts aimed at improving survivorship care.
Breast cancer, like other chronic illnesses, poses significant expense to Medicaid and other insurers.18 Better monitoring and coordination of survivorship care through primary care medical homes can help reduce additional cancer-related cost burden, lead to earlier detection of recurrences, improve patient satisfaction and quality measures, ensure a more efficient and equitable system, and eliminate costly redundancies.2,38 In addition, using novel care models like CCNC may improve patient satisfaction, enhance provider-to-provider communication, and reduce the burden of follow-up care on oncology specialists. Results from our study can be used to inform initiatives elsewhere to improve quality of survivorship care building upon the innovative medical homes approach.
Supplementary Material
Acknowledgments
The authors would like to thank and recognize their expert advisory committee, consisting of Anne Braswell, Jonathan Fischer, Bo Gamble, Eleanor Greene, Linda Kinney, Patrick Maguire, Brenda McCants, Rachel Raab, LaSonia Roberts-Melvin, and Thea Monet. Authors also would like to thank Drs Annette DuBard and Hy Muss for their insights into CCNC and cancer survivorship care and Dr Anne Marie Meyer for her insights into claims data analysis. This research was presented as an abstract to the American Society for Clinical Oncology Annual Research Meeting, held in Chicago June 1–5, 2012.
Supported by the Agency for Healthcare Research and Quality Comparative Effectiveness Research Career Development Award, 1-K-12 HS019468-01 (Weinberger); University Cancer Research Fund Health-e-NC Pilot (S.B.W.) and work was also supported by the Integrated Cancer Information and Surveillance System (ICISS), a UNC Lineberger Comprehensive Cancer Center resource.
Footnotes
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.lww-medicalcare.com.
References
- 1.Grunfeld E. Cancer survivorship: a challenge for primary care physicians. Br J Gen Pract. 2005;55:741–742. [PMC free article] [PubMed] [Google Scholar]
- 2.Burg MA, Grant K, Hatch R. Caring for patients with cancer histories in community-based primary care settings: a survey of primary care physicians in the Southeastern United States. Prim Health Care Res Dev. 2005;6:244–250. [Google Scholar]
- 3.Bowles EJA, Tuzzio L, Wiese CJ, et al. Understanding high-quality cancer care. Cancer. 2008;112:934–942. doi: 10.1002/cncr.23250. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Greenfield S, Stovall E, editors. Institute of Medicine, National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 5.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 6.Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1551. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;40:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz PA. The ‘three ps’ of cancer survivorship care. BMC Med. 2011;9:14. doi: 10.1186/1741-7015-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roundtree AK, Giordano SH, Price A, et al. Problems in transition and quality of care: perspectives of breast cancer survivors. Support Care Cancer. 2011;19:1921–1929. doi: 10.1007/s00520-010-1031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403–1410. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101–117. doi: 10.3322/caac.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao JJ, Bowman MA, Stricker CT, et al. Delivery of survivorship care by primary care physicians: the perspective of breast cancer patients. J Clin Oncol. 2009;27:933–938. doi: 10.1200/JCO.2008.18.0679. [DOI] [PubMed] [Google Scholar]
- 14.Iglehart JK. No place like home—testing a new model of care delivery. N Engl J Med. 2008;359:1200–1202. doi: 10.1056/NEJMp0805225. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal TC. The medical home: growing evidence to support a new approach to primary care. J Am Board Fam Med. 2008;21:427–440. doi: 10.3122/jabfm.2008.05.070287. [DOI] [PubMed] [Google Scholar]
- 16.Domino ME, Humble C, Lawrence WW, Jr, et al. Enhancing the medical homes model for children with asthma. Med Care. 2009;47:1113–1120. doi: 10.1097/MLR.0b013e3181adcc65. [DOI] [PubMed] [Google Scholar]
- 17.Wilhide S, Henderson T. Community Care of North Carolina: a provider-led strategy for delivering cost-effective primary care to Medicaid beneficiaries. Washington, DC: American Academy of Family Physicians; 2006. [Accessed March 8, 2013]. pp. 1–24. Available at: http://ctlawhelp.org/files/PCCM-HMO/NC-Report-AAFP-June-06.pdf. [Google Scholar]
- 18.Wender RC, Altshuler M. Can the medical home reduce cancer morbidity and mortality? Prim Care. 2009;36:845–858. doi: 10.1016/j.pop.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Goins KV, Zapka JG, Geiger AM, et al. Implementation of systems strategies for breast and cervical cancer screening services in health maintenance organizations. Am J Manag Care. 2003;9:745–757. [PubMed] [Google Scholar]
- 20.Sprandio JD. Oncology patient-centered medical home and accountable cancer care. Community Oncol. 2010;7:565–572. [Google Scholar]
- 21.Zapka JG, Taplin SH, Solberg LI, et al. A framework for improving the quality of cancer care. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 22.Gerber LH, Stout NL, Schmitz KH, et al. Integrating a prospective surveillance model for rehabilitation into breast cancer survivorship care. Cancer. 2012;118(suppl 8):2201–2206. doi: 10.1002/cncr.27472. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 24.Wheeler SB, Wu Y, Meyer A, et al. Use and timeliness of radiation therapy after breast-conserving surgery in low-income women with early-stage breast cancer. Cancer Invest. 2012;30:258–267. doi: 10.3109/07357907.2012.658937. [DOI] [PubMed] [Google Scholar]
- 25.Keating NL, Landrum MB, Guadagnoli E, et al. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 26.McClish D, Penberthy L, Pugh A. Using Medicare claims to identify second primary cancers and recurrences in order to supplement a cancer registry. J Clin Epidemiol. 2003;56:760–767. doi: 10.1016/s0895-4356(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 27.Field TS, Doubeni C, Fox MP, et al. Underutilization of surveillance mammography among breast cancer survivors. J Gen Intern Med. 2008;23:158–163. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blustein J. Medicare coverage, supplemental insurance, and the use of mammography by older women. N Engl J Med. 1995;332:1138–1143. doi: 10.1056/NEJM199504273321706. [DOI] [PubMed] [Google Scholar]
- 29.Arozullah AM, Calhoun EA, Wolf M, et al. The financial burden of cancer: estimates from a study of insured women with breast cancer. J Support Oncol. 2004;2:271–278. [PubMed] [Google Scholar]
- 30.Wheeler SB, Carpenter WR, Peppercorn J, et al. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012a;133:333–345. doi: 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler SB, Carpenter WR, Peppercorn J, et al. Predictors of timing of adjuvant chemotherapy in older women with hormone receptor-negative, stages II–III breast cancer. Breast Cancer Res Treat. 2012b;131:207–216. doi: 10.1007/s10549-011-1717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United States Department of Agriculture. Rural-Urban Community Area Codes. Economic Research Service; 2009. [Accessed March 8, 2013]. Available at: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation.aspx. [Google Scholar]
- 33.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Norton EC, Wang H, Ai C. Computing interaction effects and standard errors in logit and probit models. Stata J. 2004;4:154–167. [Google Scholar]
- 35.Gauvin JP. A quick look at the margins command, 2012. [Accessed January 14, 2013]; Available at: http://www.academia.edu.
- 36.Grunfeld E, Hodgson DC, Del Guidice ME, et al. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–181. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleicher RJ, Ruth K, Sigurdson ER, et al. Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol. 2012;30:4485–4492. doi: 10.1200/JCO.2012.41.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields D, Leshen E, Patel K. Driving quality gains and cost savings through adoption of medical homes. Health Aff (Millwood) 2010;29:819–826. doi: 10.1377/hlthaff.2010.0009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.