Abstract
Growth factors are important morphogenetic proteins that instruct cell behavior and guide tissue repair and renewal. Although their therapeutic potential holds great promise in regenerative medicine applications, translation of growth factors into clinical treatments has been hindered by limitations including poor protein stability, low recombinant expression yield, and suboptimal efficacy. This review highlights current tools, technologies, and approaches to design integrated and effective growth factor-based therapies for regenerative medicine applications. The first section describes rational and combinatorial protein engineering approaches that have been utilized to improve growth factor stability, expression yield, biodistribution, and serum half-life, or alter their cell trafficking behavior or receptor binding affinity. The second section highlights elegant biomaterial-based systems, inspired by the natural extracellular matrix milieu, that have been developed for effective spatial and temporal delivery of growth factors to cell surface receptors. Although appearing distinct, these two approaches are highly complementary and involve principles of molecular design and engineering to be considered in parallel when developing optimal materials for clinical applications.
Keywords: Regenerative medicine, growth factors, protein engineering, protein library, mutagenesis, high-throughput screening, extracellular matrix, biomaterials, drug delivery systems, controlled release
Graphical Abstract
Introduction
Regenerative medicine is an interdisciplinary field where researchers aim to replace or repair damaged cells, tissues, and organs to effectively restore normal function and circumvent the need for donation [1]. Major strategies being pursued to achieve these goals include introducing materials and modulating agents, such as extracellular matrix (ECM)-inspired biomaterial scaffolds, cells, and growth factors [2], to the damaged site to stimulate regeneration [3].
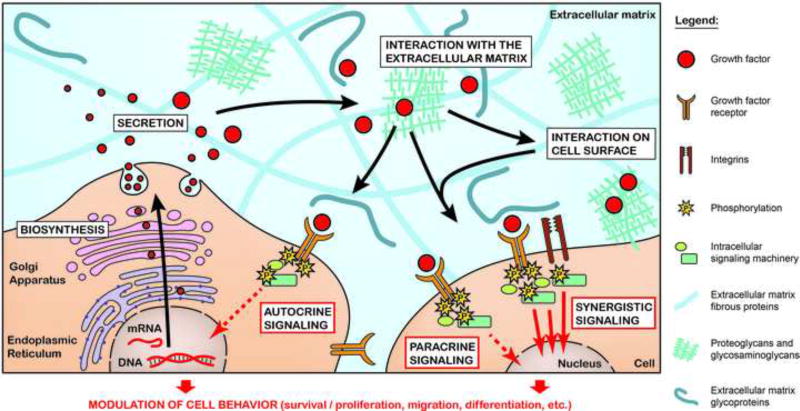
Growth factor proteins are naturally secreted from cells and directly interact with or are sequestered by the surrounding ECM for presentation to cell surface receptors. Growth factors are essential to the regenerative process. Specific growth factor receptor binding stimulates cellular signal transduction pathways that trigger events such as cell migration, survival, adhesion, proliferation, growth, and differentiation [4–6] (Fig. 1). On a larger scale, these growth factor-stimulated cellular responses are involved in organism development, angiogenesis, and wound healing [5]. Clinically-approved growth factors include human growth hormone (hGH; Humatrope® [7]), which is used to treat children of short stature, platelet-derived growth factor-BB (PDGF-BB; Regranex® [8]) which is approved to treat lower extremity diabetic neuropathic ulcers, and bone morphogenetic factor-2 (BMP-2) and BMP-7 for lumbar spine fusion (InFUSE™ Bone Graft/LT-Cage™ [9]; OP-1 Putty [10]) and open tibial fracture (INFUSE® Bone Graft [11]; OP-1 Implant [12]).
Figure 1.
From biosynthesis to cell receptor signaling, a growth factor’s journey within the physiological ECM. After their biosynthesis, growth factors are secreted into the ECM, where they interact with ECM components before binding and activating their cognate receptors. Growth factors mainly signal to cell in autocrine and paracrine fashion, to instruct their behavior during morphogenetic processes. Complexes formed between growth factors, ECM components, and cell surface receptors may lead to additive or synergistic cell signaling events.
While growth factors have had clinical success, their potential as therapeutic agents has generally been hindered by inherent limitations imposed by their native protein forms. In particular, nature has designed growth factors with properties such as low protein stability, short circulating half-life, rapid rate of cellular internalization, and localized tissue activity as mechanisms for controlling their function through restricted spatial and temporal effects. As an example, fibroblast growth factor (FGF-1) possesses intrinsically low stability, exhibiting a functional half-life of only one hour in serum at 37 °C [13]. Additional challenges arise for utilizing exogenous growth factors as therapeutics, including poor recombinant expression yield, difficulty of purification, high cost of production, and lack of appropriate delivery methods [14]. Collectively, these limitations create a significant need for new tools and technologies that will render growth factors more amenable for therapeutic use.
In this review, we discuss progress to address these needs, including the design, engineering, and development of novel proteins and protein delivery systems. Although we focus on applications related to regenerative medicine, the concepts and strategies discussed here are broadly relevant to other therapeutic applications. We first focus on different protein engineering strategies used to create growth factors with improved biochemical and biophysical properties. We then present examples of engineered microenvironments, and discuss how these strategies have been used to develop enhanced growth factor delivery systems. The concluding section provides future outlook on the critical role that design and engineering will continue to play in these efforts.
PROTEIN ENGINEERING TECHNOLOGIES APPLIED TO GROWTH FACTORS
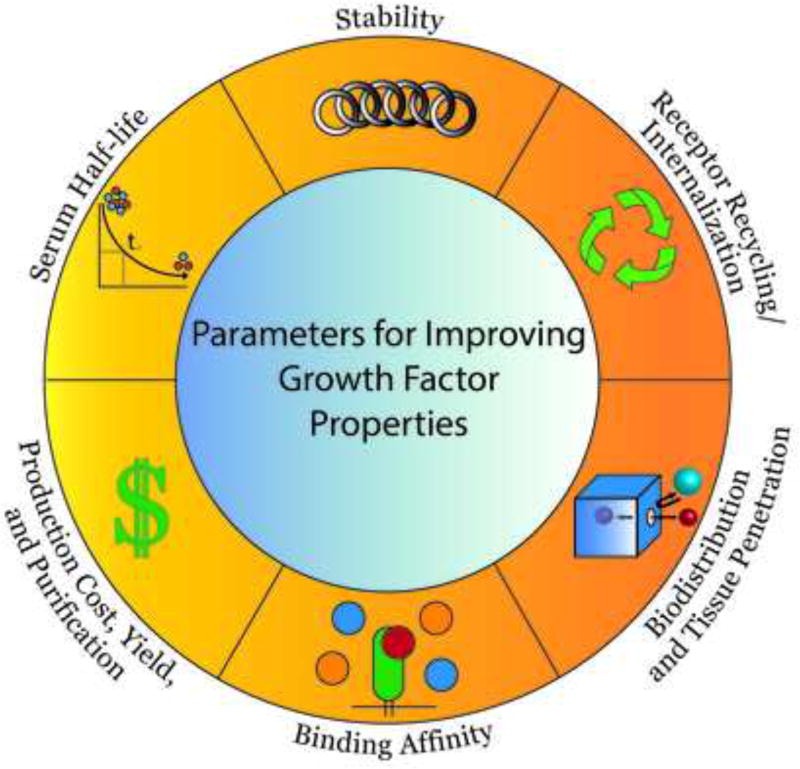
As described above, challenges exist for using natural growth factors in regenerative medicine applications that stem from their inherent limitations as proteinaceous materials; quite simply, nature designed growth factors for specific local and temporal tissue effects that do not translate well into their development or use as therapeutics. In this section, we highlight examples of combinatorial protein engineering methods, often referred to as ‘directed evolution’, that have been applied to engineer growth factors with improved properties, including increased protein stability, extended serum half-life, enhanced biodistribution, improved recombinant expression yield, and altered receptor binding affinity or internalization/recycling rate (Fig. 2).
Figure 2.
Examples of growth factor properties that can be improved using protein engineering techniques.
Overview of combinatorial protein engineering strategies
Protein library creation
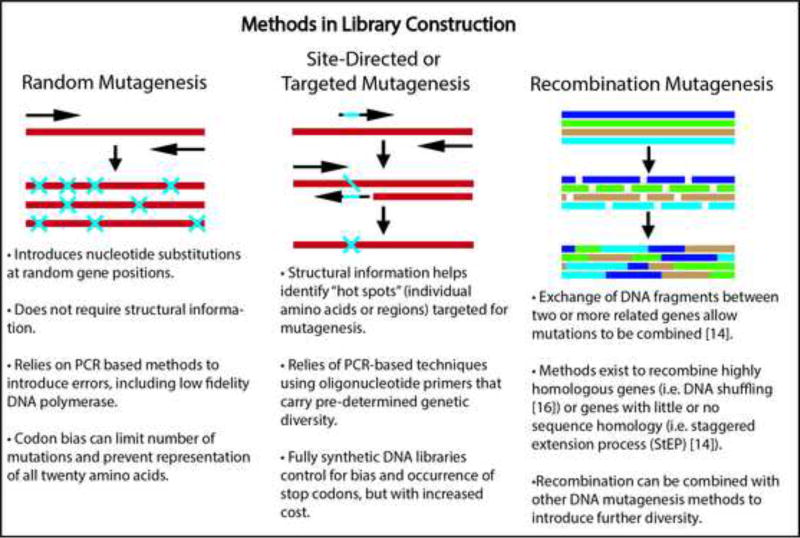
Directed evolution involves high-throughput screening of large libraries of protein variants (e.g. thousands to millions) as an efficient means to interrogate and identify beneficial growth factor mutations on a massively-paralleled scale. When constructing such libraries, a main consideration is that the protein variants should be sufficiently similar to the natural growth factor to maintain its overall structure and function, but also sufficiently divergent in sequence to impart improved functionality [15]. Commonly used library creation techniques include random mutagenesis [15, 16], site-directed mutagenesis [15], and recombination [15, 17] (Box 1), which introduce diversity into the growth factor at the gene level. The choice of mutagenesis method is governed by the amount of sequence-structure-function information available for the growth factor of interest and whether targeted or random mutations are desired. Each approach has its advantages and limitations, which are discussed more in depth elsewhere [15].
Protein library screening
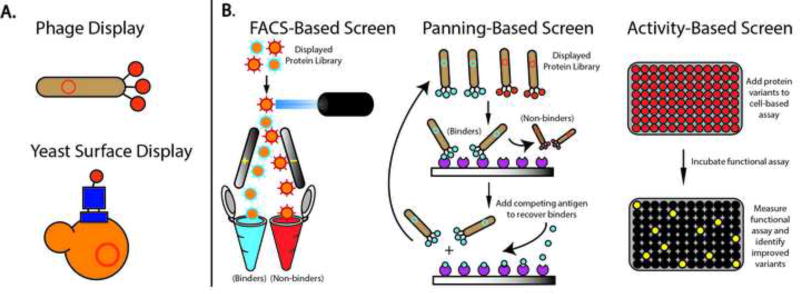
Once a mutagenesis strategy is performed, the DNA then needs to be transcribed and translated to generate a protein library. A number of systems have been developed to enable millions of diverse protein variants to be efficiently produced and screened to identify those with phenotypic improvements, including increased stability, expression yield, and altered binding affinity. Many of these approaches involve expression and tethering of individual protein variants onto the surface of cells (e.g. phage, yeast, or bacteria display), or attachment to transcription/translation machinery (e.g. ribosome or mRNA display) [18] (Fig. 3A). These fusions establish a ‘genotype-phenotype’ linkage that allows protein variants of interest to be identified through their corresponding DNA sequence. Each display technology has pros and cons specific to the type of protein and the library size that can be accommodated, which have been reviewed elsewhere [18–20].
Figure 3.
Overview of protein engineering techniques. (A) yeast and phage-based library display platforms, and (B) library screening methods.
The mantra in protein engineering is “you get what you screen for” and thus designing an appropriate library screening strategy is crucial for success. Library screening requires subjecting a diverse population of protein mutants to varying conditions and selecting the variants that are most adapted for the challenge. There is an added requirement for high-throughput methods to distinguish between millions of protein variants (Fig. 3B). Flow cytometry is often used to sort protein libraries expressed on the surface of bacteria, yeast, or mammalian cells. As an example, a library of yeast-displayed growth factor mutants was screened by flow cytometric sorting to identify variants that possess increased binding affinity to a fluorescent soluble receptor domain [21]. Alternatively, growth factor libraries displayed on the surface of phage particles have been screened by panning against a soluble receptor domain attached to a solid support [22]. These two methods both discriminate growth factor variants based on their binding affinity to a receptor of interest. Alternative screening strategies based on cellular proliferation or kinase activity have also be used to directly probe the functional activity of specific growth factor mutations [23–26].
Examples of protein engineering used to improve growth factor properties
Protein stability and half-life
A major limitation of natural growth factors is their short effective half-life due to poor stability or fast blood clearance. As a result, multiple administrations are often needed to achieve a therapeutic effect, correlating with cost and compliance considerations. Moreover, protein integrity can be compromised by fluctuations in temperature or pH, aggregation, hydrolysis of peptide bonds, or oxidation of amino acid side chains during long term storage [27].
Various protein engineering strategies have been applied to improve growth factor stability, including the introduction of stabilizing mutations or alterations, or the creation of smaller, less complex protein variants which recapitulate the functional activity of native growth factors. As an example, fibroblast growth factor-1 (FGF-1) is a potent activator of the tissue regeneration process, but suffers from poor stability [28]. Sequence analysis of FGF family members revealed a vestigial unpaired cysteine at position 83 [13]. Site-directed mutagenesis was applied to a nearby alanine residue at position 66 of FGF-1 to introduce a disulfide bond between Cys66 and Cys83. The resulting FGF-1 variant demonstrated a half-life of 14 hours in unconditioned media, a 14-fold increase in functional half-life compared to wild type FGF-1. The engineered FGF-1 variant also displayed a 10-fold increase in mitogenic activity in the absence of a thermostabalizing glycosaminoglycan, known as heparin [13].
Another example is hepatocyte growth factor (HGF), an important mitogen for cell growth, motility, and morphogenesis [29]. HGF is a large (~80 kDa) multi-domain protein notorious for its instability and tendency to aggregate in physiological buffers. For example, HGF has been shown to aggregate when incubated in saline for 3 days at 37 °C, resulting in up to a 50% loss of protein [30]. The minimally active fragment of HGF that functions as a weak agonist comprises the N-domain and first kringle domain (termed NK1), but this fragment still suffers from poor stability and low recombinant expression yield [31]. Combinatorial protein engineering methods can be used to identify protein variants with improved stability and expression yield [32]. A yeast-surface displayed NK1 library, created by random mutagenesis, was screened to identify variants with increased thermal stability; an engineered NK1 variant containing 8 mutations (termed M2.2) exhibited a 15°C increase in melting temperature compared to wild-type NK1 and functioned as a weak agonist in vitro [31]. The agonistic potency of M2.2 was significantly increased by covalently fusing NK1 monomeric subunits with a disulfide bond formed through a cysteine residue introduced at the protein N-terminus [31]. The resulting M2.2 dimer demonstrated agonistic potency in vitro that approached activity levels elicited by the native HGF protein [33], and also showed efficacy in a rat myocardial infarction model when delivered from a biomaterial scaffold constructed from ECM components [34].
Nature has improved the thermal and protease stability of small proteins by cyclizing (i.e. connecting) them through their N- and C-termini [35]. This strategy was applied to interferon alpha (IFNα2), a cytokine used to treat viral infection, through an enzyme-mediated reaction [36]. The resulting cyclized IFNα2 variant retained its ability to inhibit cell proliferation, and had an improved melting temperature of 4 °C compared to non-cyclized variants. The therapeutic properties of IFNα2 were further improved by site-specifically conjugating polyethylene glycol (PEG) to address the limitation of fast blood clearance. PEGylation, which increases the hydrodynamic radius of a protein, is a common method for enhancing the serum half-life of growth factors and cytokines to reduce dosing frequency and has been applied to human growth hormone (hGH; Somavert®; Pfizer), granulocyte colony stimulating factor (G-CSF; Neulasta®, Amgen), and interferon alpha-2a (PEGASYS®, Genentech/Roche); reviewed in [37].
Recombinant expression yield
Another important consideration when developing growth factors for clinical applications is the expression and purification methods required to properly fold complex mammalian proteins, as well as the high cost associated with production. Natural growth factors can be challenging to produce and in some cases require mammalian host expression systems [38]. Protein engineers have applied a number of strategies to improve recombinant expression yield, including the development of minimal growth factor domains which can be more easily expressed in microbial hosts. As an example, HGF must be expressed and purified from mammalian cell culture systems, compared to the NK1 fragment which can be produced in bacteria or yeast [31, 39].
Combinatorial protein engineering methods have been developed to identify protein variants with increased expression yield. Seminal studies demonstrated that increased expression levels of a protein tethered to the yeast cell surface strongly correlates with increased expression yield of that protein when expressed in soluble form [32]. This concept has been applied to improve the recombinant expression of the NK1 fragment of HGF [31]. A yeast-displayed library of NK1 mutants was created by random mutagenesis and screened to identify variants with high levels of yeast cell surface expression compared to wild-type NK1; the best variants exhibited a 40-fold improved expression yield [31–33]. Improved soluble expression yields were also achieved by targeted mutagenesis of NK1 [30] or chemical synthesis of the K1 domains of NK1 [40], studies which are described in more detail below.
Another strategy to improve protein production and stability is to remove protease cleavage sites that naturally occur within growth factors. This strategy has been applied to platelet-derived growth factor (PDGF)-BB [41]. Site directed mutagenesis was used to generate Arg32Pro and Arg28Ser PDGF-BB mutants which exhibited 5-fold increased expression levels of PDGF-BB, and also demonstrated a 2–3 fold improvement in mitogenic potency compared to wild-type PDGF-BB [41]. Similar protein engineering strategies can be applied to other growth factor families to address manufacturing challenges and would serve to effectively reduce cost and time to production.
Receptor binding affinity
Combinatorial screening approaches have also been widely used to identify protein variants that possess tighter binding to a target of interest [22, 31, 42]. The equilibrium binding affinity (KD) of a ligand/receptor interaction is defined as the ratio of the kinetic binding off-rate over the on-rate. While higher affinity binding between a ligand and its receptor has been shown to drive improved biological activity [42, 43], this correlation is not always evident, particularly for ligand agonists, and thus other design criteria are needed in protein engineering efforts. In one example, a library of human growth hormone (hGH) mutants was screened using a phage-panning approach to isolate variants which bound to hGH receptor with up to a 50-fold increase in affinity compared to wild type hGH [44]. The increased affinity of these hGH variants was mainly driven by their decreased dissociation rate constants of binding (koff). When tested for their ability to initiate JAK2 tyrosine kinase phosphorylation and subsequent cell proliferation, the hGH variants did not elicit improved biological effects compared to wild-type hGH. Furthermore, a series of hGH mutants with 5-to 500-fold reduced receptor affinity showed that the biological response was unaffected until a 30-fold increase in koff was reached [44]. These results led to the conclusion that wild-type hGH binding to hGHR surpasses the requirements for cellular activity. However, mathematical models that account for hGH binding at the cell surface, as well as induction of receptor endocytosis and downregulation, predict that the biological function of hGH is driven by the binding on-rate (kon) and the endocytic rate constant (ke) [45]. In other words, the biological potency of hGH will not be affected by decreased off-rate (koff) or tighter overall binding affinity if the hGH:hGHR complex gets internalized faster than the ligand dissociates. Collectively, these results suggest that improved potency might be achieved by engineering growth factor variants with increased binding on-rates.
Another example of engineering growth factor binding affinity involves the epidermal growth factor (EGF), which stimulates cell migration and proliferation required for regenerative applications including wound repair [46]. The short-half life of EGF due to protein degradation and fast clearance [47, 48] has limited its clinical utility. Library screening was used to identify EGF mutants with increased binding affinity to the EGF receptor (EGFR) as a means to modulate biological activity. Yeast-surface displayed libraries of EGF mutants, created by error-prone PCR and DNA shuffling, were screened to identify variants with up to a 30-fold increase in binding to the extracellular domain of EGFR [21]. Two EGF variants were found to possess faster EGFR binding on-rates and were more potent at stimulating EGFR activation [49]. In a different study, rationally created chimeric proteins consisting of EGF and a related mitogen TGFα were shown to be active at 10-fold lower concentrations than their wild-type ligand counterparts; these variants were shown to possess 3-to-5-fold higher binding on-rates and off-rates compared to natural EGFR ligands [50]. These results demonstrate that improved potency can be achieved by engineering growth factor variants with faster on-rates of receptor binding.
Receptor internalization and recycling rate
As described above for hGH, receptor internalization can potentially reduce the duration of ligand-mediated receptor signaling [45]. As further demonstration of this concept, an EGF variant containing a Y13G mutation reduces EGFR binding affinity by 50-fold, but exhibits greater potency in vitro due to decreased receptor downregulation and reduced ligand depletion [26]. These results motivate development of EGF library screens based on functional read-outs instead of binding affinity [23]. In one study, targeted mutagenesis and cell-free protein synthesis [51] were used to express a library of EGF variants in a microtiter plate format. Two EGF variants were identified that stimulated cell proliferation at concentrations 10-fold lower than wild-type EGF [23], despite having a weaker EGFR binding affinity. These results confirm that improved growth factor potency does not always correlate with increased receptor binding affinity, and highlights other parameters that should be considered when engineering growth factors for enhanced agonistic activity.
High ligand-receptor binding affinity can also lead to intracellular degradation of the complex following internalization, thus depleting the local ligand concentration and reducing regenerative potency [52]. For example, EGF drives lysosomal degradation of EGFR, effectively desensitizing the cell [53]. Hence, to increase growth factor efficacy, mutations can be introduced into a ligand to drive its receptor dissociation once the internalized complex reaches the endosomal compartment. This concept is based on TGFα, which naturally promotes EGFR recycling and is therefore a more potent mitogen than EGF [52, 53]. TGFα contains critical histidine residues that become protonated when TGFα/EGFR complexes are internalized into endosomes (pH 5.5 compared to cell surface pH of 7.4), in turn driving ligand/receptor dissociation and recycling [54]. This strategy was exploited to engineer more potent versions of G-CSF. Structural information and computational modeling were used to identify key residues on G-CSF where an introduced positive charge would disrupt the ligand/receptor complex [55]. Histidine was substituted for Asp110 or Asp113 residues using site directed mutagenesis; these mutations resulted in weaker G-CSF receptor binding at endosomal pH. Functional in vitro assays confirmed that the engineered G-CSF variants exhibited greater endocytic recycling, resulting in increased cell proliferation compared to wild type G-CSF.
Collectively, these examples demonstrate that ligand internalization and recycling can have profound effects on cell signaling, and hence, biological potency. In parallel, compounding evidence has shown that immobilized growth factors often drive greater levels of receptor-mediated signal transduction than soluble growth factors [56, 57]. This phenomenon is believed to result from reduced growth factor internalization and degradation, and has been exploited to develop novel growth factor delivery systems as discussed in detail in the sections below.
ENGINEERING GROWTH FACTORS WITHIN THEIR MICROENVIROMENT
As detailed above, combinatorial and rational protein engineering strategies have been used to engineer growth factors with the goal of improving their native properties. The second half of this review will highlight alternative approaches of engineering growth factors within their microenvironment, including designing and optimizing methods for local delivery of growth factors directly into an injured site. Growth factors act in concert with the local ECM in which they are secreted. Some growth factors are sequestered in the ECM under homeostatic conditions or after tissue injury (Fig. 1) [58, 59]. We will discuss the physiological roles of the ECM in delivering growth factors and modulating their cell signaling properties [2, 60, 61], focusing on soft (i.e. non-biomineral) components of the matrix. Taking inspiration from the ECM (Fig. 4A), we then present strategies to engineer growth factors along with their microenvironment for optimal delivery and increased therapeutic efficacy for regenerative medicine applications. We will detail important aspects of material and protein engineering to control the spatio-temporal release of growth factors for local delivery in the injured tissue, either by exogenous carrier-material or by direct targeting of the endogenous ECM. As growth factor sequestration within the ECM may also limit tissue biodistribution, we will provide examples in which lowering the affinity of growth factors to the ECM results in better therapeutic outcomes.
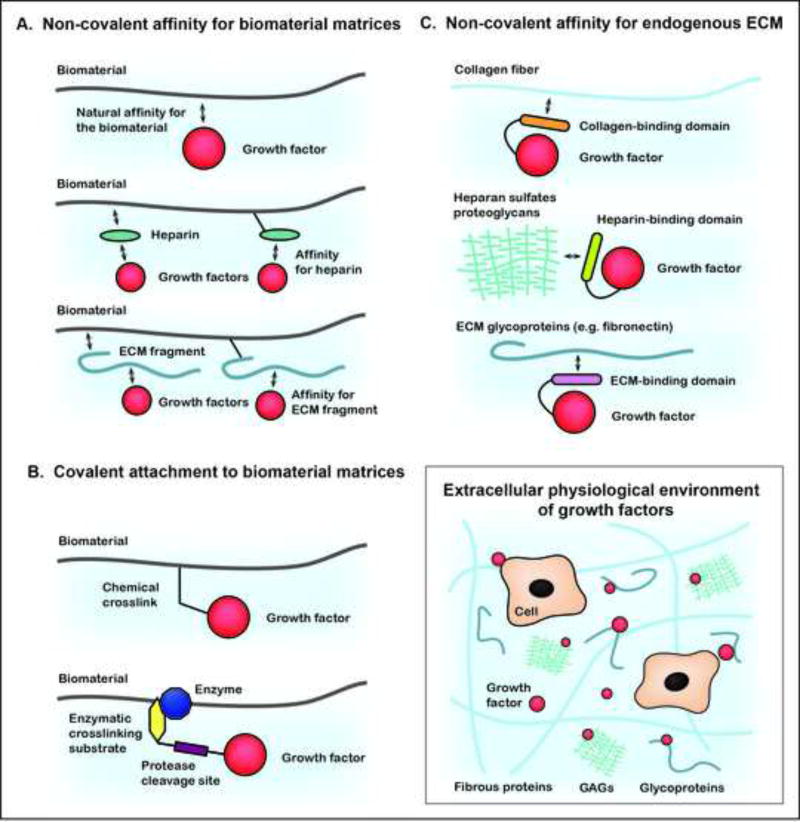
Figure 4.
ECM-inspired growth factor delivery systems. (A) Strategies for non-covalent attachment of growth factors to exogenous biomatrices, leveraging the natural affinity of growth factors to these materials. (B) Strategies to covalently attach growth factors to exogenous biomatrices using chemical or enzymatic tools. (C) Strategies to employ endogenous ECM as the delivery biomatrix by engineering fusions of growth factors with ECM binding domains. Inset, Schematic of the extracellular growth factor environment.
Roles of the ECM in the physiological delivery of growth factors
Although the ECM was initially considered to be a fibrillar network providing essential biomechanical support for cells, its important roles in molecular retention and cell signaling have been more recently highlighted [2, 62–65].
Growth factor interaction with ECM GAGs
Glycosaminoglycans (GAGs), including heparan sulfate-GAGs (HS-GAGs), are main components of the ECM that serve to effectively immobilize growth factors [66]. Growth factors that are characterized as “heparin-binding” have the ability to interact non-covalently with GAGs and particularly HS-GAGs, which share structural similarity with heparin. As an example, basic fibroblast growth factor (bFGF, FGF-2) contains amino acid residues that bind to the negatively charged sulfate and carboxyl groups of HS-GAGs through ionic and van der Waals forces [67]. Consequently, interactions between growth factors and GAGs are essential for growth factor sequestration into the ECM as well as for bioactivity, playing an important role in receptor activation and downstream intracellular signaling [68] (Fig. 1). A number of other growth factor families contain heparin-binding members, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), bone morphogenetic protein (BMP), EGF, and HGF [69], thus endowing the ECM with the general role of a growth factor reservoir.
Growth factor interaction with ECM glycoproteins
In addition to GAG binding, some growth factors interact promiscuously with ECM adhesive glycoproteins such as fibronectin, fibrinogen, tenascin C, vitronectin and osteopontin [70]. For example, VEGF-A145 [71] and the placental growth factor (PlGF)-2 [70] contain stretches of basic residues at their C-termini that confer high affinities toward matrix proteins. In addition, specific growth factor-binding domains have been identified within fibronectin [72], fibrinogen [73], tenascin C [74] and vitronectin [75]. Collagens have also been reported to directly interact with some growth factors such as HGF, however, the specific growth factor-binding epitopes in collagens have not yet been fully characterized [76]. The varying growth factor affinities for different ECM glycoproteins facilitate spatio-temporal control of growth factor release in the microenvironment, an event that is also dependent on the distribution of ECM proteins. In addition to participating in growth factor sequestration, matrix proteins also contain cell-adhesion sites, in particular, integrin-binding domains. The spatial proximity between growth factor and integrin binding sites can lead to the formation of molecular clusters at the cell surface, which are able to strongly modulate growth factor signaling and can mediate synergistic effects [75, 77, 78] (Fig. 1). As an example, α5β1 integrin binding to the 9th and 10th domains of fibronectin (FN III 9–10) and VEGF-A binding to the neighboring domains FN III 12–14, greatly enhances VEGF-A effects on endothelial cell responses, including receptor phosphorylation [77]. Similarly, simultaneous binding of αV integrins to vitronectin, and insulin-like growth factor (IGF-1) to IGF-binding proteins (IGF-BP)-3/-5, drastically increases keratinocyte migration during skin wound closure [75]. These examples highlight an additional key role of the ECM in actively presenting growth factors to cells along with appropriate co-signaling information to enhance and optimize their morphogenetic effects.
Both the reservoir function and the signaling context provided by the ECM are crucial to fulfill the physiological delivery of growth factors and provide proper guidance of cell behavior during regenerative processes (Fig. 1). A deep understanding of these natural mechanisms highlights important criteria for engineering adequate growth factor delivery systems that can be effectively designed for clinical applications in regenerative medicine.
Toward engineering ECM-inspired growth factor delivery systems
A clinical perspective on growth factor delivery
The limited success of growth factor-based therapy in the clinic is closely associated with the lack of appropriate delivery methods [14]. Growth factors that have limited interactions with the ECM in their native mature form, including VEGF-A121, PDGF-BB, BMP-2 and IGF-1 [70, 79], often exhibit short-term burst-type effects due to fast outward tissue diffusion and rapid proteolysis [2, 80]. Consequently, supraphysiological and repeated doses of these growth factors are required for therapeutic benefit, in some cases triggering life-threatening side effects including an increased cancer risk or ectopic tissue formation from systemic exposure of the administered growth factor at such high doses [8]. Due to these important safety concerns and their limited efficacy, commercially available growth factor-containing products, such as Regranex® (containing PDGF-BB, used for skin wound healing) [8] and InFUSE® (containing BMP-2, used for spinal fusion) [81], have not yet become standard-of-care therapies in regenerative medicine.
To pave the way toward safe and cost-effective growth factor-based therapies, several strategies to mimic natural ECM functions have been explored, with the goal of achieving local and sustainable delivery of bioactive growth factors, and thus allowing the reduction of therapeutic doses [2, 82].
Growth factor delivery through decellularized ECM
The important role of the ECM in instructing tissue regeneration and delivering bioactive factors, including growth factors, has been partially elucidated by research on ECM that has been stripped of cells [83, 84]. Although growth factor content of decellularized ECM is typically decreased due to the cell removal treatment, the content of native sulfated GAGs and other ECM proteins is preserved to some extent [85, 86]. Thus, decellularized ECM has been used to deliver growth factors within a close-to-native microenvironment. As examples, recent studies demonstrated that decellularized ECM-derived hydrogels containing GAGs constitute a viable platform for sequestering and delivering heparin-binding growth factors FGF-2 or an engineered HGF fragment, leading to increased neovascularization as compared to delivery in collagen in a rodent model of myocardial infarction [34, 87]. While decellularized ECMs constitute promising scaffolds for tissue engineering applications, alternative strategies focus on the development of other natural materials able to recapitulate key ECM functions, as detailed below.
Growth factor delivery through exogenous engineered biomatrices (Fig. 4A)
When engineering growth factor delivery systems, the choice of an appropriate biomaterial is central and is motivated by the material’s ability to effectively sequester growth factors. Physical and chemical properties of synthetic and natural biomaterials, such as density, porosity, viscosity, hydrophobicity, and charge can be tailored to increase growth factor retention. A detailed review of physico-chemical growth factor tethering to materials can be found elsewhere [14]. However, challenges often arise with these approaches due to the need to preserve growth factor bioactivity during incorporation into the biomaterial, as well as the need to generate cell-friendly formulations that allow material remodeling. Thus, strategies have focused on the use of natural ECM-derived materials, such as hyaluronan, chitosan, collagen/gelatin or fibrin-based scaffolds. Although the animal or human sourcing of natural biomatrices might have some limitations for clinical uses (e.g. pathogen transmission, batch-to-batch variability, availability and ethical considerations), naturally-derived biomaterials offer a physiologically relevant environment for cells [88] by their intrinsic capability to retain signaling biomolecules, display cell adhesion and proteolytic sites, and provide biomechanical support. For example, FGF-2 and VEGF-A naturally bind to fibrin [89, 90], and their controlled delivery from fibrin sealants enhance their effects on endothelial cell proliferation and blood reperfusion following myocardium infarction or limb ischemia [91]. Natural ECM-based materials can also be augmented to have increased affinity toward growth factors. For instance, gelatin-based hydrogels have been tuned to be more acidic or more basic in order to increase ionic interactions with oppositely charged growth factors [92]. The sustained delivery of FGF-2 from a negatively charged gelatin sponge, or BMP-2 from a positively charged one, have respectively shown improved tracheal cartilage and bone regeneration [93, 94].
Since heparin-binding growth factors have a natural affinity for GAGs and heparin-like molecules, another approach used to sequester growth factors relies on the introduction of heparin or heparin-binding domains in biomatrices. For example, covalent crosslinking of a heparin-binding domain into fibrin gels promotes bridging between heparin and strong heparin-binding growth factors, like FGF-2, as well as growth factors with lower affinity for heparin, like nerve growth factor (NGF)-β, which promoted neurite extension in the context of peripheral nerve regeneration [95]. Direct covalent conjugation of heparin into fibrin gels has also been implemented to sustainably deliver BMP-2, which improved bone regeneration in a calvarial bone defect mouse model [96]. Based on interactions with GAGs, another study exploited growth factor affinity for hyaluronan to efficiently deliver VEGF-C and angiopoietin-2 from hyaluronan/methylcellulose hybrid matrices to prevent lymphedema formation after lymphadenectomy [97].
As previously highlighted, growth factors also naturally bind to some ECM glycoproteins; a property that can be exploited to engineer growth factor retention into biomatrices. For example, the functionalization of fibrin hydrogels with the growth factor-binding domain of fibronectin (FN III 12–14) led to better VEGF-A and PDGF-BB retention compared to fibrin gels only, which enhanced smooth muscle cell spheroid sprouting [72]. Similarly, the incorporation of the growth factor binding-domain of fibrinogen into a synthetic polyethylene glycol (PEG) hydrogel successfully recapitulates the growth factor reservoir function of a fibrin clot. The co-delivery of FGF-2 and PlGF-2 from these fibrin-mimetic matrices greatly enhanced skin wound healing and local angiogenesis in a model of chronic wounds [73]. Interestingly, some ECM glycoproteins have the potential to actively present growth factors along with complementary signaling information, resulting in synergistic intracellular signaling and more powerful cellular responses to growth factors. These systems inspired researchers to incorporate full-length or specific fragments of ECM glycoproteins into growth factor delivery systems to further improve therapeutic outcomes. As an example, the delivery of full-length vitronectin/growth factor complexes from hyaluronan hydrogels increased dermal cell proliferation in skin wound healing [98, 99]. In an alternate study, the functionalization of a fibrin gel with recombinant fibronectin fragments (FN III 9–10/12–14), comprising the fusion of an integrin-binding domain (FN III 9–10) with a growth factor-binding domain (FN III 12–14), drastically enhanced cellular responses to VEGF-A, PDGF-BB and BMP-2, conferring therapeutic responses at very low doses in models of skin and bone regeneration [78].
Finally, growth factor-binding moieties derived from non-ECM components, found via receptor mimicry or through high-throughput screening methods, can also be used to functionalize biomaterials that specifically control growth factor release, as reviewed elsewhere [100].
Above, we described several approaches to design and engineer an environment that promotes spatio-temporal control of growth factor delivery and the proper context for cell signaling, thus recapitulating two important physiological roles of the ECM. In the following section, we focus on engineering of the growth factor itself, to tailor interactions either with the delivery system or directly with the endogenous ECM microenvironment.
Engineering growth factors for covalent interaction to exogenous delivery materials (Fig. 4B)
Covalent conjugation of growth factor onto a biomaterial used for delivery is the highest degree of immobilization, which fully abrogates growth factor diffusion. Many strategies exist to covalently attach growth factors to biomaterials [101], the most common is the carbodiimide-mediated conjugation reaction, which uses growth factor primary amines or carboxylate groups as reactive moieties. One example is given by the crosslinking of VEGF-A/angiopoietin-1 onto collagen scaffolds, thus creating a bioactive material that supports vascularization [102]. Despite these and other successes, it is likely that the inability to control conjugation sites with this approach affects growth factor bioactivity (e.g. amino-acid side chains involved in the conjugation might be ones that participate in receptor binding). In addition, covalent attachment affects internalization dynamics of growth factors and prolongs their presence much beyond their usual transient duration; two considerations that might alter the desired morphogenetic effects.
In contrast to chemical coupling, growth factors can also be enzymatically conjugated to biomatrices through an incorporated substrate sequence. The main advantage of enzymatic coupling is site-specific control of the crosslinking location within the growth factor, minimizing interference with bioactive sites. For example, the fusion of a transglutaminase substrate sequence derived from α-2-plasmin inhibitor (α2PI1–8 domain: NQEQVSPL) at the growth factor terminus allows its covalent incorporation into fibrin and PEG matrices by the coagulation factor XIIIa [103, 104]. Amongst other applications, this technology has been promising in bladder tissue engineering, in which the sustained delivery of α2PI1–8-IGF-1 enhanced smooth muscle layer regeneration [79]. In this approach, fibrin-bound growth factors are released dependent on matrix degradation and under the action of cell-secreted proteases, such as plasmin or matrix metalloproteinases. Subsequently, the addition of a plasmin-sensitive peptide sequence (Pla) between the α2PI1–8 crosslinking site and the growth factors allow local cell-mediated growth factor release, as shown by the construct α2PI1–8-Pla-VEGF-A121 in tissue revascularization [105].
As described above, growth factor engineering for covalent immobilization to exogenous delivery systems affords a high level of release control, solely based on material degradation and upon cellular demand. Alternatively, growth factors can be engineered for strong, but non-covalent interactions with biomatrices. In this case, the biomaterials that deliver the growth factor are derived from natural ECM components, thus growth factors can be engineered to bind endogenous ECM with high affinity, as detailed below.
Engineering growth factors for delivery through endogenous ECM (Fig. 4C)
Based on the observation that heparin-binding growth factors can associate with endogenous GAGs, fusions of heparin-binding domains to non-heparin-binding factors have been. As an illustration, the heparin-binding (HB) domain of HB-EGF has been added to IGF-1 to create an HB-IGF-1 fusion that preferably interacts with chondroitin sulfate (CS) GAGs within the CS-rich cartilage matrix following intra-articular knee injection [106].
ECM fibers, especially collagens, also constitute a good target for endogenous binding of growth factors due to their abundance. Similar to the previous approach, fusions between collagen-binding domains (CBD) and growth factors have been studied. As examples, CBD-NGFβ and CBD-BDNF improve peripheral nerve and spinal cord regeneration, respectively, when delivered within collagen scaffolds [107, 108]. When administrated without exogenous biomatrices, CBD-NGFβ can also bind to the endogenous collagen on the rat sciatic nerve and promote regeneration after nerve crush injury [109].
Based on similar concepts, an elegant technology was developed to simultaneously target multiples endogenous ECM proteins, with the considerable advantage of recapitulating not only the ECM function to retain growth factors, but also their appropriate presentation by ECM proteins. For this study, the required building block was based on the discovery of an PlGF-2 derived ECM-binding domain (PlGF-2123–144) which displayed high affinity for various ECM proteins and HS-GAGs [70]. The PlGF-2123–144 domain was fused to VEGF-A, PDGF-BB, and BMP-2 to endow them with high affinity toward fibronectin, fibrinogen, vitronectin, tenascin-C and osteopontin. ECM-binding variants of VEGF-A, PDGF-BB and BMP-2 significantly increased their therapeutic efficacy compared to the wild-type growth factors in models of skin chronic wound healing and non-union bone defects, when delivered at low doses either through a fibrin hydrogel or in a carrier-free manner [70].
Engineering growth factors to target endogenous ECM is a compelling strategy to mimic the physiological delivery of growth factors and optimize their therapeutic effects on morphogenetic processes. Furthermore, biomaterial-free growth factor delivery systems are effective and of reduced complexity, which significantly simplifies their translation into clinical therapies.
Engineering growth factors for decreased interactions with the ECM
As described in the above examples, ECM interactions have been exploited for effective presentation of growth factors to their cognate receptors. This mode of interaction ensures a high local concentration of growth factor with the proper spatial and temporal presentation, but can result in sequestration and suboptimal tissue biodistribution in cases where an exogenous growth factor is not locally delivered to injured tissues. To address this limitation, protein engineers have focused on ways to ablate ECM binding interactions. In one example, rational engineering was used to develop fragments of HGF with reduced affinity to HS-GAGs [30]. In this study, an NK1 variant (termed 1K1) was developed that contained point mutations at positions K132E and R134E, located within the first kringle domain. The alteration of amino acid side chains in this variant did not significantly impair proliferative and anti-apoptotic effects in vitro and in some cases enhanced the therapeutic efficacy of liver regeneration in vivo, compared to wild-type NK1 and vehicle only controls. It was further revealed that the functional activity of 1K1 was independent of HS-GAG interactions. The mutations also contributed to improved stability, demonstrating significantly less aggregation while maintaining over 95% monodispersity [30]. The N-terminal domain of HGF has been shown to possess strong interactions with the ECM. In an alternative strategy, a truncated variant of HGF, comprised of only the first kringle domain, K1, was prepared by total chemical synthesis. A biotin moiety was incorporated into this protein at the C-terminus and streptavidin was used to generate a multivalent and semisynthetic K1 construct (termed K1B/S). The K1B/S complex demonstrated potent agonistic activity in vitro, systemic activity in vivo, and efficient protection of Fas-induced liver apoptosis at doses 20-fold lower than full-length HGF [40].
Conclusion and future perspectives
Growth factors remain essential to the field of regenerative medicine, although they often possess limitations that hinder their clinical application, including poor protein stability and recombinant expression yield, suboptimal potency, and lack of robust delivery methods. As presented in this review, these constraints have driven the development of novel tools and technologies that have been used to: 1) engineer growth factor variants with improved biophysical and biological properties, and 2) engineer ECM-inspired growth factor delivery systems.
These goals, and the efforts needed to achieve them, might seem distinct, however, much synergy exists between the two approaches. First, growth factors with improved stability, that are more potent and can be produced at higher levels, are desirable regardless of how they are delivered to injured tissue. Second, both strategies involve some element of protein manipulation where the growth factor is deliberately altered or exploited to invoke a particular biological response or for optimal presentation and interaction to cells within a delivery vehicle. Thus, we suggest that the two approaches presented here need not be mutually exclusive, and should be considered together for designing the most optimal materials for regenerative medicine applications. Moreover, the design and engineering of growth factors, and in parallel, their delivery systems, involve constraints that are dictated by the natural protein properties and the microenvironment to be recapitulated. Such integrative design considerations will inform the engineering approach; for example, tethering a growth factor to a biomaterial will increase its local concentration and is expected to prevent its internalization within cells, thus in this case a strategy to engineer receptor binding affinity or ligand trafficking will be less relevant.
Bottlenecks to engineering and developing growth factors as clinical products exist in both the academic and industrial settings. While rational and combinatorial protein engineering approaches have been available for decades, their application to growth factors has been somewhat limited. Industrial interests have primarily been focused on developing biologics based on monoclonal antibodies. The high cost and inertia of retooling established infrastructure for growth factor engineering and production, and the regulatory hurdles and risks that go along with such efforts are likely deterrents. From an academic stand point, the growth factor engineering is relatively new and comprises a much smaller community compared to other fields of regenerative medicine research, such as biomaterials science and stem cell engineering, highlighting opportunities for new advances in the field. In parallel to the development of protein engineering methods, elegantly designed biomaterial-based growth factor delivery systems have also come to the forefront. The ability of these materials to uniquely leverage the properties of naturally occurring growth factors provides opportunities for clinical impact. However, challenges also exist with translating biomaterial-based therapies to the clinic that are similar to those faced by engineered growth factors, including unknown toxicity and immunogenicity profiles, as well as unchartered regulatory paths and the added complexity and heterogeneity inherent in polymeric biomaterials.
As engineered high-performance materials become increasingly more complex, research teams with expertise and knowledge spanning biology, chemistry, engineering, and clinical medicine will become an absolute necessity to help ensure that products generated from these efforts have potential to translate from benchtop to bedside. Indeed, challenges and needs for clinical adoption of these materials should be considered as early as possible in the design stage. In parallel, the excitement surrounding regenerative medicine will only continue to increase as new tools and technologies are developed and applied to drive innovation in the field.
Acknowledgments
The authors would like to acknowledge Dr. Elif Vardar, Dr. Hans M. Larsson, and Cheuk Lun (Alan) Leung for helpful discussions. Work described in this review was supported by the European Research Council through the grant Cytrix (to J.A.H.) and NIH/NCI R01 CA151706 (to J.R.C). A.M. is supported in part by a NIH NIGMS Training Grant in Biotechnology [5T32GM008412], and a Stanford-Agilent Fellowship.
Abbreviations
- BMP
Bone morphogenetic protein
- BP
Binding protein
- CBD
Collagen binding domain
- CS
Chondroitin sulfate
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFR
EGF receptor
- FGF
Fibroblast growth factor
- FN
Fibronectin
- GAG
Glycosaminoglycans
- G-CSF
Granulocytes-colony stimulating factor
- HB
Heparin binding
- HGF
Hepatocyte growth factor
- hGH
Human growth hormone
- hGH
Human growth hormone receptor
- HS
Heparan sulfate
- IFN
Interferon
- IGF
Insulin growth factor
- NGF
Nerve growth factor
- PCR
Polymerase chain reaction
- PDGF
Platelet-derived growth factor
- PEG
Polyethylene glycol
- Pla
Plasmin sensitive sequence
- PlGF
Placental growth factor
- TGF
Transforming growth factor
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mason C, Dunnill P. A brief definition of regenerative medicine. Regenerative Medicine. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Briquez PS, Hubbell JA, Martino MM. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Adv Wound Care. 2015 doi: 10.1089/wound.2014.0603. 150127064149004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Materials Today. 2010;13:14–22. [Google Scholar]
- 4.Goldman R. Growth factors and chronic wound healing: past, present, and future. Advances in Skin & Wound Care. 2004;17:24–35. doi: 10.1097/00129334-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound healing: the role of growth factors. Drugs Today. 2003;39:787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 6.Atanasova M, Whitty A. Understanding cytokine and growth factor receptor activation mechanisms. Critical Reviews in Biochemistry and Molecular Biology. 2012;47:502–530. doi: 10.3109/10409238.2012.729561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drug com. Humatrope (somatropin)- FDA prescribing information, side effects and uses. 2015:1–17. www.drugs.com/pro/humatrope.html.
- 8.U.S. Food and Drug Administration. Safety warning on becaplermin in Regranex®. Silver Spring, MD: 2008. pp. 1–10. [Google Scholar]
- 9.Medtronic. Infuse® Bone Graft/LT-Cage® Lumbar Tapered Fusion Device. 2015:1–2. www.medtronic.com/for-healthcare-professionals/products-therapies/spinal/bone-graft-options/infuse-bone-graft.
- 10.Stryker. OP-1/BMP-7 - OP-1 Putty for Spinal Fusion. 2015:1–2. www.stryker.com/cn/products/Orthobiologicals/Osteoinductive/OP-1/OP-1Putty/127025.
- 11.Medtronic. Bone Graft Surgery, Trauma Surgery, Medtronic INFUSE®. 2015:1–3. www.infusebonegraft.com/healthcare-providers/trauma-surgery/index.htm.
- 12.Stryker. OP-1/BMP-7 - OP-1 Implant for Fracture Repair. 2015:1–2. www.stryker.com/cn/products/Orthobiologicals/Osteoinductive/OP-1/OP-1Implant/020210.
- 13.Lee J, Blaber M. Increased functional half-life of fibroblast growth factor-1 by recovering a vestigial disulfide bond. Journal of Proteins & Proteomics. 2013 [Google Scholar]
- 14.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipovšek D, Mena M, Lippow SM, Basu S, Baynes BM. Library construction for protein engineering. Protein Engineering and Design. 2009 [Google Scholar]
- 16.Zaccolo M, Williams DM, Brown DM, Gherardi E. An Approach to Random Mutagenesis of DNA Using Mixtures of Triphosphate Derivatives of Nucleoside Analogues. J. Mol. Biol. 1996;255:589–603. doi: 10.1006/jmbi.1996.0049. [DOI] [PubMed] [Google Scholar]
- 17.Stemmer WP. DNA shuffling by random fragmentation and reassembly: in vitro recombination for molecular evolution. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10747–10751. doi: 10.1073/pnas.91.22.10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin AM, Weiss GA. Optimizing the affinity and specificity of proteins with molecular display. Mol. BioSyst. 2006;2:49–57. doi: 10.1039/b511782h. [DOI] [PubMed] [Google Scholar]
- 19.Moore SJ, Olsen MJ, Cochran JR, Cochran FV, Brown DN. Cell surface display systems for protein engineering. Protein Engineering and Design. 2009 [Google Scholar]
- 20.Barendt PA, Sarkar CA. Cell-free display systems for protein engineering. Protein Engineering and Design. 2009 [Google Scholar]
- 21.Cochran JR, Kim Y-S, Lippow SM, Rao B, Wittrup KD. Improved mutants from directed evolution are biased to orthologous substitutions. Protein Eng. Des. Sel. 2006;19:245–253. doi: 10.1093/protein/gzl006. [DOI] [PubMed] [Google Scholar]
- 22.Lowman HB, Bass SH, Simpson N, Wells JA. Selecting high-affinity binding proteins by monovalent phage display. Biochemistry. 1991;30:10832–10838. doi: 10.1021/bi00109a004. [DOI] [PubMed] [Google Scholar]
- 23.Lui BH, Cochran JR, Swartz JR. Discovery of Improved EGF Agonists Using a Novel In Vitro Screening Platform. J. Mol. Biol. 2011;413:406–415. doi: 10.1016/j.jmb.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Coco WM, Encell LP, Levinson WE, Crist MJ, Loomis AK, Licato LL, Arensdorf JJ, Sica N, Pienkos PT, Monticello DJ. Growth factor engineering by degenerate homoduplex gene family recombination. Nature Biotechnology. 2002;20:1246–1250. doi: 10.1038/nbt757. [DOI] [PubMed] [Google Scholar]
- 25.Souriau C, Fort P, Roux P, Hartley O, Lefranc MP, Weill M. A simple luciferase assay for signal transduction activity detection of epidermal growth factor displayed on phage. Nucleic Acids Res. 1997;25:1585–1590. doi: 10.1093/nar/25.8.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy CC, Niyogi SK, Wells A, Wiley HS, Lauffenburger DA. Engineering epidermal growth factor for enhanced mitogenic potency. Nature Biotechnology. 1996;14:1696–1699. doi: 10.1038/nbt1296-1696. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Advanced Drug Delivery Reviews. 1993;10:1–28. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 28.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 29.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 30.Ross J, Gherardi E, Mallorqui-Fernandez N, Bocci M, Sobkowicz A, Rees M, Rowe A, Ellmerich S, Massie I, Soeda J, Selden C, Hodgson H. Protein engineered variants of hepatocyte growth factor/scatter factor promote proliferation of primary human hepatocytes and in rodent liver. Gastroenterology. 2012;142:897–906. doi: 10.1053/j.gastro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Jones DS, Tsai P-C, Cochran JR. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13035–13040. doi: 10.1073/pnas.1102561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shusta EV, Kieke MC, Parke E, Kranz DM, Wittrup KD. Yeast polypeptide fusion surface display levels predict thermal stability and soluble secretion efficiency. J. Mol. Biol. 1999;292:949–956. doi: 10.1006/jmbi.1999.3130. [DOI] [PubMed] [Google Scholar]
- 33.Liu CJ, Jones DS, II, Tsai P-C, Venkataramana A, Cochran JR. An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett. 2014;588:4831–4837. doi: 10.1016/j.febslet.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnenberg SB, Rane AA, Liu CJ, Rao N, Agmon G, Suarez S, Wang R, Munoz A, Bajaj V, Zhang S, Braden R, Schup-Magoffin PJ, Kwan OL, DeMaria AN, Cochran JR, Christman KL. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials. 2015;45:56–63. doi: 10.1016/j.biomaterials.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craik DJ. Joseph Rudinger memorial lecture: Discovery and applications of cyclotides. J. Pept. Sci. 2013;19:393–407. doi: 10.1002/psc.2523. [DOI] [PubMed] [Google Scholar]
- 36.Popp MW, Dougan SK, Chuang TY, Spooner E, Ploegh HL. 2011 doi: 10.1073/pnas.1016863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parnham MJ, Bruinvels J. PEGylated Protein Drugs: Basic Science and Clinical Applications. 2009 [Google Scholar]
- 38.Park JS, Kim H, Park J, Yu S, Kim D, Lee J, Oh H, Baek K, Yoon J. Overproduction of recombinant human hepatocyte growth factor in Chinese hamster ovary cells. Protein Expression and Purification. 2010;70:231–235. doi: 10.1016/j.pep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Stahl SJ, Wingfield PT, Kaufman JD, Pannell LK, Cioce V, Sakata H, Taylor WG, Rubin JS, Bottaro DP. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem. J. 1997;326(Pt 3):763–772. doi: 10.1042/bj3260763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonneau C, Leclercq B, Mougel A, Adriaenssens E, Paquet C, Raibaut L, Ollivier N, Drobecq H, Marcoux J, Cianférani S, Tulasne D, de Jonge H, Melnyk O, Vicogne J. Semi-synthesis of a HGF/SF kringle one (K1) domain scaffold generates a potent in vivo MET receptor agonist. Chemical Science. 2015;00:1–12. doi: 10.1039/c4sc03856h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook AL, Kirwin PM, Craig S, Bawden LJ, Green DR, Price MJ, Richardson SJ, Fallon A, Drummond AH, Edwards RM, Clements JM. Purification and analysis of proteinase-resistant mutants of recombinant platelet-derived growth factor-BB exhibiting improved biological activity. Biochem. J. 1992;281(Pt 1):57–65. doi: 10.1042/bj2810057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kariolis MS, Miao YR, Jones DS, Kapur S, Mathews II, Giaccia AJ, Cochran JR. An engineered Axl “decoy receptor” effectively silences the Gas6-Axl signaling axis. Nature Chemical Biology. 2014:1–10. doi: 10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiskopf K, Ring AM, Ho CCM, Volkmer J-P, Levin AM, Volkmer AK, Özkan E, Fernhoff NB, van de Rijn M, Weissman IL, Garcia KC. Engineered SIRPα Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearce KH, Cunningham BC, Fuh G, Teeri T, Wells JA. Growth Hormone Binding Affinity for Its Receptor Surpasses the Requirements for Cellular Activity. Biochemistry. 1999;38:81–89. doi: 10.1021/bi9817008. [DOI] [PubMed] [Google Scholar]
- 45.Haugh JM. Mathematical Model of Human Growth Hormone (hGH)-Stimulated Cell Proliferation Explains the Efficacy of hGH Variants as Receptor Agonists or Antagonists. Biotechnol. Prog. 2004;20:1337–1344. doi: 10.1021/bp0499101. [DOI] [PubMed] [Google Scholar]
- 46.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 47.Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC. Sustained release of epidermal growth factor accelerates wound repair. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7340–7344. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown GL, Curtsinger L, Brightwell JR, Ackerman DM, Tobin GR, Polk HC, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J. Exp. Med. 1986;163:1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahti JL, Lui BH, Beck SE, Lee SS, Ly DP, Longaker MT, Yang GP, Cochran JR. Engineered epidermal growth factor mutants with faster binding on-rates correlate with enhanced receptor activation. FEBS Lett. 2011;585:1135–1139. doi: 10.1016/j.febslet.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenferink AE, van Zoelen EJ, van Vugt MJ, Grothe S, van Rotterdam W, van De Poll ML, O'Connor-McCourt MD. Superagonistic activation of ErbB-1 by EGF-related growth factors with enhanced association and dissociation rate constants. J. Biol. Chem. 2000;275:26748–26753. doi: 10.1074/jbc.M004489200. [DOI] [PubMed] [Google Scholar]
- 51.Jewett MC, Swartz JR. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol. Bioeng. 2004;86:19–26. doi: 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- 52.Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alwan HAJ, van Zoelen EJJ, van Leeuwen JEM. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 2003;278:35781–35790. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- 54.French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J. Biol. Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar CA, Lowenhaupt K, Horan T, Boone TC, Tidor B, Lauffenburger DA. Rational cytokine design for increased lifetime and enhanced potency using pH-activated “histidine switching,”. Nature Biotechnology. 2002;20:908–913. doi: 10.1038/nbt725. [DOI] [PubMed] [Google Scholar]
- 56.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 57.Ogiwara K, Nagaoka M, Cho C-S, Akaike T. Construction of a Novel Extracellular Matrix using a New Genetically Engineered Epidermal Growth Factor Fused to IgG-Fc. Biotechnol Lett. 2005;27:1633–1637. doi: 10.1007/s10529-005-2605-0. [DOI] [PubMed] [Google Scholar]
- 58.Schönherr E, Hausser H. Extracellular matrix and cytokines: a functional unit. Journal of Immunology Research. 2000 doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hynes RO. The Extracellular Matrix: Not Just Pretty Fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds. 2005 [Google Scholar]
- 61.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. Journal of Endocrinology. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 62.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair and Regeneration. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 64.Hubbell JA. Matrix-bound growth factors in tissue repair. Swiss Med Weekly. 2006:1–6. doi: 10.4414/smw.2006.11402. [DOI] [PubMed] [Google Scholar]
- 65.Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Advanced Drug Delivery Reviews. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 67.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Molecular and Cellular Biology. 1992;12:240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashikari-Hada S, Habuchi H, Kariya Y, Itoh N, Reddi AH, Kimata K. Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J. Biol. Chem. 2004;279:12346–12354. doi: 10.1074/jbc.M313523200. [DOI] [PubMed] [Google Scholar]
- 70.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 71.Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- 72.Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 73.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Laporte L, Rice JJ, Tortelli F, Hubbell JA, Engler AJ, editors. Tenascin C Promiscuously Binds Growth Factors via Its Fifth Fibronectin Type III-Like Domain. PLoS ONE. 2013;8:e62076. doi: 10.1371/journal.pone.0062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Upton Z, Cuttle L, Noble A, Kempf M, Topping G, Malda J, Xie Y, Mill J, Harkin DG, Kravchuk O, Leavesley DI, Kimble RM. Vitronectin: growth factor complexes hold potential as a wound therapy approach. J. Invest. Dermatol. 2008;128:1535–1544. doi: 10.1038/sj.jid.5701148. [DOI] [PubMed] [Google Scholar]
- 76.Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T, Riecken EO. Collagens in the liver extracellular matrix bind hepatocyte growth factor. YGAST. 1998;114:139–152. doi: 10.1016/s0016-5085(98)70642-0. [DOI] [PubMed] [Google Scholar]
- 77.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circulation Research. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Müller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 79.Lorentz KM, Yang L, Frey P, Hubbell JA. Engineered insulin-like growth factor-1 for improved smooth muscle regeneration. Biomaterials. 2012;33:494–503. doi: 10.1016/j.biomaterials.2011.09.088. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Molecular Biology of the Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg Neurol Int. 2013;4:343. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martino MM, Briquez PS, Maruyama K, Hubbell JA. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Advanced Drug Delivery Reviews. 2015:1–12. doi: 10.1016/j.addr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Seminars in Pediatric Surgery. 2014;23:112–118. doi: 10.1053/j.sempedsurg.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends in Molecular Medicine. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 86.Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, Huber A, Kullas KE, Tottey S, Wolf MT. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31:8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomaterialia. 2012;8:3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ige OO, Umoru LE, Aribo S. Natural Products: A Minefield of Biomaterials. ISRN Materials Science. 2012;2012:1–20. [Google Scholar]
- 89.Sahni A, Altland OD, Francis CW. FGF-2 but not FGF-1 binds fibrin and supports prolonged endothelial cell growth. J. Thromb. Haemost. 2003;1:1304–1310. doi: 10.1046/j.1538-7836.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 90.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 91.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovascular Pathology. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 92.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. Journal of Controlled Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 93.Igai H, Chang SS, Gotoh M, Yamamoto Y, Misaki N, Okamoto T, Yamamoto M, Tabata Y, Yokomise H. Regeneration of Canine Tracheal Cartilage by Slow Release of Basic Fibroblast Growth Factor from Gelatin Sponge. ASAIO Journal. 2006;52:86–91. doi: 10.1097/01.mat.0000196513.97411.3d. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12:1305–1311. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- 95.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. Journal of Controlled Release. 2000 doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 96.Yang HS, La W-G, Cho Y-M, Shin W, Yeo G-D, Kim B-S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp Mol Med. 2012;44:350. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baker A, Kim H, Semple JL, Dumont D, Shoichet M, Tobbia D, Johnston M. Experimental assessment of pro-lymphangiogenic growth factors in the treatment of post-surgical lymphedema following lymphadenectomy. Breast Cancer Res. 2010;12:R70. doi: 10.1186/bcr2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Upton Z, Wallace HJ, Shooter GK, van Lonkhuyzen DR, Yeoh Ellerton S, Rayment EA, Fleming JM, Broszczak D, Queen D, Sibbald RG, Leavesley DI, Stacey MC. Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. International Wound Journal. 2011;8:522–532. doi: 10.1111/j.1742-481X.2011.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie Y, Upton Z, Richards S, Rizzi SC, Leavesley DI. Hyaluronic acid: Evaluation as a potential delivery vehicle for vitronectin:growth factor complexes in wound healing applications. Journal of Controlled Release. 2011;153:225–232. doi: 10.1016/j.jconrel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 100.Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chemical Communications. 2014;50:15651–15668. doi: 10.1039/c4cc04317k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hajimiri M, Shahverdi S, Kamalinia G, Dinarvand R. Growth factor conjugation: Strategies and applications. J. Biomed. Mater. Res. 2014;103:819–838. doi: 10.1002/jbm.a.35193. [DOI] [PubMed] [Google Scholar]
- 102.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 103.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circulation Research. 2004;94:1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 104.Ehrbar M, Rizzi SC, Hlushchuk R, Djonov V, Zisch AH, Hubbell JA, Weber FE, Lutolf MP. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–3866. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 105.Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. Journal of Controlled Release. 2005;101:93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 106.Miller RE, Grodzinsky AJ, Cummings K, Plaas AHK, Cole AA, Lee RT, Patwari P. Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate. Arthritis Rheum. 2010;62:3686–3694. doi: 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun W, Lin H, Chen B, Zhao W, Zhao Y, Xiao Z, Dai J. Collagen scaffolds loaded with collagen-binding NGF-beta accelerate ulcer healing. J. Biomed. Mater. Res. 2010;92:887–895. doi: 10.1002/jbm.a.32445. [DOI] [PubMed] [Google Scholar]
- 108.Han Q, Sun W, Lin H, Zhao W, Gao Y, Zhao Y, Chen B, Xiao Z, Hu W, Li Y, Yang B, Dai J. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Engineering Part A. 2009;15:2927–2935. doi: 10.1089/ten.TEA.2008.0506. [DOI] [PubMed] [Google Scholar]
- 109.Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J. The effect of collagen-binding NGF-beta; on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30:4649–4656. doi: 10.1016/j.biomaterials.2009.05.037. [DOI] [PubMed] [Google Scholar]