Abstract
Background
Individuals with criminal histories have high rates of opioid dependence and mortality. Excess mortality is largely attributable to overdose deaths. Methadone maintenance treatment (MMT) is one of the best evidence-based opioid substitution treatments (OSTs), but there is uncertainty about whether methadone treatment reduces the risk of mortality among convicted offenders over extended follow-up periods. The objective of this study was to investigate the association between adherence to MMT and overdose fatality as well as other causes of mortality.
Methods and findings
We conducted a retrospective cohort study involving linked population-level administrative data among individuals in British Columbia (BC), Canada with a history of conviction and who filled a methadone prescription between January 1, 1998 and March 31, 2015. Participants were followed from the date of first-dispensed methadone prescription until censoring (date of death or March 31, 2015). Methadone was divided into medicated (methadone was dispensed) and nonmedicated (methadone was not dispensed) periods and analysed as a time-varying exposure. Hazard ratios (HRs) with 95% CIs were estimated using multivariable Cox regression to examine mortality during the study period. All-cause and cause-specific mortality rates were compared during medicated and nonmedicated methadone periods. Participants (n = 14,530) had a mean age of 34.5 years, were 71.4% male, and had a median follow-up of 6.9 years. A total of 1,275 participants died during the observation period. The overall all-cause mortality rate was 11.2 per 1,000 person-years (PYs). Participants were significantly less likely to die from both nonexternal (adjusted HR [AHR] 0.27 [95% CI 0.23–0.33]) and external (AHR 0.41 [95% CI 0.33–0.51]) causes during medicated periods, independent of sociodemographic, criminological, and health-related factors. Death due to infectious diseases was 5 times lower (AHR 0.20 [95% CI 0.13–0.30]), and accidental poisoning (overdose) deaths were nearly 3 times lower (AHR 0.39 [95% CI 0.30–0.50]) during medicated periods. A competing risk regression demonstrated a similar pattern of results. The use of a Canadian offender population may limit generalizability of results. Furthermore, our observation period represents community-based methadone prescribing and may omit prescriptions administered during hospital separations. Therefore, the magnitude of the protective effects of methadone from nonexternal causes of death should be interpreted with caution.
Conclusions
Adherence to methadone was associated with significantly lower rates of death in a population-level cohort of Canadian convicted offenders. Achieving higher rates of adherence may reduce overdose deaths and other causes of mortality among offenders and similarly marginalized populations. Our findings warrant examination in other study centres in response to the crisis of opiate-involved deaths.
In this retrospective study, Angela Russolillo and colleagues investigate mortality rates among people with a drug addiction and a criminal record during periods of methadone treatment and periods without drug substitution therapy.
Author summary
Why was this study done?
Individuals with criminal histories experience high rates of opioid dependence and premature mortality.
Deaths caused by opioids are rising acutely and offenders are at risk.
Adherence to opioid substitution treatments (OSTs), such as methadone, has been shown to reduce the risk of death during custody and immediately following release.
Little is known about the long-term association between methadone adherence and mortality.
What did researchers do and find?
This study integrated population-level data including prescriptions, convictions, and deaths in British Columbia (BC), Canada spanning 1998–2015.
We examined the risk of all-cause and cause-specific death among 14,530 people with criminal convictions who had been prescribed methadone.
Overall and cause-specific mortality rates were compared between periods when methadone was and was not dispensed.
Periods when methadone was dispensed were associated with lower risk of mortality, including overdose fatalities, after controlling for covariates.
What do these findings mean?
Practices to increase methadone adherence among opioid-dependent offenders are required and may reduce overdose-related, as well as other causes of, premature death.
Introduction
Overdoses and deaths caused by opioids have been declared a public health emergency in North America. The rising prevalence of opioid dependence [1], alongside the emergence of fentanyl in the illicit drug market [2], is contributing to premature mortality and sparking an urgent need to mobilize public health and public safety resources. Many of North America’s leading health organisations (American Medical Association, Health Canada, and Centers for Disease Control and Prevention) have set priorities in response to the escalating public health crisis [3]. Interventions emphasize prevention, education, and comprehensive care, including access to substitution treatment where indicated [4]. Particular attention has been directed toward high-risk populations, including offenders. Accidental poisoning is the most common cause of mortality among opioid-dependent individuals [5,6], with opioids present in the vast majority of drug-related deaths among ex-prisoners [7]. Several mortality-related risk factors are overrepresented among offenders (e.g., repeated incarceration, low socioeconomic status, and homelessness) [8,9], compounding the hazards associated with substance misuse. The prevalence of opioid dependence [10] and risk of death from illicit drugs [11,12], such as heroin, is higher among offenders and is acutely elevated in the weeks following prison release [13,14]. Despite evidence that prevention and treatment options (e.g., methadone) may reduce the risk of death among opioid-dependent individuals [15,16], there remain significant barriers [17,18] and underutilisation [19] of substitution treatment options for offenders. Factors such as stigma, insufficient pharmacotherapy knowledge, concerns related to medication diversion, and poor links between corrections and community-based care providers can restrict access to methadone maintenance treatment (MMT) and continuity of care for offenders with opioid dependence [20,21] whether they are sentenced to custody or community settings, as well as following the completion of sentencing.
MMT remains one of the best researched and most widely used opioid substitution treatments (OSTs) [22,23]. MMT engagement is associated with reduced illicit opioid use [24], infectious disease transmission [25], and recidivism [26,27]. While the benefits of MMT adherence are well established for a number of health and justice outcomes, including reduced health care costs [28], the role of MMT adherence in mortality among offenders is less clear. A number of observational studies in Europe and Australia have indicated that adherence to methadone reduces the risk of death during treatment compared with periods of nontreatment [29–33] in general opiate-dependent populations. In these studies, treatment effects of methadone are strongest for drug-related deaths [31] and among subpopulations of MMT users with infectious diseases (e.g., by potentiating adherence to antiretroviral treatments [ARTs]) [34]. However, these studies are not specific to offenders (in or out of custody) and are drawn from relatively small samples sizes [30,31,35], with maximum follow-up periods of 4 to 7 years [29,33,36]. Among studies that do focus on MMT among offenders, most concentrate on mortality in the initial postrelease period [16,37] or during custody [38,39]. However, in many jurisdictions, including British Columbia (BC), the majority of convictions result in sentences served exclusively in the community (versus prison) and are of relatively short duration. Little is known about the long-term course and impact of MMT among people who have served sentences at some point in their lives, although available evidence confirms that MMT adherence fluctuates over time [40]. Despite clinical and empirical support from observational research supporting a broad range of protective effects, independent systematic reviews evaluating the association between methadone treatment and mortality have concluded that the available evidence is “weak” [41] and “suggestive” [24]. Moreover, the very limited body of experimental evidence is equivocal. Mattick and colleagues [22] reported positive but nonsignificant associations between mortality and methadone compared with nontreatment. In contrast, a recent meta-analysis [32] reported greater reductions in mortality with methadone compared with buprenorphine.
Careful examination of MMT and mortality among offenders is particularly valuable due to elevated risks in this population (e.g., injection drug use, HIV) [42], periods of potential interruptions in treatment (e.g., incarceration) [43], barriers to treatment [44], and high likelihood of relapse [45]. In this study, we investigated the association between methadone and mortality in the population of convicted offenders in BC, Canada over a 17-year observation period. We describe the distribution of nonexternal and external causes of morbidity and mortality and address 2 main questions related to MMT: is the risk of all-cause mortality lower during periods of dispensed methadone compared with nondispensed periods? Is the risk of overdose mortality lower during periods of dispensed methadone?
Methods
Study design and population
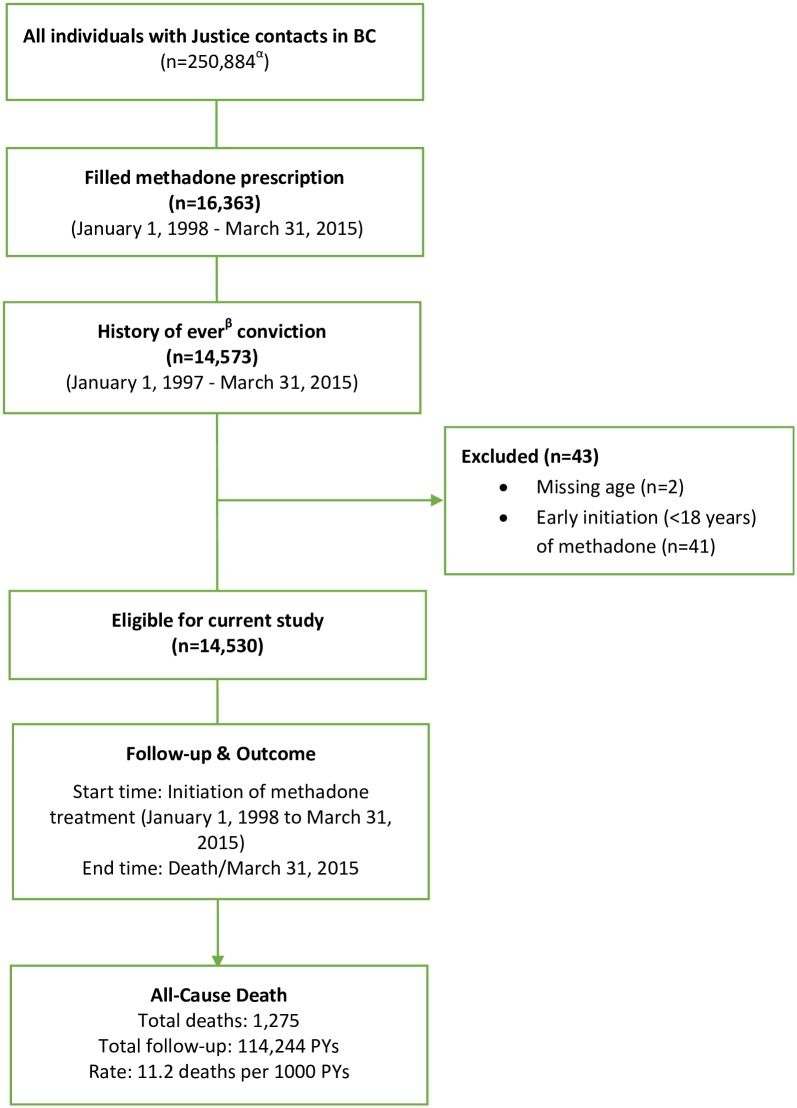
Data were obtained by linking population-level administrative records in BC, Canada under the Inter-Ministry Research Initiative (IMRI). The study cohort consisted of all individuals with provincial justice contacts (n = 250,884) in BC. Individuals with a history of conviction and who filled a methadone prescription between January 1, 1998 and March 31, 2015 were eligible for inclusion. Citizens of BC are legally required to register with the province’s publicly funded health system and are assigned a unique ID. This ID is used to link information from different program areas. We used several comprehensive data sets: the Ministry of Health’s PharmaNet, Vital Statistics, and Billing databases; and the Ministry of Justice’s registry of convictions. The IMRI serves as a resource for the development of policies and services that span health, justice, and social welfare sectors. Details of the IMRI that are not essential to the current study have been described elsewhere [46].
Follow-up extended from the date of first-dispensed methadone prescription until censoring (date of death or March 31, 2015) (Fig 1). Methadone prescription transactions were collected by the Ministry of Health. Corrections-related incidents and sociodemographic variables (age, gender, ethnicity, and education) were collected by the Ministry of Justice. Mortality data were obtained from the BC Vital Statistics Agency. Covariate information concerning medical and lab service use was extracted from the Provincial Medical Services Plan database, which details the date, diagnostic code, and cost associated with medical services to citizens in BC, including while serving sentences under provincial corrections. The study was approved by the Simon Fraser University Research Ethics Board.
Fig 1. Flow chart of offenders included in the study.
αThe cohort included participants (offenders) who had convictions (found or plead guilty and sentenced) as well as those (nonoffenders) who did not have any convictions but were under supervision of the Ministry of Justice due to remand or bail and later found not guilty. βThis time period included the study/exposure period (January 1, 1998 to March 31, 2015) for methadone as well as time prior to enrolment (from the time when justice databases became available, January 1997). BC, British Columbia; PY, person-year.
Variables
Data on the main exposure, methadone, were extracted from the PharmaNet database, a province-wide network linking all prescriptions issued by BC pharmacies. This register omits dispensing information during hospitalisation or outside the province of BC. Authorized physicians who hold an exemption from Health Canada are permitted to prescribe methadone in BC [47]. Methadone is dispensed to individuals who meet criteria for opioid dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition and/or DSM-IV-TR. Patients receiving methadone are required to comply with daily witnessed ingestion under the supervision of a pharmacist (i.e., attend pharmacy daily to receive dispensed dose of methadone), unless authorized to hold ‘carry’ privileges [47].
Methadone was treated as a time-varying exposure (i.e., medication status was not constant throughout follow-up), and each participant’s follow-up was divided into medicated (methadone was dispensed) and nonmedicated (methadone was not dispensed) periods. Following the method used in previous research [27], a participant was considered exposed to methadone based on pharmacy fill transaction dates (see S1 Text). If a participant filled their methadone prescription consistently (no gap in pharmacy transaction dates) for a period of time, this was treated as a single interval and considered a medicated period (methadone was dispensed). If a participant didn’t fill a prescription for a period of time (gap in pharmacy transaction dates), the interval was considered a nonmedicated period (methadone was not dispensed). Participants were expected to alternate between medicated and nonmedicated periods (for further details see S1 Text).
The main outcome was death during follow-up. When a person dies in BC, medical personnel (physician or nurse practitioner) or a coroner must complete a death certificate, and the death must be registered with the Vital Statistics Agency. Causes of death were coded according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). We extracted details of all-cause mortality and cause-specific mortality separated by ICD-10 chapter in accordance with the recorded cause of death. Within the category of nonexternal causes of death (ICD-10 chapter I to XVII), we separately examined deaths from infectious disease, and within external causes of death (ICD-10 chapter XX), we examined deaths by accidental poisoning and intentional self-harm (i.e., suicide).
Several covariates were included, including age (at time of methadone initiation), gender, ethnicity, education, initiation of methadone period (years), prior offences (year preceding methadone initiation), number of offences after methadone initiation (time-varying), number of custody admissions (available from 2007–2015 and used for subgroup analysis only) after methadone initiation (time-varying), severe mental illness, prior service use for non–substance-related mental disorders, prior service use for substance use disorders, and prior service use for nonpsychiatric medical reasons (see S1 Text for details).
Statistical analyses
We used descriptive statistics (counts and proportions for nominal variables; mean and SD, or median and interquartile range [IQR], for continuous variables) to characterize the study sample. We chose time-to-event, or survival, analysis because our outcome of interest was not only the occurrence of an event (death) but also when the event occurred [48]. Methadone was our primary covariate and was time-varying during the follow-up period. To address this time-varying effect, we used the extended Cox model [49]. As an estimate of effect size, we reported the hazard ratios (HRs) along with 95% CIs.
To control for potential confounding, HRs were estimated using multivariable Cox regression, with adjustment for age, gender, ethnicity, education, methadone initiation period, psychiatric diagnoses, criminal history, and health service use. In the Cox regression, we assessed the proportional hazards assumption using Kaplan Meier curve as well as the Schoenfeld residuals [50,51]. We found no violation of proportionality for methadone in the Cox models. We used the robust variance estimator to estimate SEs for the parameters [52,53]. We chose the conventional alpha level (p ≤ 0.05) to report significance for the estimated parameters. All reported p-values were 2-sided.
We used the Cox model for cause-specific deaths and also conducted competing risk regression analysis [54–56] as a sensitivity analysis. We performed competing risk regression using the method proposed by Fine and Gray [57]. Additional sensitivity analyses were conducted inflating the definition of last-dispensed methadone prescription from 1 day to 3 and 7 days and among participants whose cause of death was HIV. A subgroup analysis was conducted among participants who initiated methadone and had at least one custody admission restricted to the years 2007 to 2015, when admission and release dates were deemed to be sufficiently reliable.
Individuals with missing demographic information, including ethnicity and education level, were not excluded from the analysis but were included as separate categories titled ‘unknown’ ethnicity and ‘unknown’ education level. STATA 13.1 was used to conduct all analyses.
Results
The study cohort included 14,530 convicted offenders (mean [SD] age 34.5 [9.4] years; 71.4% male) followed from January 1, 1998 to March 31, 2015 for a total of 114,243.7 person-years (PYs). Table 1 shows baseline sociodemographic and criminological information as well as diagnostic and medical services details for the eligible sample. For methadone prescriptions, the median number of medicated and nonmedicated periods in years were 2.0 (IQR 0.5–4.9) and 3.2 (IQR 0.9–7.1), respectively, representing a total medicated time of 47,681.7 PYs and a nonmedicated time of 66,562.0 PYs.
Table 1. Sociodemographic, methadone, and crime-related characteristics of 14,530 convicted offenders from BC, 1998–2015.
| Variable | Mean (SD)/n (%) |
|---|---|
| Age at enrolment1 | |
| Mean (SD) | 34.5 (9.4) |
| Median (IQR) | 33.3 (27.0–41.0) |
| Min, Max | 18.0, 74.9 |
| Age groups (years) | |
| 18 < 25 | 2,484 (17.1) |
| 25 < 35 | 5,633 (38.8) |
| 35 < 45 | 4,242 (29.2) |
| 45 < 55 | 1,849 (12.7) |
| ≥55 | 322 (2.2) |
| Men, n (%) | 10,378 (71.4) |
| Ethnicity, n (%) | |
| White | 10,546 (72.6) |
| Indigenous | 2,180 (15.0) |
| Other | 1,300 (8.9) |
| Unknown | 504 (3.5) |
| Education level, n (%) | |
| <Grade 10 | 1,930 (13.3) |
| Grade 10/11 | 5,028 (34.6) |
| Grade 12 | 4,869 (33.5) |
| Vocational/university | 1,668 (11.5) |
| Unknown | 1,035 (7.1) |
| Follow-up period, in years | |
| Mean (SD) | 7.9 (5.1) |
| Median (IQR) | 6.9 (3.4–12.8) |
| Min, Max | <0.1, 17.2 |
| Total follow-up time (PYs) | 114, 243.7 |
| Year of methadone initiation, n (%) | |
| 1998 to 2000 | 2,844 (19.6) |
| 2001 to 2005 | 3,311 (22.8) |
| 2006 to 2010 | 4,313 (29.7) |
| 2011 to 20152 | 4,062 (27.9) |
| Medicated period, in years | |
| Mean (SD) | 3.3 (3.6) |
| Median (IQR) | 2.0 (0.5–4.9) |
| Min, Max | <0.1, 16.9 |
| Total medicated time, in PYs | 47, 681.7 |
| Number of medicated periods/episodes | |
| Mean (SD) | 44.4 (58.6) |
| Median (IQR) | 23 (7–59) |
| Min, Max | 1, 638 |
| Nonmedicated period, in years | |
| Mean (SD) | 4.6 (4.4) |
| Median (IQR) | 3.2 (0.9–7.1) |
| Min, Max | 0.0, 17.2 |
| Total nonmedicated time, in PYs | 66, 562.0 |
| Number of nonmedicated periods/episodes3 | |
| Mean (SD) | 44.0 (58.5) |
| Median (IQR) | 23 (7–58) |
| Min, Max | 0, 638 |
| Number of methadone transactions in the year after enrolment (n = 13,490)4, mean (SD) | 160.8 (116.4) |
| Received buprenorphine or buprenorphine-naloxone in follow-up period, n (%) | 1,0965 (7.5) |
| Pharmacy transactions in the year after enrolment (n = 1.055)6, mean (SD) | |
| Number of buprenorphine or buprenorphine-naloxone transactions | 6.9 (28.2) |
| Number of methadone transactions | 149.8 (109.5) |
| Severe mental illness | |
| No schizophrenia or bipolar | 9,548 (65.7) |
| Schizophrenia | 2,217 (15.3) |
| Bipolar | 2,765 (19.0) |
| Number of offences in the year prior to enrolment, mean (SD) | 1.1 (2.3) |
| Any offence in the year prior to enrolment, n (%) | |
| None | 9032 (62.2) |
| 1–2 offences | 3,373 (23.2) |
| >2 offences | 2,125 (14.6) |
| Any jail sentence in the year prior to enrolment, n (%) | 2,824 (19.4) |
| MSP services (NSMD related) in the 5-year period prior to enrolment, n (%) | |
| Low7 (≤2) | 7,388 (50.9) |
| Medium (3–10) | 3,745 (25.8) |
| High (≥11 | 3,397 (23.3) |
| MSP services (SUD related) in the 5-year period prior to enrolment, n (%) | |
| Low8 (≤4) | 7,539 (51.9) |
| Medium (5–13) | 3,427 (23.6) |
| High (≥14) | 3,564 (24.5) |
| MSP services (nonpsychiatric) in the 5-year period prior to enrolment, n (%) | |
| Low9 (≤69) | 7,132 (50.3) |
| Medium (70–139) | 3,599 (24.8) |
| High (≥140) | 3,619 (24.9) |
1Age at enrolment was based on date of initiation of methadone (between January 1, 1998 and March 31, 2015).
22015 included only 3 months (January to March) of data.
3A total of 156 (1.1%) participants did not have any nonmedicated periods and received methadone during the entire observation period.
4Restricted to participants (n = 13,490) who had at least 1 year of follow-up.
5Only a single participant received buprenorphine, and the rest received buprenorphine-naloxone.
6Restricted to participants (n = 1,055) who received buprenorphine or buprenorphine-naloxone and had at least 1 year of follow-up.
750th and 75th percentile were used to categorize into low, medium, and high groups.
850th and 75th percentile were used to categorize into low, medium, and high groups.
950th and 75th percentile were used to categorize into low, medium, and high groups.
Abbreviations: BC, British Columbia; IQR, interquartile range; Max, maximum; Min, minimum; MSP, Medical Services Plan; NSMD, Non–substance-related mental disorder; PY, person-year; SUD, substance use disorder.
During a median follow-up time of 6.9 years (IQR 3.4–12.8), 1,275 participants died (see Table 2). Median age at death was 45.1 (minimum, maximum: 21.3, 75.2). The overall all-cause mortality rate was 11.2 per 1,000 PYs, and the rate was higher during nonmedicated periods (15.0 per 1,000 PYs) compared with medicated periods (5.9 per 1,000 PYs). A total of 504 deaths (39.5%) were attributed to external causes or morbidity and mortality, which were predominantly accidental poisoning (27.8%) and intentional self-harm (4.2%). Infectious diseases (14.9%) and cancer (11.2%) were other major causes of death classified as nonexternal causes. Descriptive statistics characterizing events in the time leading up to death (n = 1,275) are available in supporting information (see S1 Table).
Table 2. Age1 at death according to ICD-10 cause of mortality among 1,275 convicted offenders from BC, 1998–2015.
| Cause of Death | N (%) | ICD-10 code2 | Mean (SD) | Median (Min, Max) |
|---|---|---|---|---|
| Nonexternal causes of morbidity and mortality (Chap I to XVIII)3 | ||||
| Certain infectious and parasitic diseases (Chap I) | 190 (14.9) | A00-B99 | 44.8 (9.4) | 51.7 (25,1, 67.2) |
| Neoplasms (Chap II) | 143 (11.2) | C00-D48 | 54.2 (8.1) | 53.8 (26.6, 74.9) |
| Endocrine, nutritional, and metabolic diseases (Chap IV) | 15 (1.2) | E00-E90 | 50.9 (8.6) | 51.0 (40.0, 66.5) |
| Mental and behavioural disorders (Chap V) | 50 (3.9) | F00-F99 | 44.1 (10.6) | 43.8 (21.4, 72.7) |
| Diseases of the nervous system (Chap VI) | 17 (1.3) | G00-G99 | 41.5 (11.0) | 41.9 (21.6, 64.7) |
| Diseases of the circulatory system (Chap IX) | 111 (8.7) | I00-I99 | 47.5 (12.1) | 46.7 (22.7, 75.2) |
| Diseases of the respiratory system (Chap X) | 81 (6.3) | J00-J99 | 51.3 (10.3) | 52.9 (22.4, 71.8) |
| Diseases of the digestive system (Chap XI) | 52 (4.1) | K00-K93 | 53.2 (7.9) | 54.9 (30.2, 70.3) |
| Symptoms, signs, and abnormal clinical and laboratory findings not elsewhere classified (Chap XVIII) | 95 (7.5) | R00-R99 | 41.3 (9.6) | 41.0 (21.6, 61.3) |
| Other nonexternal causes4 | 17 (1.3) | Chap III: D50-D89; Chap XII: L00-L99; Chap XIII: M00-M99; Chap XIV: N00-N99; Chap XVII: Q00-Q99 | 44.4 (12.6) | 44.8 (27.2, 71.8) |
| External causes of morbidity and mortality (Chap XX)5 | V01-Y98 | |||
| Transport accidents | 31 (2.4) | V01 to V99 | 41.6 (11.5) | 41.7 (21.5, 65.8) |
| Falls/accidental drowning/fire | 11 (1.0) | W00-W19; W65-W74; X00-X09 | 46.6 (9.9) | 47.0 (34.1, 63.3) |
| Accidental poisoning | 355 (27.8) | X40 to X49 | 41.7 (9.7) | 41.3 (22.2, 67.6) |
| Intentional self-harm | 53 (4.2) | X60 to X84 | 40.4 (10.0) | 41.1 (22.4, 65.8) |
| Assault | 28 (2.2) | X85-Y09 | 34.6 (6.7) | 35.0 (22.9, 43.9) |
| All other external causes | 26 (2.0) | W20-W64; W75-W99; X10-X39; X50-X59; Y10-Y89 | 42.3 (10.7) | 42.5 (21.3, 59.5) |
| Total | 1,275 (100) | 45.2 (10.9) | 45.1 (21.3, 75.2) |
1Age at the time of death.
2lCD-10 codes were used to classify 1,268 deaths, and ICD-9 codes were used to classify 7 deaths, whose comparable ICD-10 group was as follows: Chap 5: 1; Chap X: 1; Chap XVIII: 1; and Chap XX, Accidental poisoning: 4.
3This group represents 771 (60.5%) deaths (ICD-10: 768 and ICD-9: 3).
4Deaths included: Chap III: 2; Chap VII: 0; Chap VIII: 0; Chap XII: 4; Chap XIII: 5; Chap XIV: 5; Chap XV: 0; Chap XVI: 0; and Chap XVII: 1.
5This group represents 504 (39.5%) deaths (ICD-10: 500 and ICD-9: 4).
Abbreviations: BC, British Columbia; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision.
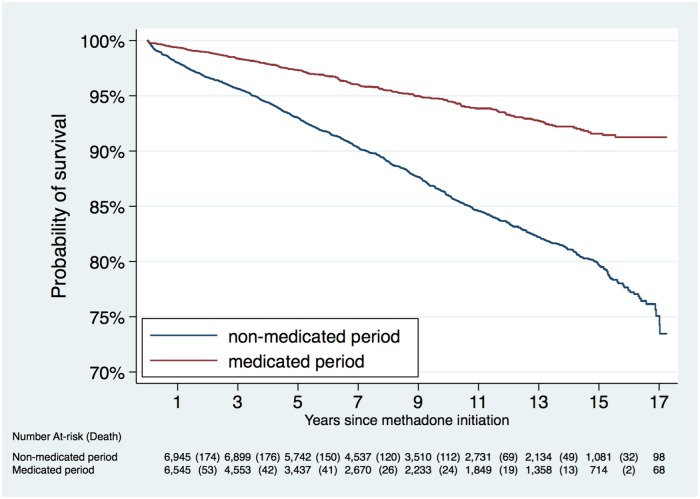
We compared rates of death during dispensed methadone periods and nondispensed periods. Fig 2 shows the Kaplan-Meier curve for all-cause mortality throughout the follow-up period. Participants were significantly more likely to die during nonmedicated methadone periods than during medicated periods (Fig 2).
Fig 2. Kaplan-Meier curve for all-cause mortality among 14,530 convicted offenders from BC, 1998–2015.
BC, British Columbia.
As shown in Table 3, participants were more likely to die from both nonexternal (adjusted hazard ratio [AHR] 0.27 [0.23–0.33]) and external (AHR 0.41 [0.33–0.51]) causes during nonmedicated periods. The risk of death due to infectious diseases was 5 times lower (AHR 0.20 [0.13–0.30]) during medicated methadone periods compared with nonmedicated periods. Similarly, for deaths caused by accidental poisoning and intentional self-harm, the AHRs were 0.39 (0.30–0.50) and 0.36 (0.18–0.70), respectively, representing a roughly 2.75 times lower risk of death during medicated methadone periods. All other external (AHR 0.54 [0.34–0.85]) and nonexternal (AHR 0.30 [0.25–0.37]) causes of morbidity and mortality were associated with significantly lower mortality risk during periods when methadone was dispensed. The effects (AHR) of all other covariates included in the multivariable model are available in Supporting information (S2–S4 Tables).
Table 3. HR estimates of dispensed methadone on mortality among 14,530 convicted offenders from BC, 1998–2015.
| Cause of Death | Medicated Methadone Period | Number of Deaths | Total PYs | Death Rate per 1,000 PYs (95% CI) | UHR (95% CI)1 | AHR2 (95% CI) |
|---|---|---|---|---|---|---|
| All-cause mortality3 | No | 996 | 66,562.0 | 15.0 (14.1–15.9) | Reference | Reference |
| Yes | 279 | 47,681.7 | 5.9 (5.2–6.6) | 0.37 (0.32–0.42)5 | 0.32 (0.28–0.37) | |
| Total4 | 1,275 | 114,243.7 | 11.2 (10.6–11.8) | |||
| Nonexternal causes | No | 623 | 66,562.0 | 9.4 (8.7–10.1) | Reference | Reference |
| Yes | 148 | 47,681.7 | 3.1 (2.6–3.7) | 0.32 (0.26–0.38) | 0.27 (0.23–0.33) | |
| Total | 771 | 114,243.7 | 6.8 (6.3–7.2) | |||
| Infectious diseases | No | 162 | 66,562.0 | 2.4 (2.1–2.8) | Reference | Reference |
| Yes | 28 | 47,681.7 | 0.6 (0.4–0.9) | 0.23 (0.15–0.35) | 0.20 (0.13–0.30) | |
| Total | 190 | 114,243.7 | 1.7 (1.4–1.9) | |||
| Other nonexternal causes | No | 461 | 66,562.0 | 6.9 (6.3–7.6) | Reference | Reference |
| Yes | 120 | 47,681.7 | 2.5 (2.1–3.0) | 0.35 (0.28–0.43) | 0.30 (0.25–0.37) | |
| Total | 581 | 114,243.7 | 5.1 (4.7–5.5) | |||
| External causes | No | 373 | 66,562.0 | 5.6 (5.1–6.2) | Reference | Reference |
| Yes | 131 | 47,681.7 | 2.8 (2.3–3.3) | 0.45 (0.37–0.55) | 0.41 (0.33–0.51) | |
| Total | 504 | 114,243.7 | 4.4 (4.0–4.8) | |||
| Accidental poisoning | No | 266 | 66,562.0 | 4.0 (3.5–4.5) | Reference | Reference |
| Yes | 89 | 47,681.7 | 2.0 (1.9–2.3) | 0.43 (0.33–0.55) | 0.39 (0.30–0.50) | |
| Total | 355 | 114,243.7 | 3.1 (2.8–3.5) | |||
| Intentional self-harm | No | 41 | 66,562.0 | 0.6 (0.5–0.8 | Reference | Reference |
| Yes | 12 | 47,681.7 | 0.3 (0.1–0.4) | 0.40 (0.21–0.77) | 0.36 (0.18–0.70) | |
| Total | 53 | 114,243.7 | 0.5 (0.4–0.6) | |||
| Other external causes | No | 66 | 66,562.0 | 1.0 (0.8–1.3) | Reference | Reference |
| Yes | 30 | 47,681.7 | 0.6 (0.4–0.9) | 0.57 (0.37–0.90) | 0.54 (0.34–0.85) | |
| Total | 96 | 114,243.7 | 0.8 (0.7–1.03) |
1Robust estimator was used to calculate SE and the CIs for both UHR and AHR estimates.
2Separate multivariable Cox regression was conducted for all-cause and for each cause-specific death. Each multivariable model was controlled for the following: age (18 < 25 years, 25 < 35 years, 35 < 45 years, 45 < 55 years, and ≥55), gender (men and women), ethnicity (white, indigenous, and other), education (<grade 10, grade 10/11, grade 12, vocational/university), initiation period (1998 to 2000, 2001 to 2005, 2006 to 2010, and 2011 to 2015), prior offences (none, 1–2 offences, and >2 offences), number of current offences (continuous), severe mental illness (no schizophrenia or bipolar; schizophrenia and bipolar), prior NSMD-related services (low, medium, and high), prior SUD-related services (low, medium, and high), and prior nonpsychiatric services (low, medium, and high).
3Nonexternal and external causes represent 2 broad subcategories of all-cause mortality (771+ 504 = 1,275). Nonexternal and external causes are further subdivided into 2 (infectious diseases and other nonexternal causes) and 3 (accidental poisoning, intentional self-harm, and other external causes) groups, respectively.
4The total represents the sum of deaths for medicated (methadone dispensed) and nonmedicated (methadone not dispensed) periods.
5Bold indicated significance of HR estimates at p < 0.05.
Abbreviations: AHR, adjusted HR; BC, British Columbia; HR, hazard ratio; NSMD, non–substance-related mental disorder; PY, person-year; SUD, substance use disorder; UHR, unadjusted HR.
A competing risk regression demonstrated a similar pattern of results for both nonexternal (AHR 0.32 [0.27–0.39]) and external causes (AHR 0.46, [0.38–0.57]) of death as well as for other types of cause-specific deaths (S5 Table). When restricted to participants who initiated methadone between 2007 and 2015 (when custody data became available), the rate of custody admission was 0.3 per PY following methadone initiation. The subgroup analysis (S6 Table) among participants (n = 2,905) with at least one custody admission (AHR 0.27 [0.15–0.51]) produced findings consistent with our primary results. Similarly, the HIV cause-specific sensitivity analysis (S7 Table) produced comparable methadone treatment effects (AHR 0.19 [0.11–0.33]). Sensitivity analyses (S8 Table) involving alternate definitions for methadone periods (from 1 day to 3 and 7 days) confirm the same overall pattern of results for nonexternal (3-day AHR 0.41 [0.34–0.48]; 7-day AHR 0.52 [0.45–0.52]) and external causes (3-day AHR 0.54 [0.44–0.66); 7-day 0.59 [0.48–0.71]] of death and remain significant although, as expected, the effect decays when medicated time is inflated to 3 and 7 days.
Discussion
In this longitudinal cohort study, dispensed methadone was associated with significantly lower risk of both all-cause and cause-specific mortality among patients diagnosed with opioid dependence and with prior convictions. To our knowledge, this is the first study to investigate the association between MMT and mortality in a large sample over an extended period (i.e., greater than 10 years) with adjustment for diverse covariates. The majority of our sample did not commit an offence in the year preceding methadone initiation (62%), and few received sentences that included time in custody (19%). Therefore, our observation period overwhelmingly corresponds to events occurring in community settings while participants were not under correctional supervision.
Our study has several implications for the treatment of opioid dependence and prevention of premature mortality in populations with complex health and social needs including exposure to corrections. The statistically significant relationship between dispensed methadone and lower risk of all-cause mortality is particularly relevant in the context of North America’s current opioid overdose crisis. The World Health Organization has recognised methadone as an essential medicine for over a decade [58] and recommended access to methadone (or other agonist treatments) for all opioid-dependent prisoners [59], acknowledging the role of untreated substance dependence as a contributor to mortality [19,60]. Despite global awareness of the importance of methadone in treating opioid dependence, a number of barriers limit the optimisation of methadone programs, including high physician patient loads [61], lack of education and training [20], and stigma [62]. These challenges are often amplified among criminal justice populations [63] and among individuals residing in remote and rural areas [64] even when offered through low-barrier services within a universal healthcare system [65]. In addition, agonist treatment options are not routinely offered alongside psychosocial or counselling interventions despite recommendations that support their importance in care [66] and their relationship to mitigating overdose risk in criminal justice populations [67]. Nevertheless, our results indicate that when methadone is administered, despite current limitations, it can reduce mortality among marginalized patients with opioid dependence. Previous research has demonstrated the substantially elevated risk of mortality among offenders during the period immediately following release from custody [13,68]. Our results expand on this work to show that mortality risk is elevated among methadone recipients with any exposure to the corrections system—where the majority are not exposed to custody—and over periods of time that greatly exceed their time under correctional supervision. Efforts to make methadone treatment more accessible, integrated, and comprehensive may yield additional life-saving benefits.
Consistent with other research [11], overdose deaths accounted for nearly one-third of mortality in our cohort. In addition to the reduced risk of all-cause mortality, our results demonstrate that adherence to methadone was associated with a lower risk of death from accidental poisoning compared with nonmedicated periods. Despite evidence that methadone adherence decreases the risk of fatal overdose [32], poor retention undermines this potential benefit. Furthermore, the potential elevated risk for overdose associated with treatment adjustments (i.e., induction and cessation) has raised concerns regarding the effectiveness of methadone as a harm-reduction measure. However, this concern is weakly supported by evidence [36] and should not be a deterrent when offering treatment with methadone because fatalities are more strongly related to other causes [35], including the illicit use of nonprescription methadone [69]. On average, participants in our cohort spent more time in nonmedicated periods than medicated periods, signalling an urgent need to substantially improve adherence.
An emerging body of evidence has highlighted the positive impact of MMT for populations with infectious disease. Patients with HIV/AIDS using methadone are associated with earlier ART initiation and higher levels of adherence [70,71]. Potential explanations are that MMT adherence is associated with increased stability and decreased risks (e.g., drug injecting), enabling increased engagement in HIV treatment. Our results are consistent with the finding that methadone may potentiate ART adherence in patients with opioid dependence, demonstrating a 5-fold lower risk of infectious disease mortality compared with nonmedicated methadone periods (in HIV subgroup analyses, this finding remained consistent).
Researchers examining deaths among offenders have focused on the risk immediately following release from custody and have consistently found significant mortality during this critical period [14]. Although the transition from prison warrants close attention to prevent mortality, a narrow focus on prison release ‘…fails to capture the ongoing elevation of risk among ex-prisoners, and directs attention away from the ongoing health needs of this chronically marginalized and unwell group’ [72] (p. 1555). Current harm reduction and addictions literature advocates for the treatment and management of substance misuse as a chronic disease [40] rather than an acute episodic illness requiring detoxification [73]. This approach is supported by observations that a majority of opioid-dependent individuals receiving methadone have repeated treatment episodes, with continuous and/or longer treatment duration typically occurring after several failed attempts [74]. The paucity of literature evaluating mortality risk over extended time periods limits our understanding of risk related to the chronic and relapsing nature of opioid dependence among individuals with criminal justice involvement. Our research design aimed to address this gap by investigating treatment as it fluctuates over a relatively long follow-up period.
Our study offers the advantages of a complete population of convicted offenders accessing methadone, with specific objective measurement of exposure and outcome while controlling for several key covariates. Our sample included individuals who were exposed to provincial corrections, typically for short periods of time, serving sentences in noncustodial settings. Therefore, our observation period overwhelmingly represents community-based methadone prescribing. Despite its strengths, this study has limitations. The use of a Canadian provincial offender population may limit the generalizability of results to other settings, jurisdictions, and patient groups. Our outcome is restricted to recorded deaths and does not account for undetected mortality. Also, our findings may have been subject to compliance bias because adherence to MMT may indicate unmeasured behaviours that, in turn, have an influence on mortality. The magnitude of the protective effects of methadone from nonexternal causes of death should be interpreted with caution because our results and analyses do not take into account methadone prescribed in hospitals or other care facilitates (e.g., hospice); however, this limitation does not affect the magnitude of effect for external causes of mortality. Receipt of methadone treatment may be accompanied by psychosocial supports (e.g., counselling supports, Alcoholics Anonymous [AA], Narcotics Anonymous [NA], etc.) with varying degrees of participation by individuals. Involvement with ancillary supports was not accounted for in our analyses and may have altered treatment adherence. Methadone prescribing in BC almost universally involves witnessed methadone ingestion, and therefore our use of pharmacy dispensing records provides a strong basis for inferring methadone adherence. Disruptions to treatment, such as access or relocation, were not assessed and may have influenced our results. The influence of other opioid prescriptions (buprenorphine and buprenorphine-naloxone) prior to or during the observation period was minimal (approximately 1% of the total opiate agonist prescriptions between 2008 and 2015); however, their exclusion is a limitation to our study. Lastly, we did not account for methadone dose, which has been shown to be related to mortality outcomes [75]; therefore, research examining the relationship between dose and mortality is needed, as is research investigating additional opiate treatments (e.g., suboxone).
Conclusion
In a large cohort of Canadian convicted offenders, rates of mortality were significantly lower during periods when individuals were dispensed methadone compared with periods in which they were not dispensed methadone. Our findings strongly indicate that efforts to increase methadone adherence may reduce mortality in high-risk populations such as opioid-dependent offenders. Our findings warrant examination in other study centres in response to the crisis of opiate-involved deaths.
Supporting information
(DOCX)
(DOCX)
BC, British Columbia.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
BC, British Columbia; HR, hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio; BC, British Columbia.
(DOCX)
(DOCX)
Acknowledgments
The authors gratefully acknowledge support from the British Columbia Inter-Ministry Research Initiative (IMRI) and members of the IMRI Steering Committee.
Abbreviations
- AA
Alcoholics Anonymous
- AHR
adjusted HR
- ART
antiretroviral treatment
- BC
British Columbia
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- HR
hazard ratio
- IMRI
Inter-Ministry Research Initiative
- IQR
interquartile range
- ICD-10
International Statistical Classification of Diseases and Related Health Problems 10th Revision
- MMT
methadone maintenance treatment
- NA
Narcotics Anonymous
- OST
opioid substitution treatment
- UHR
unadjusted HR
- PY
person-year
Data Availability
Aggregate data are provided in the paper and its Supporting Information. Researchers can seek access to individual-level data via the British Columbia Data Stewardship Committee (https://www2.gov.bc.ca/gov/content/health/conducting-health-research-evaluation/data-access-health-data-central/requesting-access).
Funding Statement
This research was supported by funds provided by the Canadian Institutes of Health Research (AR: GSD-14620; JMS: 2009 s0231), the British Columbia Ministry of Justice (JMS: 2014 s0040), and Health Canada (JMS:2009s0231). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382: 1564–1574. 10.1016/S0140-6736(13)61530-5 [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Noonan RK, Houry D. Underlying Factors in Drug Overdose Deaths. JAMA. 2017;318: 2295–2296. 10.1001/jama.2017.15971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gostin LO, Hodge JG, Noe SA. Reframing the Opioid Epidemic as a National Emergency. JAMA. 2017;318: 1539–1540. 10.1001/jama.2017.13358 [DOI] [PubMed] [Google Scholar]
- 4.Bonnie RJ, Kesselheim AS, Clark DJ. Both Urgency and Balance Needed in Addressing Opioid Epidemic: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA. 2017;318: 423–424. 10.1001/jama.2017.10046 [DOI] [PubMed] [Google Scholar]
- 5.Merrall ELC, Bird SM, Hutchinson SJ. Mortality of those who attended drug services in Scotland 1996–2006: record-linkage study. Int J Drug Policy. 2012;23: 24–32. 10.1016/j.drugpo.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darke S, Mills KL, Ross J, Teesson M. Rates and correlates of mortality amongst heroin users: Findings from the Australian Treatment Outcome Study (ATOS), 2001–2009. Drug and Alcohol Dependence. 2011;115: 190–195. 10.1016/j.drugalcdep.2010.10.021 [DOI] [PubMed] [Google Scholar]
- 7.Andrews JY, Kinner SA. Understanding drug-related mortality in released prisoners: a review of national coronial records. BMC Public Health. 2012;12: 270 10.1186/1471-2458-12-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariminia A, Law MG, Butler TG, Corben SP, Levy MH, Kaldor JM, et al. Factors associated with mortality in a cohort of Australian prisoners. Eur J Epidemiol. 2007;22: 417–428. 10.1007/s10654-007-9134-1 [DOI] [PubMed] [Google Scholar]
- 9.King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104: e32–42. 10.2105/AJPH.2014.301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101: 181–191. 10.1111/j.1360-0443.2006.01316.x [DOI] [PubMed] [Google Scholar]
- 11.Hakansson A, Berglund M. All-cause mortality in criminal justice clients with substance use problems—A prospective follow-up study. Drug and Alcohol Dependence. 2013;132: 499–504. 10.1016/j.drugalcdep.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 12.Chang Z, Lichtenstein P, Larsson H, Fazel S. Substance use disorders, psychiatric disorders, and mortality after release from prison: a nationwide longitudinal cohort study. The Lancet Psychiatry. 2015;2: 422–430. 10.1016/S2215-0366(15)00088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105: 1545–1554. 10.1111/j.1360-0443.2010.02990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356: 157–165. 10.1056/NEJMsa064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degenhardt L, Larney S, Randall D, Burns L, Hall W. Causes of death in a cohort treated for opioid dependence between 1985 and 2005. Addiction. 2013;109: 90–99. 10.1111/add.12337 [DOI] [PubMed] [Google Scholar]
- 16.Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction. 2017;112: 1408–1418. 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- 17.Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O’Grady KE, et al. Why don’t out-of-treatment individuals enter methadone treatment programmes? International Journal of Drug Policy. 2010;21: 36–42. 10.1016/j.drugpo.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vashishtha D, Mittal ML, Werb D. The North American opioid epidemic: current challenges and a call for treatment as prevention. Harm Reduct J. BioMed Central; 2017;14: 7 10.1186/s12954-017-0135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-Assisted Therapies—Tackling the Opioid-Overdose Epidemic. N Engl J Med. 2014;370: 2063–2066. 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- 20.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag. 2009;5: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouyoumdjian FG, Patel A, To MJ, Kiefer L, Regenstreif L. Physician prescribing of opioid agonist treatments in provincial correctional facilities in Ontario, Canada: A survey. PLoS ONE. 2018;13(2): e0192431 10.1371/journal.pone.0192431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Mattick RP, editor. Cochrane Database Syst Rev. 2009;72: CD002209 10.1002/14651858.CD002209.pub2 [DOI] [PubMed] [Google Scholar]
- 23.Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. N Engl J Med. 1969;280: 1372–1375. 10.1056/NEJM196906192802502 [DOI] [PubMed] [Google Scholar]
- 24.Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65: 146–157. 10.1176/appi.ps.201300235 [DOI] [PubMed] [Google Scholar]
- 25.Dolan K, Moazen B, Noori A, Rahimzadeh S, Farzadfar F, Hariga F. People who inject drugs in prison: HIV prevalence, transmission and prevention. Int J Drug Policy. 2015;26 Suppl 1: S12–5. 10.1016/j.drugpo.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 26.Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction. 2012;107: 372–380. 10.1111/j.1360-0443.2011.03618.x [DOI] [PubMed] [Google Scholar]
- 27.Russolillo A, Moniruzzaman A, McCandless LC, Patterson M, Somers JM. Associations between methadone maintenance treatment and crime: a 17-year longitudinal cohort study of Canadian provincial offenders. Addiction. 2017;382: 1564 10.1111/add.14059 [DOI] [PubMed] [Google Scholar]
- 28.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11: 1–171. [DOI] [PubMed] [Google Scholar]
- 29.Cousins G, Boland F, Courtney B, Barry J, Lyons S, Fahey T. Risk of mortality on and off methadone substitution treatment in primary care: a national cohort study. Addiction. 2016;111: 73–82. 10.1111/add.13087 [DOI] [PubMed] [Google Scholar]
- 30.Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103: 462–468. 10.1111/j.1360-0443.2007.02090.x [DOI] [PubMed] [Google Scholar]
- 31.Ledberg A. Mortality related to methadone maintenance treatment in Stockholm, Sweden, during 2006–2013. Journal of Substance Abuse Treatment. 2017;74: 35–41. 10.1016/j.jsat.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 32.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357: j1550 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans E, Li L, Min J, Huang D, Urada D, Liu L, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction. 2015;110: 996–1005. 10.1111/add.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Shi CX, McGoogan JM, Rou K, Zhang F, Wu Z. Methadone maintenance treatment and mortality in HIV-positive people who inject opioids in China. Bull World Health Organ. 2013;91: 93–101. 10.2471/BLT.12.108944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fugelstad A, Stenbacka M, Leifman A, Nylander M, Thiblin I. Methadone maintenance treatment: the balance between life-saving treatment and fatal poisonings. Addiction. 2007;102: 406–412. 10.1111/j.1360-0443.2006.01714.x [DOI] [PubMed] [Google Scholar]
- 36.Pierce M, Bird SM, Hickman M, Marsden J, Dunn G, Jones A, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction. 2016;111: 298–308. 10.1111/add.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014;109: 1306–1317. 10.1111/add.12536 [DOI] [PubMed] [Google Scholar]
- 38.Larney S, Gisev N, Farrell M, Dobbins T, Burns L, Gibson A, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open. 2014;4: e004666 10.1136/bmjopen-2013-004666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gisev N, Larney S, Kimber J, Burns L, Weatherburn D, Gibson A, et al. Determining the impact of opioid substitution therapy upon mortality and recidivism among prisoners: A 22 year data linkage study. May 31, 2015 pp. 1–7. https://aic.gov.au/publications/tandi/tandi498. [cited 4 Apr 2018].
- 40.Scott CK, Dennis ML, Laudet A, Funk RR, Simeone RS. Surviving drug addiction: the effect of treatment and abstinence on mortality. Am J Public Health. 2011;101: 737–744. 10.2105/AJPH.2010.197038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28: 321–329. 10.1016/j.jsat.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Treviño L, Saiz PA, García-Portilla MP, Díaz-Mesa EM, Sánchez-Lasheras F, Burón P, et al. A 25-year follow-up of patients admitted to methadone treatment for the first time: mortality and gender differences. Addict Behav. 2011;36: 1184–1190. 10.1016/j.addbeh.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 43.Lim S, Harris TG, Nash D, Lennon MC, Thorpe LE. All-Cause, Drug-Related, and HIV-Related Mortality Risk by Trajectories of Jail Incarceration and Homelessness Among Adults in New York City. Am J Epidemiol. 2015;181: 261–270. 10.1093/aje/kwu313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon MS, Kinlock TW, Miller PM. Medication-assisted treatment research with criminal justice populations: challenges of implementation. Behav Sci Law. 2011;29: 829–845. 10.1002/bsl.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. American Medical Association; 2008;300: 2303–2305. [DOI] [PubMed] [Google Scholar]
- 46.Rezansoff SN, Moniruzzaman A, Gress C, Somers JM. Psychiatric diagnoses and multiyear criminal recidivism in a Canadian provincial offender population. Psychology, Public Policy, and Law. 2013;19: 443–453. 10.1037/a0033907 [DOI] [Google Scholar]
- 47.Physicians CO, Columbia SOB. Methadone and Buprenorphine: Clinical Practice Guideline for Opioid Use Disorder [Internet]. 2016. July pp. 1–68. https://www.cpsbc.ca/files/pdf/MB-WS-101-2017-04-01-P01-Intro.pdf. [cited 15 Dec 2017]. [Google Scholar]
- 48.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Springer New York; 1972;B34: 86–94. [Google Scholar]
- 49.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. The Annals of Statistics. Institute of Mathematical Statistics; 1982;10: 1100–1120. 10.1214/aos/1176345976 [DOI] [Google Scholar]
- 50.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81: 515 10.2307/2337123 [DOI] [Google Scholar]
- 51.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Statistics in Medicine. 1995;14: 1707–1723. 10.1002/sim.4780141510 [DOI] [PubMed] [Google Scholar]
- 52.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Berkeley, CA; 1967. pp. 221–233. [Google Scholar]
- 53.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48: 817 10.2307/1912934 [DOI] [Google Scholar]
- 54.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133: 601–609. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. International Journal of Epidemiology. 2012;41: 861–870. 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Statistics in Medicine. 2007;26: 2389–2430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 57.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94: 496 10.2307/2670170 [DOI] [Google Scholar]
- 58.WHO Model List of Essential Medicines [Internet]. 20 ed. World Health Organization. http://www.who.int/medicines/publications/essentialmedicines/en/. [cited 15 Jan 2018].
- 59.United Nations Office in Drugs and Crime [internet]. HIV/AIDS Prevention, Care, Treatment and Support in Prison Settings: A Framework for an Effective National Response. www.who.int/hiv/pub/prisons/prison_framework/en/. [cited 15 Dec 2017].
- 60.Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36: 559–574. 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- 61.Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of Substance Abuse Treatment. 2010;39: 22–31. 10.1016/j.jsat.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 62.Earnshaw V, Smith L, Copenhaver M. Drug Addiction Stigma in the Context of Methadone Maintenance Therapy: An Investigation into Understudied Sources of Stigma. Int J Ment Health Addict. 2013;11: 110–122. 10.1007/s11469-012-9402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Legal Action Center. Confronting an Epidemic: The Case for Eliminating Barriers to Medication- Assisted Treatment of Heroin and Opioid Addiction [Internet]. 2015 Feb pp. 1–8. www.lac.org. [cited 10 Nov 2017].
- 64.Eibl JK, Morin K, Leinonen E, Marsh DC. The State of Opioid Agonist Therapy in Canada 20 Years after Federal Oversight. Can J Psychiatry. 2017;62: 444–450. 10.1177/0706743717711167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nosyk B, Sun H, Evans E, Marsh DC, Anglin MD, Hser Y-I, et al. Defining dosing pattern characteristics of successful tapers following methadone maintenance treatment: results from a population-based retrospective cohort study. Addiction. 2012;107: 1621–1629. 10.1111/j.1360-0443.2012.03870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruneau J, Ahamad K, Goyer M-È, Poulin G, Selby P, Fischer B, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018;190: E247–E257. 10.1503/cmaj.170958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization Regional Office for Europe [internet]. Preventing overdose deaths in the criminal-justice system. http://www.euro.who.int/en/publications/abstracts/preventing-overdose-deaths-in-the-criminal-justice-system-2014. [cited 10 Dec 2017].
- 68.Zlodre J, Fazel S. All-cause and external mortality in released prisoners: systematic review and meta-analysis. Am J Public Health. 2012;102: e67–75. 10.2105/AJPH.2012.300764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wikner BN, Öhman I, Seldén T, Druid H, Brandt L, Kieler H. Opioid-related mortality and filled prescriptions for buprenorphine and methadone. Drug Alcohol Rev. 2014;33: 491–498. 10.1111/dar.12143 [DOI] [PubMed] [Google Scholar]
- 70.Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105: 907–913. 10.1111/j.1360-0443.2010.02905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nosyk B, Min JE, Colley G, Lima VD, Yip B, Milloy MJS, et al. The causal effect of opioid substitution treatment on HAART medication refill adherence. AIDS. 2015;29: 965–973. 10.1097/QAD.0000000000000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kinner SA. Commentary on Merrall et al. (2010): understanding mortality and health outcomes for ex-prisoners—first steps on a long road. Addiction. Blackwell Publishing Ltd; 2010;105: 1555–1556. 10.1111/j.1360-0443.2010.03030.x [DOI] [PubMed] [Google Scholar]
- 73.National Institute on Drug Abuse. Principles of Drug Addiction Treatment. 3rd ed. National Institutes of Health U.S. Department of Health and Human Services; 2012 Dec pp. 1–44. Report No.: 12–4180. https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/principles-effective-treatment. [cited 8 Jan 2018].
- 74.Nosyk B, MacNab YC, Sun H, Fischer B, Marsh DC, Schechter MT, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol. 2009;170: 783–792. 10.1093/aje/kwp186 [DOI] [PubMed] [Google Scholar]
- 75.Liao D-L, Chen P-C, Chen C-H, Hsieh C-J, Huang Y-F, Shih W-Y, et al. Higher methadone doses are associated with lower mortality in patients of opioid dependence in Taiwan. J Psychiatr Res. 2013;47: 1530–1534. 10.1016/j.jpsychires.2013.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
BC, British Columbia.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
BC, British Columbia; HR, hazard ratio.
(DOCX)
AHR, adjusted hazard ratio.
(DOCX)
AHR, adjusted hazard ratio; BC, British Columbia.
(DOCX)
(DOCX)
Data Availability Statement
Aggregate data are provided in the paper and its Supporting Information. Researchers can seek access to individual-level data via the British Columbia Data Stewardship Committee (https://www2.gov.bc.ca/gov/content/health/conducting-health-research-evaluation/data-access-health-data-central/requesting-access).