Abstract
Fusarium verticillioides, an important maize pathogen produces fumonisins and causes stalk and ear rot; thus, we are aimed to clarify its infection cycle by assessing enhanced green fluorescent protein (EGFP) expression in stalk and ear rot strains. Maize seeds were inoculated with stable and strongly pathogenic transformants. To investigate the degree of infection, inoculated plants were observed under a stereo fluorescence microscope, and affected tissue strains were detected using PCR. We found that both transformants infected maize. Hyphae infected the plants from radical to the stem and extended to the ear and infected ear kernels caused a second infection. This process formed the infection cycle.
Introduction
Fusarium verticillioides (teleomorph Gibberella moniliformis) is one of the most common causal agents of diseases in maize (Zea mays L.) and a wide range of crops and plants worldwide [1]. This fungus produces fumonisins, a group of mycotoxins that can accumulate in the kernels making them toxic to animals and humans [2–7]. In addition to their negative health impacts, studies have revealed that the fumonisins produced by F. verticillioides cause significant yield losses [8] and also threaten the health of humans and animals [9–11].
Maize is particularly susceptible to F. verticillioides infection due to the large amounts of fumonisins produced [12, 13].
Fusarium stalk rot reduces output in maize by 10% typically and by 30–50% in severely affected areas [14], while Fusarium ear rot, characterized by discolored and a reduced number of grains, not only reduces yield, but also influences the quality of the seeds.
As the policy of straw returning (i.e., leaving straw in the field instead of burning to protect the environment) becomes more common in China, maize stalk and ear rot are spreading because F. verticillioides can survive in crop residues, which initiates subsequent infection [15, 16]. Thus, these diseases have become widespread in maize production, which is significantly influencing yield and causing increasing concern.
Green fluorescent protein (GFP) discovered in Aequorea victoria has the advantages of being stable, convenient to use, and easily observed in living cells [17]. Thus, GFP has been used in many studies, including those on the filamentous fungi of Fusarium. For example, Ronald and Thomas (2004) used a strain of F. graminearum transformed with GFP to investigate growth in barley spike and the patterns of seed infections [18]. Additionally, Li et al. (2010) created transformants with GFP to investigate growth in wheat and the pattern of seed infection [19], Qi et al. (2014) evaluated wheat cultivars using the GFP transformants of F. graminearum to track its initial infection and spread [20], and Murillo et al. (1999) investigated F. moniliforme infected maize seedling in histology [21]. To precisely monitor the progress of F. verticillioides infection, transformed pathogens containing the enhanced green fluorescent protein (EGFP) marker gene were used in this study. Yu et al. (2017) successfully transformed the EGFP gene in Fusarium (eGFP-Tag) and use it to follow the infection process of maize stalks in northeast China [22].
There are few studies on the relationship between the infection cycle in stalk rot and ear rot, so this study aimed to clarify the infection cycle of these diseases in maize. To do this, the EGFP gene was expressed in the F. verticillioides strains Fv-s1 and Fv-e1 obtained from stalk and ear rot respectively, using the PEG-CaCl2-mediated transformation method [23] as described by Yelton et al. (1984), and three transformants of each strain were selected for subsequent experimentation, and pathogenicity assays of wild strains and transformants were conducted to test differences between the strains. An inoculation test was conducted on maize seedlings using the toothpick method [24] and the transformed isolates Fv-eGFPs1 and Fv-eGFPe1 were inoculated on different maize seeds and then hyphal colonization and degree of infection in the maize plants were confirmed with a stereo fluorescence microscope and by subsequent molecular detection.
Materials and methods
Ethics statement
Samples in this study were obtained from public areas that no specific permissions were required for these locations. Field sites are public access and F. verticillioides is not endangered or protected species.
Plant material
The maize variety used in this assay was ‘Xianyu 335’, a line that is susceptible to both stalk and ear rot [25]. Plants were grown in a greenhouse maintained at 26°C with a photoperiod of 16 h created using a fluorescent lamp.
Fusarium strains and plasmids
Stalk and ear rot samples were collected from 42 locations in northeastern China during 2013 and 2014. In total, 29 stalk isolates of F. verticillioides (18.6%) and 60 ear isolates (42.6%) were obtained [26]. Pathogenicity assay was conducted of all the F. verticillioides strains, that evaluated by assessing the degree of decay in the seedling radicles and coleoptiles. To further investigate the degree of infection in maize, the most pathogenic isolates from each disease Fv-s (GenBank accession number MF803750) from stalk rot and Fv-e (GenBank accession number MF803751) from ear rot, were selected.
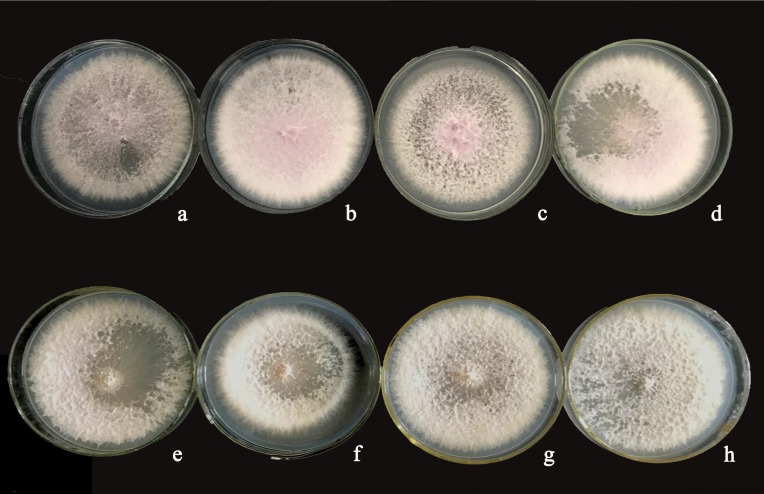
The plasmid pBARGPE1-Hygro-EGFP was modified from the original plasmid pBARGPE1 [obtained from Miaoling Biotechnology Company (Wuhan, China; http://www.miaolingbio.com)] to contain the EGFP gene. The fragment containing the EGFP gene, isolated from pBARKS1-egfp [27], was ligated to the pBARGPE1 vector, which is regulated by the Aspergillus nidulans gpdA promoter (PgpdA) and trpC terminator (TtrpC), and the bar coding region of the gene was replaced by HygB, which is controlled by the TrpC promoter. The EGFP gene was then transformed into F. verticillioides using the PEG-CaCl2-mediated transformation method [28] that according to Wang et al. (2013) [29]. The transformants that showed stable resistance against hygromycin B and fluoresced after continuous culturing for five generations were selected. Finally, three transformed strains were selected for each disease. For stalk rot the strains were Fv-eGFPs1, Fv-eGFPs2, and Fv-eGFPs3, and for ear rot, they were Fv-eGFPe1, Fv-eGFPe2, and Fv-eGFPe3 (Fig 1).
Fig 1. Observation of Fusarium verticillioides wild strains and their enhanced green fluorescent protein (EGFP) transformants.
Colony growth (5-day-culture) of each strain. (a) stalk rot wild strain Fv-s, (b) Fv-eGFPs1, an EGFP transformant of Fv-s, (c) Fv-eGFPs2, an EGFP transformant of Fv-s, (d) Fv-eGFPs3, an EGFP transformant of Fv-s, (e) ear rot wild strain Fv-e, (f) Fv-eGFPe1, an EGFP transformant of Fv-e, (g) Fv-eGFPe2, an EGFP transformant of Fv-e, (h) Fv-eGFPe3, an EGFP transformant of Fv-e.
Pathogenicity assays between wild strains and transformants
Pathogenicity assays between wild strains and their transformants were performed using corn seedlings following the methods of Gai et al. (2017) [26] in which the seedlings were evaluated by assessing the degree of decay in the radicles. Plants were placed in an incubator maintained at 26°C, and negative controls were conducted by inoculating plants with sterile inocula. The experiment was repeated twice with three replicates for each treatment.
The disease severity index (DSI) was calculated using the Townsend-Heuberger formula [30], and then subjected to an analysis of variance (ANOVA) to evaluate differences between strains. To determine which groups were statistically different, a least significant difference test (LSD; significance designated at P < 0.05) was performed using the program SPSS v16 (IBM Co., North Castle, NY, USA).
Inoculation test
The transformed isolates Fv-eGFPs1 and Fv-eGFPe1 were used in an assay to observe the degree of hyphae infection degree throughout maize plant growth. The inoculation test was conducted in a greenhouse at Shenyang Agriculture University in May 2016. Corn kernels was loaded into plastic bags and inoculated with strain after sterilized by an autoclaving, then cultured 30 days when the mycelia overgrow with the corn kernels. Inocula were grown to the seedling stage following the method [27] of Sun et al. (2012), with slight modifications. Twenty grams of inoculum was added to the center of a pot (40 cm in diameter), and then five maize seeds were placed approximately 3 cm apart around the inoculum and covered with 3 cm of sterilized soil. A negative control was set up by inoculating seeds with sterilized inoculum. After germination, three plants were left in each pot, and the other seedlings that sprouted were removed. All the material mentioned in the assay was sterilized before using.
Sufficient water was provided once a week throughout the growing season.
A slightly modified version of the toothpick method [24] was used to inoculate adult plants. Toothpicks were boiled for 30 minutes, sterilized in an autoclave, dried, and inserted into the cultures of each strain, which had been growing on PDA (potato dextrose agar) at 26°C for 5 days. Each plant was then inoculated by inserting three toothpicks into the stem 5 cm above the soil.
In all, 200 plants, divided into two groups of 100 each (group 1 was inoculated with Fv-eGFPs1 and group 2 with Fv-eGFPe1), were tested. We collected maize plants at two points in time. The first collection comprised 20 plants from each group after 3 months, and the second, comprised the remaining 80 plants from each group when the maize was fully grown.
To demonstrate the infection cycle, the fluorescent kernels collected from the field assay were cultured on the PDA medium at 28°C for 5 days, 1×107 spore suspension with maize seedlings soaked, and sterile water as control.
Microscopic examination
Infected tissues were split lengthwise and observed for fluorescence with a stereo fluorescence microscope (Leica MZ 16F; Leica, Germany) under blue light with a 450–490 nm excitation filter and 500–550 nm emission filter. Fluorescent hyphae were viewed with a Nikon Eclipse 80i fluorescence microscope (Nikon, Japan) using an FITC (465–495 nm excitation and 510–555 nm emission) filter set and photographed with a Nikon DS-Fi1c camera and NIS-Elements software.
Molecular detection
To determine whether the infected parts of the plants were infected with F. verticillioides strains transformed by the EGFP gene, a PCR was performed. The fluorescent tissue was cut and cultured on PDA medium, and then 7-day-old fungal mycelia were freeze-dried and ground into powder for genomic DNA extraction using the MiniBEST Plant Genomic DNA Extraction Kit (Takara Bio, Inc., Kusatsu, Japan).
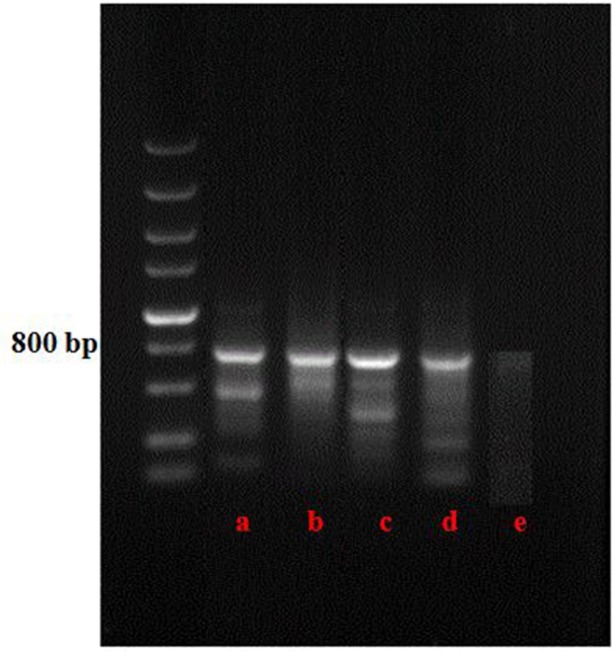
The target EGFP gene was examined with the primers egfp-f 5′-AGT GCT TCA GCC GCT AC-3′ and egfp-r 5′-CGT CCT CCT TGA AGT CG-3′ (Nai et al. 2015) [28]. The PCR program included an initial denaturation at 95°C for 5 min; 30 cycles at 94°C for 30 s, 50°C for 30s, and 72°C for 2 min; and a final extension step at 72°C for 10 min. The amplified fragments were analyzed using electrophoresis on a 0.8% (w/v) agarose gel in 1× TBE buffer (Fig 2).
Fig 2. Detection of target EGFP gene of the strains by PCR amplification.
a,b,c,d) were the transformants from different maize tissues, and e) was the wild type as negative control.
Results
Pathogenicity assays between wild strains and transformants
The degree of decay in seedling radicles showed no significant differences in the DSI (disease severity index) values between wild strains and their transformants, although the transformants had slightly weaker virulence compared to the original wild strain (Table 1).
Table 1. The disease severity index of pathogenicity test between wild-type strains and transformants.
| Strain | Disease severity index | Disease severity index | ||
|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | Mean (P<0.05) | |
| Fv-s | 20.0 | 33.3 | 50.0 | 34.4a |
| Fv-eGFPs1 | 40.0 | 30.0 | 26.7 | 32.2a |
| Fv-eGFPs2 | 20.0 | 30.0 | 20.0 | 23.3a |
| Fv-eGFPs3 | 20.0 | 30.0 | 50.0 | 33.3a |
| Fv-e | 40.0 | 40.0 | 30.0 | 36.7a |
| Fv-eGFPe1 | 40.0 | 40.0 | 26.7 | 35.6a |
| Fv-eGFPe2 | 20.0 | 20.0 | 30.0 | 23.3a |
| Fv-eGFPe3 | 30.0 | 30.0 | 20.0 | 26.7a |
Degree of infection
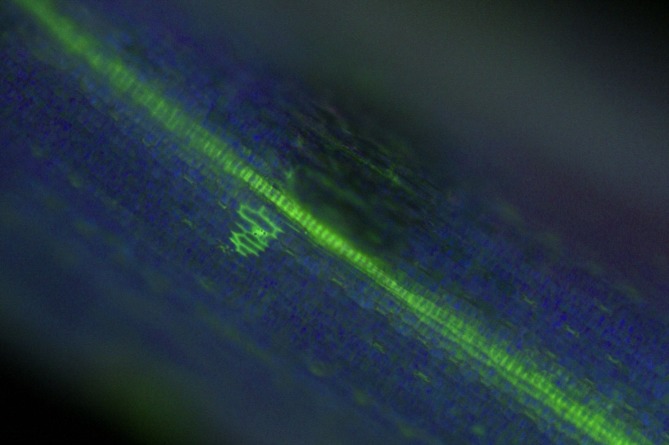
At the initial seed germination stage, F. verticillioides hyphae were typically attached to the maize radicle. Some hyphae were observed penetrating the radicle into the cells, and these hyphae spread the infection to other cells with a rectangular extension (Fig 3). Next, the mycelia spread throughout the tissues infecting the rest of the plants continuously.
Fig 3. Hyphae of Fusarium verticillioides transformant spread the infected across cells with a rectangular extension.
At the adult stage, inoculated plants were cut lengthwise revealing that most plants were infected from the first to the second stalk internode above the ground and that infected piths had turned brown and emitted green fluorescence under the stereo fluorescence microscope. Only three plants had observable green fluorescence at the third internode, and no fluorescence appeared above the third stalk internode in any plant.
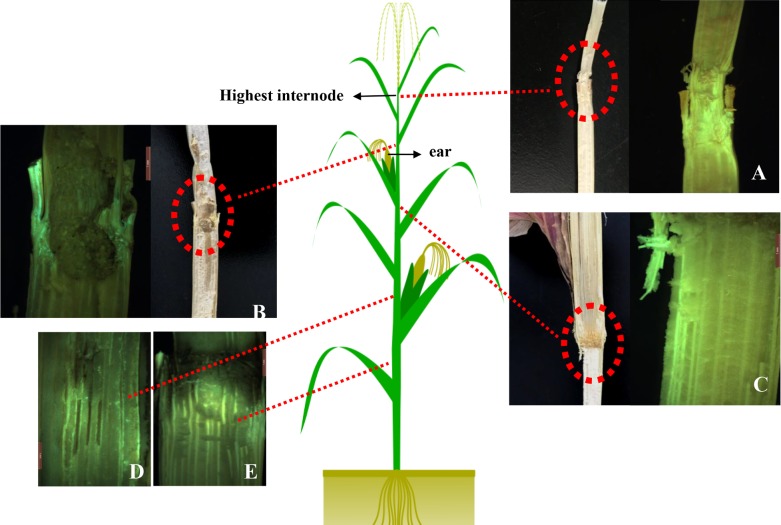
Fully ripened plants showed various degrees of infection throughout the stalk. More than half of these plants had discolored piths and fluoresced green under the stereo fluorescence microscope from the fourth to fifth stalk internode aboveground. Further, 13 plants (group 1 n = 5; group 2 n = 8) had detectable green in the tissue at higher internodes (Fig 4), and nine maize ears fluoresced (group 1 n = 3; group 2 n = 6; Fig 5). We found that necrosed piths were almost non-fluorescent, but we typically observed stronger green fluorescence above 3 cm approximately in the affected stem. It appeared that the normal pith may have already been infected by the pathogen, but not all of the infected tissue could be seen visually. Moreover, we frequently observed green fluorescence in stalks that were damaged by insects.
Fig 4. Different degrees of enhanced green fluorescent protein (EGFP)-tagged Fusarium verticillioides infection in stalks of maize.
A) The highest infected internode under the natural light and blue light (Fv-eGFPe1); B) the internode above the ear under the natural light and blue light (Fv-eGFPs1); C) the internode below the ear under the natural light and blue light (Fv-eGFPe1); D and E) the below infected internode under blue light (Fv-eGFPs1).
Fig 5. Maize ears infected with EGFP-tagged Fusarium verticillioides under blue light.
A) a healthy kernel; B and C), ears infected by Fv-eGFPs1; D, E and F) ears infected by Fv-eGFPe1. Bars indicate 1 mm.
Infection cycle pattern
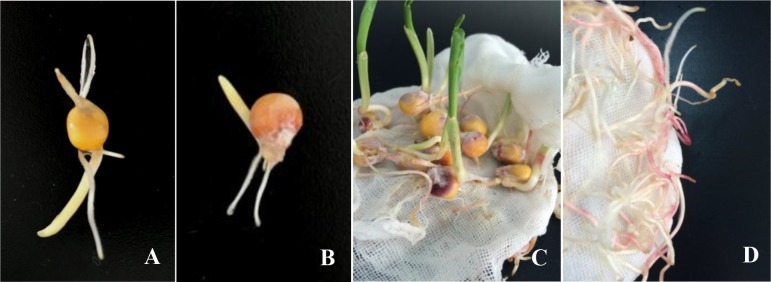
Discolored seedlings and radicles exhibited decay symptoms after 24h, which the parts of symptoms became increasingly over time (Fig 6), and the seedlings as negative control shown normal growth without decay or discolored. In the test, both Fv-eGFPs1 and Fv-eGFPe1, which were obtained from stalk rot and ear rot, respectively, can infect the maize kernels that cause a second infection, and formed a infection cycle.
Fig 6. Infection cycle of maize seeds inoculated with Fusarium verticillioides strains cultured from fluorescent kernels.
A) a healthy maize seed (negative control); B) a seed infected with the re-separated strain of fluorescent kernels; C) discolored seedlings (5-day-growth) with the re-separated strain of fluorescent kernels; D) decayed and discolor radical (5-day-growth) infected with the re-separated strain of fluorescent kernels.
Discussion
We found that all transformants had slightly weaker virulence compared to the original wild strains, but these differences were not significantly. This may have been caused by the continuous culturing on PDA, leading to a slightly reduction in the pathogenicity of the strains.
Regarding the degree of infection, more than half of the plants had observable green fluorescence from the fourth to fifth stalk internodes above the ground, and green fluorescence was detected in more plants infected with ear rot than in those infected with stalk rot, which means that there was a higher incidence of ear rot pathogens of stalk rot. This interpretation is corroborated by the occurrence of fluorescence at the highest internode. Additionally, we observed fluorescence in some stalks that lacked rot symptoms altogether. This result might have been caused by passive movement (eg. transpiration) which pulled the pathogen through the tissue. A mechanism for this type of movement has also been suggested by Duncan and Howard (2009) [31].
Furthermore, we found that insect damage influenced the severity of ear rot: the high incidence of insect damage often led to severe ear rot on maize [32]. Therefore, to prevent disease and control ear rot, pest control needs to be enhanced.
Some scholars had reported Fusarium from stalk rot and ear rot that exists a relationship on etiology between these two diseases [33–35]. The same Fusarium species from these two diseases showed high homology and was able to cross-infect.
From the assay of inoculated maize seeds, it can be concluded that F. verticillioides obtained from both diseases (stalk rot and ear rot on maize) can infect maize seeds and extend to the kernel, causing systematic infection, and the infected kernels are also pathogenetic to maize, which occurs a infection cycle from seeds to stem to ear and to seeds again.
To our knowledge, this study is the first to compare how the two pathogens from stalk rot and ear rot differed by inoculating seeds with each pathogen and investigating the infection cycle. Our results indicated that F. verticillioides obtained from either stalk rot or ear rot on maize can infect maize plants by gaining access to the plant through the radicle and then infecting the stalk upward through various tissues to the ear. Further, the infected kernels can cause a second infection when they become seeds thus completing the infection cycle. The results of the present work defined the infection cycle of F. verticillioides obtained from stalk rot and ear rot and clarified the relationship between these two diseases. Additionally, our results provide a basis for assessing the resistance of stalk rot and ear rot disease in the analyzed maize variety.
Acknowledgments
The authors thank Dr. Dong Huaiyu and Dr. Wang Lijuan at the Academy of Agricultural Sciences of Liaoning for their help in the plasmid transformation.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was supported by the National Key Technology Research and Development Program (2016YFD0300704; 2017YFD0300704) and Research Projects in Liaoning Province (2014201003).
References
- 1.Bacon CW, and Nelson PE. Fumonisin production in corn by toxigenic strains of Fusarium moniliforme and Fusarium proliferatum. Journal of Food Protection. 1994; 57: 514–521. [DOI] [PubMed] [Google Scholar]
- 2.Desjardins AE, Plattner RD, Lu M, and Claflin LE. Distribution of fumonisins in maize ears infected with strains of Fusarium moniliforme that differ in fumonisin production. Plant Disease. 1998; 82:953–958. [DOI] [PubMed] [Google Scholar]
- 3.Bacon CW, Yates IE, Hinton DM, and Meredith F. Biological control of Fusarium moniliforme in maize. Environ Health Perspect. 2001; 109:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascale M, Visconti A, and Chelkowski J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. European Journal Plant Pathology. 2002; 108:645–651. [Google Scholar]
- 5.Janse van Rensburg B, Mc Laren NW, Schoeman A, and Flett BC. The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop Protection. 2016; 79:56–63. [Google Scholar]
- 6.Zhang LP, Wang JS, Zhang CL, and Wang QM. Analysis of potential fumonisin-producing Fusarium species in corn products from three main maize-producing areas in eastern China. Journal of the Science of Food and Agriculture. 2013; 93:693–701. 10.1002/jsfa.5794 [DOI] [PubMed] [Google Scholar]
- 7.Desjardins AE. Fusarium Mycotoxins: Chemistry, Genetics and Biology. American Phythopathological Society Press; 2006; 79–108. [Google Scholar]
- 8.Silva JJ, Viaro HP, Ferranti LS, Oliveira ALM, Ferreira JM, Ruas CF, et al. Genetic structure of Fusarium verticillioides populations and occurrence of fumonisins in maize grown in Southern Brazil. Crop Protection. 2017; 99:160–167. [Google Scholar]
- 9.Marasas WFO. Discovery and occurrence of the fumonisins: a historical perspective. Health Perspectives. 2001; 109:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shim WB, Flaherty JE, and Woloshuk CP. Comparison of fumonisin B1 biosynthesis in maize germ and degermed kernels by Fusarium verticillioides. Journal of Food Protection. 2003; 66:2116–2122. [DOI] [PubMed] [Google Scholar]
- 11.Rheeder JP, Marasas WFO, and Vismer EF. Production of fumonisins analogs by Fusarium species. Applied and Environmental Microbiology. 2002; 68:2101–2105. 10.1128/AEM.68.5.2101-2105.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesterházy A, Lemmens M, and Reid LM. Breeding for resistance to ear rots caused by Fusarium spp. in maize a review. Plant Breeding. 2012; 131:1–19. [Google Scholar]
- 13.Lanubile A, Maschietto V, Borrelli VM, Stagnati L, Logrieco AF and Marocco A. Molecular Basis of Resistance to Fusarium Ear Rot in Maize Frontiers in Plant Science. 2017; 8–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li WJ, He P and Jin JY. Effect of potassium on ultrastructure of maize stalk pith and young root and their relation to stalk rot resistance. Agricultural Sciences in China. 2010; 9:1467–1474. [Google Scholar]
- 15.Munkvold GP, and Carlton WM. Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds. Plant Disease. 1997; 81:211–216. [DOI] [PubMed] [Google Scholar]
- 16.Blacutt AA, Gold SE, Voss KA, Gao M and Glenn AE. Fusarium verticillioides: Advancements in Understanding the Toxicity, Virulence, and Niche Adaptations of a Model Mycotoxigenic Pathogen of Maize. Phytopathology. 2018; 108:312–326. 10.1094/PHYTO-06-17-0203-RVW [DOI] [PubMed] [Google Scholar]
- 17.Chalfie M, Tu Y, Euskirchen G, Ward WW, and Prashar DC. Green fluorescent protein as a marker for gene expression. Science. 1994; 263:802–805. [DOI] [PubMed] [Google Scholar]
- 18.Ronald WS, and Thomas MH. Use of Fusarium graminearum transformed with gfp to follow infection patterns in barley and Arabidopsis. Physiological and Molecular Plant Pathology. 2004; 64:45–53. [Google Scholar]
- 19.Li H, Zhang J, and Liao Y. Expression and characterization of a green fluorescence protein gene in a transgenic Fusarium graminearum from wheat. Journal of Triticeae Crops. 2010; 30:824–828. [Google Scholar]
- 20.Qi JX, Liu TG, Xu Y, Chen HG, Gao L, Liu B, et al. Jellyfish green fluorescent protein (GFP) as a reporter for Fusarium gramminearum development on wheat. Journal of Integrative Agriculture. 2014; 13:2177–2183. [Google Scholar]
- 21.Murillo I, Cavalarin L, and Segundo BS. Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRms protein. Phytopathology. 1999; 89:737–747. 10.1094/PHYTO.1999.89.9.737 [DOI] [PubMed] [Google Scholar]
- 22.Yu CJ, Saravanakumar K, Xia H, Gao JX, Fu KH, Sun JN, et al. Occurrence and virulence of Fusarium spp. associated with stalk rot of maize in northeast China. Physiological and Molecular Plant Pathology. 2017; 98:1–8. [Google Scholar]
- 23.Yelton MM, Hamer JE, and Timberlake WE. Transformation of Aspergillus nidulans by using a trpC plasmid. Proceedings of the National Academy of Sciences. 1984; 81:1470–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munkvold GP, McGee DC, Carlton WM. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology. 1997; 87:209–217. 10.1094/PHYTO.1997.87.2.209 [DOI] [PubMed] [Google Scholar]
- 25.Yao Y. Revelation of Xianyu335 as major cultivar in China. Seed Development. 2014; 4:11 10.3969/j.issn.1000-8071.2014.04.005 [DOI] [Google Scholar]
- 26.Gai XT, Xuan YH, and Gao ZG. Diversity and pathogenicity of Fusarium graminearum species complex from corn stalk and ear rot strains in northeast China. Plant Pathology. 2017; 66:1267–1275. [Google Scholar]
- 27.Sun SQ, Yang XR, and Tian T. Study on inoculation method of corn stalk rot resistance identification. Tianjin Agricultural Sciences. 2012; 18:160–162. [Google Scholar]
- 28.Nai YS, Lee SJ, Kim S, Ju HJ, Je YH, and Kim JS. Expression of egfp gene based on Agrobacterium tumefaciens-mediated transformation in Beauveria bassiana sensu lato ERL836. Journal of Asia-Pacific Entomology. 2015; 18:677–681. [Google Scholar]
- 29.Wang YL, Xiao SX, Xiong DG, and Tian CM. Genetic transformation, infection process and qPCR quantification of Verticillium dahliae on smoke-tree Cotinus coggygria. Australasian Plant Pathology. 2013; 42:33–41. [Google Scholar]
- 30.Townsend GR, and Heuberger JW. Methods for estimating losses caused by diseases in fungicide experiments. Plant Disease Reporter. 1943; 27:340–343. [Google Scholar]
- 31.Duncan KE, and Howard RJ. Biology of maize kernel infection by Fusarium verticillioides. Plant Microbe Interact. 2010; 23:6–16. [DOI] [PubMed] [Google Scholar]
- 32.Mazzoin E, Scandolara A, Giorni P, Pietri P. Field control of Fusarium ear rot, Ostrinia nubilalis (Hubner), and fumonisins in maize kernels. Pest Management Science. 2011; 67(4):458–465. 10.1002/ps.2084 [DOI] [PubMed] [Google Scholar]
- 33.Ren JP, Wu X., and Sun X H. A study on infection cycle of pathogen of corn ear rot and stalk rot in Jilin Province. Maize Science. 1995; 10:25–28. [Google Scholar]
- 34.Chen J. Status and perspective on research of ear rot and stalk rot in maize. Journal of Shenyang Agricultural University. 2000; 31:393–401. [Google Scholar]
- 35.Zou QD, Chen J, Xu Y, and Wang L. Relationship of infection cycle between ear rot and stalk rot in maize caused by Fusarium. Journal of Shenyang Agricultural University. 2000; 31:487–489. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.