Abstract
Lameness is a leading cause of welfare and culling issues in cattle, with claw lesions accounting for the majority of these issues. Although the treatment of claw lesions in cattle is a daily activity for hoof trimmers, veterinarians, and livestock producers, there is surprisingly little information in the peer-reviewed literature on which to base strong evidence-based conclusions. As a consequence, many treatment modalities used are empirical and, in some cases, may be counterproductive to rapid lesion healing. Furthermore, many of these empirical treatment modalities fail to fully consider the underlying pathogenesis of the disease process and the implications that it has on lesion healing. For example, sole ulcers are largely a consequence of metabolic disorders and mechanical overloading. Therapeutic interventions that fail to address the weight-bearing issues are unlikely to be successful. Likewise, white line disease is believed to be predisposed by rumen acidosis and laminitis, and interventions need to include in them appropriate measures to prevent further cases through nutritional management. The goal of this review paper is to review the pathogenesis of claw lesions in the context of the published literature and allow the reader to arrive at rational treatment interventions based on the best available information. The use of an orthopedic block applied to the healthy claw of a lame foot, judicious use of bandage or wrap, careful selection of parenteral or topical therapy, and a treatment protocol to manage pain and promote recovery are key components of responsible management of lameness disorders in cattle.
Keywords: lameness, treatment, claw lesions, sole ulcer, white line disease
Pathogenesis of claw lesions
The most common claw lesions observed in cattle are ulcers (sole, toe, and heel), white line disease (WLD), traumatic injury of the sole caused by excessive wear, thinning of the sole with subsequent ulcer formation, and penetration of the sole by foreign bodies. In the following, we take a moment to review the pathogenesis of each of these conditions beginning with a discussion of laminitis (coriosis).
Laminitis (coriosis)
Laminitis is considered to be an important underlying cause of lameness disorders commonly encountered as claw horn lesions. However, it has been suggested that the term laminitis is insufficient as the lesion descriptor since this condition affects far more than just the suspensory tissues of the laminar corium. Coriosis has been proposed as a better term because it more accurately describes the condition as an inflammatory insult affecting all regions of the corium.1 For example, coriosis involving the coronary corium accelerates the growth of wall horn; however, since altered blood flow reduces keratinization of horn cells, hoof walls are weaker causing the claw horn capsule to become abnormally deformed. Dorsal walls become concave and axial, and abaxial walls flatten as they reach the weight bearing surface. Coincident with these are effects on the laminar corium which predispose to rotation and sinking of the third phalanx (P3) and thus, widening of the white line (WL). Coriosis may also occur as a subclinical condition in which claw horn produced by all regions of the corium is soft and yellowish or reddish in color as a consequence of poor keratinization and staining by transudates that leak into the extravascular tissues during horn formation.1
The term laminitis more accurately describes the pathology of the laminar corium that leads to a weakness and a failure of its connection to the lamellae of the wall. An important distinction in the discussion of laminitis regards its pathogenesis as an inflammatory or a degenerative condition. In equines, inflammation associated with laminitis results in a complete separation at the dermal–epidermal junction permitting downward displacement and rotation of the apex of P3. On occasion, the apex of P3 rotates sufficiently to perforate the sole. In cattle, laminitis is thought to be primarily a degenerative process affecting the dermal–epidermal junction and basal cell layer of the epidermis. In contrast to the equine, the significance of inflammation in the pathogenesis of laminitis in cattle is unclear. Inflammation may be largely a secondary event occurring subsequent to an increase in interstitial tissue pressure resulting from vascular events associated with vasodilatation, congestion, transudation, and diapedesis within the corium.2
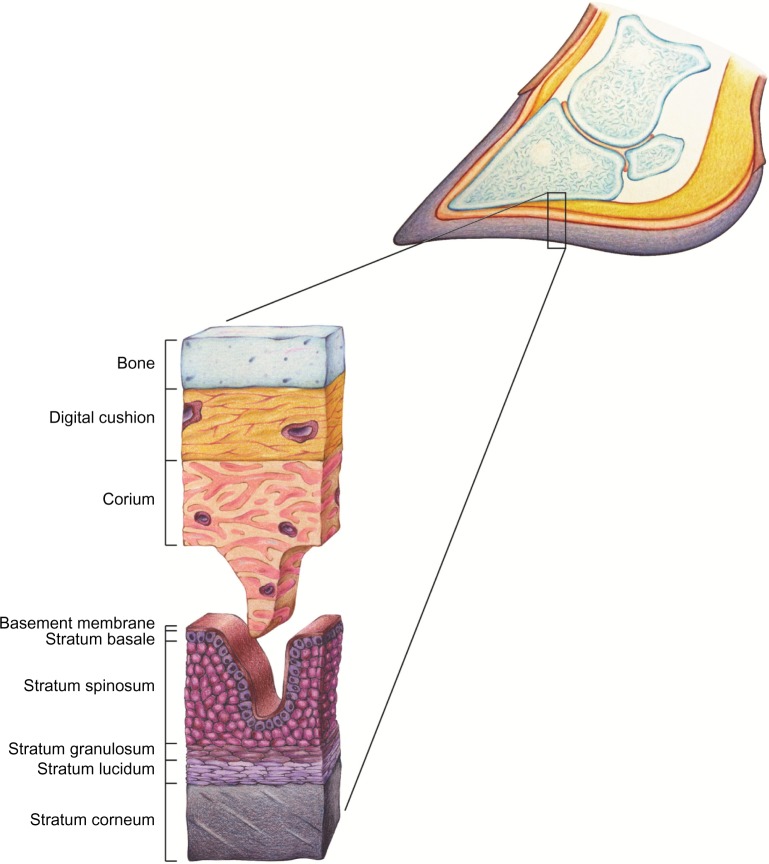
Effects on the basal cell layer or stratum basale are equally important (Figure 1). Cells within this layer are in a continuous state of proliferation and differentiation into keratinocytes destined to become claw horn or basal cells that remain within the stratum basale. As a consequence, vascular compromise interfering with the metabolic exchange occurring between the corium and developing keratinocytes and basal cells within this layer not only reduces horn quality but also integrity of the dermal–epidermal junction.2
Figure 1.
Histology of the corium and epithelium of the bovine claw.
Note: The diagram illustrates the digital cushion, corium layers of the epithelium that comprise the claw horn capsule including the basement membrane, stratum basale (basal cell layer), stratum spinosum, stratum granulosum, stratum lucidum and stratum corneum. Reproduced from Shearer JK, van Amstel SR. Manual of Foot Care in Cattle. 2nd ed. Ft Atkinson, WI: WD Hoards and Sons Company; 2013.20
A primary difference with respect to laminitis in cattle versus that which is observed in the horse involves changes that occur within the dermal–epidermal connection and the dermal segment. In contrast to the horse, in which case separation of this connection is normally observed, in cattle, the suspensory tissues of the corium are more likely to elongate or stretch in response to breakdown of the collagen fiber bundles within these structures.3–5 As a result extreme compression of the corium beneath the apex of P3 in the toe is less likely, instead the downward displacement of P3 and compression of the digital cushion and corium at the heel–sole junction (ie, zone 4 on the claw zone diagram in Figure 2) are more likely to be the outcome. It is presumably for these reasons that sole and heel ulcers are more likely to occur subsequent to laminitis in cattle than toe ulcers.
Figure 2.
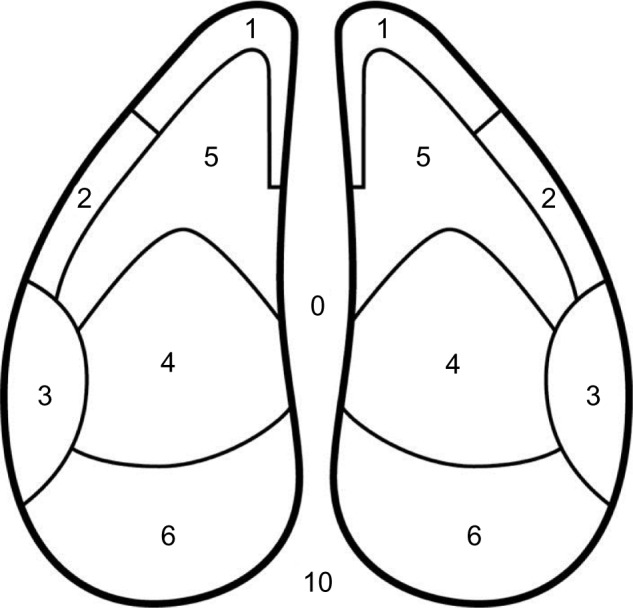
Claw zone diagram.
Notes: Diagram showing zones used for recording claw lesions. Reprinted from Journal of Dairy Science. Vol 92(7). Sanders AH, Shearer JK, De Vries A. Seasonal incidence of lameness and risk factors associated with thin soles, white line disease, ulcers, and sole punctures in dairy cattle. Pages 3165–3174., Copyright 2009, with permission from Elsevier.19
Laminitis: phases 1, 2, and 3
Ossent and Lischer describe laminitis as a disorder occurring in three distinct but overlapping phases.2 Phase 1 is initiated by the release of vasoactive substances that impair blood flow to the corium and may require only a matter of hours to days to cause significant damage to the tissues of the corium. Degeneration of the dermal–epidermal junction and basal cell layer leads to failure of the suspensory apparatus of P3 and entry into phase 2. The second phase of laminitis is characterized by the sinking or the downward displacement of P3 and compression-related injury of the corium and digital cushion beneath. Injury to the solar corium and digital cushion from compression causes hemorrhage, thrombosis, and variable amounts of necrosis similar to that observed in phase 1, but in this case occurring from contusion-related trauma caused by the sinking of P3. Although the animal may experience pain during phases 1 and 2, lesions of the claw horn capsule that characterize phase 3 (eg, hemorrhages, WLD, and sole ulcers) are not apparent for 8–9 weeks after the initial events of phase 1.2
Insight relative to the timing of phases 1–3 may be gleaned from the work of Leach et al who studied the development of claw hemorrhages in first-calving heifers. They observed that hemorrhages were few in number prior to calving, but increased following calving and movement into confinement housing. Lesions were most severe in the WL at 9 weeks and in the sole at 14 weeks. These data suggest that the initial insult (phase 1) occurs in the laminar corium which is also the site of WL horn formation and therefore hemorrhages in the WL precede those observed in the sole. In the study cited here, the WL hemorrhages preceded those observed in the sole by 5 weeks. The sinking of P3 (phase 2) and damage to the solar corium predisposes to hemorrhages in the sole that likely occur secondary to trauma to the solar corium from compression by the sinking of P3.6
Ulcers of the sole, toe, and heel
Skin ulcers are one of the most common types of lesions in humans, and the majority of these are associated with pressure necrosis whereby tissues are compressed between bony prominences and hard surfaces.7 Sole ulcers in cattle have a very similar pathogenesis whereby the corium becomes compressed between the flexor tuberosity of the P3 from above and the sole in contact with the floor beneath.8 Disturbances in the microvasculature of the contused area of involved tissue lead to ischemia, hypoxia, and the development of an ulcer that typically occurs in zone 4 of the claw zone diagram in Figure 2.
An ulcer may be defined as a full thickness defect in the epithelium that exposes the underlying corium. In cattle, sole ulcers (and to some extent WLD) are largely a consequence of metabolic disorders and mechanical loading that contributes to injury of the solar, perioplic (corium of the heel), and laminar corium.2 Most common metabolic conditions predisposing to claw lesions include rumen acidosis, coriosis/laminitis, activation of metalloproteinases, and hormonal changes, specifically relaxin (or relaxin-like hormone) and estrogen in the peripartum period.9–12 Enzyme degradation of the strong collagen fiber bundles within the laminar corium coupled with weakening of these structures by peripartum hormonal changes leads to sinking and rotation of the P3 and compressive damage of the solar and perioplic corium tissues that lie beneath. Displacement of P3 and subsequent damage to the underlying corium is compounded by mechanical loading secondary to claw horn overgrowth and unbalanced weight bearing.8
Ventral to P3 are tissues which make up the underlying support structure for P3. This tissue is composed of loose connective tissue from the solar and perioplic corium and caudally by the digital cushion (Figure 1). The digital cushion is composed of loose connective tissue and varying amounts of adipose tissue in a series of three pads that run in a longitudinal direction beneath P3. This structure has become the object of attention by several researchers in recent years as observation suggests that the size and type of fat within the digital cushion may have important implications for the occurrence of lameness.9,13–15 Researchers at Cornell University determined that the prevalence of sole ulcers and WLD was significantly associated with thickness of the digital cushion. They also found that the thickness of the digital cushion was positively associated with body condition score. In other words, thinner animals had smaller or thinner digital cushions and were at a higher risk of developing sole ulcers.15
White line disease
Details of the anatomy of the laminar corium and WL were presented previously by Mulling.10 These details are reviewed here because understanding the anatomy and production of the WL is important to understanding the development of WLD.
The WL (or zona alba) is a three-part structure (outer, intermediate, and inner zones) formed by epidermal cells in the distal wall or laminar region (Figure 3). Horn of the WL is produced by epidermis overlying the dermal laminae, by cap papillae on the crests of the laminae, and by terminal papillae at the distal end of the dermal laminae. The dermal laminae are positioned in a proximal–distal orientation (parallel with) to the dorsal surface of P3. At the distal end, the dermal laminae bend underneath the tip of the apex of the toe where they merge with thick terminal papillae and eventually transition into rows of dermal papillae that produce the horn of the sole. The outermost region of the WL is formed in the uppermost part of the dermal laminae that lies adjacent to the wall. It consists of nonpigmented and nontubular horn. The intermediate zone of the WL consists of cap horn produced by the cap papillae and the inner zone contains tubular horn which is formed by the epidermis of the terminal papillae. The differences in origin and thus the heterogeneity of horn within each of these zones represent a point of weakness within the WL and weight-bearing surface of the claw. The vulnerability of the WL is improved by the proper keratinization and cornification of horn cells within these zones.10
Figure 3.
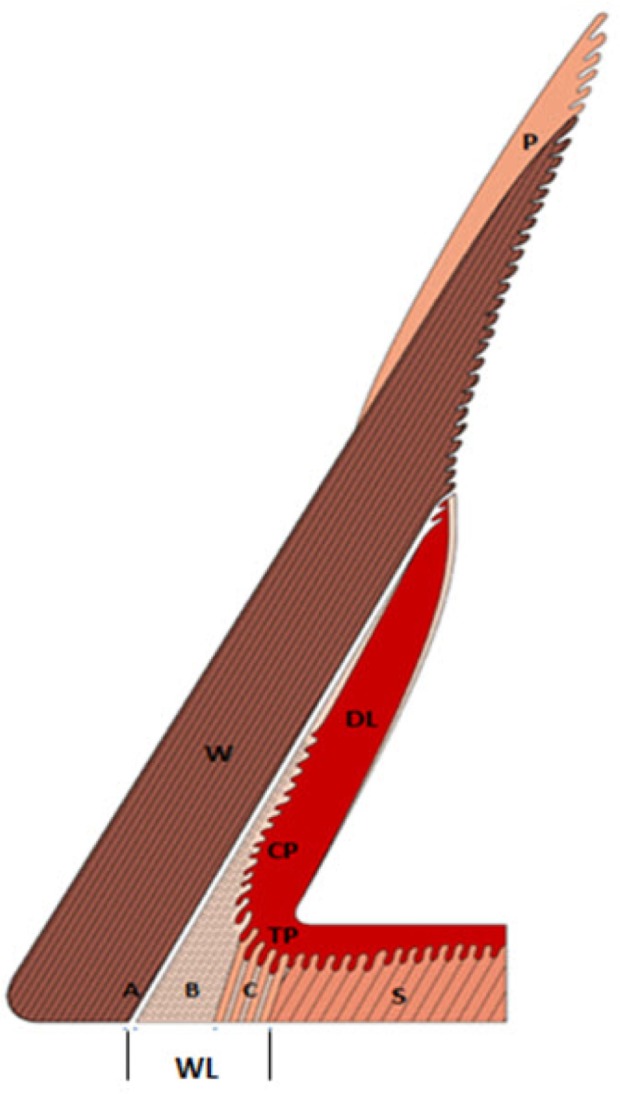
Sagittal view of the bovine hoof illustrating the white line (WL) in relation to the periople (P), wall (W), sole (S) and deeper structures of the laminar corium (dermal laminae [DL]).
Notes: The WL is a three-part structure formed by epidermal cells in the distal wall and laminar region. It consists of an outer (A), intermediate (B), and inner zone (C). The outermost region (A) of the WL is formed in the upper most part of the dermal laminae (DL) that lies adjacent to the wall (W). The outer zone (A) consists of non-pigmented and non-tubular horn. The intermediate zone (B) of the WL consists of cap horn produced by the cap papillae (CP) and the inner zone (C) contains tubular horn which is formed by the epidermis of the terminal papillae (TP) they bend under the tip of the toe to merge with the sole. Adapted from Mulling C. Theories on the pathogenesis of white line disease – an anatomical perspective. In: 12th International Symposium on Lameness in Ruminants; 2002; Orlando, Florida.10
The laminar region where the dermal laminae originate is also the laminar corium or suspensory apparatus of P3. Damage to these tissues during the period of horn formation is likely to be exhibited later as abnormalities in the WL (hemorrhages, widening, etc) when this horn reaches the weight-bearing surface. The WL is predisposed to penetration by foreign material from the environment and to separation for at least two reasons: 1) the heterogeneous makeup of horn in the WL and 2) the vascular architecture and microcirculation of blood in the distal laminar region which is easily disturbed.10,16 These attributes are complicated by mechanical stresses particularly those encountered on certain types of flooring surfaces that increase sheering forces on both the axial and abaxial walls.17
WLD occurs as a consequence of the penetration of WL horn by foreign material with or without prior weakening.10 However separation within the WL greatly increases the potential for the entrapment of foreign bodies, such as a stone or other extraneous material. Once they become lodged within the WL, they are gradually pushed deeper toward the corium. Bacteria associated with the foreign material colonize within WL horn, causing further decay as it migrates toward the live tissues of the corium. Upon reaching the corium, an abscess forms that causes pain and thus lameness.
Laminitis disrupts blood flow to the corium and is believed to be an important underlying cause of WLD. When blood flow to the corium is adversely altered, the result is the production of poorly keratinized (dyskeratotic) horn that is weaker and less resistant to invasion by foreign material. Weakened, defective, or poor quality horn is more prone to separation and colonization by bacteria and fungi. Other contributing causes are tensional or mechanical forces associated with prolonged standing or walking on hard, rough, and uneven flooring surfaces that increase laminar corium inflammation. Cattle may be particularly susceptible during the transition period when the suspensory tissues are more likely to be weakened by metabolic and hormonal factors described earlier. Tarlton et al suggested that swelling and distortion of the laminae and corium are likely to increase lateral pressure on the hoof wall, and therefore may predispose to WL separation.11 Inflammation and degeneration of the laminar corium, occurring at the time of WL horn formation results in hemorrhage that becomes visible within the WL when it reaches the weight-bearing surface. Although these hemorrhages are historical information, they are, nonetheless, useful indicators of previous insult to the laminar corium and a potential underlying cause of the formation of weaker horn in the WL.
The most common site for WLD is the abaxial heel area (zone 3 on the claw zone; Figure 2). This region is predisposed to WLD for a couple of reasons: 1) the lateral claw bears more weight and 2) this region bears the physical impact associated with heel strike as the cow places her foot to the floor during locomotion.
Traumatic lesions of the sole
Traumatic lesions of the sole are frequently associated with excessive wear on abrasive flooring surfaces that leads to thin soles and in worst-case scenarios, thin sole toe ulcers.18,19 The lesion frequently occurs as a separation of the sole away from the WL in zone 5 near the junctions of zones 1 and 2 on the claw zone diagram (Figure 2). Because of their location near the WL, these lesions are frequently misdiagnosed as WLD. Toe ulcers are an important secondary complication to thin soles and many have a tendency to become chronic nonhealing lesions. They are an increasingly common disorder in large herds where cows are required to walk long distances on abrasive concrete flooring surfaces. As will be described in later sections of this review, there is growing evidence that chronic toe lesions may be complicated by an atypical form of digital dermatitis.
Sole lesions also occur as a consequence of foreign bodies such as a nail, stone, or other sharp object encountered on floors and walkways. Depending upon the depth of penetration, these types of lesions can have serious consequences. For example, when the foreign body is able to penetrate sufficiently deep to make contact with the P3, a severe osteitis is likely to develop. In cases where the foreign object penetrates only the more superficial tissues of the corium and digital cushion, the prognosis is more favorable.17,20
We emphasize that a clear understanding of the pathogenesis of these lesions is important for the development of a reasonable treatment as well as control strategies. Claw lesions are metabolic and mechanically induced conditions. Although abscess formation with claw lesions is a common feature in the presentation of these disorders, it is a secondary complication and should not necessarily drive decisions to employ antibiotic therapy.
Histology of the claw capsule
The modified epidermis of the claw horn capsule is a multilayered structure of cornified epithelium that overlies the corium (Figure 1). The corium is a highly vascular nerve-rich tissue consisting of a dense matrix of connective tissue. The function of the claw horn capsule is to protect these sensitive and more fragile tissues of the underlying corium. Although it has no vascular supply, keratinocytes within the stratum basale and stratum spinosum receive nutrients by diffusion across the basement membrane from capillaries within the corium. The primary structural protein of the epidermis is keratin. In claw horn, the keratins in greatest abundance are “hard” keratins (ie, those with a greater amount of disulfide bonding). In contrast, skin contains more “soft” keratins which contain a greater amount of sulfhydryl bonds.
As described earlier, any condition that disturbs or reduces the flow of blood to the corium will adversely affect the quality of horn produced. In addition to providing nourishment for the growth and development of keratinocytes destined to become claw horn, it also serves as the connection between the basement membrane and the periosteal surface of the P3.
Wound healing as it pertains to claw lesions
Claw lesions affecting the dermal–epidermal tissues of the bovine claw heal by second intention with granulation tissue. Healing by first intention, that is, by suturing the wound margins is reserved for fresh, uncontaminated lesions. Lesions affecting the bovine claw do not fit these criteria. Depending upon severity, lesions of the claw capsule are left to simply re-epithelize, or when lesions are sufficiently deep to disrupt the basement membrane, they must heal by granulation and re-epithelialization. Wound healing is a dynamic and complicated process generally described in terms of three (some do not include hemostasis) or four overlapping phases including hemostasis, inflammation, proliferation, and maturation.7,21 An understanding of these events and what conditions may interfere with the process are useful in terms of guiding the treatment procedures.
Hemostasis
The first response to injury affecting the dermis or corium is hemorrhage. In cases of acute injury, hemorrhage plays a key role in wound repair as the source of blood platelets essential for clot formation. Within minutes platelets enter the site of injury and begin to clump, forming a clot that is later reinforced by fibrin proteins that ultimately create an insoluble blockage essential for long-term hemostasis. Coincident with the clotting process is the release of numerous cytokines and vasoactive mediators such as epinephrine, norepinephrine, and prostaglandins that cause vasoconstriction to reduce blood loss and also activate inflammatory cells in preparation for the second phase of the healing process.7,21
Inflammation
The inflammatory phase is characterized by the influx of white blood cells that phagocytize bacteria and cellular debris within the site of injury. In acute lesions, it is sometimes described as a process involving two overlapping stages: the vascular and cellular stages. The vascular stage begins with vasoconstriction (within 10–15 minutes postinjury) of the arterioles and venules near the site of injury. Vasoconstriction is followed by a period of vasodilatation and increased vascular permeability whereby fluids move from the blood stream into the injured tissue. The transudation of fluid into and around the site of injury contributes to pain, heat, redness, edema, and loss of function of the affected tissues. The cellular stage is marked by the movement of white blood cells from the blood stream to the site of injury. Neutrophils are the predominant white cells early in the inflammatory process. Their role is the engulfment of bacteria. Neutrophils are later replaced by macrophages that enter the site and continue to engulf residual bacteria and debride devitalized tissues.
Proliferation
The proliferative phase is characterized by angiogenesis, fibroplasia, and granulation tissue formation, epithelialization, and tissue contraction. Any interference in these events generally results in prolonged wound healing and the potential for the development of a chronic lesion.
Angiogenesis
Wound healing requires a rich blood supply in order to keep pace with the high metabolic demand associated with the synthesis of new tissues. It often begins with endothelial cells originating from uninjured blood vessels adjacent to the site of injury that send cytoplasm-filled projections of cell wall into the extracellular matrix. Hypoxia, lactate, and low pH all serve as stimulants for the process of angiogenesis.7,21
Fibroplasia and granulation tissue formation
The wound cell population is dominated by fibroblasts during the proliferation phase that normally begins within the first 2–3 days after injury has occurred. These cells have multiple functions some of which are the production of ground substance and collagen (primarily collagen type III) within the wound site. With sufficient neovascularization, granulation tissue develops rapidly and fills in the defects caused by injury. Although less resistant to external factors than intact skin, it provides an early, though imperfect, barrier to injurious agents from the environment.7,21
Epithelialization
Epithelialization normally occurs from the level of the basal cell layer atop the basement membrane. Basal cells within the germinal (or basal cell) layer are in a constant state of proliferation and differentiation into keratinocytes or basal cells. Those that differentiate into basal cells remain within the stratum basale, whereas cells that differentiate into keratinocytes will gradually migrate outward from the germinal layer to the stratum spinosum, stratum granulosum, stratum lucidum, and ultimately the stratum corneum (Figure 1).7,21
In the case of claw lesions, keratinocytes positioned superficial to the basement membrane facilitate early repair by migrating to the surface of the intact granulation tissue bed. In a few days, keratinocytes at the wound edges are freed from their attachments to other cells and their basement membranes and begin a slow migration toward the center of the wound. The speed of re-epithelialization depends upon the severity and type of injury suffered. For example, re-epithelialization is rapid when the injury is superficial (ie, such as an abrasion), and the basement membrane and basal cell layer are intact or minimally damaged. On the other hand, when a full thickness defect of the epithelium occurs, the recovery process will likely be prolonged. In this circumstance, residual keratinocytes at the wound site are not immediately available for recruitment to start the healing process. Instead, re-epithelialization must occur from the wound edges requiring centripetal movement of keratinocytes from the wound margins.22
Wound contraction is an important stage in wound healing. It usually begins within 1–2 weeks after the initial injury has occurred and may continue for several weeks or months thereafter. Histologically, it is associated with the differentiation of fibroblasts into myofibroblasts. By forming connections or adhesions to the extracellular matrix at wound edges, myofibroblasts essentially pull wound edges toward the center of the lesion thereby gradually reducing the size of the wound. This coupled with the centripetal movement of healthy adjacent dermal and epithelial tissues reduces the total surface area of exposed corium.7,21
Maturation (and remodeling)
The final phase of wound healing is that which leads to the formation of a scar. This phase usually begins after a few weeks and may continue for several months. Histologically, collagen type III fibers that were part of early wound granulation tissue formation are gradually replaced with type I fibers, the most abundant type of collagen in scar tissue. Collagen fibers that were once rather irregularly arranged become realigned and cross-linked in a series of organized fiber bundles. These changes result in an increase of the tensile strength of scar tissue, an important objective in the maturation process.7,21
Methods applied to treat and manage claw disorders in cattle
In the following, we review the methods applied to treat and manage claw lesions in cattle. Some are beneficial and some are potentially detrimental. Trimming and debridement of claw lesions is the most common approach to therapy, but ideas differ as to how extensive or aggressive this therapy should be. Foot blocks are an important adjunct to therapy because they prevent continued trauma of injured tissues by the removal of weight bearing on affected claws. Bandages would seem to be an obvious part of therapy, but some information suggests that they may actually delay healing in claw lesions. Parenteral therapy is occasionally used, but its value as a routine treatment for claw lesions has not been determined. Topical therapy is commonly applied, but compounds or medications that increase inflammation may be counterproductive. Lameness is painful, therefore, managing the discomfort associated with these conditions is important. Finally, posttherapy monitoring and management are often overlooked, but clinical observation and research indicate these are important considerations in the recovery of animals from lameness. We begin with a discussion of functional and corrective claw trimming.
Functional and corrective trimming (the Dutch method)
Functional trimming
The relative size and shape of the claw capsule is determined by the rate of horn growth versus wear. When the rate of horn growth exceeds the rate of wear, claws become overgrown, and weight bearing within and between the claws is adversely altered. This disparity in weight load is normally greatest for the lateral claw of rear feet and the medial claw of front feet whereby pressure is concentrated at the heel–sole junction (ie, the typical site for sole ulcers).
The objectives of functional trimming, the method developed by Toussaint Raven, are to correct overgrowth and altered weight bearing by 1) reducing the dorsal wall length of the toe to approximately 75–80 mm (depending upon size of the animal), 2) creating a uniform sole thickness (approximately 6–7 mm) throughout the weight-bearing surface of each claw, and 3) balancing load between the weight-bearing surfaces of each claw. Conducted as needed this procedure improves function and reduces the potential for development of claw lesions.8,23
Corrective trimming
Functional trimming as described above is a three-step process used to restore function to the foot by returning overgrown claws to their normal size and proportions. Corrective or therapeutic trimming procedures are applied as necessary to correct and treat claw lesions. These procedures include 1) the adjustment of weight bearing to reduce weight load on injured claws and 2) the correction of early claw lesions by removing loose and undermined horn without causing damage to underlying tissues of the corium. In all cases, efforts to reduce damage to epithelial and corium tissues are most likely to encourage prompt healing.8,24
For most lesions (including ulcers, WLD, traumatic lesions of the sole and foreign bodies trapped within the claw capsule) corrective trimming is sufficient to affect a cure. A UK survey of veterinary surgeons (N=270) and cattle foot trimmers (N=135) found that 47% of veterinarians and 89% of trimmers used the functional and corrective trimming techniques described by Toussaint Raven.25 It is unclear how many veterinarians and trimmers specifically use these techniques in the US; however, a recent survey of these groups indicated that most use a corium-sparing approach described as removing all loose and damaged horn adjacent to ulcers to avoid damage to the corium.26 So while techniques may vary, the objectives are indeed the same or at least very similar. And finally, a telephone survey of 84 UK dairy farmers designed to investigate on-farm treatment practices for sole ulcers, sole bruising, and WLD found that trimming of the affected claw was a consistent treatment procedure.27
Corrective trimming is an essential step in the treatment of claw lesions. By removing all necrotic and loose or undermined horn, an aerobic microenvironment is created that greatly reduces the possibility of further complication associated with abscess formation. When these procedures are conducted carefully to avoid damage to healthy peripheral tissues of the corium, postprocedural pain is minimized, and the rate of recovery is more rapid.8,20,24,28
In the US, claw trimmers and on-farm employees do both preventive maintenance trimming and corrective trimming associated with lameness. Veterinarians do primarily corrective trimming work in the context of treating lameness. Most claw trimmers prefer to schedule their work on a weekly, biweekly, or monthly basis. Cows that develop lameness between visits are scheduled for examination and treatment at the trimmer’s next visit. As a consequence, delays in treatment of lame cows are inevitable. This has increased the popularity of training on-farm employees in foot care and trimming so that care of lame animals can be provided in a timelier manner. In a survey of farmers in the UK, 75% reported treating lame cows within 48 hours and 13% reported treating lame animals within a week of detection.27 Farmers reported several barriers to timely treatment including lack of time 23%, a need for better equipment 29%, and the need for a better restraint system 30%. Leach et al reported that treatment of cows within 48 hours reduced the prevalence of lameness 4 weeks after treatment as well as the number of serious lameness cases.29
In addition to the lack of equipment and facilities, 69% of UK farmers indicated that they had received little or no formal training in cattle foot care.27 In the US, this lack of formal training was the stimulus for development of the Master Hoof Care Program that began at the University of Florida in 1996, to provide training for on-farm employees in foot care and claw trimming.20,30 When farms prioritize care of lame cows and people have the knowledge, equipment, and facilities to properly care for lameness, it is far more likely to get done correctly and in a timely manner. A recent incentive for timely foot care is the need to comply with requirements of welfare audits. Present-day welfare assessments and audits require prompt treatment of lame cows.31 Since lameness is a near daily occurrence and a prominent welfare issue, herds must have the capability to treat lameness promptly (within 24–48 hours).
Orthopedic foot blocks
The primary objective in treatment of sole ulcers is to relieve pressure (ie, remove weight bearing) from damaged tissues. The use of an orthopedic block applied to the healthy claw to relieve weight bearing on a claw affected by a sole ulcer is one way to achieve this objective. Relief from weight bearing is also advantageous as a means to relieve pain and promote recovery. There is support in the literature in the form of controlled studies to support this conclusion.
Pyman32 evaluated three methods of treatment for sole lesions, two of which involved relieving pressure on injured claws with an orthopedic foot block and a third through the use of a padded bandage. Specifically, the treatments consisted of the following: 1) a wooden block glued on to the unaffected claw (39 cows); 2) a rubberized block applied to the unaffected claw (42 cows); and 3) a padded bandage applied to the whole foot (31 cows). Lameness recovery rates based upon evaluation of lameness (scores 1–3, with three being severe lameness) for cows treated with wooden blocks, rubberized blocks, and the padded bandage were 48.7%, 45.2%, and 19%, respectively, at day 3 posttreatment. By day 7 recovery rates were 65.8%, 76.2%, and 32%, respectively. However, by 2 weeks posttreatment, there was no significant difference in recovery rate between the three treatments.32 In this study, cows treated with either the wooden or rubberized block had a more rapid return to a normal gait than cows fitted with the padded bandage. These observations highlight the importance of providing relief from weight bearing during the early recovery period.32
Similar results were obtained in a study of 24 animals treated for sole ulcers, 16 of which were treated with either a wood block fitted with Technovit® (Technovit-2-Bond, Heraeus-Kulzer GmbH, Wehrheim, Germany) adhesive or a CowSlip® (Giltspur Scientific Ltd, Ballyclare, Northern Ireland, UK) plastic block. A third group was treated with Solka Hoofgel® (Kanters Special Products BV, Lieshout, the Netherlands), a bandage and no block on the healthy claw. Evaluation at day 28 following the application of treatments found that seven of eight (87.5%) cows treated with the wood block applied with Technovit® had completely healed. Six of eight (75%) cows in the CowSlip® treated group had also completely healed compared with four of eight (50%) bandage-only group.33
Few would dispute the fact that claw blocks are an important part of the therapy for claw lesions, particularly sole ulcers. Nonetheless, the use of orthopedic blocks is inconsistent among veterinarians and trimmers. Foot blocks were used by 79% of veterinary surgeons compared with 97% of trimmers responding in a UK survey.25 In a US survey 76% of veterinarians reported using blocks as a routine in foot care compared with 86% of trimmers.26 These differences were highly significant in both surveys and are most likely related to the fact that veterinarians are less well-equipped to restrain and treat foot problems compared with trimmers. For example, in the US survey 61% of veterinarians reported using manual restraint (ropes) for foot care. By comparison, 100% of trimmers reported using some type of restraint device: manual chute 6%, hydraulic standing-type chute 45%, and a tilt table 49%.26
Bandages and wraps
The use of bandages or wraps on claw lesions is a fairly standard practice for many trimmers and veterinarians. In fact, the US survey results suggest that more than half of the US veterinarians and hoof trimmers use a bandage when treating claw lesions.26 Anecdotally, recommendations on the use of bandages vary from those who believe it is important to wrap all claw lesions to those who advise not wrapping any. Theoretically, the application of a bandage would protect the lesion and prevent contamination of the wound. In a controlled environment this is probably true, but in most confinement-type dairy environments any benefit from preventing contamination through the use of a bandage is lost within a matter of minutes to hours. Manure slurry, moisture, and the use of a foot bath at least once a day quickly results in a bandage that becomes heavily contaminated with organic matter and footbath solutions. A bandage soaked in contaminated formalin and copper sulfate solutions is likely of little benefit and may cause additional irritation to healing lesions. Additionally, serious complications can occur when bandages are applied too tightly or gradually tighten on their own overtime, particularly as they become wet. In these situations bandages can become a significant liability and can result in the development of new lesions associated with the bandage. In such cases, the adage of “first do no harm” is compromised.
Researchers at Cornell University conducted a controlled study to evaluate the effects of bandaging uncomplicated sole ulcers (referred to by the authors as “sole abscesses”) on treatment outcome.34 Cows were scored for lameness and assigned to one of three groups: 1) not lame; 2) lame but bearing weight on the affected foot; and 3) lame and not bearing weight on the affected foot. After assignment to one of the three lameness groups, each foot was randomly allocated to either a bandage (B) or no bandage (NB) group. There was no mention of the use of foot blocks for either treatment group. Feet of cows in the NB group were given no additional treatment. The feet of cows in the B group received a topical treatment consisting of sulfanilamide powder on a piece of cotton that was placed over areas of exposed corium followed by wrapping the foot with roll gauze and a layer of pine tar over the gauze. Feet were examined at 5–7 days (at which time the bandage was removed) and then re-examined again at 30 days posttreatment. There were no significant differences between the B or NB groups at the 1 month examination; however, a higher proportion of the NB treated feet were classified as not lame at the 5–7 day evaluation. Researchers concluded that the extra expense of placing a bandage on the foot after corrective trimming of the lesion was unnecessary. These researchers also noted that bandaging small claw lesions in stanchion barn-housed cows delayed healing.34
Clinical experience and research suggests that the development of exuberant granulation tissue in bovine claw lesions can delay wound healing.35 Very little information is available in the peer-reviewed literature regarding the role of bandages in the development of exuberant granulation tissue in the bovine foot. Delayed healing of lower limb lesions and excessive granulation tissue formation following the use of bandages has been reported in equines.36,37 Although controversial, some have suggested that these observations may be a consequence of reduced oxygen tension and low pH at the lesion surface beneath the bandage.38 In contrast, others have shown that oxygen supplementation under metatarsal bandages failed to result in improved wound healing.39 The development of exuberant granulation tissue is a complex process and is very poorly understood in bovines. Given the significant differences between bovine claw lesions and equine lesions (location of common lesions, environmental cleanliness, and the ability to observe and manage bandages) care should be taken in extrapolating recommendations across species. Nonetheless, this is an area in need of additional research.
On occasion, there are situations where a topical dressing under a loose wrap or a bandage is desired in cattle. Examples might be situations where corrective trimming has led to excessive hemorrhage or in cases where large areas of the corium have been exposed. In these cases, a protective bandage may be advised as long as it can be removed or changed after 24 hours or when it becomes excessively soiled.
Research on therapy of claw lesions
In addition to the studies described earlier (Pyman,32 Sala et al,33 and White et al34), there are five others worthy of mention. In the following, we briefly summarize each of these in chronological order.
Lischer et al40 conducted a controlled field study designed to evaluate the effect of biotin on healing of uncomplicated sole ulcers in dairy cattle. Twenty-four cows with uncomplicated mild sole ulcer on the lateral claw of a rear foot were placed into either a treatment or a control group. Cattle assigned to the treatment group received 40 mg of biotin per day for a period of 50 days, whereas animals in the control group received a placebo. All cattle received an orthopedic shoe fitted onto the healthy medial claw of the lame foot. A sample of horn was collected on day 0 for baseline histological information. Exposed corium was disinfected with chlorhexidine and protected with a bandage which was changed at days 5, 10, 15, and 20. The bandage was removed (lesion permitting) on day 30. At the conclusion of the study, lesions in both the groups were observed to have a solid layer of horn and none of the cows were lame. However, histological evaluation of horn samples at day 50 demonstrated a significant improvement in horn quality for the biotin-supplemented group. Researchers concluded that although there was no evidence that healing occurred more rapidly, supplementation of biotin may improve horn quality and offer benefits to reducing the possibility of lesion reoccurrence.40
Van Amstel et al35 evaluated the healing response of 17 dairy cows with sole ulcers. Of these, nine cows had mature uncomplicated ulcers, defined as full-thickness sole horn defects with visible or protruding corium; six of the nine with corium characterized as slightly damaged and three of the nine had ulcers in which the corium was considered to be more seriously damaged. The remaining eight animals had complicated ulcers all of which had a draining tract and swelling of the heel and tissues proximal to the coronet extending above the fetlock. Treatment of the uncomplicated ulcers consisted of corrective trimming and the application of a foot block to the healthy claw. At 1 week posttreatment, there was a progressive reduction in size of the lesion and no response to digital pressure applied to elicit pain in eight of the nine animals treated for uncomplicated ulcers. At 30 days posttreatment, 66% of ulcers were covered by a layer of new horn, and 44% were still flexible to digital pressure. Ulcers with excessive granulation tissue were observed to heal more slowly requiring 41–60 days compared with those free of excessive granulation tissue which required 12–28 days.35
Durmus41 conducted a study on treatment observations in 42 cattle with a total of 68 claws affected with sole ulcers. Eleven animals had lesions classified as slight (corium exposed but not determined to need topical treatment), 28 animals with lesions characterized as moderate (undermined sole and protruding granulation tissue) and three animals with lesions described as severe (corium severely damaged with several areas of necrosis). Treatment consisted of preemptive parenteral antibiotic therapy for 7 days followed by corrective trimming and the application of a “wet antiseptic dressing”. Exposed granulation tissue was excised with a sharp hoof knife. Researchers did not report details of their observations relative to treatment outcome; they simply reported that most of the sole ulcers were recovered within 4–5 months.41 It is not possible to draw significant conclusions from this study since the paper did not contain sufficient information to make comparisons of healing response rates for claw lesions of differing severities. Nonetheless, it does describe a rather unique approach to treatment of claw lesions.
Azarabad et al42 studied 16 milking cows aged 2–5 years with uncomplicated sole ulcers. Cows were treated by application of a wooden block to the healthy claw and topical treatment of the ulcer with Solka Hoofgel® (Kanters Special Products BV). The primary objective of the study was to assess the progress of wound healing of sole ulcers during the lesion recovery phase; therefore, biopsies were obtained from lesions for histopathological evaluation on days 1, 10, and 21. Histopathology of day 1 lesions confirmed changes consistent with the inflammatory stage of wound healing. Day 10 histopathology revealed granulation tissue and epidermal regeneration from wound margins and a thin layer of epidermal cells covering the wound surface. By day 21 the lesion surfaces were completely covered by a moderate layer of cornified epithelium. These researchers concluded that the healing of sole ulcers as observed in this study followed a pattern similar to that described for wound healing of other cutaneous lesions. Furthermore, they suggested that these data indicated that corrective trimming, use of an orthopedic block, and Solka Hoofgel® were an effective treatment regimen for sole ulcers.42
Nguhiu-Mwangi et al43 analyzed 625 hospital case records of dairy cows treated for foot conditions from 1981 to 2006 at the Large Animal Hospital, Faculty of Veterinary Medicine at the University of Nairobi. The treatment protocol consisted of cleansing the foot with soap and water followed by hoof trimming. Sole abscesses were treated by corrective trimming of the necrotic horn surrounding the lesion and drainage of enclosed purulent material in the case of abscessed lesions. Severely eroded and necrotic horn of the sole was debrided and lesions treated topically with either a 10% copper sulfate or 5% formalin solution. Feet that became infected were treated parenterally with trimethoprim-potentiated sulfonamides at a dosage of 150–200 mg/kg body weight one time a day for 3 days. An alternative treatment used was 10% oxytetracycline at a dosage of 7 mg/kg body weight once a day for 3–5 days depending on severity. Both drugs were administered either intravenously or intramuscularly. Authors reported that only a small percentage of animals failed to recover and those that did not had regressed to become complicated as deep digital sepsis (DDS) conditions.43
These studies attest to the fact that approaches to therapy of claw lesions varies considerably depending upon the clinical presentation (ie, swollen or abscessed) of the condition and personal preferences or beliefs regarding the treatment of contaminated or infected lesions. As discussed in the following, parenteral therapy is sometimes preferred because it is more convenient or easier to do. Topical therapy of claw lesions is used presumably because “it seems like the right thing to do” for an open contaminated lesion.
Parenteral therapy
Parenteral antimicrobial therapy for the treatment of claw lesions is not a common practice on dairy farms in the US unless swelling indicates soft tissue involvement above the hoof. In these cases, parenteral therapy is indicated and may be effective if instituted early. On the contrary, in feedlots most cattle pulled for foot lameness are given a diagnosis of foot rot and frequently treated with a long-acting antibiotic or combination of antibiotics.44,45 In fact, many of these animals do not have foot rot; rather a claw lesion or digital sepsis condition for which parenteral therapy is unlikely to be effective. Feedlot cattle rarely have the benefit of having claw lesions treated by corrective trimming and an orthopedic block. Many in the feedlot cattle industry are either not convinced these procedures are economically justifiable or they are willing to accept salvage value for lame animals that do not recover. Research is needed to determine if it is cost-effective to renovate or invest in facilities, equipment, time, and employee training in order to apply more effective corrective trimming procedures and foot block use.
As with any lesion (claw lesions included) that has progressed to the point of abscess formation, antibiotic therapy alone rarely proves to be beneficial. Cattle have an exceptional ability to wall-off infections. On the other hand, curetting or corrective trimming that permits drainage of purulent material, debridement of necrotic horn associated with a claw lesion, and application of a block to the sound claw appear to be of greatest importance in effecting a cure. Systemic therapy adds significant cost to the treatment process with little or no apparent benefit. There are no drugs labeled for the treatment of uncomplicated claw lesions in the US. At a time of increasing concern with respect to antibiotic resistance and no scientific information to support parenteral therapy as effective, there seems little justification for systemic therapy in a claw lesion treatment protocol.
Topical therapy
A US survey indicated that topical treatments for claw horn lesions were used by 59% of veterinarians and 53% of hoof trimmers. The medication used most frequently was the soluble powder form of tetracycline or oxytetracycline (48% by veterinarians and 81% by hoof trimmers) followed by copper sulfate for veterinarians and ichthammol ointment (a sulfurous, tarry compound with mild antiseptic properties used primarily as a drawing agent) for trimmers.26 The Cornell University study by White et al34 reported using Sulfanilamide under a wrap and Pyman32 reported using 20 g of copper sulfate under a padded bandage in research trials designed to evaluate the effectiveness of bandages and orthopedic blocks, respectively. Azarabad et al reported using a compound marketed as Solka Hoofgel, a product described as a combination of minerals (copper and zinc) in a chelated form, organic acids, and adhesive components.42 Van Amstel et al used tetracycline and a tetracycline–dexamethasone combination for treatment of claw lesions and atypical digital dermatitis affecting exposed corium.35,46 Finally, Nguhiu-Mwangi et al used either 10% copper sulfate or 5% formalin as topical treatment for claw lesions observed in their study.43
Irrespective of the antimicrobial compound used in topical therapy of claw lesions, one must question if such a treatment approach makes sense. As explained earlier (under Pathogenesis of Claw Lesions), claw lesions are a consequence of metabolic and mechanical or physical factors. Thus, abscess formation associated with these lesions is not a primary condition; rather a secondary condition that occurs in response to the development of a microenvironment at the dermal–epidermal interface that supports the colonization and propagation of anaerobic bacteria. Corrective trimming provides an exit for the drainage of purulent material surrounding claw lesions and also changes the microenvironment to one that is less likely to support anaerobic bacterial growth and thus further abscess formation. It seems unlikely that topical treatment provides any great benefit and may even complicate recovery. As discussed later in this review, results of recent work at Iowa State University (JK Shearer et al, unpublished data, 2014) suggest that treatments that increase inflammation may also increase granulation tissue formation and delay healing.
Nonirritating topical treatment options
There are several options that one might consider for topical treatment of claw lesions. Triple antibiotic ointments and silver sulfadiazine are sometimes used because of their broad spectrum of activity. These may also be advantageous since they may actually stimulate epithelialization.21 Petrolatum-based ointments, such as Aquaphor Ointment (Beiersdorf Inc., Wilton, CT, USA) and white petroleum jelly are used in human medicine for treatment of minor cutaneous lesions or to keep surgical wounds moist during the postoperative period. A similar product used in humans and animals is Vitamin A and D ointment, which is 93.5% white petrolatum in combination with Vitamins A and D and a small amount of corn oil and light mineral oil. These ointments limit surface bacterial growth and prevent dressings from sticking to wounds.47
A product more commonly used on farms for minor skin wounds and irritation is Bag Balm (Dairy Association Co, Lyndonville, VT, USA). It consists of 8-hydroxyquinoline sulfate 0.3% in a petroleum jelly and lanolin base. The primary application for Bag Balm in the US is for the topical treatment of chapped and irritated skin on the udder and teats of cows. Bag Balm is frequently confused with Udder Balm (MBMA Corporation, Des Moines, IA, USA), a product containing petrolatum, propylene glycol, Aloe vera, lanolin, and vitamins A, D, and E and multiple other ingredients. Udder Balm has no specific antibacterial ingredients, but rarely causes irritation to sensitive tissues and helps to prevent the loss of moisture from the skin which is helpful in wound healing.
Anecdotal reports suggest that granular sugar and honey are also used as topical wound dressings on claw lesions. The theory of sugar’s effectiveness in wound healing is based upon its high osmolarity which draws moisture from the wound, thus inhibiting the growth of bacteria. Sugar has also been used in infected lesions for the debridement of necrotic tissue. Honey’s antibacterial benefits are likely related to its low pH, high osmolarity, and peroxide activity.48 The value of sugar or honey as treatments for claw lesions needs further investigation.
Healing rates for claw lesions
Wound healing is a spontaneous and continuous process, but as described earlier, lameness detection and treatment is often delayed. As a consequence, claw lesions in cattle are generally encountered in various stages of wound repair. Corrective trimming that avoids damage to peripheral tissues allows lesions to heal rapidly. Careless trimming techniques can also reverse wound healing progress that delays the resolution of claw lesions. There are several factors that may adversely influence the rate of wound repair. Therefore the range in days from treatment to complete recovery may be 1–2 months or more.
Lischer et al evaluated healing rates on 74 cows with 105 claw lesions over a 6-month period.49 Their data indicated that the mean time for the formation of a closed layer of new epithelium was 25 days for lesions causing slight corium alterations, 33 days for moderate corium alterations, and 42 days for lesions causing severe alterations of the corium. Investigators noted that 68% of all lesions were completely covered by a solid layer of new horn at 30 days following the initial treatment.49 Observations by Van Amstel et al were very similar whereby 66% of lesions were covered by a layer of new horn at 30 days.35 In the study by Azarabad et al, of 16 cows, lesions were observed to have a layer of new horn covering the exposed corium by 21 days following treatment.42 The latter studies were conducted on smaller numbers of animals but it is safe to say that mild-to-moderate lesions are likely to require somewhere around 21–30 days and more severe lesions a minimum of 40 days and potentially as long as 60 days.
Infection as a cause of nonhealing claw lesions
There is little chance of avoiding posttreatment contamination of lesions in the environment of most dairy cows, particularly since the foot is continually exposed to manure and moisture. Presumably, this is why many people elect to utilize a topical medication and bandage for treating claw lesions. However, as discussed earlier, the benefits of this practice are of questionable value in preventing contamination and under certain conditions may be contraindicated for wound healing.
In recent years, several authors have described an increase in “nonhealing claw lesions” particularly in herds suffering a high prevalence of digital dermatitis.17,46,50–53 The lesions are granular in appearance, extremely painful, and often observed on the corium under loose and undermined horn in association with WLD and sole ulcers. In some cases the lesions occur as a secondary complication on claw lesions where corium was previously exposed in the process of corrective trimming. Histopathology of these lesions indicates they are characterized by hyperkeratotic epithelium overlying dense zones of necrosis and pyogranulomatous inflammation in association with exuberant granulation tissue.46 Researchers also found spirochetes on surface as well as deep within the lesions.46
Effective treatments reported include tetracycline as a topical spray under a bandage and a paste of dexamethasone and oxytetracycline in combination applied under a loose wrap.46,54 In the study by Van Amstel et al, dexamethasone was added to oxytetracycline powder to form a paste and applied topically under a bandage and changed every 5–7 days. No spirochetes were observed in follow-up biopsies after 19 days in two cows. Clinically, lesions showed progressive improvement and after 8 weeks appeared to have undergone complete clinical resolution.46
Chronic toe lesions
One of the most common of nonhealing lesions observed in dairy cattle is chronic toe lesions which are almost invariably infected with spirochetes associated with digital dermatitis.46,50,51,53,55–59 Affected animals walk in such a way as to put most of the limb’s weight toward the heel. This allows the toe of the claw to overgrow becoming long and also very thick as a consequence of less wear of the sole at the toe.
Alternatives to a topical treatment approach are amputation of either the affected claw55,59,60 or the apex of the affected digit.61 A study of 122 cattle over a 4-year period found that claw lesions occurring in the apex of P3 had the potential to rapidly spread to the distal phalanx.61 Early stage treatment was conservative with corrective trimming and a block applied to the healthy claw, but once osteomyelitis was detected, the apex of P3 was resected. Researchers concluded that although complete recovery required more than 2 months, results were better than those obtained with complete amputation of the claw.61
Research on topical treatment of claw lesions
The early care and treatment of wounds has a significant impact on time required for healing. The objective in wound healing is a rapid unimpeded re-epithelialization of the lesion and anything that prolongs the inflammatory or proliferative phases of wound healing is contraindicated. The clinical indication of an interference with wound healing is the formation of exuberant granulation tissue.21 This is corroborated by the observations of Van Amstel et al, who found that ulcers with excessive granulation tissue healed more slowly compared with lesions free of granulation tissue.35
The equine literature on wound healing suggests multiple materials and other factors are capable of adversely affecting healing of cutaneous lesions including full strength antiseptics (such as chlorhexidine diacetate and strong iodines), irritating topical antimicrobials, copper sulfate, nitric acid, acetic/malic acid mixtures, silver nitrate, triple dye, supersaturated potassium permanganate, hypochlorite, lye, and many other home remedies.21,38 Any product or topical with low pH is likely to cause cellular toxicity. When used as topical medication in the acute stage of wound healing these compounds cause necrosis which interferes with cellular migration and epithelial cell proliferation. The result is granulation tissue formation and inhibited epithelialization and wound contraction.21
Similar observations were made in a recent study by our research team at Iowa State University (JK Shearer et al, unpublished data, 2014). Eighteen cows with claw lesions were randomly allocated into a treatment or a control group. Cows assigned to the treatment group received corrective trimming, a claw block applied to the healthy claw, topical treatment with either oxytetracycline soluble powder, or a powdered formulation of copper sulfate and a loose bandage. Cows assigned to the control group had claw lesions treated by corrective trimming and a foot block applied to the healthy claw. Lesions were visually examined and photographed on day 0, at 24–36 hours posttreatment and again at 21–30 days posttreatment. A subsample of cows was also monitored in the immediate posttreatment period for behavioral evidence of posttreatment discomfort. At the conclusion of the trial, photographs of lesions at 24–36 hours posttreatment were compared with lesions at day 21–30 posttreatment by two independent observers. Neither of the observers had knowledge of treatment assignments and simply scored the lesions based upon visual evidence of inflammation and excessive granulation tissue formation (JK Shearer et al, unpublished data, 2014).
Lesions from cows treated with oxytetracycline or copper sulfate on day 1 following treatment were observed to have an inflamed surface surrounded by varying amounts of black necrotic debris (Figure 4). Although there was none of the black-colored debris in lesions from control cows, it was not possible to determine if the degree of inflammation in treated lesions differed from untreated controls based upon visual observation. Conversely, there was a significant difference in the presence of excessive granulation tissue observed at day 21 for lesions treated with oxytetracycline or copper sulfate and a bandage (Figure 5). Speculation is that the granulation tissue observed was a result of increased inflammation associated with the topical treatments applied in this study (JK Shearer et al, unpublished data, 2014).
Figure 4.
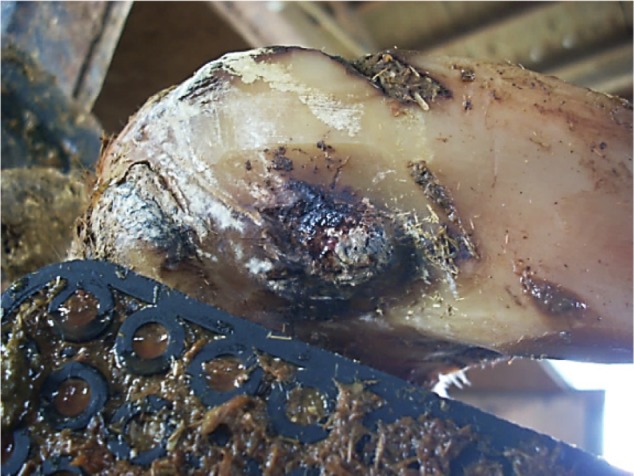
Cow with sole ulcer 24 hours posttreatment with oxytetracycline soluble powder.
Figure 5.
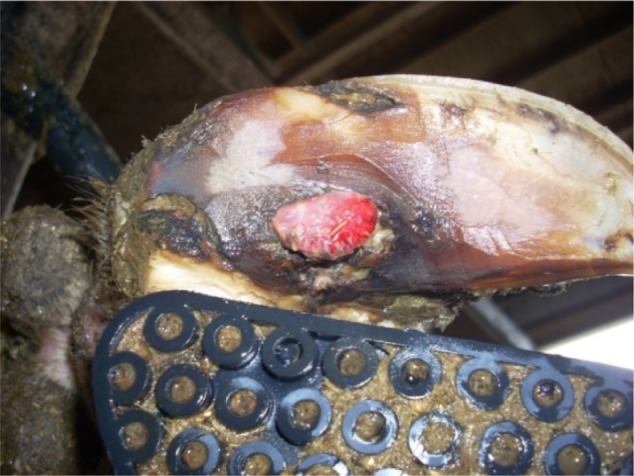
Cow with sole ulcer and excessive granulation tissue at 21 days posttreatment with oxytetracycline soluble powder.
Moreover, anecdotal observation of animals with claw lesions topically treated with tetracycline or copper sulfate also suggests that these compounds cause significant irritation and pain in the immediate posttreatment period. To objectively assess this observation, researchers monitored a subset of cows from both groups for a period of 15 minutes in the immediate posttreatment period. Results indicated that cows treated topically with either oxytetracycline or copper sulfate exhibited nearly three times as many pain-related behaviors (mean of 4.5/15 minutes for cows with no topical treatment compared with a mean of 13.6/15 minutes for cows treated with Oxytet or copper sulfate) in the posttreatment period (JK Shearer et al, unpublished data, 2014). For pain reasons alone, one may conclude that unless there is clear evidence of a therapeutic benefit from the application of these compounds their use is difficult to justify.
Posttreatment drug residue in topically treated animals
It is a common belief that a drug residue from topical treatment of claw lesions using tetracycline or oxytetracycline is highly unlikely. This was corroborated by the US survey of veterinarians and claw trimmers that found 84% of respondents (81% American Association of Bovine Practitioners and 86% Hoof Trimmers Association) did not recommend withholding milk following topical treatment with tetracycline or oxytetracycline compounds.26
In order to determine the likelihood of creating a detectable residue, our research group collected blood and milk samples from eleven cows with topically treated claw lesions. Seven cows (Farm 1) were treated with oxytetracycline soluble powder (7.3 g) and four (Farm 2) with one scoop (equivalent to 25.5 g) of tetracycline soluble powder. Blood and milk samples were collected pre- and posttreatment (oxytetracycline-treated cows sampled three times per day and the tetracycline treated cows were sampled two times per day for 3 days posttreatment). Serum and milk samples were frozen after collection and submitted to the Pharmacology Analytical Support Team (PhAST) at Iowa State University’s College of Veterinary Medicine. Drug concentrations were quantified using liquid chromatography-mass spectrometry with a level of detection for the assays at 1 ng/mL. Lesion surface area (exposed corium) was also calculated using ImageJ software (available from National Institute of Health).62
Results of the assays for tetracycline in plasma demonstrated a Cmax (maximum concentration) of 4.78±2.82 ng/mL; for milk, Cmax was 20.64±14.51 ng/mL (recorded at the third milking on day 2). Cmax for oxytetracycline in plasma was 2.15±1.20 ng/mL (recorded at 48 hours posttopical application); for milk, Cmax was 20.81±19.90 ng/mL recorded at the seventh milking (milking three times per day). Regulatory action for oxytetracycline and tetracycline are >300 ppb, which is well above levels observed in this study, but all samples had detectable levels of drug. We also observed that lesions with larger surface areas tended to have higher log-transformed drug concentrations in both plasma and milk. We conclude that topical treatment with either tetracycline or oxytetracycline derivatives is likely to result in detectable residues, but concentrations are well below actionable levels.62
To the knowledge of these authors, until now there has been only one other report in the scientific literature documenting drug residue following topical treatment of a claw lesion. That study was conducted in two lactating cows using a topical product containing 5% chloramphenicol in an alcohol solution, commercially registered as not intended for use in food producing animals. One of the study cows had teat lesions and another had skin lesions on the shoulders and claws. Topical treatment consisted of four consecutive applications of the chloramphenicol solution within a 41-hour period. Milk, blood, and urine samples were taken immediately before the first treatment and at regular intervals thereafter for a period of 12 days following the last application. Detectable residues were obtained from blood plasma, urine, and milk from the treated animals. Despite a very low dose, residues of Chloramphenicol were detectable in plasma and urine for 2 days and milk for 5 days beyond the day of the final treatment, respectively. Chloramphenicol levels detected in milk were slightly above the minimum required performance limit (MRPL) of 0.3 µg/kg on days 1 and 5 following treatment.63
Pain and pain management
The subject of pain management in cattle and specifically that associated with lameness has been reviewed recently.64 Hyperalgesia (increased sensitivity to pain) is one of the most significant challenges in managing lameness-related pain in cattle. Since lameness often goes undetected until gait is markedly affected, hyperalgesia is more the norm than the exception when dealing with pain associated with lameness.
The perception of pain in animals and humans occurs by way of multiple tissue receptors designed to sense mechanical or physical types of pain (mechanoreceptors), heat or cold temperatures (thermoreceptors), and tissue damage caused by injury (nocireceptors). These receptors are well-distributed throughout the body permitting the sensation of pleasurable as well as noxious types of stimuli. Pain associated with lameness affects primarily nocireceptors.
Tissue damage at the site of injury results in a barrage of impulses that are sent from the site of injury to the spinal cord and brain which interprets them as pain. Inflammation accompanies these events and causes the release of multiple pain mediators such as substance P, various peptides, inflammatory cells, among other chemical substances.21 These substances cause their own type of damage and inflammation by diffusing into the tissues that surround the site of injury thereby expanding the total area involved. Nerve fibers in the vicinity of these expanding inflammatory mediators become sensitized and spontaneously send impulses to the spinal cord and brain thus amplifying the pain. So while the original site of injury may be small, as the inflammatory process progresses pain is felt over a much larger area.
Hyperalgesic or neuropathic (wind-up) pain is particularly common in cattle suffering from chronic lameness disorders. It is characterized by an animal that exhibits an exaggerated pain reaction in response to a lesser stimulus. Persistent pain, regardless of whether it occurs by injury or disease, results in inflammation and hyper-excitability or “winding-up” of the sensory neurons in the spinal cord and brain. As neurons become “wound-up” they become more sensitive to painful stimuli whereby something that would normally be only mildly uncomfortable becomes extremely painful. Neuropathic pain is normally quite resistant to analgesic therapy; therefore, lame animals generally require an extended length of time for recovery to a nonlame pain-free state on their injured limbs.64 Whay et al reported significant decreases in the nociceptive threshold of lame animals lasting through 28 days beyond the point of detection.65 This is one of the reasons for the recommendation that lameness be treated promptly. This is especially important since the natural stoic behavior of cows and the fact that lameness disorders are generally present long before they are identified results in hyperalgesia and prolonged recovery from lameness despite effective treatment.
Pain management using a multi-modal approach
The management of pain associated with lameness is best accomplished through the use of a multi-modal approach including 1) use of intravenous regional or ring block anesthesia when needed for corrective trimming and treatment of painful conditions, 2) careful and thorough corrective trimming without damage to adjacent healthy corium tissues, 3) use of an orthopedic foot block to relieve weight bearing on injured claws, 4) avoidance of topical therapies that increase discomfort and prolong recovery, 5) administration of analgesics including local anesthetics, nonsteroidal anti-inflammatory drugs (NSAIDs), and sedative-analgesics, and finally 6) comfortable housing and thoughtful management of lame cows in the posttreatment period.64
Intravenous regional anesthesia or ring block using 2% lidocaine has been described in detail elsewhere.64 These techniques are easy to perform and very effective when painful procedures are necessary. When these procedures are used in cattle affected with DDS, bacteremia is a potential secondary complication with or without debridement of the digital lesions. This suggests that systemic or local antimicrobial treatment may be warranted either prior to or concurrently with the use of intravenous regional anesthesia procedures in cattle with DDS.66
Sedative-analgesics
For animals requiring surgery or for those that may be particularly painful, anxious, or fractious, the preemptive use of a sedative-analgesic such as xylazine is able to reduce the stress response.67 Twenty-four cows affected with claw disorders were randomly allocated into two groups of 12 animals each and treated with either xylazine (0.05 mg/kg body weight) or sterile saline by intramuscular administration. Animals were placed in lateral recumbency on a tilt chute type trimming table and administered regional anesthesia with 2% procaine. Cows treated with xylazine exhibited mild signs of sedation lasting approximately 1–2 hours. Xylazine-treated cows also demonstrated reduced behavioral responses to the insertion of needles for local anesthesia, ear flicking during treatment, and reduced lameness scores once released from the tilt table. Researchers concluded that xylazine is an effective component of a multimodal analgesic protocol.67
Nonsteroidal anti-inflammatory drugs
Studies on nonsteroidal anti-inflammatories for management of pain associated with lameness in cattle have yielded conflicting results or evidence of only mild-to-moderate benefit. Cows treated with ketoprofen had mild improvements in gait and less variation in weight distribution between feet on all four limbs.68–70 Flunixin meglumine resulted in a significant reduction in pain when used at the time of lameness induction and again 12 hours later in an Amphotericin B-induced-lameness model. Researchers determined that animals were less likely to be lame, placed more weight on the affected foot 12 hours posttreatment and spent less time lying compared with control animals.71 Conflicting results were observed in another study of cows administered flunixin meglumine (2.2 mg/kg) immediately before and 24 hours after corrective claw trimming. In this study, there was no effect of treatment on gait scores; but untreated controls spent more time lying down than treated cows.70 Part of the reason for the variability in results is likely due to the heterogeneity (both acute and hyperalgesic pain was represented in treatment groups) of pain observed under field conditions. As described earlier, in many cases by the time animals undergo treatment, the pain has progressed to the neuropathic form whereby animals are quite refractory to pain management therapies.
Possibly more promising are observations and results of recent studies of the combined use of gabapentin and meloxicam. Meloxicam is a NSAID with potent analgesic properties in cattle. Gabapentin, originally developed for the treatment of epilepsy in humans, has been found to have benefits for the mitigation of chronic neuropathic and acute inflammatory pain72 that may be present in cattle as well.73 In a recent study, Coetzee et al found that the administration of gabapentin at 15 mg/kg combined with meloxicam at 0.5 mg/kg once daily for 4 days increased the amount of force applied on the lame claw in calves subjected to an experimental lameness model.74
Attitudes of veterinarians toward pain management
The attitudes of cattle veterinarians toward pain and pain management have been reported previously.25,26,74,75–79 The most recent of these a survey of veterinarians who do foot care in cattle indicating that they considered sole ulcers to be a painful lesion, but most (76%) did not recommend the use of analgesics.26 Reasons cited were concerns that available drug options were not efficacious (41%) and second, there was a lack of approved analgesics for use (26%) in cattle. A more comprehensive survey of analgesic use by bovine practitioners in the US found that most considered acute lameness more painful than chronic lameness, and practitioners in the youngest age group were much more likely to use analgesics in cattle with lameness compared with older practitioners.78 Canadian researchers conducted a mail survey to assess analgesic usage for surgeries and medical conditions in cattle, pigs, and horses. More than 90% of veterinarians reported using analgesic drugs for equine surgeries, for cesarean section in sows and cows, and for bovine claw amputation and omentopexy. However, analgesics used were often inadequate, and many veterinarians did not give analgesics to young animals. Results of these surveys indicate that the veterinary profession in North America needs to continue improving its pain management in livestock.77
A survey of veterinarians in the UK identified claw amputation as the most painful procedure for cattle, but only 61% reported using NSAIDs to control the postsurgical pain. One reason for the lack of analgesic therapy may have been an unwillingness or inability to appreciate the pain cattle experienced with these conditions. This was supported by data demonstrating that those respondents who did not use analgesics also assigned lower pain scores to the various health conditions and treatment procedures. Other factors were age and sex; recent graduates and females were more likely to score the pain associated with lameness conditions higher (ie, more painful).75 Similar results were obtained in a survey of dairy veterinarians in New Zealand. Claw amputation was perceived to be the most painful procedure, followed by caesarian section and surgery for abomasal displacement. Highest median pain scores for procedures/conditions were observed for graduates from the year 2000 onward and female veterinarians.76 In some cases, veterinarians are reluctant to use analgesia fearing the increased cost may not be acceptable to farm clients.69
One would have to conclude that there is a growing need for veterinary continuing education on pain and pain management in animals and continued development of more efficacious and cost-effective analgesics for use in food animals.
Posttherapy housing and management
When investigating a herd experiencing a high prevalence of lameness one is tempted to assume that the most likely cause is an increase in the number of new cases. In fact, it may be due to poor recovery rates whereby animals that get lame stay lame because animals are not responding to therapy.79 While it is important to review herd records and treatment procedures, one must also review housing and management of lame cows during the posttreatment period.
At the first sign of lameness affected animals should be identified and pulled from their respective groups, relocated to a pen near the foot care and trimming area and scheduled for examination and treatment at the earliest possible convenience, preferably within 48 hours of detection. It is important to understand that cows experiencing lameness may have difficulty lying or rising in a stall. Cook and Nordlund,79 proposed that cows housed in free-stall barns with firm unyielding stall surfaces were less likely to lie down for fear of pain and slipping on the stall surface. They suggested that sand was a superior stall surface since it permitted a better cushion and more traction for the rear foot when cows were attempting to lie down or rise. It was their view that this was an important reason for the lower prevalence of lameness observed in sand compared with mattress bedded free stall barns.79,80
Obstacles presented by stalls can also be overcome by moving animals to pasture, dry lot, or a bedded pack where natural behaviors associated with lying down and rising are unrestricted. Canadian researchers found that lame cows offered a 4-week period on pasture had improved gait scores, despite spending less time lying down.81 Similar observations were reported by Endres and Barberg of cows on bedded packs.82 They observed freer movement and the ability of cows to assume all natural lying positions when housed on bedded packs.
Housing for lame cows should be within close proximity to the milking parlor to reduce the distance animals are required to walk each day. Instead of three times per day milking, some herds will put lame cows in two times per day milking groups to reduce requirements for walking to and from the milking parlor. Extra effort should be made to insure that flooring surfaces are clean and secure to prevent slipping. Properly textured surfaces or rubber flooring may improve comfort and also footing. It is also advantageous to house lame cows near the hospital where animals can be observed and treated more conveniently by health and foot care personnel. Regardless of whether all trimming is conducted by outside people, having a trim chute near these areas is useful so that animals may be conveniently examined or retreated as needed.
Summary
Claw disorders represent some of the most common and debilitating of lameness conditions in cattle; however, despite the frequency with which they occur, specific information on their treatment is lacking. Therefore, treatment is largely empirical and likely most effective when protocols are based upon our understanding of the pathogenesis of claw lesions and wound healing. Claw disorders in cattle are associated with metabolic disorders that are common around the time of calving and to mechanical factors related to overgrowth of claw horn that contributes to overloading particularly on the lateral claw of rear feet. One of the objectives of functional claw trimming is to correct this weight bearing disparity thereby reducing the likelihood of sole ulcer or WLD. Claw lesions in cattle heal by second intention and follow a pattern common to that observed in cutaneous wounds. Based on available information and current understanding of the pathogenesis of claw lesions and wound healing, the treatment of claw lesions should include functional and corrective claw trimming that avoids damage to peripheral healthy tissues and the adjustment of weight bearing by the application of an orthopedic block. The use of bandages or wraps has not been studied extensively. At least one well-controlled study suggests that they offer little or no benefit. Others would argue that bandages and wraps are necessary to keep lesions clean and to protect tender delicate tissues in exposed lesions. Another area of controversy is that regarding the use of parenteral and topical antimicrobial therapy for claw lesions. While there is little or no data to support either treatment practice previous research and survey information indicate that these are common modes of therapy. A recent study at Iowa State University suggests that topical treatments commonly used to treat claw lesions may increase the rate of granulation tissue formation and thus could delay healing. This is most surely an important area for further study. Current information indicates that mild-to-moderate uncomplicated sole ulcers can be expected to develop a layer of new horn by 21–30 days after initial treatment with more severe lesions requiring somewhere between 40 and 60 days. The purpose of the claw horn capsule is to protect the underlying corium, therefore when lesions result in exposure of the corium the objective is rapid re-epithelialization of the lesion. The management of pain in lame cattle is often complicated by the fact that much of it is neuropathic (wind-up type) pain. Based on the authors’ knowledge and experience the best strategy to mitigate lameness pain in cattle may be accomplished by implementing a multi-modal approach including the following: 1) use of intravenous regional or ring block anesthesia when needed for painful conditions, 2) careful and thorough corrective trimming without damage to adjacent healthy corium tissues, 3) use of an orthopedic foot block to relieve weight bearing on injured claws, 4) avoidance of topical therapies that increase discomfort and prolong recovery, 5) administration of analgesics including local anesthetics, NSAIDs, and sedative-analgesics, and finally 6) comfortable housing and thoughtful management of lame cows in the posttreatment period. Finally, many lame cows may fail to recover not because of poor corrective trimming or therapeutic management of their condition; instead, they may not improve because they are unable to cope with housing and management conditions that restrict natural behaviors for rest and recuperation.
Footnotes
Disclosure
Trial work described in this review was supported in part by a grant from the American Association of Bovine Practitioners and the Hoof Trimmers Association; however, neither had a role in the study design, data analysis, decision to publish, or preparation of this manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Blowey R, Weaver D. Color Atlas of Diseases and Disorders of Cattle. 3rd ed. London, UK: Mosby Elsevier Ltd; 2011. [Google Scholar]
- 2.Ossent P, Lischer Ch J. Bovine lamninitis: the lesions and their pathogenesis. Practice. 1998;20:415–427. [Google Scholar]
- 3.Ossent P. Subclinical bovine laminitis. Cattle Pract. 1999;7:193–195. [Google Scholar]
- 4.Lischer Ch J, Ossent P. The significance of the suspensory mechanism of the third phalanx and its fat bodies in the pathogenesis of sole ulcers in cattle. Part I: Macroscopic findings. III International Conference on Bovine Lameness; Parma, Italy. 2000. [Google Scholar]
- 5.Ossent P, Lischer Ch J. The significance of the suspensory mechanism of the third phalanx and its fat bodies in the pathogenesis of sole ulcers in cattle. Part II: Microscopic findings. III International Conference on Bovine Lameness; Parma, Italy. 2000. [Google Scholar]
- 6.Leach KA, Logue DN, Kempson SA, Offer JE, Ternent HE, Randalis JM. Claw lesions in dairy cattle: development of sole and white line haemorrhages during the first lactation. Vet J. 1997;154(3):215–225. doi: 10.1016/s1090-0233(97)80024-x. [DOI] [PubMed] [Google Scholar]
- 7.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 8.Raven T. Cattle Footcare and Claw Trimming. Farming Press Ltd; Ipswich, UK: 1989. [Google Scholar]
- 9.Lischer CJ, Ossent P, Räber M, Geyer H. Suspensory structures and supporting tissues of the third phalanx of cows and their relevance to the development of typical sole ulcers (Rusterholz ulcers) Vet Rec. 2002;151(23):694–698. [PubMed] [Google Scholar]
- 10.Mulling C. Theories on the pathogenesis of white line disease – an anatomical perspective. 12th International Symposium on Lameness in Ruminants; Orlando, Florida. 2002. [Google Scholar]
- 11.Tarlton JF, Holah DE, Evans KM, Jones S, Pearson GR, Webster AJ. Biomechanical and histopathological changes in the support structures of bovine hooves around the time of first calving. Vet J. 2002;163(2):196–204. doi: 10.1053/tvjl.2001.0651. [DOI] [PubMed] [Google Scholar]
- 12.Webster AJ. Effect of environment and management on the development of claw and leg diseases. XXII World Buiatrics Congress; Hanover, Germany. 2002. [Google Scholar]
- 13.Raber M, Lischer Ch J, Geyer H, Ossent P. The bovine digital cushion – a descriptive anatomical study. Vet J. 2004;167(3):258–264. doi: 10.1016/S1090-0233(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 14.Raber M, Scheeder MR, Ossent P, Lischer Ch J, Geyer H. The content and composition of lipids in the digital cushion of the bovine claw with respect to age and location – a preliminary report. Vet J. 2006;172(1):173–177. doi: 10.1016/j.tvjl.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Bicalho RC, Machado VS, Caixeta LS. Lameness in dairy cattle: a debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence and thickness of the digital cushion. J Dairy Sci. 2009;92(7):3175–3184. doi: 10.3168/jds.2008-1827. [DOI] [PubMed] [Google Scholar]
- 16.Hirschberg RM, Mülling CKW, Budras K-D. Pododermal angioarchitecture of the bovine claw in relation to form and function of the papillary body: a scanning electron microscopic study. Microsc Res Tech. 2001;54(6):375–385. doi: 10.1002/jemt.1150. [DOI] [PubMed] [Google Scholar]
- 17.Blowey R. Cattle Lameness and Hoofcare. Ipswich, UK: Farming Press; 1993. [Google Scholar]
- 18.Van Amstel SR, Shearer JK. Clinical report – characterization of toe ulcers associated with thin soles in dairy cows. Bovine Pract. 2008;42(2):189–196. [Google Scholar]
- 19.Sanders AH, Shearer JK, De Vries A. Seasonal incidence of lameness and risk factors associated with thin soles, white line disease, ulcers, and sole punctures in dairy cattle. J Dairy Sci. 2009;92(7):3165–3174. doi: 10.3168/jds.2008-1799. [DOI] [PubMed] [Google Scholar]
- 20.Shearer JK, van Amstel SR. Manual of Foot Care in Cattle. 2nd ed. Ft Atkinson, WI: WD Hoards and Sons Company; 2013. [Google Scholar]
- 21.Auer J, Stick J. Equine Surgery. 2nd ed. St Louis: Saunders Elsevier; 2012. [Google Scholar]
- 22.O’Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001;26(6):525–530. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 23.Manske T, Hultgren J, Bergsten C. The effect of claw trimming on the hoof health of Swedish dairy cattle. Prev Vet Med. 2002;54(2):113–129. doi: 10.1016/s0167-5877(02)00020-x. [DOI] [PubMed] [Google Scholar]
- 24.Shearer JK, van Amstel SR. Functional and corrective claw trimming. Vet Clin North Am Food Anim Pract. 2001;17(1):53–72. doi: 10.1016/s0749-0720(15)30054-2. [DOI] [PubMed] [Google Scholar]
- 25.O’Callaghan-Lowe KA, Murray RD, Cripps PJ, Ward WR. Working practices of cattle foot trimmers used for footcare in dairy cattle compared with those of veterinary surgeons for treatment of lameness in large animal practice. J Vet Med A Physiol Pathol Clin Med. 2004;51(9–10):429–434. doi: 10.1111/j.1439-0442.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- 26.Kleinhenz K, Plummer P, Danielson J, et al. Survey of veterinarians and hoof trimmers on methods applied to treat claw lesions in dairy cattle. Bovine Pract. 2014;48:47–52. [Google Scholar]
- 27.Horseman SV, Whay HR, Huxley JN, Bell NJ, Mason CS. A survey of the on-farm treatment of sole ulcer and white line disease in dairy cattle. Vet J. 2013;197(2):461–467. doi: 10.1016/j.tvjl.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 28.van Amstel SR, Shearer JK. Manual for the Treatment and Control of Lameness in Cattle. Ames, IA: Blackwell Publishing; Professional: 2006. [Google Scholar]
- 29.Leach KA, Tisdall DA, Bell NJ, Main DC, Green LE. The effects of early treatment for hindlimb lameness in dairy cows on four commercial UK farms. Vet J. 2012;193(3):626–632. doi: 10.1016/j.tvjl.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Shearer JK, van Amstel S, Gonzalez-Sagues A, Shearer L. The master hoof care program: an international program designed to teach foot care and claw trimming procedures. XXI World Buiatrics Congress; Punta del Este, Uruguay. 2000. [Google Scholar]
- 31.Program NDF Farmers assuring responsible management. 2012. [Accessed January 17, 2015]. http://www.nationaldairyfarm.com/
- 32.Pyman MF. Comparison of bandaging and elevation of the claw for the treatment of foot lameness in dairy cows. Aust Vet J. 1997;75(2):132–135. doi: 10.1111/j.1751-0813.1997.tb14173.x. [DOI] [PubMed] [Google Scholar]
- 33.Sala A, Igna C, Schuszler L. Comparative aspects of pododermatitis circumscripta (sole ulcer) treatment in dairy cow. Bull Univ Agric Sci Vet Med Cluj Napoca. 2008;65(2):207–211. [Google Scholar]
- 34.White ME, Glickman LT, Embree IC, Powers PM, Pearson EG. A randomized trial for evaluation of bandaging sole abscesses in cattle. J Am Vet Med Assoc. 1981;178(4):375–377. [PubMed] [Google Scholar]
- 35.Van Amstel SR, Shearer JK, Palin FL. Case report – clinical response to treatment of pododermatitis circumscripta (ulceration of the sole) in dairy cows. Bovine Pract. 2003;37:143–150. [Google Scholar]
- 36.Berry DB, 2nd, Sullins KE. Effects of topical application of antimicrobials and bandaging on healing and granulation tissue formation in wounds of the distal aspect of the limbs in horses. Am J Vet Res. 2003;64(1):88–92. doi: 10.2460/ajvr.2003.64.88. [DOI] [PubMed] [Google Scholar]
- 37.Dart AJ, Perkins NR, Dart CM, Jeffcott LB, Canfield P. Effect of bandaging on second intention healing of wounds of the distal limb in horses. Aust Vet J. 2009;87(6):215–218. doi: 10.1111/j.1751-0813.2009.00428.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilmink J, VanWeeren P. Treatment of exuberant granulation tissue. Clin Tech Equine Pract. 2004;3(2):141–147. [Google Scholar]
- 39.Tracey AK, Alcott CJ, Schleining JA, et al. The effects of topical oxygen therapy on equine distal limb dermal wound healing. Can Vet J. 2014;55(12):1146–1152. [PMC free article] [PubMed] [Google Scholar]
- 40.Lischer Ch J, Koller U, Geyer H, Mulling C, Schulze J, Ossent P. Effect of therapeutic dietary biotin on the healing of uncomplicated sole ulcers in dairy cattle – a double blinded controlled study. Vet J. 2002;163(1):51–60. doi: 10.1053/tvjl.2001.0627. [DOI] [PubMed] [Google Scholar]
- 41.Durmus A. Management of sole ulcer in cattle. Indian Vet J. 2005;82:1096–1097. [Google Scholar]
- 42.Azarabad H, Nowrouzian I, Soleymani E, Vakilgilani G, Seyedjavadi S. Wound healing process of uncomplicated “Rusterholz” ulcer following treatment by Wooden Block and Hoofgel® in Bovine Hoof: histopathological aspects. Am J Anim Vet Sci. 2006;1(2):27–30. [Google Scholar]
- 43.Nguhiu-Mwangi J, Mbithi P, Wabacha J, Mbuthia P. Retrospective study of foot conditions in dairy cows in urban and periurban areas of Kenya. Isr J Vet Med. 2008;63:40–45. [Google Scholar]
- 44.Stokka GL, Lechtenberg K, Edwards T, et al. Lameness in feedlot cattle. viii. Vet Clin North Am Food Anim Pract. 2001;17(1):189–207. doi: 10.1016/s0749-0720(15)30062-1. [DOI] [PubMed] [Google Scholar]
- 45.Griffin D, Perino L, Hudson D. G93-1159 Feedlot Lameness. http://digitalcommons.unl.edu/extensionhist/196.
- 46.van Amstel SR, Shearer JK, Cooper VL. 16th Symposium and 8th Conference on Lameness in Ruminants. Rotorua, New Zealand: 2011. Clinical presentation, treatment approach and histopathology of atypical digital dermatitis lesions. [Google Scholar]
- 47.Morales-Burgos A, Loosemore MP, Goldberg LH. Postoperative wound care after dermatologic procedures: a comparison of 2 commonly used petrolatum-based ointments. J Drugs Dermatol. 2013;12(2):163–164. [PubMed] [Google Scholar]
- 48.Winkler K. Topics in Wound Management. The Merck Veterinary Manual. Whitehouse Station, NJ: Merck Sharp and Dohme Corp, a subsidiary of Merck and Co; 2012. [Google Scholar]
- 49.Lischer CJ, Dietrich-Hunkeler A, Geyer H, Schulze J, Ossent P. Healing process of uncomplicated sole ulcers in dairy cows kept in tie stalls: clinical description and blood chemical investigations. Schweiz Arch Tierheilkd. 2001;143(3):125–133. [PubMed] [Google Scholar]
- 50.Cook NB, Burgi K. “Hairy Attack” A new lesion affecting the corium of the white line – a case report. 15th Symposium and 7th Conference of Lameness in Ruminants; Kuopio, Finland. 2008. [Google Scholar]
- 51.Holzhauer M, Dopfer D, de Boer J, van Schaik G. Effects of different intervention strategies on the incidence of papillomatous digital dermatitis in dairy cows. Vet Rec. 2008;162(2):41–46. doi: 10.1136/vr.162.2.41. [DOI] [PubMed] [Google Scholar]
- 52.Holzhauer M. Non-healing white line lesion, advanced experience. 16th Symposium and 8th Conference of Lameness in Ruminants; Rotorua, New Zealand. 2011. [Google Scholar]
- 53.Evans NJ, Blowey RW, Timofte D, et al. Association between bovine digital dermatitis treponemes and a range of “non-healing” bovine hoof disorders. Vet Rec. 2011;168(8):214. doi: 10.1136/vr.c5487. [DOI] [PubMed] [Google Scholar]
- 54.Kofler J, Reichert J, Dietrich J. Evaluation of a treatment regime for digital dermatitis-associated white line lesions and sole ulcers (“non-healing” bovine hoof disorders) in dairy cows. 17th International Symposium and 9th International Conference on Lameness in Ruminants; Bristol, UK. 2013. [Google Scholar]
- 55.Atkinson O. Non-healing hoof lesions in dairy cows. Vet Rec. 2011;169(21):561–562. doi: 10.1136/vr.d7426. [DOI] [PubMed] [Google Scholar]
- 56.Acevedo J, Chesterton R, Hurtado C. Bovine digital dermatitis and non-healing lesions and toe necrosis in grazing dairy herds in Chile. 17th International Symposium and 9th International Conference on Lameness in Ruminants; Bristol, UK. 2013. [Google Scholar]
- 57.Minini S, Crowhurst F, Nicolas J. Toe necrosis and non-healing hoof lesions in commercial dairy herds in Argentina. 17th International Symposium and 9th International Conference on Lameness in Ruminants; Bristol, UK. 2013. [Google Scholar]
- 58.Blowey R. Non-healing hoof lesions in dairy cows. Vet Rec. 2012;170(1):26–27. doi: 10.1136/vr.e32. [DOI] [PubMed] [Google Scholar]
- 59.Blowey R. Non-healing hoof lesions in dairy cows. Vet Rec. 2011;169(20):534. doi: 10.1136/vr.d7267. [DOI] [PubMed] [Google Scholar]
- 60.Blowey R. Changing indications for digit amputation in cattle. Vet Rec. 2011;169(9):236–237. doi: 10.1136/vr.d5428. [DOI] [PubMed] [Google Scholar]
- 61.Nuss K, Kostlin R, Bohmer H. Significance of septic, traumatic ungulocoriitis (pododermatitis) at the tip of the bovine claw. Tierarztl Prax. 1990;18(6):567–575. [PubMed] [Google Scholar]
- 62.Coetzee JF, Kleinhenz K, Pingsterhaus B, Schleining J, Wulf P, Shearer J. Effect of topical treatment of claw horn lesions with tetracycline-derivatives on plasma and milk concentrations. 47th Annual Conference of the American Association of Bovine Practitioners; Albuquerque, NM. 2014. [Google Scholar]
- 63.Pengov A, Flajs VC, Zadnik T, Marinšek J, Pogačnik M. Distribution of chloramphenicol residues in lactating cows following an external application. Anal Chim Acta. 2005;529(1–2):347–351. [Google Scholar]
- 64.Shearer JK, Stock ML, Van Amstel SR, Coetzee JF. Assessment and management of pain associated with lameness in cattle. Vet Clin North Am Food Anim Pract. 2013;29(1):135–156. doi: 10.1016/j.cvfa.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Whay HR, Waterman AE, Webster AJ, O’Brien JK. The influence of lesion type on the duration of hyperalgesia associated with hindlimb lameness in dairy cattle. Vet J. 1998;156:23–29. doi: 10.1016/s1090-0233(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 66.Simpson KM, Streeter RN, Taylor JD, Gull TB, Step DL. Bacteremia in the pedal circulation following regional intravenous perfusion of a 2% lidocaine solution in cattle with deep digital sepsis. J Am Vet Med Assoc. 2014;245(5):565–570. doi: 10.2460/javma.245.5.565. [DOI] [PubMed] [Google Scholar]
- 67.Rizk A, Herdtweck S, Offinger J, Meyer H, Zaghloul A, Rehage J. The use of xylazine hydrochloride in an analgesic protocol for claw treatment of lame dairy cows in lateral recumbency on a surgical tipping table. Vet J. 2012;192(2):193–198. doi: 10.1016/j.tvjl.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Flower FC, Sedlbauer M, Carter E, von Keyserlingk MAG, Sanderson DJ, Weary DM. Analgesics improve the gait of lame dairy cattle. J Dairy Sci. 2008;91(8):3010–3014. doi: 10.3168/jds.2007-0968. [DOI] [PubMed] [Google Scholar]
- 69.Whay HR, Webster AJ, Waterman-Pearson AE. Role of ketoprofen in the modulation of hyperalgesia associated with lameness in dairy cattle. Vet Rec. 2005;157(23):729–733. doi: 10.1136/vr.157.23.729. [DOI] [PubMed] [Google Scholar]
- 70.Chapinal N, de Passille AM, Rushen J, Wagner S. Automated methods for detecting lameness and measuring analgesia in dairy cattle. J Dairy Sci. 2010;93(5):2007–2013. doi: 10.3168/jds.2009-2803. [DOI] [PubMed] [Google Scholar]
- 71.Schulz KL, Anderson DE, Coetzee JF, White BJ, Miesner MD. Effect of flunixin meglumine on the amelioration of lameness in dairy steers with amphotericin B-induced transient synovitis-arthritis. Am J Vet Res. 2011;72(11):1431–1438. doi: 10.2460/ajvr.72.11.1431. [DOI] [PubMed] [Google Scholar]
- 72.Hurley RW, Chatterjea D, Rose Feng M, Taylor CP, Hammond DL. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97(5):1263–1273. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 73.Coetzee JF, Mosher RA, Kohake LE, et al. Pharmacokinetics of oral gabapentin alone or co-administered with meloxicam in ruminant beef calves. Vet J. 2011;190(1):98–102. doi: 10.1016/j.tvjl.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Coetzee JF, Mosher RA, Anderson DE, et al. Impact of oral meloxicam administered alone or in combination with gabapentin on experimentally induced lameness in beef calves. J Anim Sci. 2014;92(2):816–829. doi: 10.2527/jas.2013-6999. [DOI] [PubMed] [Google Scholar]
- 75.Huxley JN, Whay HR. Current attitudes of cattle practitioners to pain and the use of analgesics in cattle. Vet Rec. 2006;159(20):662–668. doi: 10.1136/vr.159.20.662. [DOI] [PubMed] [Google Scholar]
- 76.Laven RA, Huxley JN, Whay HR, KJ S. Results of a survey of attitudes of dairy veterinarians in New Zealand regarding painful procedures and conditions in cattle. N Z Vet J. 2009;57(4):215–220. doi: 10.1080/00480169.2009.36904. [DOI] [PubMed] [Google Scholar]
- 77.Hewson CJ, Dohoo IR, Lemke KA, Barkema HW. Canadian veterinarians’ use of analgesics in cattle, pigs, and horses in 2004 and 2005. Can Vet J. 2007;48(2):155–164. doi: 10.4141/cjas68-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fajt VR, Wagner SA, Norby B. Analgesic drug administration and attitudes about analgesia in cattle among bovine practitioners in the United States. J Am Vet Med Assoc. 2011;238(6):755–767. doi: 10.2460/javma.238.6.755. [DOI] [PubMed] [Google Scholar]
- 79.Cook NB, Nordlund KV. The influence of the environment on dairy cow behavior, claw health and herd lameness dynamics. Vet J. 2009;179(3):360–369. doi: 10.1016/j.tvjl.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Cook NB. Prevalence of lameness among dairy cattle in Wisconsin as a function of housing type and stall surface. J Am Vet Med Assoc. 2003;223(9):1324–1328. doi: 10.2460/javma.2003.223.1324. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez-Mendo O, von Keyserlingk MA, Veira DM, Weary DM. Effects of pasture on lameness in dairy cows. J Dairy Sci. 2007;90(3):1209–1214. doi: 10.3168/jds.S0022-0302(07)71608-9. [DOI] [PubMed] [Google Scholar]
- 82.Endres MI, Barberg AE. Behavior of dairy cows in an alternative bedded-pack housing system. J Dairy Sci. 2007;90(9):4192–4200. doi: 10.3168/jds.2006-751. [DOI] [PubMed] [Google Scholar]