Abstract
Background
Products from Ficus carica have been used in traditional medicine to treat many diseases. This study aimed to analyze anticancer effects of extracts of F. carica leaves on the triple-negative breast cancer cell line MDA-MB-231.
Materials and methods
The human breast cancer cell line MDA-MB-231 was used to evaluate effects of F. carica extracts. Effects of F. carica on cell viability were evaluated using MTT assays. Cell-cycle distribution was examined using cell-cycle analysis. Wound-healing assays were used to evaluate migration of MDA-MB-231. Quantitative reverse-transcription polymerase chain reaction was used to detect levels of Bax, p53, p21, GATA3, ELF5, cyclin-dependent kinases, MMP2, and tissue inhibitors of metalloproteinase.
Results
We investigated the mechanism of anti-growth effects, and found that the expressions of genes that promote apoptosis were increased. In addition, the treated cells illustrated increased portion at S phase and changed expression of cyclin-dependent kinases, demonstrating cell-cycle arrest at the S phase. Furthermore, treated cells showed decreased cell mobility, which is essential for metastasis. Two of the active components of F. carica leaves, bergapten and psoralen, had similar anticancer effects as F. carica leaf extracts, indicating that these two components might play important roles in anticancer effects of F. carica leaves.
Conclusion
Our findings suggest that F. carica leaves might be a good source to develop drugs for suppressing cancer-cell growth and migration to treat triple-negative breast cancers.
Keywords: Ficus carica, breast cancer, MDA-MB-231, migration
Introduction
Ficus carica (fig) is an important member of the family Moraceae, which has been cultivated by humans for a long time. F. carica is originally from the Middle East and West Asia, but spread to many other regions in the world. Products of F. carica are widely used as food sources and medicine to treat various diseases.1 For example, extract of fig fruit exerts hypotensive and antihypertensive effects in glucose-induced hypertensive rats2 and exhibits moderate cytotoxic activities against human cancer cell lines, eg, MCF7, HepG2, and U2OS,3 and exhibits antiproliferation against cervical cancer cell lines.4 Leaf decoctions affect lipid catabolism in hypertriglyceridemic rats and had hypoglycemic action in type 1 diabetic patients, while fig latex also shows in vitro inhibitory effects on proliferation of various cancer cell lines.5–7
Fig leaves, fruit, and latex all contain anticancer components, among which bergapten and psoralen are two important ones.8 Bergapten has inhibitory effects on the liver cancer cell line, stomach cancer cell line, and NPC cells, the mechanism of which may include direct killing, arresting the cell cycle, and inducing apoptosis.9,10 It has been proved that psoralen can inhibit the proliferation and migration abilities of MCF7/ADR cells,11 as well as inhibit breast cancer-cell growth in the bone microenvironment and regulate the function of osteoblasts and osteoclasts in tumor-bearing mice.12
Breast cancer has substantially higher incidence than any other cancer among diseases in women,13 and are categorized into three basic groups: estrogen receptor (ER) positive, human epidermal growth factor receptor-2 (HER2/ERBB2) and triple-negative breast cancer (TNBC, also known as basal-like breast cancer). TNBC is the most difficult subtype of breast cancer to treat. Some key molecules in breast cancer are tightly involved in proliferation or apoptosis of breast cancer cells, including GATA3, p53, Bax, p21, ELF5, and cyclin-dependent kinases (CDKs), which can affect the viability of cancer cells by influencing the cell cycle, repairing damaged DNA, or inducing apoptosis.14–16 Metastasis is the main cause of death caused by breast cancer, and many molecules are involved in the process, including MPP2, TIMP1, and TIMP2.17–19 In this study, we explored whether extracts from F. carica leaves and their bergapten and psoralen components have anticancer effects on TNBC cell line MDA-MB-231, and investigated the molecular mechanisms underlying this effect by analyzing the expression of key breast cancer biomarkers crucial to cell proliferation, the cell cycle, and migration.
Materials and methods
Materials and reagents
F. carica leaves were collected from Jilin Agriculture University in September 2017 and taxonomically confirmed by Professor Yaohui Hu at the School of Food Science and Engineering. 40 g fig leaves was macerated in 450 mL water at 25°C for 1 hour, and boiled for 2 hours in distillation apparatus. The extract of fig leaves was concentrated to 50 mL, and filtered through a 0.22 μm filter. To calculate the concentration of the extract of fig leaves, the residue of fig leaves was dried and weighed, and totally 20 g of the residue of fig leaves was obtained. Therefore, the concentration of the extract was calculated as following, (40 g–20 g)/50 mL = 400 mg/mL. Psoralen (66-97-7; Meilun Biotechnology, Dalian, China), bergapten (484-20-8; Meilun Biotechnology), an MTT cell-proliferation and cytotoxicity-assay kit (BB-4201; BestBio, Shanghai, China), cell-cycle-detection kit (BB4104; BestBio, Nanjing, China), Trizol Plus RNA-purification kit (12183555; Thermo Fisher Scientific, Waltham, MA, USA), Super RT kit (PR-6601; BioTek, Winooski, VT, USA), and SYBR reverse-transcription polymerase chain reaction (RT-PCR) premixture (PR7701; BioTek) were used in this study.
Cell culture
The human breast epithelial cell line MCF-10A and human breast cancer cell line MDA-MB-231 were a generous gift from Professor Zhe Li (Harvard University, Boston, MA, USA). Use of the gifted MCF-10A and MDA-MB-231 cells was approved by the School of Life Sciences, Jilin University, Changchun, China. MCF-10A cells were cultured with Ham’s F12 medium and DMEM with 2.5 mM L-glutamine (Thermo Fisher Scientific) and supplemented with 5% horse serum (Thermo Fisher Scientific), 0.5 mg/mL hydrocortisone (Sigma-Aldrich, St Louis, MO, USA), 100 ng/mL cholera toxin (Sigma-Aldrich), 20 ng/mL epidermal growth factor (PeproTech, Rocky Hill, NJ, USA), 10 μg/mL insulin (Sigma-Aldrich), and 100 IU/mL penicillin–streptomycin (Sigma-Aldrich). MDA-MB-231 cells were cultured with DMEM (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific) and 100 IU/mL penicillin–streptomycin (Sigma-Aldrich). The culture environment was maintained at 37°C with saturated humidity and 5% CO2.
MTT assays
Cells were seeded at 5,000/well in a 96-well plate and incubated in media with different concentrations of F. carica leaf extract, psoralen, and bergapten for 24, 48, and 72 hours, respectively. The effect of F. carica on cell viability was assessed by MTT (BestBio) solution. After incubation for each period, 10 μL MTT solution (5 mg/mL) was added to each well and incubated for 4 hours at 37°C. Then, the medium was removed and replaced by 150 μL dimethyl sulfoxide (BestBio). Plates were read at 490 nm wavelength using a microplate reader (ELx808; BioTek). All experiments were repeated three times.
Wound-healing assays
MDA-MB-231 cells were seeded in six-well plates at a density such that after 24 hours of incubation, they reached about 90% confluence as a monolayer. A wound was generated by scratching the monolayer with a 200 μL pipette tip across the center of the well. After scratching, wells were gently washed twice to remove the detached cells and replaced with fresh DMEM (without serum). These MDA-MB-231 cells were treated with various graded concentrations of F. carica leaf extract, psoralen, and bergapten. Microscopy images were taken at indicated time points 0, 24, and 48 hours to measure the width of the gaps. Widths of gaps were quantified and analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). Nine duplicate photos were taken for each treatment. All experiments were repeated three times. The average wound size represented relative cell migration.
Cell-cycle analysis
MDA-MB-231 cells were exposed to graded concentrations of F. carica leaf decoction (0, 4 mg/mL) for 48 or 72 hours and collected. Cell-cycle distribution was evaluated using the cell-cycle-detection kit according to the manufacturer’s instructions. Briefly, cells were washed twice with cold PBS and fixed in 70% ethanol at −20°C overnight. Cells were resuspended in cold PBS containing 1% BSA and 20 μL RNase incubated at 37°C for 30 minutes, and then stained with a propidium iodide solution at 4°C for 30 minutes. Cell cycles were analyzed by flow cytometry (FACScan; BD, Franklin Lakes, NJ, USA). Cell-cycle distribution was calculated using ModFit cell-cycle-analysis software (version 2.01.2; BD).
Quantitative RT-PCR
MDA-MB-231 cells were treated with graded concentrations of an F. carica leaf decoction (0, 4, 8 mg/mL) for 48 or 72 hours and collected. RNA isolation and quantitative RT-PCR (qRT-PCR) assays were performed according to the manufacturer’s instructions. Total RNA from cells was extracted with Trizol reagent following the manufacturer’s protocol, and complementary DNA was synthesized using Moloney murine leukemia virus (M-MLV) RT with random primers. Complementary DNA was generated with the Super RT kit according to the manufacturer’s protocol. qRT-PCR was performed using the SYBR RT-PCR premixture. qRT-PCR primer sequences are listed in Table 1.
Table 1.
Quantitative RT-PCR primer sequences (all sequences from 5′ to 3′)
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | GCCGCCAGCTCACCAT | TCGATGGGGTACTTCAGGGT |
| Bax | CCCTTTTGCTTCAGGGTTTC | CCCTTTTGCTTCAGGGTTTC |
| p53 | CCTCAGCATCTTATCCGAGTGG | TGGATGGTGGTACAGTCAGAGC |
| p21 | GTGAGCGATGGAACTTCGACTT | AGAGGTTTACAGTCTAGGTGGA |
| GATA3 | GCCGTTGAGGGTTTCAGAGA | TCCGAGCACAACCACCTTAG |
| ELF5 | CTCTTTGGACCTAGCCACCG | AGCAGTTGGAATCAGCATCTGT |
| CDK1 | CTGGCTGATTTTGGCCTTGC | CCACTTCTGGCCACACTTCA |
| CDK5 | CTCCGGGAGATCTGCCTACT | AGCTCCCCATTCTTTACAATCTCA |
| CDK9 | CAGTACGACTCGGTGGAGTG | TGTAATGGGGAACCCCTCCT |
| CDK10 | TGGACAAGGAGAAGGATG | CTGCTCACAGTAACCCATC |
| MMP2 | GAGTGCATGAACCAACCAGC | GTGTTCAGGTATTGCATGTGCT |
| TIMP1 | GCAATTCCGACCTCGTCATC | TAGACGAACCGGATGTCAGC |
| TIMP2 | CTGCGAGTGCAAGATCACG | TGGTGCCCGTTGATGTTCTT |
Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Statistical analysis
All data analyses were performed with SPSS 18.0 software (SPSS, Chicago, IL, USA). Results are reported as mean ± SD of at least three independent experiments. Student’s t-test was used to compare differences between two groups. Tukey’s post hoc test was used to validate the ANOVA for comparing measurement data between groups. P<0.05 was considered statistically significant.
Results
Treatment with extracts and components of F. carica leaves inhibited proliferation of MDA-MB-231 cells, but not MCF10A cells
To investigate the anticancer effects of extracts and components of F. carica leaves, MTT assays were performed to evaluate the proliferation of the breast cancer cell line MDA-MB-231 and normal breast epithelial cell line MCF10A. MDA-MB-231 and MCF10A cells were incubated with different concentrations of an aqueous extract of F. carica leaves. Analysis indicated that the F. carica leave extract treatment decrease the viability of the MDA-MB-231 cell in a dose- and time-dependent manner (Figure 1A), but did not have any effect on the viability of normal epithelial MCF10A cells (Figure 1B), demonstrating that the antiproliferation effect of F. carica leaf extract was specific to MDA-MB-231 cells. To verify further the observation of the antiproliferation effect of F. carica leaves, MDA-MB-231 cells were treated with psoralen (Figure 1C) and bergapten (Figure 1D), two major active components of F. carica leaves. Treatment with psoralen and bergapten reduced the viability of MDA-MB-231 cells (Figure 1E and F), but not the viability of MCF10A cells (Figure S1).
Figure 1.
Extracts of Ficus carica leaves, psoralen, and bergapten inhibited the proliferation of MDA-MB-231 cells.
Notes: (A) MDA-MB-231 and (B) MCF10A cells were treated with extracts of F. carica leaves (0, 1, 2, 4, 8 mg/mL) at different time points (24, 48, 72 hours). Viability of treated cells evaluated by MTT assays. (C) Chemical structure of psoralen. (D) Chemical structure of bergapten. (E) MDA-MB-231 cells were treated with different concentrations of psoralen (0, 20, 40, 60, 80, 100 μg/mL) and (F) bergapten (0, 5, 10, 15, 20, 25, 30 μg/mL) at different time points (24 hours, 48 hours, 72 hours). Viability of treated cells evaluated by MTT assays. Viability of control cells at 24 hours represented as 100%. Data presented as mean ± SD (n=3). *P<0.01; **P<0.05; ***P<0.001.
Treatment with extracts and components of F. carica suppressed expression of key genes involved in the regulation of cell viability
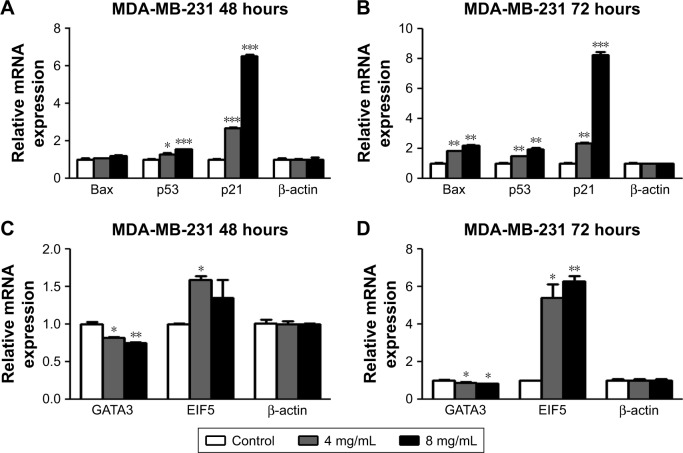
To investigate the mechanism underlying the inhibitory effects of F. carica leaf extract on the proliferation of MDA-MB-231 cells, the expression of genes involved in proliferation and antiapoptosis was examined. F. carica leaf-extract treatment increased the expression levels of BAX, TP53, and TP21 (Figure 2A and B), which were involved in the promotion of apoptosis and regulation of cell viability, supporting the possibility that the inhibitory effects on cell viability might be caused by the promotion of apoptosis. Furthermore, the activation of proteins that were involved in the regulation of apoptosis was examined by Western blotting, and caspase 3 was cleaved upon treatment with extracts of F. carica leaves (Figure S2). In addition, the expressions of the breast cancer-marker gene GATA3 and proto-oncogene ELF5 were also examined by RT-PCR. The results showed that the mRNA level of GATA3 was decreased, while the expression of ELF5 was increased upon treatment (Figure 2C and D). These observations suggest the antitumor proliferation effect of F. carica leaves might be caused by the decreased expression of the breast cancer-marker gene GATA3.
Figure 2.
Extracts of Ficus carica leaves affected the expression of genes that regulate cell viability.
Notes: (A, B) MDA-MB-231 cells were treated with different concentrations of extracts of F. carica leaves (0, 4, 8 mg/mL) at different time points (48, 72 hours). Expression of key genes involved in regulation of cell viability measured by quantitative polymerase chain reaction. (C, D) MDA-MB-231 cells were treated with different concentrations of extracts of F. carica leaves (0, 4, 8 mg/mL) at different time points (48, 72 hours). Expression of key genes of breast cancer biomarkers measured by quantitative polymerase chain reaction. Data presented as mean ± SD (n=3). *P<0.01; **P<0.05; ***P<0.001.
Extract of F. carica leaves induced cell-cycle arrest in MDA-MB-231 cells at the Sphase
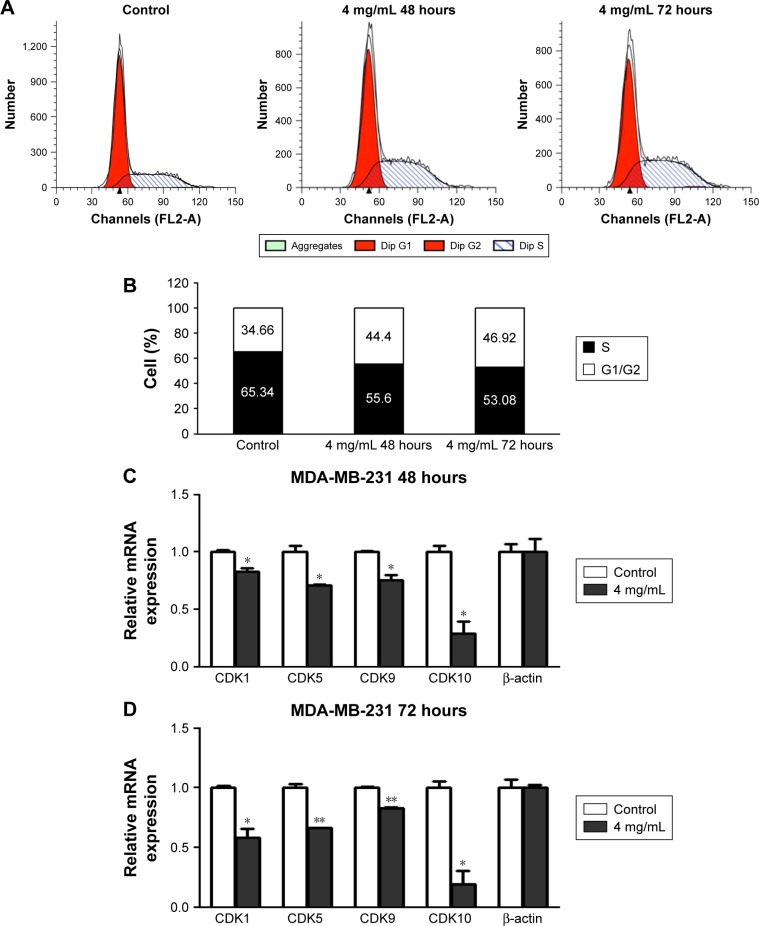
To clarify the mechanism of how F. carica leaf extract inhibited the growth of MDA-MB-231 cells, flow cytometry was performed to examine the effect of F. carica leaf extract on the cell cycle. We found that the percentage of MDA-MB-231 cells in the S phase was significantly increased upon F. carica leaf treatment (Figure 3A and B). Meanwhile, CDK-family protein transcription, which is involved in cell-cycle arrest, was also detected by RT-PCR. The results showed that mRNA levels of the CDK family were downregulated upon treatment with F. carica leaves (Figure 3C and D). These results demonstrated that F. carica leaf extract can induce cell-cycle arrest in MDA-MB-231 cells at the S phase, maybe partly through inhibiting the expression of genes in the CDK family.
Figure 3.
Extracts of Ficus carica leaves induced cell-cycle arrest.
Notes: (A) Cells were incubated with extracts of F. carica leaves at concentrations of 0 or 4 mg/mL for 48 hours or 72 hours, stained with propidium iodide, and numbers of cells at different phases of cell cycle analyzed by flow cytometry. (B) Percentage of cells at different phases of cell cycle shown. Reverse-transcription polymerase chain-reaction analysis showed decreased expression of genes in CDK family in MDA-MB-231 upon treatment with extracts of F. carica leaves for 48 hours (C) or 72 hours (D). Data presented mean ± SD (n=3). *P<0.01; **P<0.05.
Aqueous extract of F. carica leaves and its major active components suppressed migration of MDA-MB-231 cells
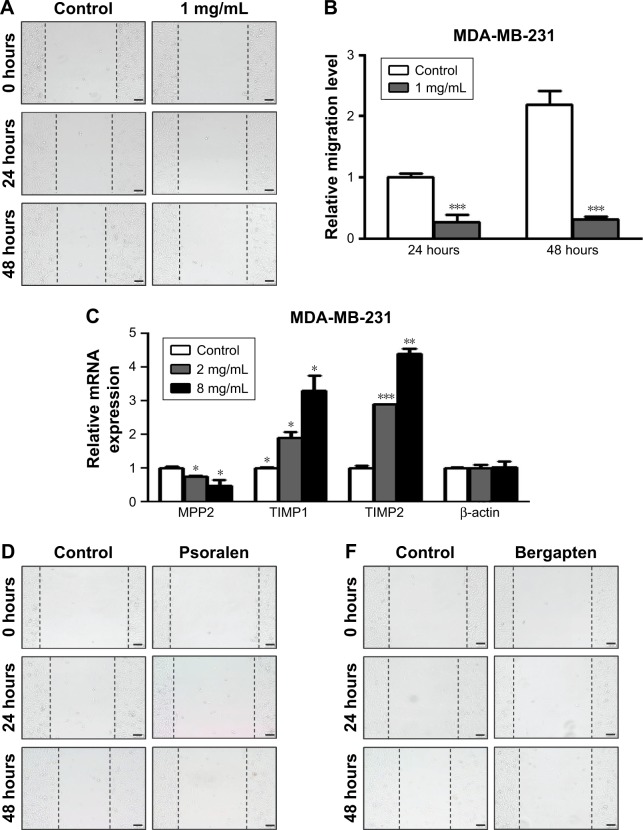
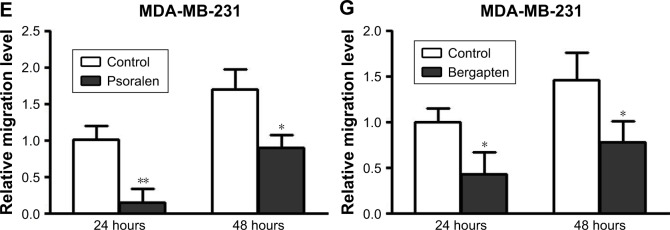
To investigate further the antitumor effects of F. carica leaf extract, we conducted wound-healing assays to examine whether the treatment with F. carica leaf extract could affect the migration of MDA-MB-231 cells. The results showed that migration of MDA-MB-231 cells was significantly reduced by treatment with F. carica leaf extract (Figure 4A and B). Moreover, expressions of genes involved in cell migration were examined by RT-PCR. We found that expression of MPP2, a gene involved in promotion of migration, was significantly decreased upon F. carica leaf treatment. On the other hand, the expressions of genes involved in suppression of migration, eg, TIMP1 and TIMP2, were increased upon F. carica leaf treatment (Figure 4C). The effects of psoralen and bergapten on the mobility of MDA-MB-231 cells were consistent on wound-healing assays: both suppressed the migration of MDA-MB-231 cells (Figure 4D–G).
Figure 4.
Extracts of Ficus carica leaves, psoralen, and bergapten suppressed migration of MDA-MB-231 cells.
Notes: (A) MDA-MB-231 cells were treated with 1 mg/mL extracts of F. carica leaves and mobility of MDA-MB-231 cells evaluated by wound-healing assays. After treatment for indicated times (0, 24, or 48 hours), cell migration in the denuded zone was photographed. Representative images of migrated cells shown. Scale bars represent 100 μm (B) Relative migration of the treated MDA-MB-231 cells was analyzed. Data presented as mean ± SD (n=9, ***P<0.001). (C) MDA-MB-231 cells were treated with 2 or 8 mg/mL extracts of F. carica leaves for 48 hours and expression levels of MMP2, TIMP1, and TIMP2 measured by reverse-transcription polymerase chain reaction. Data presented as mean ± SD (n=3, *P<0.05, **P<0.01, ***P<0.001). (D) MDA-MB-231 cells were treated with 20 μg/mL psoralen for indicated time points (0, 24, or 48 hours), and cell migration in the denuded zone was photographed. Representative images of migrated cells are shown. Scale bars represent 100 μm (E) Relative migration level of psoralen-treated MDA-MB-231 cells was analyzed. Data presented as mean ± SD (n=9, *P<0.05, **P<0.01). (F) MDA-MB-231 cells were treated with 5 μg/mL bergapten for indicated times (0, 24, or 48 hours) and cell migration in the denuded zone photographed. Representative images of migrated cells are shown. Scale bars represent 100 μm (G) Relative migration level of bergapten-treated MDA-MB-231 cells was analyzed. Data presented as mean ± SD (n=9, *P<0.05).
Discussion
In this study, we report that treatment with F. carica extract inhibits the proliferation of MDA-MB-231 cells, but not MCF10A cells. MDA-MB-231 is a TNBC cell line, and MCF10A is a normal breast epithelial cell line. This result suggests that extracts and components of F. carica have specific growth-inhibitory effects on breast cancer cells. In agreement with our result, previous studies have found that fig leaves, fruit, and latex all contain anticancer components.3–8
Previous studies have shown that of fig fruit extracts exhibited moderate cytotoxic activities against MCF7, HepG2, and U2OS cells,3,4 and fig latex has also shown inhibitory effects on proliferation of various cancer cell lines, eg, the Raji B-cell lymphoma stomach cancer line.5–7 Little was known about the antiproliferation effect of the leaf decoction in cancer. We found that of F. carica leaf extract had an antiproliferation effect on TNBC cell MDA-MB-231 and could upregulate the expression of genes involved in apoptosis, such as BAX, TP53, and TP21. Moreover, we also found that F. carica leaf decoction perhaps inhibited the viability of MDA-MB-231 cells partly by enhancing cell-cycle arrest in the S phase. Consistently with the role of CDKs in the cell cycle,15 our results also demonstrated mRNA levels of CDK1, CDK5, CDK9, and CDK10 were down-regulated upon treatment with F. carica leaves. Additionally, we demonstrated that F. carica leaves and the plant’s major components psoralen and bergapten significantly inhibited the migration ability of MDA-MB-231. Consistently with suppressed migration, our results showed an increased expression level of MPP2 and decreased expression level of TIMP1 and TIMP2. To summarize, F. carica L leaves have multiple anticancer effects on breast cancer.
F. carica products have been widely used as both food and medicine, and the plant’s use in multiple cancer prevention, cancer therapy, and anti-inflammation may even have originated in ancient and medieval times.19 Previous research has also shown that F. carica products can inhibit proliferation of various cancer cell lines, such as cervical cancer cell line HeLa. Fig products contain anticancer components, such as bergapten, psoralen, benzaldehyde, amylose, and selenium.8
Although the present study provided some interesting results, there were also a few limitations. First, we did not employ high-enough concentrations of psoralen and ber-gapten find IC50 levels, since these substances are difficult to dissolve, even in dimethyl sulfoxide. Second, cell-cycle analysis has not been done for the compounds, which will be performed in the following study. In conclusion, extracts of F. carica leaves showed anticancer effects in TNBC cells, and thus might be a potential source to develop cancer medicine.
Supplementary materials
Psoralen and bergapten did not affect the survival of the normal mammary epithelial cell line MCF10A.
Notes: MCF10A cells were treated with different concentrations of (A) psoralen (0, 20, 40, 60, 80, 100 μg/mL) and (B) bergapten (0, 5, 15, 20, 25, 30 μg/mL) at different time points (24, 48, 72 hours). Viability of treated cells evaluated by MTT assays. Viability of control cells at 24 hours represented as 100%. Data presented as mean ± SD (n=3).
Extracts of Ficus carica leaves affect the activation of proteins involved in the regulation of apoptosis.
Notes: MDA-MB-231 cells were treated with different concentrations of extracts of F. carica leaves (0, 4, 8 mg/mL) for 72 hours. Protein levels of key apoptosis markers measured by Western blotting with antibodies indicated.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (31471356) and Jilin Province Science and Technology Development Project (20180101240JC).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barolo MI, Mostacero NR, López SN. Ficus carica L. (Moraceae): an ancient source of food and health. Food Chem. 2014;164:119–127. doi: 10.1016/j.foodchem.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 2.Alamgeer GA, Iman S, Asif H, Saleem M. Evaluation of antihypertensive potential of Ficus carica fruit. Pharm Biol. 2017;55(1):1047–1053. doi: 10.1080/13880209.2017.1278611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jing L, Zhang YM, Luo JG, Kong LY. Tirucallane-type triterpenoids from the fruit of Ficus carica and their cytotoxic activity. Chem Pharm Bull. 2015;63(3):237–243. doi: 10.1248/cpb.c14-00779. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Zhang Y, Yang X, et al. Ficus carica polysaccharides promote the maturation and function of dendritic cells. Int J Mol Sci. 2014;15(7):12469–12479. doi: 10.3390/ijms150712469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, He D, Peng Y, et al. Matrine suppresses the migration and invasion of NSCLC cells by inhibiting PAX2-induced epithelial-mesenchymal transition. Onco Targets Ther. 2017;10:5209–5217. doi: 10.2147/OTT.S149609. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Hashemi SA, Abediankenari S, Ghasemi M, Azadbakht M, Yousefzadeh Y, Dehpour AA. The effect of fig tree latex (Ficus carica) on stomach cancer line. Iran Red Crescent Med J. 2011;13(4):272–275. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Wang X, Jiang S, et al. Cytotoxicity of fig fruit latex against human cancer cells. Food Chem Toxicol. 2008;46(3):1025–1033. doi: 10.1016/j.fct.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Ikegami H, Nogata H, Inoue Y, et al. Expression of FcFT1, a flowering locus T-like gene, is regulated by light and associated with inflorescence differentiation in fig (Ficus carica L.) BMC Plant Biol. 2013;13:216. doi: 10.1186/1471-2229-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro M, Guido C, de Amicis F, et al. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol Rep. 2016;35(1):568–576. doi: 10.3892/or.2015.4327. [DOI] [PubMed] [Google Scholar]
- 10.Panno ML, Giordano F. Effects of psoralens as anti-tumoral agents in breast cancer cells. World J Clin Oncol. 2014;5(3):348–358. doi: 10.5306/wjco.v5.i3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Cheng K, Han Y, et al. Effects of psoralen as an anti-tumor agent in human breast cancer MCF-7/ADR cells. Biol Pharm Bull. 2016;39(5):815–822. doi: 10.1248/bpb.b15-00957. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Sun Z, Ye Y, Han X, Song X, Liu S. Psoralen inhibits bone metastasis of breast cancer in mice. Fitoterapia. 2013;91:205–210. doi: 10.1016/j.fitote.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 13.del Pup L, Peccatori FA. Is ovulation induction with letrozole in breast cancer patients still safe even if it could increase progesterone levels? Eur Rev Med Pharmacol Sci. 2018;22(1):246–249. doi: 10.26355/eurrev_201801_14125. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Guo Y, Fu X, et al. Dendrobium candidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers. Onco Targets Ther. 2016;9:21–30. doi: 10.2147/OTT.S93305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 16.Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol. 2009;329(2):227–241. doi: 10.1016/j.ydbio.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Fu X, Wang Y, et al. Erianin inhibits the proliferation of T47D cells by inhibiting cell cycles, inducing apoptosis and suppressing migration. Am J Transl Res. 2016;8(7):3077–3086. [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansky EP, Paavilainen HM, Pawlus AD, Newman RA. Ficus spp. (fig): ethnobotany and potential as anticancer and anti-inflammatory agents. J Ethnopharmacol. 2008;119(2):195–213. doi: 10.1016/j.jep.2008.06.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Psoralen and bergapten did not affect the survival of the normal mammary epithelial cell line MCF10A.
Notes: MCF10A cells were treated with different concentrations of (A) psoralen (0, 20, 40, 60, 80, 100 μg/mL) and (B) bergapten (0, 5, 15, 20, 25, 30 μg/mL) at different time points (24, 48, 72 hours). Viability of treated cells evaluated by MTT assays. Viability of control cells at 24 hours represented as 100%. Data presented as mean ± SD (n=3).
Extracts of Ficus carica leaves affect the activation of proteins involved in the regulation of apoptosis.
Notes: MDA-MB-231 cells were treated with different concentrations of extracts of F. carica leaves (0, 4, 8 mg/mL) for 72 hours. Protein levels of key apoptosis markers measured by Western blotting with antibodies indicated.