Abstract
Purpose
Therapies with novel mechanisms of action are needed for multiple myeloma (MM). T cells can be genetically modified to express chimeric antigen receptors (CARs), which are artificial proteins that target T cells to antigens. B-cell maturation antigen (BCMA) is expressed by normal and malignant plasma cells but not normal essential cells. We conducted the first-in-humans clinical trial, to our knowledge, of T cells expressing a CAR targeting BCMA (CAR-BCMA).
Patients and Methods
Sixteen patients received 9 × 106 CAR-BCMA T cells/kg at the highest dose level of the trial; we are reporting results of these 16 patients. The patients had a median of 9.5 prior lines of MM therapy. Sixty-three percent of patients had MM refractory to the last treatment regimen before protocol enrollment. T cells were transduced with a γ-retroviral vector encoding CAR-BCMA. Patients received CAR-BCMA T cells after a conditioning chemotherapy regimen of cyclophosphamide and fludarabine.
Results
The overall response rate was 81%, with 63% very good partial response or complete response. Median event-free survival was 31 weeks. Responses included eradication of extensive bone marrow myeloma and resolution of soft-tissue plasmacytomas. All 11 patients who obtained an anti-MM response of partial response or better and had MM evaluable for minimal residual disease obtained bone marrow minimal residual disease–negative status. High peak blood CAR+ cell levels were associated with anti-MM responses. Cytokine-release syndrome toxicities were severe in some cases but were reversible. Blood CAR-BCMA T cells were predominantly highly differentiated CD8+ T cells 6 to 9 days after infusion. BCMA antigen loss from MM was observed.
Conclusion
CAR-BCMA T cells had substantial activity against heavily treated relapsed/refractory MM. Our results should encourage additional development of CAR T-cell therapies for MM.
INTRODUCTION
Multiple myeloma (MM) is an almost always incurable malignancy of plasma cells. In recent years, several new therapies for MM have prolonged survival of patients with MM, but cure for MM remains elusive. MM therapies with novel mechanisms of action continue to be needed.1-4
A chimeric antigen receptor (CAR) is a fusion protein containing T-cell–signaling domains and an antigen-recognition moiety.5-9 T cells transduced with CARs directed against the B-cell antigen CD19 have established efficacy in leukemia10-14 and lymphoma.15-19 The success of anti-CD19 CAR T-cell therapies against leukemia and lymphoma has encouraged development of CARs targeting MM.5,20-23
B-cell maturation antigen (BCMA) is a member of the tumor necrosis factor superfamily; BCMA is found on MM cells, normal plasma cells, and a small subset of normal B cells; BCMA is not expressed on other normal cells.5,20,24-28 This favorable expression pattern led us to develop the first reported anti-BCMA CARs.20 We tested one of the anti-BCMA CARs that we designed (CAR-BCMA) in the first-in-humans clinical trial, to our knowledge, of an anti-BCMA CAR.22 Here, we report final results of this first in humans study.
PATIENTS AND METHODS
Clinical Trial and Patient Information
All enrolled patients gave informed consent. The study was approved by the Institutional Review Board of the National Cancer Institute and was registered as ClinicalTrials.gov NCT02215967. The US Food and Drug Administration permitted an Investigational New Drug Application for CAR-BCMA T cells. BCMA expression on MM was required for study enrollment.
Preparation of CAR-BCMA T Cells
The CAR-BCMA chimeric antigen receptor was encoded by the gamma-retroviral mouse stem cell-based splice-gag vector and contained a murine anti-BCMA single-chain variable fragment, hinge and transmembrane regions from human CD8α, the CD28 costimulatory domain, and the CD3ζ T-cell activation domain.20,22 Peripheral blood mononuclear cells were collected from patients by leukapheresis, and whole peripheral blood mononuclear cells were cultured and transduced. T cells were infused a median of 9 (range, 9 to 10) days after initiation of culture. Additional cell production details are available in the Data Supplement.
Patient Treatment Plan
Patients received cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 daily on days −5 to −3 before CAR-BCMA T-cell infusion on day 0. Chemotherapy was administered to enhance the activity of adoptively transferred T cells.29-31 The dose levels tested were 0.3, 1, 3, and 9 × 106 CAR+ T cells/kg. MM response assessment was conducted according to the International Uniform Response Criteria for Multiple Myeloma.32 Cytokine-release syndrome (CRS) was graded as described.33
Ex Vivo Assays
Immunohistochemistry, flow cytometry including minimal residual disease (MRD) detection by eight-color flow cytometry, quantitative polymerase chain reaction, cytokine assays, and statistical comparisons were performed as described in the Data Supplement and as previously performed.22 Comparisons were made with nonparametric statistics, as described in the figure legends.
RESULTS
Patient Characteristics
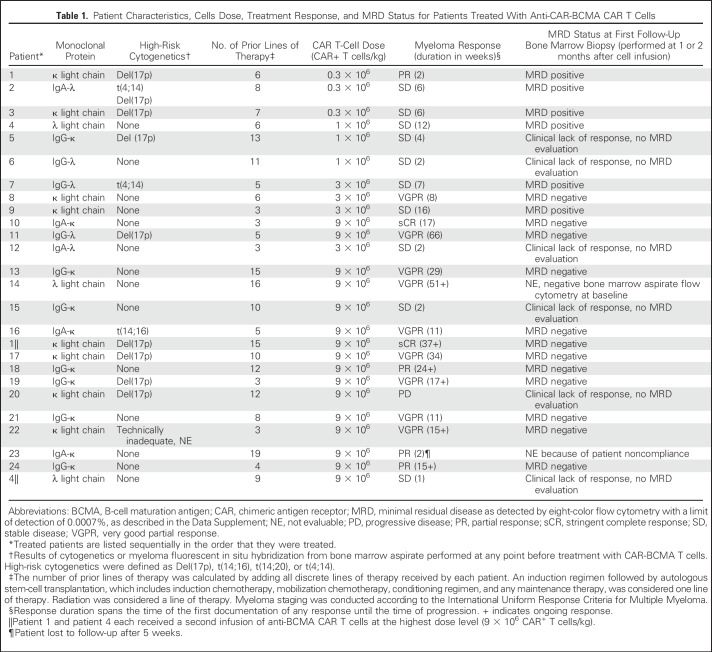
CAR-BCMA T-cell infusions were administered to 24 patients in this clinical trial. Ten patients received CAR-BCMA T-cell infusions at doses of 0.3 to 3 × 106 CAR+ T cells/kg, as previously reported (Table 1).22 Short-term follow-up on patients 10 and 11, who received 9 × 106 CAR+ T cell/kg, was also reported.22 Sixteen patients received CAR-BCMA T-cell infusions at the highest dose level of 9 × 106 CAR+ T cells/kg (Table 1). Patients 1 and 4 were treated twice, once at the highest dose level and once at lower dose levels (Table 1). This report is focused on the 16 patients treated at the highest dose level. Unless specifically noted, statements in this report refer to the 16 patients receiving the highest dose level. Patients had a median of 9.5 (range, 3 to 19) lines of therapy before protocol enrollment (Data Supplement). Sixty-three percent of patients were refractory to their last treatment regimen, defined as response less than partial response (PR) after most recent myeloma therapy or progressive disease (PD) within 60 days after most recent myeloma therapy. Forty percent of evaluable patients receiving the highest dose level had high-risk cytogenetics, including 33% with Del(17p) (Table 1). Pretreatment characteristics were similar among patients treated with the lower dose levels and the 9 × 106 CAR+ T cells/kg dose level (Table 1; Data Supplement).22 For the patients receiving the highest dose level, a median of 40% (range, 23.2% to 66.0%) of the infused T cells expressed CAR-BCMA (Data Supplement). All patients treated at the 9 × 106 CAR+ T cells/kg dose level are included in this report. Three patients were enrolled but did not receive CAR-BCMA T cells, one because of rapid progression of MM, one because of lack of BCMA expression on MM, and one because of possible bacterial contamination of the cell product.
Table 1.
Patient Characteristics, Cells Dose, Treatment Response, and MRD Status for Patients Treated With Anti-CAR-BCMA CAR T Cells
Anti-BCMA CAR T Cells Induce Remissions in Patients With Heavily Pretreated MM
At doses of 0.3 to 3 × 106 CAR+ T cells/kg, only two of 10 patients experienced responses of PR or better (Table 1).22 Of the 16 patients who received 9 × 106 CAR+ T cells/kg, 13 patients had responses of PR or better (Table 1). The overall response rate for patients receiving 9 × 106 CAR+ T cells/kg was 81%, and all patients except patient 20 had a substantial decrease in their serum MM marker (Fig 1A). The median event-free survival for patients treated at a cell dose of 9 × 106/kg was 31 weeks (Fig 1B). Currently, six patients, all treated with the highest dose level, have ongoing responses. The other 10 patients receiving the highest dose level have experienced myeloma progression.
Fig 1.
Chimeric antigen receptor (CAR)–B-cell maturation antigen (BCMA) T cells have antimyeloma activity. (A) Waterfall plot with each bar representing the maximum percentage change of the myeloma marker for each patient. We compared pretreatment and nadir post-treatment values of involved free κ or λ light chains or the intact monoclonal antibody (M protein). The intact immunoglobulin M protein was used if it was measurable. If the M protein was not measurable, free light chains were used. (B) Event-free survival in weeks for patients treated with 9 × 106 CAR+ T cells/kg. Median event-free survival was 31 weeks. Seven patients were censored. One patient was censored because he refused to come to appointments starting 5 weeks after CAR-BCMA infusion. The other six patients were censored for being in an ongoing response at last follow-up. Only five censor marks are present because two patients each were censored at 17 and 26 weeks after CAR-BCMA infusion, and the censor marks are too close to be distinguished. Events were all progressive multiple myeloma except for patient 13, in whom the event was initiation of new multiple myeloma therapy without clear progression of myeloma. (C) Before CAR-BCMA T-cell infusion, patient 14 had a large left abdominal soft tissue plasmacytoma, as seen on computed tomography imaging. (D) This abdominal mass significantly decreased in size 4 weeks after CAR-BCMA T-cell infusion. (E) The mass continued to decrease in size 9 weeks after CAR-BCMA T-cell infusion. (F) The mass was not appreciable on computed tomography 55 weeks after CAR-BCMA T-cell infusion. (G) Patient 14’s λ light chains decreased quickly after CAR-BCMA T-cell infusion. Twenty-five weeks after CAR-BCMA T-cell infusion, λ light chains began to increase, but the ratio of λ to κ light chains remained normal, indicating recovery of normal plasma cells. (H) Patient 14 received intravenous immunoglobulin infusions for hypogammaglobulinemia after CAR-BCMA T-cell infusion, as indicated by the gold arrows. His IgG, IgA, and IgM subsequently all increased. He has not received intravenous immunoglobulins since 13 weeks after CAR-BCMA T-cell infusion. (I) Plasma cells were evaluated in bone marrow core biopsies by CD138 immunohistochemistry (IHC) staining before CAR-BCMA treatment and 2 months after CAR-BCMA T-cell infusion. The percentage of plasma cells decreased in all nine evaluable patients. P = .0039 by Wilcoxon matched-pairs signed rank test. Only nine of 16 patients were evaluable for plasma cell eradication by IHC, because three patients had levels of bone marrow myeloma too low to evaluate by IHC pretreatment, three patients did not have a post-treatment bone marrow biopsy because of lack of clinical response, and one patient did not have a post-treatment bone marrow biopsy because of patient noncompliance.
Patient 14 had a large abdominal mass that resolved on computed tomography imaging (Figs 1C-1F). His λ light chains decreased precipitously and became undetectable after CAR T-cell infusion (Fig 1G). Six months after cell infusion, λ light chains began to increase, but the κ to λ ratio remained normal, and IgG, IgA, and IgM immunoglobulins increased (Figs 1G and 1H); this pattern indicated recovery of normal plasma cells. Patient 14’s anti-MM response is very good PR (VGPR) ongoing at 51 weeks.
CAR-BCMA T Cells Effectively Deplete the Bone Marrow of Plasma Cells
Nine patients were evaluated with bone marrow biopsies and immunohistochemistry staining for CD138 before and 2 months after CAR-BCMA infusion. In all nine patients, bone marrow plasma cells decreased between these time points (Fig 1I). Eleven of 11 evaluated patients were found to be MRD-negative by bone marrow flow cytometry. Five patients were not evaluated for MRD: three patients because of clinical lack of response, one patient because of an MRD-negative bone marrow before protocol enrollment, and one patient because of patient noncompliance. (Table 1).
Patient 1’s bone marrow biopsy showed eradication of plasma cells by CD138 and BCMA staining (Figs 2A and 2B). Her κ light chains became undetectable 14 days after CAR-BCMA T-cell infusion (Fig 2C), and she obtained a stringent complete response (sCR), ongoing at 37 weeks. Twenty-nine months before patient 1 was treated with 9 × 106 CAR-BCMA T cells/kg, she had been treated with 0.3 × 106 CAR-BCMA T cells/kg, which yielded a PR lasting 2 weeks.22 A second patient, patient 4, also received two CAR-BCMA T-cell treatments. Patient 4 received a first treatment with 1 × 106 CAR-BCMA T cells/kg, followed 34 months later by a second treatment with 9 × 106 CAR-BCMA T cells/kg.22 Patient 4 obtained responses of stable disease (SD) after both treatments.
Fig 2.
Chimeric antigen receptor (CAR)–B-cell maturation antigen (BCMA) T cells eradicated bone marrow myeloma, and serum BCMA declined with myeloma elimination. (A) Patient 1’s bone marrow biopsy before infusion of CAR-BCMA T cells demonstrated 20% to 25% involvement with multiple myeloma by CD138 staining. The multiple myeloma cells expressed BCMA. (B) Eleven weeks after CAR-BCMA T-cell infusion, Patient 1’s bone marrow biopsy showed no evidence of multiple myeloma by CD138 or BCMA staining. (C) Patient 1’s κ light chains decreased quickly after CAR-BCMA T-cell infusion and remained undetectable 38 weeks after CAR-BCMA T-cell infusion. (D) BCMA can be detected in patient serum. Patients with responses of partial response, very good partial response, and stringent complete response had substantially decreased levels of serum BCMA after CAR-BCMA T-cell infusion. P < .001 for the comparison of before treatment and after treatment. (E) Serum BCMA levels remained stable or only slightly decreased in patients with responses of stable disease or progressive disease after CAR-BCMA T-cell infusion (P = .75, not significant). For D and E, before treatment samples were collected just before the start of conditioning chemotherapy. After treatment samples were collected 26 to 39 days after CAR T-cell infusion, except for patient 20, whose sample was collected 15 days after CAR T-cell infusion, and patient 23, whose sample was collected 14 days after CAR T-cell infusion. Statistical comparison was by the Wilcoxon matched-pairs signed rank test. (F) Patient 21 had a myeloma response of very good partial response. Thirteen weeks after CAR-BCMA infusion, the patient had myeloma progression with increased κ light chains and progression of bone lesions. Simultaneously, serum BCMA increased. (G) Compared with patients experiencing less than grade 3 cytokine-release syndrome (CRS), patients experiencing grade 3 or 4 CRS had a significantly higher percentage of bone marrow plasma cells before CAR-BCMA T-cell infusion. P = .04 by Mann-Whitney test. H and E, hematoxylin and eosin.
Serum BCMA Declines Along With Myeloma Burden
BCMA is elevated in the serum of patients with MM.34 In patients obtaining anti-MM responses, serum BCMA decreased (Fig 2D). In contrast, serum BCMA only underwent minor changes in patients who did not obtain anti-MM responses (Fig 2E). Serum BCMA decreased as patient 21’s MM entered a VGPR; when MM progressed in multiple boney sites and serum free light chains started to increase, her serum BCMA also increased, indicating that BCMA could be a tumor marker for MM (Fig 2F).
Toxicities of Anti-BCMA CAR T Cells
CAR-BCMA T-cell toxicity was mild at lower dose levels, with no cytokine-release syndrome (CRS) of grades 3 or 4 at cell doses of 0.3 to 3 × 106 CAR+ T cells/kg (Data Supplement).22 At a cell dose of 9 × 106 CAR+ T cells/kg, CRS-related toxicities were substantial (Table 2). The first two patients treated with 9 × 106 CAR+ T cells/kg, patients 10 and 11, had 90% and 80% of bone marrow cells that were MM plasma cells, respectively. Both patients 10 and 11 experienced severe CRS toxicities. Because of the toxicities experienced by patients 10 and 11, the latter 14 patients treated with 9 × 106 CAR-BCMA T cells/kg were required to have lower MM burdens. Each of the latter 14 patients had < 30% bone marrow plasma cells before CAR-BCMA infusion.
Table 2.
Adverse Events, Cytokine Release Syndrome Grade, and Immunosuppression After Anti-BCMA CAR T-Cell Infusion at a Dose of 9.0 × 106 CAR+ T cells/kg
Six of 16 patients receiving 9 × 106 CAR-BCMA T cells/kg (38%) required vasopressor support for hypotension, and one patient required mechanical ventilation. Five patients (31%) received tocilizumab, and four patients (25%) received corticosteroids for adrenal insufficiency or CRS-related toxicities (Table 2). Patients with CRS of grade 3 or 4 had higher levels of bone marrow plasma cells compared with patients who had less than grade 3 CRS, which indicates an increased chance of severe CRS in patients with high MM burdens (Fig 2G).
Neurologic toxicities were limited to confusion or delirium in the setting of severe CRS, except for patient 15 who experienced encephalopathy and muscle weakness of all extremities. This patient was severely ill with grade 4 CRS at the time of his neurologic toxicity, and his extremity weakness was consistent with critical illness polyneuropathy/polymyopathy.35 Extensive neurologic defects, as seen in clinical trials of anti-CD19 CAR T cells, were not observed.15-17
Most cytopenias occurred early after CAR-BCMA T-cell infusion and were attributable to the cyclophosphamide and fludarabine conditioning chemotherapy, but some periods of cytopenia were delayed until after recovery of blood counts from chemotherapy. Patient 14 developed pancytopenia with grade 4 thrombocytopenia 28 days after CAR-BCMA T-cell infusion. Patient 16 developed pancytopenia with grade 4 thrombocytopenia 25 days after CAR-BCMA T-cell infusion. Neutropenia rapidly resolved with filgrastim administration in both cases. Both patients received the thrombopoietin receptor agonist eltrombopag and prednisone for thrombocytopenia (Table 2). Platelets recovered to > 50,000/µL without transfusion support by 63 and 53 days after CAR-BCMA T-cell infusion in patients 14 and 16, respectively.
Association of Serum Cytokines With CRS
We assessed the serum levels of 19 cytokines before CAR-BCMA T-cell infusions and at multiple time points after CAR-BCMA infusions. The peak fold-increase over the before-treatment level was calculated for each cytokine in each patient (Fig 3A). The cytokines with the largest median fold-increases were interferon-γ, interleukin (IL)-6, IL-10, and granulocyte-macrophage colony-stimulating factor. The cytokines in which high peak blood levels were most closely associated with grade 3 and 4 CRS were IL-15, IL-10, and IL-8 (Figs 3B-3D). IL-6, IL-4, and tumor necrosis factor-α were also associated with grade 3 and 4 CRS. For all tested cytokines, P values and fold-difference for comparisons of cytokine levels with CRS grades are in the Data Supplement.
Fig 3.
Many cytokines were prominently increased after anti-BCMA CAR (CAR-BCMA) T cell infusions, and peak CAR cell levels were associated with anti-myeloma responses. (A) Serum levels of 19 cytokines were determined at multiple time points after CAR-BCMA infusion for all patients. For each cytokine, the peak fold-increase over pretreatment cytokine levels was calculated. The peak fold-increase is shown as a blue dot, and the median peak fold-increase for each cytokine is shown as a gold bar. (B-D) The peak absolute serum levels of each of 19 cytokines were compared between patients with a maximum CRS grade of 3 or 4 at any time point versus patients with a maximum CRS grade less than 3 at all time points. The three cytokines with the lowest P value for the comparison were (B) IL-15, (C) IL-10, and (D) IL-8. For these comparisons, all 16 patients were included. Comparison of peak cytokine levels was made by the Mann-Whitney test, and correction for multiple comparisons was performed with the Bonferroni-Dunn test. (E and F) The percentage of peripheral blood mononuclear cell that were CAR+ cells was determined by quantitative polymerase chain reaction, and the absolute number of CAR+ cells/μL of blood was determined by multiplying the percentage of CAR+ cells by the sum of blood lymphocytes plus monocytes/μL. The time courses of CAR+ cell levels in the blood of each patient obtaining responses of stringent complete response, very good partial response, or partial response (responders) were determined. The patients are divided among two graphs to allow viewing of the lines for each patient. Day 0 is the day of CAR-BCMA infusion. (G) The time courses of CAR+ cell levels in the blood of the three patients with treatment outcomes of stable disease or progressive disease (nonresponders) were determined by quantitative polymerase chain reaction. The y-axis scale is the same as in (A) to emphasize the lower level of blood CAR+ cells in nonresponders. (H) Peak blood CAR+ cell levels were higher in responders versus nonresponders. (P = .013, Mann-Whitney test; n = 16). GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; TNF, tumor necrosis factor.
High Blood CAR+ Cell Levels Were Associated With Antimyeloma Responses
Peak CAR+ cell levels have previously been associated with antimalignancy responses in patients treated with CAR T cells.17,36 In this study, peak levels of CAR T cells occurred between 7 and 14 days after CAR-BCMA T-cell infusion for all patients (Figs 3E and 3F). The peak CAR+ cell level and CAR+ cell level 1 month after infusion are in the Data Supplement. The peak CAR T-cell levels of patients who obtained antimyeloma responses of sCR, VGPR, or PR (responders) were higher than the peak CAR T-cell levels of patients who obtained outcomes of SD or PD (nonresponders), (Figs 3E-3H). Notably, peak CAR T-cell levels were not different between patients experiencing grade 3 to 4 CRS and patients experiencing less than grade 3 CRS (Data Supplement). CAR+ cells were detected in the bone marrow at similar levels as in blood (Data Supplement).
CAR-BCMA T Cells Became More Differentiated After Infusion
We saw a striking decrease in the CD4:CD8 ratio of CAR-BCMA T cells after infusion (Fig 4A). The predominantly CD8+ T cells present after infusion had acquired a more differentiated phenotype. Senescence is defined as a loss of T-cell proliferative capacity, and T-cell exhaustion refers to a loss of functional capabilities, such as the ability to make cytokines.37 Both CD4+ and CD8+ CAR-BCMA T cells had a higher fraction of cells expressing the T-cell senescence marker killer cell lectin-like subfamily receptor G1 after infusion compared with before infusion (Fig 4B).38 CD57 is another marker of T-cell senescence that has been shown to be an indicator of limited T-cell proliferative capacity.38,39 The fraction of both CD4+ and CD8+ CAR-BCMA T cells expressing CD57 increased after infusion, but the magnitude of increase was greater for CD8+ T cells (Fig 4C). The fraction of CAR-BCMA T cells expressing programed cell death protein 1 (PD-1), a marker of T-cell exhaustion and activation,37,38 also increased after infusion (Fig 4D). T cells can be divided into four groups on the basis of T-cell memory phenotype.40 CAR-BCMA T cells contained a larger fraction of cells with the more differentiated effector memory and effector memory RA phenotypes after infusion compared with before infusion. There was a corresponding decrease in the fraction of CAR-BCMA T cells with the less differentiated naïve and central memory T-cell phenotypes after infusion (Figs 4E and 4F). The fraction of CAR-BCMA T cells, especially CD8+ T cells, expressing CD28 decreased after infusion (Data Supplement).
Fig 4.
After infusion, CD8+ highly differentiated T cells dominate the chimeric antigen receptor (CAR)–B-cell maturation antigen (BCMA) T-cell population, and multiple myeloma (MM) cells can lose BCMA expression after anti-BCMA CAR T cells (CAR-BCMA) T-cell infusions. (A) The ratio of CD4+ to CD8+ CAR-BCMA T cells decreased after infusion (P < .001). For A, data were from CD4 versus CD8 flow cytometry plots gated on CD3+CAR+ lymphocytes. For A through F, infusion means a cell sample obtained just before infusion, and ex vivo means cells from a patient blood sample obtained 6 to 9 days after infusion. For A through F, all comparisons were made by the Wilcoxon matched-pairs signed rank test (n = 14). Patients 4 and 20 are not included, because their postinfusion blood CAR+ cell levels were too low for accurate flow cytometry analysis. (B) The fraction of CAR+ T cells expressing KLRG-1 increased significantly on CAR-BCMA–expressing T cells after infusion. P < .001 for the comparison of CD4+ infusion versus ex vivo CAR-BCMA T cells. P < .001 for the comparison of CD8+ infusion versus ex vivo CAR-BCMA T cells. (C) The fraction of CAR+ T cells expressing CD57 increased significantly on CAR-BCMA–expressing T cells after infusion. P = .004 for the comparison of CD4+ infusion versus ex vivo CAR-BCMA T cells. P < .001 for the comparison of CD8+ infusion versus ex vivo CAR-BCMA T cells. (D) The fraction of CAR+ T cells expressing PD1 increased significantly on CAR-BCMA–expressing T cells after infusion. P < .001 for the comparison of CD4+ infusion versus ex vivo CAR-BCMA T cells. P < .001 for the comparison of CD8+ infusion versus ex vivo CAR-BCMA T cells. For B through D, data are from plots gated on either CD4+CD3+ lymphocytes or CD8+CD3+ lymphocytes. (E and F) After infusion, the fraction of CAR+ T cells that had a phenotype consistent with either naïve or central memory (CM) T cells decreased, and the fraction of CAR+ T cells with a phenotype consistent with either effector memory (EM) T cells or effector memory RA (TEMRA) T cells increased. Naïve was defined as C-C chemokine receptor 7 (CCR7)+ CD45RA+. CM was defined as CCR7+ CD45RA-negative. EM was defined as CCR7-negative CD45RA-negative. TEMRA was defined as CCR7-negative CD45RA+. These markers were evaluated on CD3+ lymphocytes that expressed CAR-BCMA and either CD4 (E) or CD8 (F). For CD4+ T cells, there were statistically significant differences between infusion and ex vivo Naïve + CM (P < .001) and infusion and ex vivo EM + TEMRA (P < .001). For CD8+ T cells, there were statistically significant differences between infusion and ex vivo Naïve + CM (P < .001) and infusion and ex vivo EM + TEMRA (P < .001). (G) Surface expression of BCMA was measured by flow cytometry before CAR-BCMA therapy. Wide variability in BCMA expression between patients was observed. There was a nonstatistically significant trend (NS by Mann-Whitney test) toward higher BCMA T-cell expression, as measured by antibody binding capacity assay, among patients obtaining responses of stringent complete response, very good partial response, or partial response (responders) compared with patients with outcomes of stable disease or progressive disease (nonresponders). (H) Patient 11 had an MM response of VGPR. For all plots, red indicates MM cells, green indicates normal plasma cells, and purple indicates B cells. Before CAR-BCMA T-cell infusion, the MM population expressed high levels of BCMA. Fifty-six weeks after CAR-BCMA T-cell infusion, the small number of MM cells that were present lacked BCMA expression. Sixty-eight weeks after CAR-BCMA T-cell infusion, the MM cells displayed mixed BCMA expression, with some cells negative for BCMA expression. Multiple myeloma cells were defined as CD138+ CD38+ CD19-negative CD81-negative cells. Normal plasma cells were defined as CD138+ CD38+ CD19+ CD81+ cells. B cells were defined as CD138-negative CD19+ CD20+ cells.
BCMA Loss After CAR-BCMA T-Cell Infusion
We assessed pretreatment cell-surface BCMA expression by flow cytometry and found widely variable BCMA expression between patients (Fig 4G). We demonstrated loss of BCMA from myeloma cells of patient 11 56 weeks after CAR-BCMA T-cell infusion. Patient 11’s bone marrow MM cells had mixed BCMA expression, with some BCMA+ cells and some BCMA-negative cells at the time of myeloma progression (Fig 4H).
DISCUSSION
This study is the first-in-humans clinical trial of CAR T cells targeting BCMA. CAR T cells have the advantage of being mechanistically different from all other MM treatment modalities, and this trial has demonstrated anti-MM activity by CAR-BCMA T cells against MM resistant to other therapies, including MM with the Del(17p) chromosome abnormality. Thirteen of the 16 patients treated with the highest dose level of the study obtained responses of sCR, VGPR, or PR. Unlike most other MM therapies,2,3 CAR T-cell therapy is either a one-time or an infrequent intervention. The infrequent nature of CAR T-cell therapy allows some patients treatment-free intervals, when they often enjoy a high quality of life without therapy-related toxicities; however, the short-term CRS toxicities after CAR T-cell infusions are a disadvantage for CAR T-cell therapies.
This work highlights many areas for improvement of anti-BCMA CAR T-cell therapies. In our current work (Fig 3H) and previous work,12,17,36 high blood peak CAR+ cell levels were associated with clinical antimalignancy responses; therefore, efforts to enhance in vivo CAR T-cell proliferation and survival are important areas for future research. We have shown that long-term persistence of anti-CD19 CAR T cells is not necessary to maintain remissions in at least some patients with lymphoma.18 The importance of persistence of CAR T cells in treating MM requires additional study. Most CARs in clinical use today, including the CAR used in this study, have antigen-recognition domains derived from mouse antibodies that are potentially immunogenic and possibly susceptible to immunologic rejection. The immunogenicity of CARs might be reduced by using human antigen-recognition domains.41 Reducing immunogenicity might be especially important if multiple doses of CAR T cells are administered. In vivo survival of CAR T cells might be improved by finding methods to delay acquisition of the highly differentiated, senescent phenotype that we found in ex vivo CAR T cells (Figs 4B-4F). This highly differentiated T-cell phenotype was also detected in prior studies.36,42
Toxicity was substantial in this study, with six of 16 patients receiving the highest dose level requiring vasopressors. Similar to prior reports of an association between high bone marrow leukemia burden and severe CRS,11,12 we found that grade 3 or 4 CRS was associated with a high level of bone marrow plasma cells (Fig 2G). We analyzed the cytokines in the blood of all patients in the trial. The cytokines most closely associated with CRS were IL-15, IL-10, and IL-8 (Figs 3B-3D). We previously reported BCMA loss in a patient obtaining a response of SD after CAR-BCMA treatment.22 BCMA loss was demonstrated in a second patient who obtained a long VGPR after CAR-BCMA therapy (Fig 4H). BCMA expression is highly variable in MM; the MM of some patients expresses low levels of BCMA (Fig 4G). MM might be especially susceptible to escape from single-antigen therapies, because MM has been shown to be a phenotypically heterogeneous malignancy, with numerous subclones within the same patient.43-45 An example of heterogeneous BCMA expression is patient 11’s BCMA expression 68 weeks after treatment (Fig 4H). The sometimes low or absent BCMA expression on MM cells provides a rationale for targeting antigens other than BCMA. In addition to BCMA, there are other promising antigens for CAR T-cell therapy of MM, but appropriate antigens will likely be rare, because few proteins are limited to only nonessential tissues.5 When CAR T cells are used, avoiding antigens expressed in essential tissues is probably necessary, because using CARs to target antigens that were expressed by normal organs has led to severe toxicities.46,47
This work demonstrated that CAR-BCMA T cells have substantial anti-MM activity. The small number of patients treated is a weakness of this study. Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity. These results should encourage efforts to increase the efficacy of anti-BCMA CAR T-cell therapies by increasing blood CAR T-cell levels, increasing CAR T-cell survival in vivo, targeting antigens other than BCMA, combining CAR T cells with other anti-MM therapies, and administering multiple doses of CAR T cells. Improving durability of responses will be the main measure for improvement of efficacy of CAR T-cell therapies. It is possible that durability of response will be best increased by a more complete eradication of myeloma cells early after CAR T-cell infusion. Because most patients in this study did not obtain CRS, improvement of eradication of MM is clearly needed. Another area for improvement of CAR T-cell therapies is prevention and management of toxicity. Clinical improvement in all CAR T-cell therapies will involve increasing the ratio of antimalignancy activity to toxicity. Anti-BCMA CAR T cells are a novel and powerful therapy for MM, with many promising avenues for improvement.
ACKNOWLEDGMENT
We thank the staff of the National Institutes of Health (NIH) Clinical Center Department of Transfusion Medicine, including Jolynn Procter, Naoza Collins-Johnson, Vicki Fellowes, and Minh Tran, for chimeric antigen receptor B-cell maturation agent T-cell production; Steven Rosenberg, Surgery Branch, National Cancer Institute, NIH, for assistance with γ-retroviral vector production; the Experimental Transplantation and Immunology Branch, NCI clinical inpatient service staff, Shayla Duncan, for clinical specimen processing; the NIH Clinical Center 3 Northeast inpatient unit nursing staff; and the NIH Clinical Center intensive care unit medical staff.
Footnotes
Clinical trial information: NCT02215967.
Listen to the podcast by Dr Becker at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer N. Brudno, Michael Wang, Steven A. Feldman, Ronald E. Gress, James N. Kochenderfer
Collection and assembly of data: Jennifer N. Brudno, Irina Maric, Steven D. Hartman, Jeremy J. Rose, Michael Wang, Norris Lam, Maryalice Stetler-Stevenson, Dalia Salem, Constance Yuan, Steven Pavletic, Jennifer A. Kanakry, Syed Abbas Ali, Lekha Mikkilineni, Steven A. Feldman, David F. Stroncek, Brenna G. Hansen, Judith Lawrence, Rashmika Patel, Frances Hakim, James N. Kochenderfer
Data analysis and interpretation: Jennifer N. Brudno, Irina Maric, Steven D. Hartman, Jeremy J. Rose, Michael Wang, Norris Lam, Maryalice Stetler-Stevenson, Dalia Salem, Constance Yuan, Steven Pavletic, Steven A. Feldman, Frances Hakim, James N. Kochenderfer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jennifer N. Brudno
Stock or Other Ownership: Gilead Sciences: Prior stock ownership by spouse in previous 2 years, now divested, no current conflict (I)
Irina Maric
No relationship to disclose
Steven D. Hartman
No relationship to disclose
Jeremy J. Rose
No relationship to disclose
Michael Wang
Consulting or Advisory Role: Pharmacyclics, Acerta Pharma, Nordic Bioscience, MoreHealth, Adienne, Celgene, Janssen Research & Development
Research Funding: Pharmacyclics, Janssen Research & Development, Kite Pharma, Juno Therapeutics, Acerta Pharma, AstraZeneca, Celgene, BeiGene, Oncoceutics, Asana Biosciences, Novartis
Norris Lam
No relationship to disclose
Maryalice Stetler-Stevenson
No relationship to disclose
Dalia Salem
No relationship to disclose
Constance Yuan
No relationship to disclose
Steven Pavletic
Research Funding: Eli Lily (Inst), Celgene (Inst), AstraZeneca (Inst), Actelion (Inst)
Jennifer A. Kanakry
No relationship to disclose
Syed Abbas Ali
Consulting or Advisory Role: Takeda, Amgen, Aduro Biotech, Juno Therapeutics
Research Funding: Celgene, Aduro Biotech, Poseida Therapeutics
Lekha Mikkilineni
No relationship to disclose
Steven A. Feldman
Patents, Royalties, Other Intellectual Property: Patent royalties for CAR-T manufacturing process
David F. Stroncek
No relationship to disclose
Brenna G. Hansen
No relationship to disclose
Judith Lawrence
No relationship to disclose
Rashmika Patel
No relationship to disclose
Frances Hakim
No relationship to disclose
Ronald E. Gress
No relationship to disclose
James N. Kochenderfer
Research Funding: Bluebird Bio (Inst), Gilead Sciences/Kite Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I have three patents pending on chimeric antigen receptors (CARs) and one granted patent on anti–B-cell maturation antigen CARs. Companies involved: Bluebird Bio, Novartis, Gilead Sciences/Kite Pharma
Travel, Accommodations, Expenses: Gilead Sciences/Kite Pharma
REFERENCES
- 1.Palumbo A, Anderson K: Multiple myeloma. N Engl J Med 364:1046-1060, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Sonneveld P: Management of multiple myeloma in the relapsed/refractory patient. Hematology (Am Soc Hematol Educ Program) 2017:508-517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laubach J, Garderet L, Mahindra A, et al. : Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia 30:1005-1017, 2016 [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1038/leu.2011.196. Kumar SK, Lee JH, Lahuerta JJ, et al: Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 26:149-157, 2012 [Erratum: Leukemia 26:1153, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkilineni L, Kochenderfer JN: Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 130:2594-2602, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudno JN, Kochenderfer JN: Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15:31-46, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Sadelain M, Brentjens R, Rivière I: The basic principles of chimeric antigen receptor design. Cancer Discov 3:388-398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turtle CJ, Hudecek M, Jensen MC, et al. : Engineered T cells for anti-cancer therapy. Curr Opin Immunol 24:633-639, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus MV, June CH: Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res 22:1875-1884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi LA, Berger C, et al. : CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126:2123-2138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila ML, Riviere I, Wang X, et al. : Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brentjens RJ, Davila ML, Riviere I, et al. : CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turtle CJ, Hanafi LA, Berger C, et al. : Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8:355ra116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Kassim SH, et al. : Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33:540-549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Somerville RPT, Lu T, et al. : Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 35:1803-1813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Somerville RPT, Lu T, et al. : Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 25:2245-2253, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kochenderfer JN, Wilson WH, Janik JE, et al. : Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116:4099-4102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. : B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 19:2048-2060, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garfall AL, Maus MV, Hwang WT, et al. : Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 373:1040-1047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali SA, Shi V, Maric I, et al. : T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128:1688-1700, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos CA, Savoldo B, Torrano V, et al. : Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest 126:2588-2596, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laabi Y, Gras MP, Brouet JC, et al. : The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res 22:1147-1154, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor BP, Raman VS, Erickson LD, et al. : BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199:91-98, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng LG, Sutherland APR, Newton R, et al. : B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 173:807-817, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Novak AJ, Darce JR, Arendt BK, et al. : Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood 103:689-694, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Thompson JS, Schneider P, Kalled SL, et al. : BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med 192:129-135, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochenderfer JN, Yu Z, Frasheri D, et al. : Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116:3875-3886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RJ: Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 155:1063-1074, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. : Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907-912, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. doi: 10.1038/sj.leu.2404284. Durie BGM, Harousseau JL, Miguel JS, et al: International uniform response criteria for multiple myeloma. Leukemia 20:1467-1473, 2006 [Erratum: Leukemia 20:2220, 2006; and Leukemia 21:1134, 2007] [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1182/blood-2014-05-552729. Lee DW, Gardner R, Porter DL, et al: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [Erratum Blood 126:1048, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez E, Smith EJ, Yashar MA, et al. : The role of B-cell maturation antigen in the biology and management of, and as a potential therapeutic target in, multiple myeloma. Target Oncol 13:39-47, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Kramer CL: Intensive care unit-acquired weakness. Neurol Clin 35:723-736, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Brudno JN, Somerville RPT, Shi V, et al. : Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 34:1112-1121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbar AN, Henson SM: Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol 11:289-295, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Larbi A, Fulop T: From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytometry A 85:25-35, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Brenchley JM, Karandikar NJ, Betts MR, et al. : Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Lenig D, Förster R, et al. : Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Alabanza L, Pegues M, Geldres C, et al. : Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther 25:2452-2465, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochenderfer JN, Dudley ME, Carpenter RO, et al. : Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122:4129-4139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melchor L, Brioli A, Wardell CP, et al. : Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 28:1705-1715, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Bolli N, Avet-Loiseau H, Wedge DC, et al. : Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun 5:2997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paíno T, Paiva B, Sayagués JM, et al. : Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 29:1186-1194, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Lamers CHJ, Sleijfer S, Vulto AG, et al. : Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J Clin Oncol 24:e20-e22, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Morgan RA, Yang JC, Kitano M, et al. : Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18:843-851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]