Abstract
Purpose
Incidental cardiac irradiation can cause cardiac injury, but little is known about the effect of radiation on specific cardiac segments.
Methods
For 456 women who received breast cancer radiotherapy between 1958 and 2001 and then later experienced a major coronary event, information was obtained on the radiotherapy regimen they received and on the location of their cardiac injury. For 414 women, all with documented location of left ventricular (LV) injury, doses to five LV segments were estimated. For 133 women, all with documented location of coronary artery disease with ≥ 70% stenosis, doses to six coronary artery segments were estimated. For each segment, numbers of women with left-sided and right-sided breast cancer were compared.
Results
Of women with LV injury, 243 had left-sided breast cancer and 171 had right-sided breast cancer (ratio of left v right, 1.42; 95% CI, 1.17 to 1.73), reflecting the higher typical LV radiation doses in left-sided cancer (average dose left-sided, 8.3 Gy; average dose right-sided, 0.6 Gy; left minus right dose difference, 7.7 Gy). For individual LV segments, the ratios of women with left- versus right-sided radiotherapy were as follows: inferior, 0.94 (95% CI, 0.70 to 1.25); lateral, 1.42 (95% CI, 1.04 to 1.95); septal, 2.09 (95% CI, 1.37 to 3.19); anterior, 1.85 (95% CI, 1.39 to 2.46); and apex, 4.64 (95% CI, 2.42 to 8.90); corresponding left-minus-right dose differences for these segments were 2.7, 4.9, 7.2, 10.4, and 21.6 Gy, respectively (Ptrend < .001). For women with coronary artery disease, the ratios of women with left- versus right-radiotherapy for individual coronary artery segments were as follows: right coronary artery proximal, 0.48 (95% CI, 0.26 to 0.91); right coronary artery mid or distal, 1.69 (95% CI, 0.85 to 3.36); circumflex proximal, 1.46 (95% CI, 0.72 to 2.96); circumflex distal, 1.11 (95% CI, 0.45 to 2.73); left anterior descending proximal, 1.89 (95% CI, 1.07 to 3.34); and left anterior descending mid or distal, 2.33 (95% CI, 1.19 to 4.59); corresponding left-minus-right dose differences for these segements were −5.0, −2.5, 1.6, 3.5, 9.5, and 38.8 Gy (Ptrend = .002).
Conclusion
For individual LV and coronary artery segments, higher radiation doses were strongly associated with more frequent injury, suggesting that all segments are sensitive to radiation and that doses to all segments should be minimized.
INTRODUCTION
Radiotherapy with curative intent is given to many patients with cancer. In breast cancer, radiotherapy reduces the risks of recurrence and death,1,2 but incidental cardiac irradiation may increase the risk of heart disease.3-5 Thoracic radiotherapy can also increase heart disease risk in Hodgkin lymphoma, childhood cancer, esophageal cancer, and lung cancer.6,7 Ischemic heart disease (IHD) is the most common radiation-related heart disease, and radiation-related risk increases approximately linearly with mean whole-heart radiation dose.6,8,9
Radiation-related IHD may be caused by microvascular myocardial disease or macrovascular coronary artery disease.7 Doses from radiotherapy to individual myocardial or coronary artery segments differ substantially depending on regimen, and regimens differ by tumor type, stage, and location and, for breast and lung cancer, laterality.10,11 Considerable resources are being invested in reducing cardiac exposure from radiotherapy.12,13 At present, however, little is known about the long-term effects of irradiating specific segments of the left ventricle (LV) or coronary arteries. Such knowledge may help guide the adoption of cardiac-sparing techniques, and help oncologists to identify the optimal radiotherapy plan for each individual patient.
Several studies have provided insight into the risk of radiation-related heart disease after breast cancer radiotherapy by comparing the numbers of women with left- and right-sided breast cancer and calculating the likely difference in cardiac dose between the two groups.7,8 Here, we extend this technique by considering women irradiated for breast cancer who subsequently developed IHD and for whom the location (segment) of cardiac injury and the radiotherapy regimen, including cancer laterality, were documented. Any differences between women irradiated for left-sided compared with right-sided breast cancer in the distribution of cardiac injuries across the different cardiac segments is likely to reflect differences in the spatial distribution of radiation received by different segments during left-sided and right-sided radiotherapy.
In this study, for each cardiac segment, we calculated the ratio of the number of women with injury to that segment after left-sided radiotherapy to the number of women with injury to that segment after right-sided radiotherapy. We related these ratios to differences in the typical doses delivered to the various segments from left-sided and right-sided radiotherapy.
METHODS
All women who received adjuvant breast cancer radiotherapy in Stockholm from 1958 to 2001 or Denmark from 1977 to 2000 and who subsequently had a major coronary event (defined as myocardial infarction [International Classification of Diseases, 10th Revision, codes I21 to I24], coronary revascularization [Nordic Medico-Statistical Committee Classification of Surgical Procedures, version 1.9, code FN], or death from IHD [hospital or community; International Classification of Diseases, 10th Revision, codes I20 to I25]) were identified from Swedish national patient and cause of death registers and the Danish Breast Cancer Cooperative Group, patient discharge, and cause of death registers.8 Each woman’s radiotherapy regimen and medical history before breast cancer diagnosis were abstracted from her hospital oncology record. Women without histopathologic confirmation of cancer, with bilateral or metastatic disease, with previous cancer (except nonmelanoma skin cancer), with previous thoracic radiotherapy, or whose breast cancer recurred before their major coronary event were excluded. A total of 963 eligible women were identified. This study was approved by the Danish Data Protection Agency and by the Ethics Review Board of the Karolinska Institutet in Stockholm.
Location and Type of Cardiac Injury
Hospital cardiology notes were sought for all 963 women. Information on the location, nature, and extent of any LV myocardial injury and on any disease of the left anterior descending (LAD), right, and circumflex coronary arteries was abstracted by four research nurses. Two cardiologists and an oncologist (K.R., B.G., and C.C.) who were blinded to the cancer laterality used this information to code site of injury. Injury location included five LV segments (anterior, inferior, apex, lateral, and septal; Data Supplement) and two segments (proximal, mid/distal, or distal) of each of the LAD, right, and circumflex coronary arteries.14 LV injury was defined as evidence of: myocardial infarction on ECG, permanent perfusion defects on multiple-gated acquisition or myocardial perfusion scan, regional wall motion abnormalities on echocardiogram, or LV infarction on autopsy record. Coronary artery disease was defined as ≥ 70% coronary artery stenosis at angiogram or autopsy. Coronary artery disease with < 70% stenosis was excluded because it is often subclinical and thus would be under-reported. Information on other locations (eg, right ventricle) was rarely reported in cardiology notes and thus was not included.
Radiation Dosimetry
Radiation doses were estimated for five LV segments and two segments each of the LAD, right, and circumflex coronary arteries (Figs 1 and 2; Data Supplement).14 First, each woman’s radiotherapy chart was used to categorize her according to regimen. Second, a typical computed tomography (CT) scan was selected. Third, all regimens were reconstructed on the typical CT scan to derive regimen-specific doses for each segment. Fourth, each woman was allocated segment doses according to regimen category and total dose. Fifth, dosimetry uncertainties were assessed.
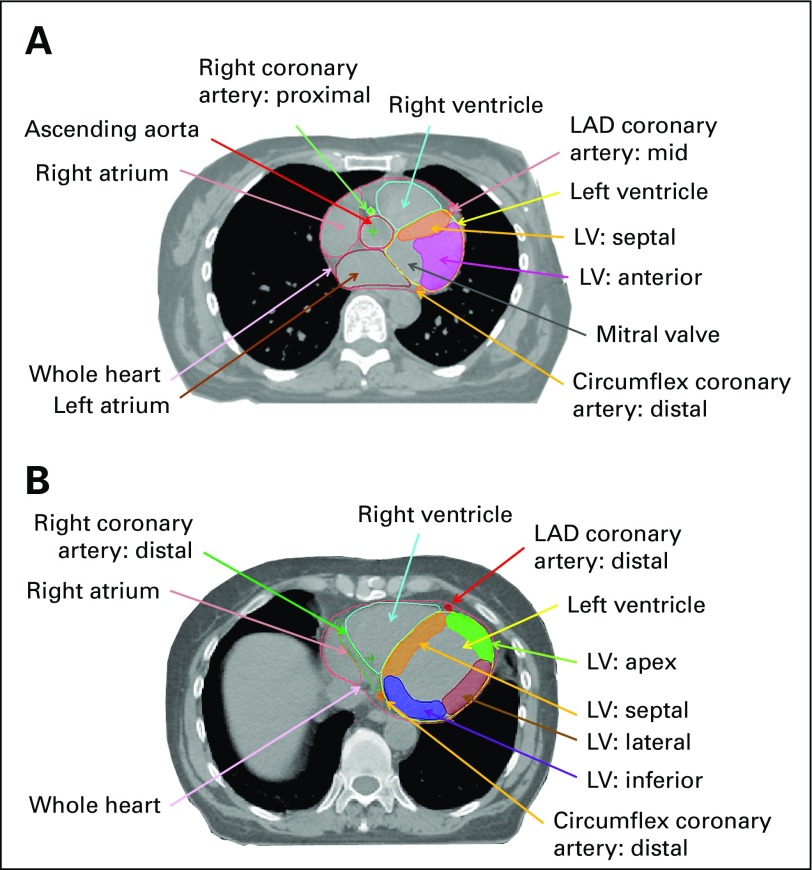
Fig 1.
Axial computed tomography images illustrating left ventricle (LV) and coronary artery segments. (A) Level of proximal (superior) LV. (B) Level of mid LV. As is usual in radiotherapy planning, the patient's right is on the reader’s left. LAD, left anterior descending coronary artery.
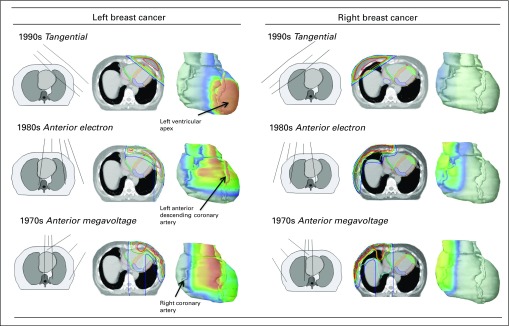
Fig 2.
Spatial distribution of radiation dose in the heart from breast cancer regimens commonly used in different decades for women in the study. Isodoses were as follows: red, 50 Gy; orange, 48 Gy; yellow, 44 Gy; green, 40 Gy; blue, 25 Gy; and purple, 10 Gy. Field borders were usually 25 Gy. Left ventricular segments are as follows: orange, septal; green, apex; brown, lateral; and purple, inferior. Anterior electron included an electron field to chest wall and internal mammary lymph nodes, and a photon field to lateral thorax and axillary and supraclavicular nodes. Anterior megavoltage included a cobalt-60 field to internal mammary lymph nodes and an oblique electron field to chest wall projecting to contralateral side.
Statistical Methods
Radiation dose estimates for individual cardiac segments were available only for women with recorded cardiac injury and not for otherwise comparable women in the population without a major coronary event. Therefore, it was not possible to assess dose-response relationships in terms of the percentage increase per gray in segment injury rate.
Separate analyses were conducted for LV and coronary arteries. The ratio of the number of women receiving left-sided radiotherapy to the number receiving right-sided radiotherapy was calculated (termed left-versus-right ratio). Tests for heterogeneity in the left-versus-right ratio with various characteristics were conducted using logistic regression.
For each injured LV or coronary artery segment, the typical dose to that segment was calculated based on radiotherapy regimen, including cancer laterality. The average of the typical doses was then calculated separately for left-sided and right-sided cancer, and the difference between these average typical doses was derived (termed left-minus-right dose difference). The segments were then ranked according to left-minus-right dose differences. A test for trend in the left-versus-right ratios was conducted using logistic regression with rank as the independent variable (Data Supplement). Calculations were performed using Stata statistical software version 13.0 (StataCorp, College Station, TX).
RESULTS
Location of cardiac injury was identified for 456 women, and these women were included in the study. The other 507 eligible women were not included because their cardiology record was unavailable (n = 250), their injury location was not documented (n = 243), or their regimen was identical in left-sided and right-sided breast cancer (n = 14; Data Supplement).
LV Injury
Information on location of LV injury was obtained for 712 LV segments in 414 women. The case-defining event was myocardial infarction in 91% of women (376 of 414 women), coronary artery disease in 7% (29 of 414 women), and death certificate information in 2% (nine of 414 women; Table 1). For 57% of women (234 of 414 women), available information included at least one of the following: echocardiogram, multiple-gated acquisition or myocardial perfusion scan, or autopsy. For the remaining 43% of women (180 of 414 women), information on LV injury location was available only from ECGs.
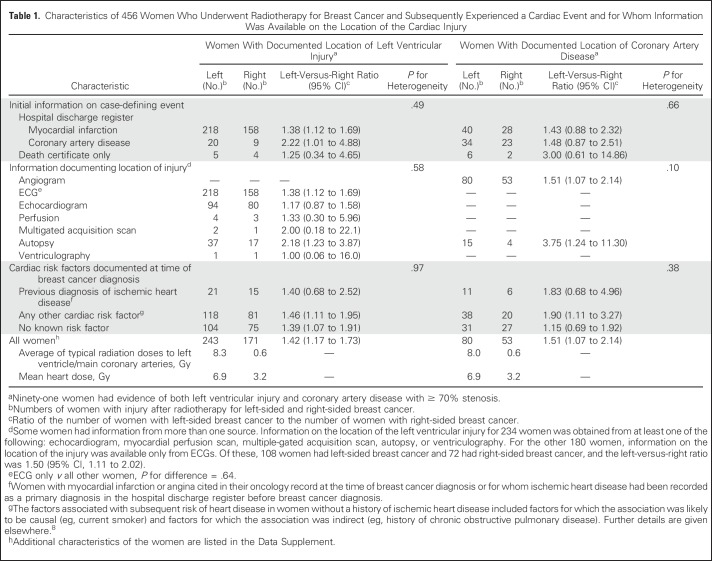
Table 1.
Characteristics of 456 Women Who Underwent Radiotherapy for Breast Cancer and Subsequently Experienced a Cardiac Event and for Whom Information Was Available on the Location of the Cardiac Injury
Radiotherapy was for left-sided cancer in 243 women and right-sided cancer in 171 women, giving a left-versus-right ratio of 1.42 (95% CI, 1.17 to 1.73), resulting from the larger LV radiation doses in left-sided cancer (average of typical LV doses in women with LV injury: left-sided, 8.3 Gy; right-sided, 0.6 Gy; left-minus-right dose difference, 7.7 Gy). The left-versus-right ratio did not vary significantly according to initial information on case-defining event, type of information documenting the injury location, or presence of cardiac risk factors at time of cancer diagnosis (P for heterogeneity > .10 for all three factors).
Average whole-heart doses were 6.9 Gy for left-sided radiotherapy and 3.2 Gy for right-sided radiotherapy. Exposure of the heart was nonuniform for all regimens, with substantial variation in doses received by different cardiac segments (Fig 2). For left-sided breast cancer, the LV apex received the highest doses for most regimens. For right-sided breast cancer, the entire LV was outside the fields for most regimens.
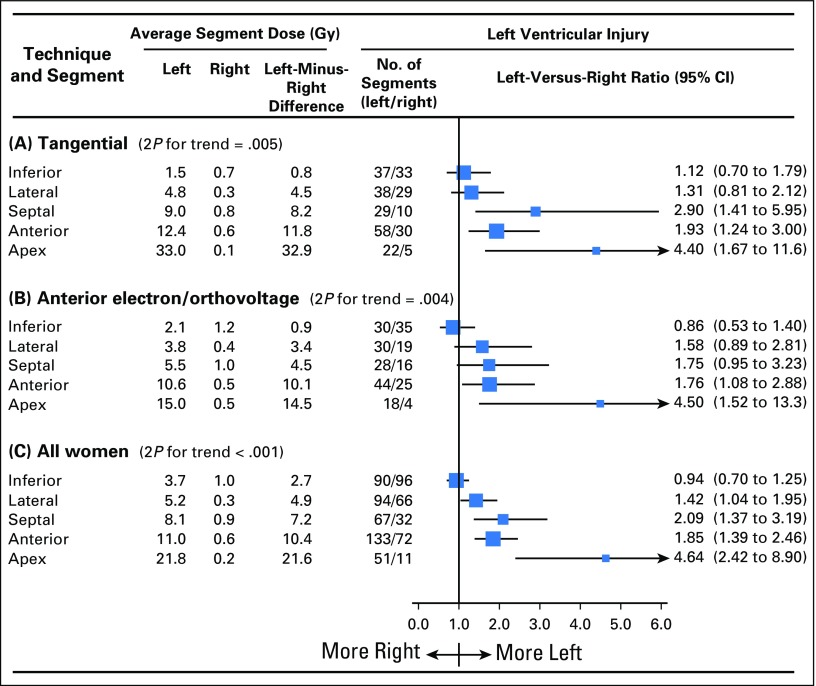
Ninety women had injury to the inferior LV segment after left-sided radiotherapy compared with 96 women after right-sided radiotherapy, giving a left-versus-right ratio of 0.94 (95% CI, 0.70 to 1.25; Fig 3C). The average typical inferior segment doses were 3.7 Gy for left-sided radiotherapy and 1.0 for right-sided radiotherapy, giving a left-minus-right difference of 2.7 Gy. For other segments, the left-versus-right ratios were as follows: lateral, 1.42 (95% CI, 1.04 to 1.95); septal, 2.09 (95% CI, 1.37 to 3.19); anterior, 1.85 (95% CI, 1.39 to 2.46); and apex, 4.64 (95% CI, 2.42 to 8.90); the corresponding left-minus-right differences in segment dose were 4.9, 7.2, 10.4, and 21.6 Gy, respectively (Ptrend across all segments < .001).
Fig 3.
Left ventricular segment injury by radiotherapy technique. Average typical doses to ventricular segments in radiotherapy for left-sided and right-sided breast cancer and numbers of women with ventricular injury in left-sided and right-sided breast cancer are shown. In each panel, segments are listed in order of difference in the average of the typical segment doses received in left-sided and right-sided breast cancer for women with injury to the segment concerned (ie, left-minus-right difference). Some women had injury to more than one segment. Mean times to cardiac events were as follows: (A) tangential left, 10.8 years; tangential right, 12.4 years; (B) anterior electron or orthovoltage left, 14.2 years; anterior electron or orthovoltage right, 14.4 years; (C) all women left, 13.7 years; and all women right, 14.5 years.
Twenty radiotherapy techniques were used (Data Supplement). For the two most common (tangential and anterior electron or orthovoltage), the left-versus-right ratios of women with injury to individual LV segments increased with increasing left-minus-right segment dose difference (tangential, Ptrend = .005; anterior electron or orthovoltage, Ptrend = .004; Figs 3A and 3B; Data Supplement).
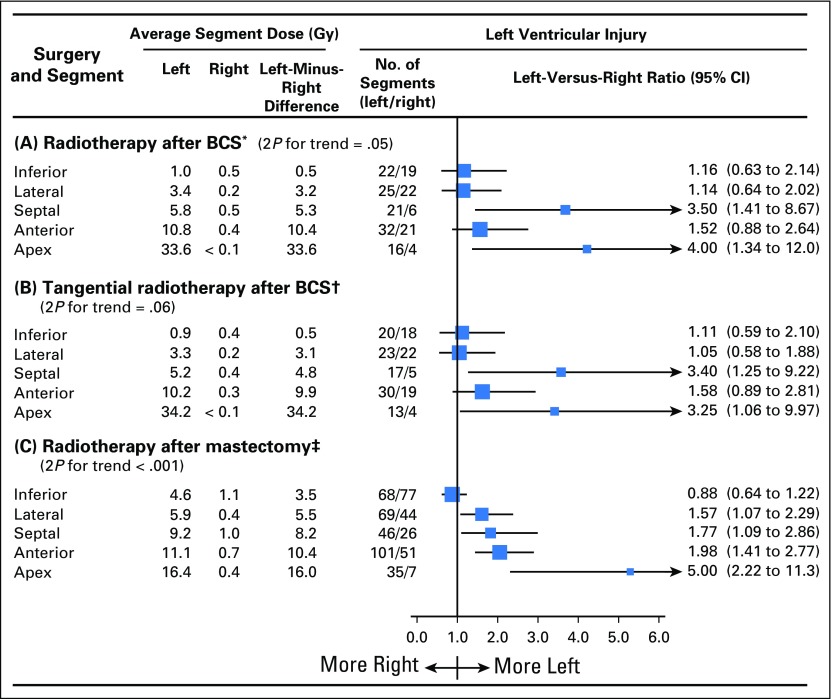
The left-versus-right ratio of injury increased with the left-minus-right difference in typical dose both for the 99 women irradiated after breast-conserving surgery (Ptrend = .05; Fig 4A) and for the 315 women irradiated after mastectomy (Ptrend < .001; Fig 4C). Ninety of the 99 women who underwent breast-conserving surgery received tangential radiotherapy, and in these women, the left-versus-right ratio of injury still tended to increase with the left-minus-right difference in typical dose (Ptrend = .06; Fig 4B).
Fig 4.
Left ventricular (LV) segment injury by type of surgery and radiotherapy technique. Average typical doses to ventricular segments in radiotherapy for left-sided and right-sided breast cancer and numbers of women with ventricular injury in left-sided and right-sided breast cancer are shown. In each panel, segments are listed in order of difference in the average of the typical segment doses received in left-sided and right-sided breast cancer for women with injury to the segment concerned (ie, left-minus-right difference). Some women had injury to more than one segment. (*) Radiotherapy after breast-conserving surgery (BCS) included tangential radiotherapy in 90 of 99 women and anterior electron or orthovoltage radiotherapy in nine of 99 women. (†) Tangents delivered after BCS involved smaller left-minus-right dose differences to most LV segments than tangents after mastectomy because the fields were not as wide, with the medial border typically midline rather than contralateral. (‡) Techniques included tangential in 76 of 315 women, anterior electron or orthovoltage in 136 of 315 women, cobalt chain in 66 of 315 women, and anterior megavoltage in 37 of 315 women. Mean times to cardiac events were as follows: (A) radiotherapy after BCS left, 7.6 years; radiotherapy after BCS right, 9.6 years; (B) tangential radiotherapy after BCS left, 7.5 years; tangential radiotherapy after BCS right, 9.7 years; (C) radiotherapy after mastectomy left, 15.6 years; and radiotherapy after mastectomy right, 16.0 years.
Coronary Artery Disease
Information on location of coronary artery stenosis was obtained for 221 segments in 133 women. The initial case-defining event was myocardial infarction in 51% of the women (68 of 133 women), coronary artery disease in 43% (57 of 133 women), and death certificate information in 6% (eight of 133 women; Table 1). Coronary angiography was available for all 133 women, and for 19 women, an autopsy report was also available. Eighty women were irradiated for left-sided cancer and 53 for right-sided cancer (left-versus-right ratio, 1.51; 95% CI, 1.07 to 2.14), with typical doses to the main coronary arteries combined of 8.0 and 0.6 Gy, respectively. As with LV injury, the left-versus-right ratio did not vary significantly according to the initial case-defining event information, type of information documenting location of injury, or presence of cardiac risk factors at breast cancer diagnosis (P for heterogeneity ≥ .10 for all three factors).
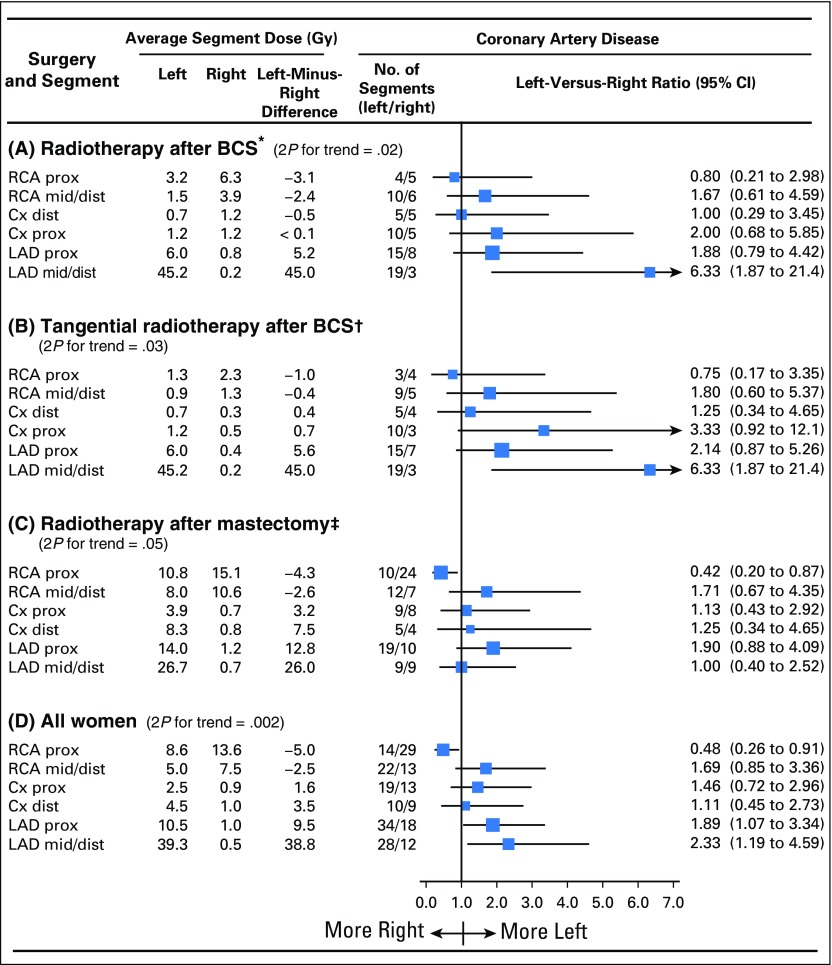
Average whole-heart doses were 7.1 Gy for left-sided radiotherapy and 3.1 Gy for right-sided radiotherapy. For left-sided radiotherapy, part of the LAD coronary artery was included in the fields for most regimens, whereas part of the right coronary artery was usually irradiated in right-sided radiotherapy (Fig 2). Left-versus-right ratios of women with disease in any of the six individual coronary artery segments were as follows: proximal right, 0.48 (95% CI, 0.26 to 0.91); mid or distal right, 1.69 (95% CI, 0.85 to 3.36); proximal circumflex, 1.46 (95% CI, 0.72 to 2.96); distal circumflex, 1.11 (95% CI, 0.45 to 2.73); proximal LAD, 1.89 (95% CI, 1.07 to 3.34); and mid or distal LAD, 2.33 (95% CI, 1.19 to 4.59); the corresponding left-minus-right dose differences were −5.0, −2.5, 1.6, 3.5, 9.5, and 38.8 Gy respectively (Ptrend = .002; Fig 5D).
Fig 5.
Coronary artery disease by type of surgery and radiotherapy technique. Average typical doses to arterial segments in radiotherapy for left-sided and right-sided breast cancer and numbers of women with coronary artery disease in left-sided and right-sided breast cancer are shown. Coronary artery disease was defined as ≥ 70% stenosis. In each panel, segments are listed in order of difference in the average of the typical doses received in left-sided and right-sided breast cancer for women with injury to the segment concerned (ie, left-minus-right difference). Some women had injury to more than one segment. One hundred twenty-eight women had information on diseased coronary artery segment and are included here. Five additional women had disease with known coronary artery location but not known segment. All 133 women are included in the Data Supplement. (*) Radiotherapy after breast-conserving surgery (BCS) included tangential radiotherapy in 48 of 53 women and anterior electron or orthovoltage radiotherapy in five of 53 women. (†) Tangents delivered after BCS involved smaller left-minus-right dose differences to most left ventricular segments than tangents after mastectomy because the fields were not as wide, with the medial border typically midline rather than contralateral. (‡) Techniques included tangential in 16 of 75 women, anterior electron or orthovoltage in 38 of 75 women, cobalt chain in 11 of 75 women, and anterior megavoltage in 10 of 75 women. Mean times to cardiac events were as follows: (A) radiotherapy after BCS left, 7.0 years; radiotherapy after BCS right, 9.8 years; (B) tangential radiotherapy after BCS left, 7.1 years; tangential radiotherapy after BCS right, 9.6 years; (C) radiotherapy after mastectomy left, 19.3 years; radiotherapy after mastectomy right, 17.2 years; (D) all women left, 13.5 years; and all women right, 14.7 years. Cx, circumflex; dist, distal; LAD, left anterior descending artery; prox, proximal; RCA, right coronary artery.
When women given radiotherapy after breast-conserving surgery and mastectomy were considered separately, the left-versus-right ratio of segment injury increased with left-minus-right segment dose difference for both (breast-conserving surgery, Ptrend = .02; mastectomy, Ptrend = .05; Figs 5A and 5C). Notably, for radiotherapy after mastectomy, typical doses to the right coronary artery proximal segment were higher for right-sided than left-sided radiotherapy (left-sided dose, 10.8 Gy; right-sided dose, 15.1 Gy; left-minus-right dose difference, −4.3 Gy), and more women with right-sided than left-sided breast cancer had disease of this segment (left-versus-right ratio, 0.42; 95% CI, 0.20 to 0.87). Results for whole coronary arteries were similar (Data Supplement).
DISCUSSION
We have extended the commonly used technique of comparing the numbers of women with heart disease after radiotherapy for left-sided and right-sided breast cancer to provide insight into the effect of radiotherapy on individual segments of the LV and coronary arteries. We have shown that, for segments where there was little difference in the typical dose received from radiotherapy for left-sided and right-sided breast cancer, the left-versus-right ratios of the numbers of women with injury were close to 1. However, as the differences in typical segment dose between left-sided and right-sided cancer increased, so did the left-versus-right ratios of the numbers of women with injury to the segment concerned. Most increases were statistically significant when the left-minus-right difference in typical dose was greater than approximately 4 Gy. Notably, when the typical dose from right-sided radiotherapy was > 4 Gy higher than from left-sided radiotherapy, the left-versus-right ratio of the number of women with injury was significantly lower than 1. Radiation-related increases in injury were seen throughout the cardiac structures studied, including the lateral, septal, anterior, and apex LV segments; the proximal right coronary artery segment; and the proximal and mid or distal LAD segments. These findings strongly suggest a close and direct relation between radiation exposure and injury to different segments of the LV and coronary arteries.
The 963 women eligible for this study formed the patient cases in a population-based case-control study. Any differences between the 456 women included in this study and the remaining 507 women who were not included are unlikely to have biased our results (Data Supplement). For the women studied, it is unlikely that breast cancer laterality affected the decision to give radiotherapy or the regimen used; in the population from which the women in our study were drawn, the ratio of the number of women irradiated for left-sided versus right-sided cancer was 1.1 (left breast cancer incidence is slightly higher than that of right breast cancer), and the characteristics of the women irradiated for left-sided and right-sided cancer were virtually identical, as was their subsequent mortality from all causes other than heart disease.5 Hence, it is likely that the increases in segment injury reported are causally related to radiation.
A limitation of our study was that individual CT information was unavailable because the women were irradiated before the era of three-dimensional CT radiotherapy planning. Therefore, it was necessary to estimate cardiac doses retrospectively using a typical CT scan. Reassuringly, our cardiac dose estimates are similar to other estimates for these regimens.15,16 Furthermore, we showed that dosimetric uncertainties had little effect on the use we made of our segment dose estimates (Data Supplement).
A second limitation is that for most women with LV injury, we did not have information on possible disease of the coronary artery supplying the segment concerned. Nevertheless, for 91 women with information on both coronary artery disease and LV injury, LV injury tended to occur in segments supplied by the diseased coronary arteries (Data Supplement). This may be a result of radiation-related coronary artery disease causing LV ischemia downstream. Alternatively, it may be a result of proximity of the coronary arteries to the LV segments they supply, resulting in similar radiation doses being received by both. Doses to many LV and coronary artery segments were highly correlated (Data Supplement). Hence, in our study, we could not tell whether radiation-related LV injury was caused directly by LV irradiation or indirectly by radiation-related coronary artery disease.
Myocardial perfusion defects (ischemic areas of the LV) after breast cancer radiotherapy have been demonstrated in studies involving a total of approximately 600 women. In some studies, women had cardiac imaging before and then months or years after left-sided radiotherapy, and each woman’s pre- and postradiotherapy images were compared. In other studies, cardiac imaging was performed between 5 and 19 years after radiotherapy, and images of women given left-sided, right-sided, or no radiotherapy were compared.17,18 The results of these studies are consistent with our study, although the clinical implications of the abnormalities are unknown. The myocardial perfusion studies did not provide segment doses, but several of them showed that the location of LV perfusion defects was determined by the borders of radiotherapy fields, rather than the distribution of major coronary vessels. This suggests defects were caused by damage to the myocardial microvasculature rather than coronary artery damage.19 In two echocardiography studies including 70 women who received tangential radiotherapy, LV segment doses were related to subsequent segment function both before and a few weeks after radiotherapy.20,21 The LV apex received the highest doses from left tangential radiotherapy and had poorer function after radiotherapy than other segments. Function was significantly reduced in LV segments that received > 3 Gy.
Disease of the main coronary arteries has been demonstrated in three studies of patients referred for angiography some years after radiotherapy.22-24 The studies, which were based on a total of 149 irradiated patients, found that coronary stenoses occurred preferentially in arterial segments likely to have received high radiation doses and are consistent with our findings.
In breast cancer radiotherapy, cardiac radiation doses have decreased over recent years. Women in the present study were irradiated between 1958 and 2001 and received mean heart doses of approximately 7 and 3 Gy for left-sided and right-sided breast cancer, respectively. In a systematic review of regimens in studies published from 2003 to 2013, average mean heart doses were 5.4 Gy (range, < 0.1 to 28.6 Gy) in 398 left-sided regimens and 3.3 Gy (range, 0.4 to 21.6 Gy) in 45 right-sided regimens.11
Currently, most women irradiated for breast cancer have tangential radiotherapy after breast-conserving surgery,11 which is often considered risk free. However, in some countries, modern left tangential radiotherapy still delivers heart doses of several Gy,11 and the LV apex and mid or distal LAD coronary artery segments are still in the radiation fields for some women.12 In our study, the left-versus-right ratios for injury to these segments from tangential radiotherapy after breast-conserving surgery were approximately 3 for the LV apex and approximately 6 for the mid or distal LAD segment, indicating that irradiating these segments causes injury and that, where possible, they should be excluded from fields using cardiac-sparing techniques.12,13
In breast cancer, cardiac exposure from radiotherapy may increase in the future, because recent studies have shown that internal mammary radiotherapy improves breast cancer survival25-27 and it is difficult to irradiate the internal mammary chain without incidentally irradiating the heart.11 In addition, some women have unfavorable anatomy where incidental cardiac irradiation is unavoidable. In Hodgkin lymphoma, lung cancer, and esophageal cancer, the tumor can lie close to the heart,10,13 rendering it difficult to achieve full tumor dose without exposing the heart. With modern three-dimensional CT-based radiotherapy planning, doses to small regions (eg, cardiac segments) can be modified by changing beam angles or using different techniques, so oncologists may have choice over exposure to individual structures. However, there is lack of consistency in radiotherapy guidelines on which cardiac structures are sensitive to radiation and should therefore be avoided. In radiotherapy for breast cancer and lymphoma, this is reflected by differing cardiac dose constraints. For example, in some countries, but not in others, the LAD coronary artery is considered a separate organ at risk, with more stringent dose constraints than the heart.28-30 We demonstrated associations between radiation dose and injury for both LV and coronary artery segments. Therefore, the safest strategy, based on current knowledge, is to minimize dose to all segments.
ACKNOWLEDGMENT
We thank research nurses Ann-Sofie Andersson and Milka Krestelica in Sweden and Liselotte Jeppesen in Denmark, and Ulrich H. Koehler for data management in Denmark. Procedures for accessing the data for this study are available on https://www.ndph.ox.ac.uk/about/data-access-policy.
Footnotes
Supported by Cancer Research UK (Grant No. C8225/A21133) and by a research contract to the University of Oxford under the Department of Health Policy Research Programme (Studies of Ionising Radiation and the Risk of Heart Disease, 091/0203); by core funding from Cancer Research UK, the UK Medical Research Council, and the British Heart Foundation to the Oxford University Clinical Trial Service Unit (Grant No. MC_U137686858); and by the British Heart Foundation Centre for Research Excellence at the University of Oxford (Grants No. RE/08/04 [D.C.] and RE/13/1/30181 [S.C.D.]).
C.T. and P.M. contributed equally to this work, and S.C.D., P.H., and M.E. contributed equally to this work.
Listen to the podcast by Dr Wazer at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Carolyn Taylor, Paul McGale, David Cutter, Maj-Britt Jensen, Kazem Rahimi, Sarah C. Darby, Per Hall, Marianne Ewertz
Collection and assembly of data: Dorthe Brønnum, Candace Correa, Frances K. Duane, Bruna Gigante, Maj-Britt Jensen, Kazem Rahimi, Per Hall
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study With Individual Patient Data
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Carolyn Taylor
No relationship to disclose
Paul McGale
No relationship to disclose
Dorthe Brønnum
No relationship to disclose
Candace Correa
No relationship to disclose
David Cutter
No relationship to disclose
Frances K. Duane
No relationship to disclose
Bruna Gigante
No relationship to disclose
Maj-Britt Jensen
Honoraria: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Celgene
Ebbe Lorenzen
No relationship to disclose
Kazem Rahimi
No relationship to disclose
Zhe Wang
No relationship to disclose
Sarah C. Darby
No relationship to disclose
Per Hall
Research Funding: Atossa Genetics (Inst)
Marianne Ewertz
No relationship to disclose
REFERENCES
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, et al. : Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707-1716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGale P, Taylor C, Correa C, et al. : Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127-2135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehammar JC, Jensen MB, McGale P, et al. : Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977-2005. Radiother Oncol 123:299-305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C, Correa C, Duane FK, et al. : Estimating the risks of breast cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 35:1641-1649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGale P, Darby SC, Hall P, et al. : Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 100:167-175, 2011 [DOI] [PubMed] [Google Scholar]
- 6.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. : Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 34:235-243, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Cutter D, Taylor C, Rahimi K, et al. : Effects of radiation therapy on the cardiovascular system, in Ewer E, Yeh ETH. (eds): Cancer and the Heart (ed 2). Shelton, CT, People’s Medical Publishing House, 2013, pp 83-131 [Google Scholar]
- 8.Darby SC, Ewertz M, McGale P, et al. : Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:987-998, 2013 [DOI] [PubMed] [Google Scholar]
- 9.van den Bogaard VAB, Ta BDP, van der Schaaf A, et al. : Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 35:1171-1178, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maraldo MV, Brodin NP, Vogelius IR, et al. : Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 83:1232-1237, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Taylor CW, Wang Z, Macaulay E, et al. : Exposure of the heart in breast cancer radiation therapy: A systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys 93:845-853, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Taylor CW, Kirby AM: Cardiac side-effects from breast cancer radiotherapy. Clin Oncol (R Coll Radiol) 27:621-629, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Boda-Heggemann J, Knopf AC, Simeonova-Chergou A, et al. : Deep inspiration breath hold- based radiation therapy: A clinical review. Int J Radiat Oncol Biol Phys 94:478-492, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Duane F, Aznar MC, Bartlett F, et al. : A cardiac contouring atlas for radiotherapy. Radiother Oncol 122:416-422, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorsen LB, Thomsen MS, Overgaard M, et al. : Quality assurance of conventional non-CT-based internal mammary lymph node irradiation in a prospective Danish Breast Cancer Cooperative Group trial: The DBCG-IMN study. Acta Oncol 52:1526-1534, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Taylor CW, Brønnum D, Darby SC, et al. : Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977-2001. Radiother Oncol 100:176-183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaidar-Person O, Zagar TM, Oldan JD, et al. : Early cardiac perfusion defects after left-sided radiation therapy for breast cancer: Is there a volume response? Breast Cancer Res Treat 164:253-262, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Taylor CW, McGale P, Darby SC: Cardiac risks of breast-cancer radiotherapy: A contemporary view. Clin Oncol (R Coll Radiol) 18:236-246, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lind PA, Pagnanelli R, Marks LB, et al. : Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 55:914-920, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Erven K, Jurcut R, Weltens C, et al. : Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys 79:1444-1451, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Lo Q, Hee L, Batumalai V, et al. : Strain imaging detects dose-dependent segmental cardiac dysfunction in the acute phase after breast irradiation. Int J Radiat Oncol Biol Phys 99:182-190, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Nilsson G, Holmberg L, Garmo H, et al. : Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 30:380-386, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Correa CR, Litt HI, Hwang WT, et al. : Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 25:3031-3037, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moignier A, Broggio D, Derreumaux S, et al. : Coronary stenosis risk analysis following Hodgkin lymphoma radiotherapy: A study based on patient specific artery segments dose calculation. Radiother Oncol 117:467-472, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Poortmans PM, Collette S, Kirkove C, et al. : Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 373:317-327, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Whelan TJ, Olivotto IA, Parulekar WR, et al. : Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373:307-316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorsen LB, Offersen BV, Danø H, et al. : DBCG-IMN: A population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol 34:314-320, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Gagliardi G, Constine LS, Moiseenko V, et al. : Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 76:S77-S85, 2010. (suppl 3) [DOI] [PubMed] [Google Scholar]
- 29.Hoskin PJ, Díez P, Williams M, et al. : Recommendations for the use of radiotherapy in nodal lymphoma. Clin Oncol (R Coll Radiol) 25:49-58, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen MH, Berg M, Pedersen AN, et al. : Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: National guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol 52:703-710, 2013 [DOI] [PubMed] [Google Scholar]