Abstract
Purpose
Addition of imatinib to intensive chemotherapy improved survival for children and young adults with Philadelphia chromosome–positive acute lymphoblastic leukemia. Compared with imatinib, dasatinib has increased potency, CNS penetration, and activity against imatinib-resistant clones.
Patients and Methods
Children’s Oncology Group (COG) trial AALL0622 (Bristol Myers Squibb trial CA180-204) tested safety and feasibility of adding dasatinib to intensive chemotherapy starting at induction day 15 in patients with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia age 1 to 30 years. Allogeneic hematopoietic stem-cell transplantation (HSCT) was recommended for patients at high risk based on slow response and for those with a matched family donor regardless of response after at least 11 weeks of therapy. Patients at standard risk based on rapid response received chemotherapy plus dasatinib for an additional 120 weeks. Patients with overt CNS leukemia received cranial irradiation.
Results
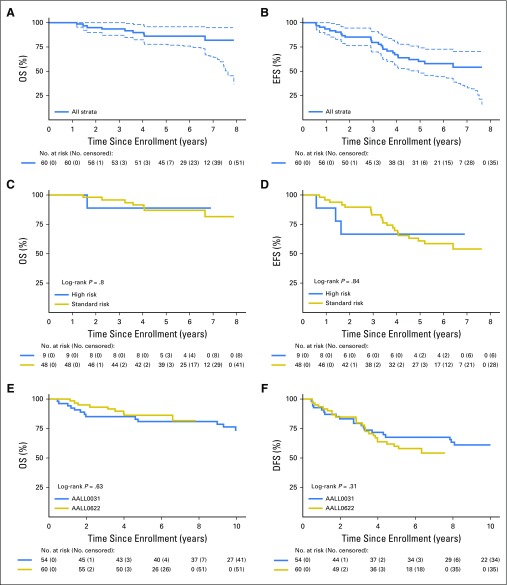
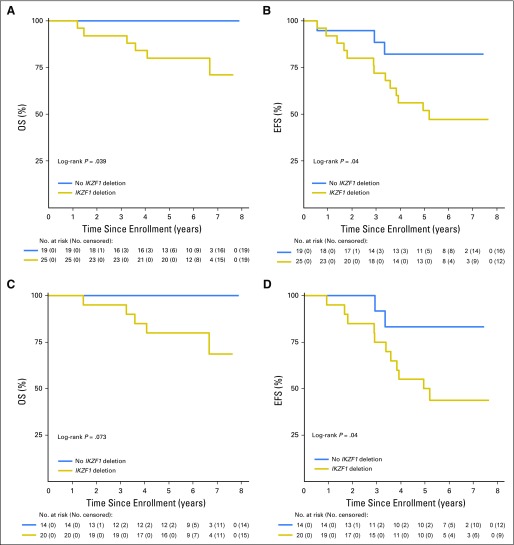
Sixty eligible patients were enrolled. Five-year overall (OS) and event-free survival rates (± standard deviations [SD]) were 86% ± 5% and 60% ± 7% overall, 87% ± 5% and 61% ± 7% for standard-risk patients (n = 48; 19% underwent HSCT), and 89% ± 13% and 67% ± 19% for high-risk patients (n = 9; 89% underwent HSCT), respectively. Five-year cumulative incidence (± SD) of CNS relapse was 15% ± 6%. Outcomes (± SDs) were similar to those in COG AALL0031, which used the same chemotherapy with continuous imatinib: 5-year OS of 81% ± 6% versus 86% ± 5% (P = .63) and 5-year disease-free survival of 68% ± 7% versus 60% ± 7% (P = 0.31) for AALL0031 versus AALL0622, respectively. IKZF1 deletions, present in 56% of tested patients, were associated with significantly inferior OS and event-free survival overall and in standard-risk patients.
Conclusion
Dasatinib was well tolerated with chemotherapy and provided outcomes similar to those with imatinib in COG AALL0031, where all patients received cranial irradiation. Our results support limiting HSCT to slow responders and suggest a potential role for transplantation in rapid responders with IKZF1 deletions.
INTRODUCTION
Survival for children with acute lymphoblastic leukemia (ALL) exceeds 85%,1-5 and Philadelphia chromosome (Ph), t(9;22)(q34;q11.2), and BCR-ABL1 fusion are present in 3% to 5% of children with ALL. Historically, fewer than half of children with Ph-positive ALL survived when treated with chemotherapy with or without hematopoietic stem-cell transplantation (HSCT).6,7 Expression of the BCR-ABL1 fusion protein, a constitutively activated ABL1 tyrosine kinase, leads to transformation.8 Secondary cytogenetic abnormalities9,10 and cooperative mutations such as IKZF1 deletions11-13 contribute to inferior outcomes in Ph-positive ALL.
Children’s Oncology Group (COG) trial AALL0031 in Ph-positive ALL demonstrated that adding the tyrosine kinase inhibitor (TKI) imatinib to intensive chemotherapy dramatically improved survival compared with that in patients receiving chemotherapy alone.14,15 AALL0031 patients treated with HSCT had outcomes similar to those receiving chemotherapy plus imatinib. Similarly, the EsPhALL (European Study of Postinduction Treatment of Ph-Positive ALL) group showed improved outcomes in patients receiving imatinib plus chemotherapy compared with chemotherapy alone in good-risk patients with Ph-positive ALL.16
Although imatinib improves survival in Ph-positive ALL, outcomes are still inferior to those in children with Ph-negative ALL. Furthermore, AALL0031 used cranial irradiation in every patient. Cranial irradiation can adversely affect learning and cognition and cause brain tumors.17-20 The dual ABL/SRC TKI dasatinib is 300 times more potent than imatinib at blocking ABL kinase activity21 and is active in most patients with imatinib resistance.22,23 Dasatinib accumulates in the CNS, a sanctuary site for leukemia where penetration of imatinib is poor.24
We hypothesized that substituting dasatinib for imatinib and starting TKI therapy earlier (at day 15 rather than day 35) would lead to more rapid clearance of leukemia and improved survival, while abrogating the need for cranial irradiation. The objectives of the COG AALL0622 trial (Bristol Myers Squibb trial CA180-204) were to determine the feasibility and toxicity of adding dasatinib to AALL0031 chemotherapy and to determine whether dasatinib plus AALL0031-style chemotherapy would lead to 3-year event-free survival (EFS) of at least 60% in patients with good early response to therapy.
PATIENTS AND METHODS
Patients
AALL0622 enrolled patients age 1 to 30 years with Ph-positive ALL from July 14, 2008, through February 3, 2012. This study was approved by the National Cancer Institute and the institutional review boards of COG and Dana-Farber Cancer Institute member institutions. Informed consent and assent were obtained in accordance with federal guidelines. Dasatinib was supplied by the National Cancer Institute. Inclusion and exclusion criteria were similar to those in AALL0031 except for the inclusion of young adults age 22 to 30 years (Data Supplement).
Risk Stratification
Minimal residual disease (MRD) was assessed by flow cytometry at one of two central reference laboratories at end of induction and after two consolidation cycles.25 Patients were stratified as high risk (HR) if end-of-induction MRD levels were ≥ 1% and/or MRD level was ≥ 0.01% at end of consolidation 2; the remaining patients were standard risk (SR). Allogeneic HSCT was recommended after at least 11 weeks of therapy for HR patients and for SR patients with a matched family donor. The remaining SR patients received chemotherapy plus dasatinib for an additional 120 weeks. Patients who underwent HSCT came off protocol-directed therapy at the time of HSCT. The AALL0622 chemotherapy plan was the same as that used in COG AALL0031,14 with minor modifications (Data Supplement). Only patients with overt CNS leukemia received 18-Gy cranial irradiation.
Dasatinib Therapy
In cohort 1, dasatinib 60 mg/m2 once daily was administered starting on induction day 15 for 2 weeks of each 3- to 4-week treatment block (discontinuous dasatinib; Data Supplement). By study design, patients continued to be enrolled in cohort 1 until six completed the first intensification block (week 23). If five of six patients completed therapy through week 23 without dose-limiting toxicity (Data Supplement), then patients would be enrolled in cohort 2 and receive dasatinib 60 mg/m2 per day continuously throughout therapy starting at induction day 15. Dasatinib was not recommended after HSCT, and data were not collected regarding whether patients received TKI post-HSCT or after completion of chemotherapy.
IKZF1 Deletion Analysis
IKZF1 deletions were assessed using the Illumina 2.5 Exome Array (San Diego, CA) as previously described.26
Statistical Analysis
Data are current as of December 2016. EFS and overall survival (OS) were calculated based on the date of study enrollment to date of first event or last follow-up. Events included induction failure, relapse at any site, second malignancy, or death. Patients for whom induction did not fail were censored as of the date of last contact. Cumulative incidence rates (CIRs) were estimated using the method of Fine and Gray.27 Survival curves were constructed using the Kaplan-Meier life table method,28 with standard errors computed using the method of Peto and Peto.29 Log-rank tests were used to compare survival curves between groups.30 Fisher’s exact and two-sample χ2 tests across available categories were used in analyses involving proportions (remission and MRD-positive rates). A two-sided Wilcoxon rank sum test was used to compare times to event for those receiving HSCT or chemotherapy alone. Differences with P values ≤ .05 were considered significant.
RESULTS
Patient Characteristics
Sixty-three patients from 47 institutions were enrolled from July 2008 to February 2012 (Data Supplement). Patient characteristics are summarized in Table 1. Two patients were ineligible because they had chronic myeloid leukemia in lymphoid blast crisis. One patient was ineligible because an ECG was not performed before enrollment.
Table 1.
Patient Characteristics
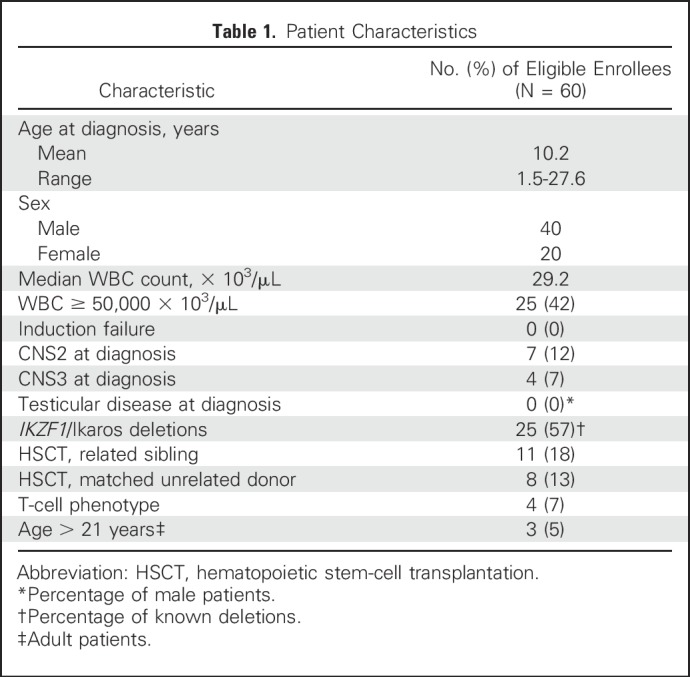
Of 60 eligible patients, 34 completed protocol therapy, which consisted of seven intensive blocks and 10 maintenance blocks of chemotherapy plus dasatinib. Of the patients who did not complete protocol therapy, 19 underwent HSCT; four discontinued protocol therapy for posterior reversible encephalopathy syndrome, poor patient compliance, leukoencephalopathy, or prolonged corrected QT interval, and three experienced events during therapy. The remaining events were relapses that occurred after patients completed therapy or went off protocol for HSCT. Patients were observed for outcome data until they went off study for reasons such as death, loss to follow-up, withdrawal of consent, or enrollment in another study with therapeutic intent after being in follow-up.
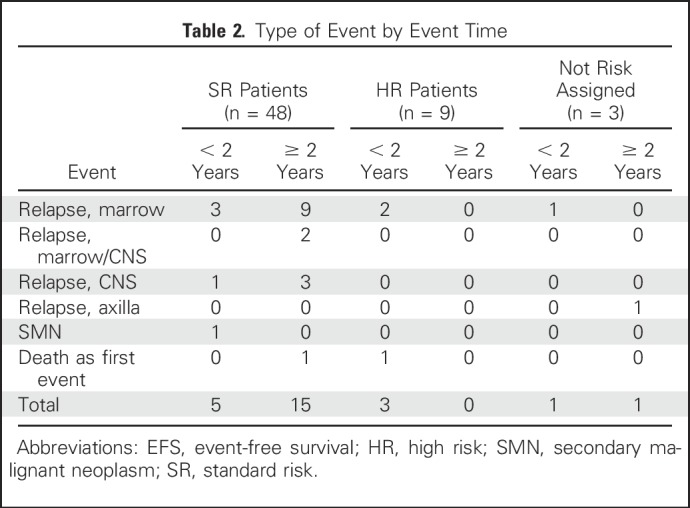
Thirty-nine patients were assigned discontinuous dasatinib (cohort 1), and 21 were assigned continuous dasatinib (cohort 2). Four had T-cell immunophenotype, and 56 had B-cell immunophenotype. Table 2 summarizes outcomes and events by risk group.
Table 2.
Type of Event by Event Time
Summary of Toxicities
The combination of dasatinib plus intensive chemotherapy was found to be safe and feasible. A summary of the toxicities is provided in the Data Supplement. Importantly, no deaths resulting from toxicity occurred in this study.
Impact of Adding TKI Mid-Induction on Early Response Rates
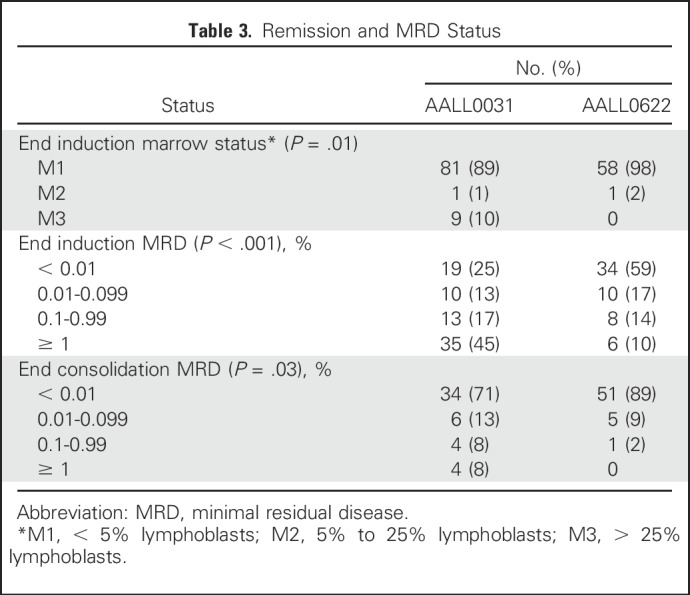
AALL0622 introduced dasatinib on induction day 15, compared with imatinib therapy initiated on day 1 of consolidation 1 in AALL0031. End-of-induction (day 29) complete remission rate was 98% (n = 59) in AALL0622 compared with 89% (n = 91) in AALL0031 (P = .01; Table 3). Furthermore, 59% of AALL0622 versus 25% of AALL0031 patients had MRD < 0.01% at end of induction (P < .001; Table 3). Despite two thirds of AALL0622 patients being treated with discontinuous dasatinib, 89% of AALL0622 patients (n = 57) had MRD < 0.01% at end of consolidation 2 versus 71% treated with continuous imatinib in AALL0031 (n = 48; P = .03; Table 3).
Table 3.
Remission and MRD Status
Impact of Dasatinib on EFS and OS
The primary outcome end point of AALL0622 was achievement of 3-year EFS ≥ 60% among SR patients. Three-year EFS (± standard deviation [SD]) in SR patients was 84.6% ± 5.7%. Because relapses continued after 3 years, we continued to observe patients to report 5-year outcomes. For the 60 evaluable patients, 5-year OS and EFS rates (± SDs) were 86% ± 5% and 60% ± 7%, respectively (Figs 1A and 1B). SR patients (n = 48; 19% underwent HSCT in first complete remission [CR1]) had 5-year OS and EFS rates (± SDs) of 87% ± 5% and 61% ± 7%, respectively, and HR patients (n = 9; 89% underwent HSCT in CR1) had 5-year OS and EFS rates (± SDs) of 89% ± 13% and 67% ± 19%, respectively (Figs 1C and 1D). Table 2 summarizes events and whether they occurred early (< 2 years) or late (> 2 years).
Fig 1.
Long-term survival in AALL0622. (A) Overall survival (OS) and (B) Event-free survival (EFS) in whole cohort. (C) OS and (D) EFS by risk group. (E) OS and (F) disease-free survival (DFS) comparison between AALL0031 and AALL0622.
Among 60 patients, four (6.7%) had CNS3 status at diagnosis. Despite the addition of dasatinib and substantial CNS-directed chemotherapy, non-HSCT and non-CNS3 patients in AALL0622 had a trend toward increased 5-year CIR (± SD) of isolated or combined CNS relapse of 15.4% ± 5.9% (n = six of 40), compared with 6.6% ± 4.6% (n = two of 31; P = .30) in those treated with continuous imatinib and 12-Gy cranial irradiation in AALL0031.14,15 Three CNS relapses occurred in each cohort of AALL0622. All CNS relapses occurred in SR patients receiving chemotherapy plus dasatinib. No patient undergoing transplantation, most of whom received CNS irradiation as part of their conditioning, experienced CNS relapse. No AALL0622 patients had testicular leukemia at diagnosis or relapse. Five-year CIR (± SD) for isolated marrow relapse was similar between the two trials (18.0% ± 6.3% in AALL0622 and 23.7% ± 8.0% in AALL0031; P = .97).
There was no difference in OS or disease-free survival between patients receiving imatinib in AALL0031 (cohorts 4 and 5, including patients undergoing HSCT; n = 54) and AALL0622 (cohorts 1 and 2, including those undergoing HSCT; n = 60). Five-year OS (± SD) was 81% ± 6% for AALL0031 versus 86% ± 5% for AALL0622 (P = .63); 5-year disease-free survival (± SD) was 68% ± 7% for AALL0031 versus 60% ± 7% in AALL0622 (P = .31; Figs 1E and 1F). Outcomes remained similar between the two trials when analyses were limited to patients age ≤ 21 years. Of the four patients with T-cell ALL, three were SR and one was not assigned to a risk group (HSCT). Two experienced events; one SR patient had a CNS relapse, and the patient not assigned to a risk group had a relapse involving lymph nodes.
Impact of HSCT
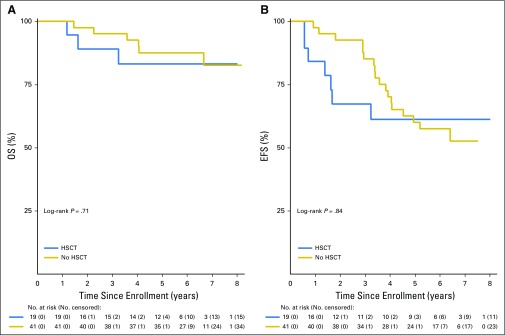
AALL0622 recommended HSCT for patients with a matched sibling donor or with high-risk features based on MRD. Nineteen patients underwent HSCT, including eight HR (42%), nine SR (47%, six who had sibling donors), and two non–risk assigned patients (11%) because of lack of MRD data. Four patients did not undergo transplantation according to protocol guidelines; three SR patients underwent unrelated HSCT, and one patient underwent HSCT in maintenance rather than after consolidation 2. Transplantation-preparative regimens and supportive care were provided at the discretion of the local centers.
Outcomes for patients receiving HSCT and chemotherapy alone are compared in Figure 2. There were a total of 48 patients deemed SR based on response, nine of whom underwent HSCT based on the presence of a matched sibling donor (n = 6) or physician or patient choice (n = 3). Among the remaining 39 SR patients, 33 completed protocol therapy in CR1 (dropouts, n = 3; relapses, n = 2; and secondary malignant neoplasm during therapy, n = 1). Of these 33 patients, 13 subsequently relapsed (and another died as a result of unrelated cause).
Fig 2.
Outcomes comparing patients who underwent and did not undergo bone marrow transplantation: (A) Overall survival (OS) and (B) event-free survival (EFS).
OS and EFS rates were similar in patients receiving chemotherapy plus dasatinib and chemotherapy plus dasatinib before HSCT (5-year OS [± SD], 88% ± 5% v 83% ± 10%; P = .71 and EFS, 60% ± 8% v 61% ± 13%; P = .84). Importantly, nearly half of the patients undergoing HSCT were HR based on MRD (n = 8 of 19). However, 5-year EFS (± SD) was 63% ± 19% (n = 8) in HR and 76% ± 17% (n = 9) in SR patients who received HSCT, compared with 59% ± 8% (n = 39) in SR patients receiving chemotherapy plus dasatinib, although these analyses were hindered by small patient numbers. Only one HR patient received dasatinib plus chemotherapy, and this patient remained in CR1. Events occurred significantly earlier in patients undergoing HSCT versus those receiving chemotherapy, with median time to first event of 506 versus 1,275 days, respectively (P = .002).
Impact of IKZF1 Deletions on Outcome
We analyzed diagnostic specimens from 44 patients who had adequate banked material for the presence of IKZF1 deletions. An IKZF1 deletion was present in 56.8% of tested AALL0622 patients (n = 25 of 44) and was associated with significantly inferior outcomes (5-year OS [± SD], 80% ± 8% v 100%; P = .04 and EFS [± SD], 52% ± 10% v 82% ± 10%; P = .04, respectively; Figs 3A and 3B). Among the SR patients, 58.8% (20 of 34) had IKZF1 deletions, which were associated with inferior OS and EFS (5-year OS [± SD], 80% ± 9% v 100%; P = .07 and EFS [± SD], 50% ± 11% v 83% ± 13%; P = .04; Figs 3C and 3D). Half (four of eight) of tested HR patients had IKZF1 deletions, which were not predictive of outcome, with 5-year OS of 100% for both groups and 5-year EFS (± SD) of 75% ± 22% for those with deletions and 75% ± 38% for those without deletions.
Fig 3.
Outcomes based on presence or absence of an Ikaros deletion. (A) Overall survival (OS) and (B) event-free survival (EFS) in whole cohort based on presence of IKZF1 deletion. (C) OS and (D) EFS in standard-risk patients based on presence of IKZF1 deletion.
DISCUSSION
AALL0622, the first pediatric cooperative group study to our knowledge of using dasatinib in Ph-positive ALL, met its primary safety and efficacy aims. Two additional pediatric cooperative group Ph-positive ALL trials, COG AALL003114,15 and the EsPhALL trial,16 used imatinib as the TKI. All three trials had small patient numbers, the largest being EsPhALL with 178 participants.16 Given the limitations of small size, the outcomes in these three trials were similar. EFS rates were 60% for AALL0622 (5 years), 61.9% for EsPhALL (4 years),16 and 58% overall for AALL0031 (5 years).15 For AALL0031 cohort 5, which used continuous imatinib, 5-year EFS was 63% for those undergoing unrelated-donor HSCT, 64% for those undergoing related-donor HSCT, and 71% for those receiving chemotherapy alone. Overall survival rates for these trials were 86% for AALL0622 (5 years), 72.1% for EsPhALL (4 years),16 and 70% for AALL0031 overall (5 years).15 The apparent better OS rate in AALL0622 may reflect that only approximately one third of patients underwent HSCT in first remission, compared with 80% in the EsPhALL trial. It is notable that 5-year OS rate in AALL0622 approaches that of all children with ALL treated in COG trials from 2000 to 2005 (90%).2 However, the 4- and 5-year EFS rates of approximately 60% in these three Ph-positive ALL trials are much lower than the expected approximately 85% EFS rate for unselected patients with ALL.31,32 The discussion will focus on ways to potentially improve EFS rates for children with Ph-positive ALL.
In AALL0622, early responses to dasatinib plus chemotherapy based on MRD by flow cytometry were impressive. The improved induction success rate and reduced rate of detectable MRD at the end of consolidation may reflect in part the addition of TKI earlier in treatment in AALL0622 compared with AALL0031 (week 3 v 5, respectively). Detection of MRD at the end of induction is usually a powerful predictor of relapse in children with ALL.25,33-37 However, improved early response did not translate to improved EFS. Why did the improved early response not lead to better outcomes in AALL0622 versus AALL0031 or EsPhALL? Most patients in AALL0622 received dasatinib discontinuously. Continuous rather than discontinuous imatinib exposure led to better outcomes in AALL0031. However, AALL0622 was closed before we could fully test the effect of providing dasatinib continuously. A second possibility that could explain the lack of improvement is the reduction from 9 to 7 high-dose methotrexate doses. We consider this to be less likely, because this is almost double the number of high methotrexate doses used in other pediatric trials that have had better leukemia control and much lower CNS relapse rates.38,39
Our study posited that dasatinib would replace CNS irradiation to prevent CNS relapse. However, the cumulative incidence of isolated and combined CNS relapses for patients treated without radiotherapy in AALL0622 was surprisingly high at 15%, compared with 6% in AALL0031, where all patients received radiotherapy. Most patients in the original EsPhALL cohort underwent HSCT, and so these data cannot be compared easily. Results from the recently completed COG AALL1122 trial will more definitively explore the consequence of eliminating radiotherapy in CNS relapse in a larger cohort of children with Ph-positive ALL treated with dasatinib.
AALL0622 used HSCT to control resistant leukemia in HR (MRD-positive) patients but also recommended transplantation in SR patients with sibling donors. Five-year OS in SR patients, most of whom did not undergo transplantation, was 87%, suggesting that if relapse occurred, salvage by HSCT in second remission may have been possible in many of these patients. Thus, the results of our trial suggest that HSCT may not be necessary in SR patients, and the availability of a sibling donor should not be considered an indication for HSCT. For HR patients, HSCT was associated with OS and EFS comparable to SR patients, suggesting that HSCT abrogated the adverse prognostic significance of high MRD in these patients.
We made no recommendations regarding clearing MRD before HSCT in this study. At the time this study was conducted, it was not known that MRD levels before HSCT significantly predicted outcomes in ALL.40-43 Although patients undergoing HSCT received dasatinib for only 6 to 10 weeks before transplantation, additional TKI exposure pre- or post-HSCT may not improve outcomes in patients with suboptimal early responses. Immuotherapy such as blinotumomab44,45 or rapid reduction of immunosuppression post-transplantation42 could improve outcomes in HR patients.
Our data support measuring IKZF1 deletions to identify HR patients. Patients harboring IKZF1 deletions had significantly inferior outcomes. Conversely, those with wild-type IKZF1 had 5-year OS of 100%. These data are consistent with other studies showing that IKZF1 deletion confers poor prognosis in Ph-positive ALL.12,13 Furthermore, IKZF1 deletions were associated with a higher risk of relapse in patients who were SR based on MRD. Additional data from more recent trials in which fewer patients received HSCT will be necessary to see if this is a consistent finding that merits a change in risk stratification.
AALL0622 compared outcomes with historical controls. AALL0622 closed early to open COG AALL1122 (ClinicalTrials.gov identifier: NCT01460160), using dasatinib with EsPhALL chemotherapy backbone, leading to a smaller number of patients enrolled in cohort 2 than planned.
In conclusion, AALL0622 established that dasatinib was well tolerated with intensive chemotherapy. The addition of dasatinib during induction led to better early response rates but not improved EFS relative to AALL0031, in part because of increased CNS relapse. We cannot yet conclude that the current dasatinib plus chemotherapy combination is better than imatinib plus chemotherapy, but results using dasatinib were at least similar to those of AALL0031 using imatinib. The favorable 5-year OS of 88% in patients receiving chemotherapy plus dasatinib without HSCT demonstrates that most children with Ph-positive ALL should not undergo transplantation in first remission. Our study suggests that screening for IKZF1 deletions might be added to assessment of early response rates to identify low-risk Ph-positive patients who do not need transplantation and high-risk Ph-positive patients who may be suitable candidates for HSCT and/or alternative therapies.
Footnotes
Supported by Children’s Oncology Group (COG) Group Operations Grants No. U10 CA098543 and U10 CA180886 and COG Statistics and Data Center Grants No. U10 CA098413 and U10 CA180899. The National Cancer Institute (NCI) provided dasatinib. W.B.S. is the Stop Children’s Cancer of Palm Beach County Chair of Pediatric Hematology/Oncology at the University of Florida; S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at the Children’s Hospital of Philadelphia; and C.M. is supported by the Leukemia and Lymphoma Society Specialized Center of Research, NCI Cancer Center Support Grant No. CA021765, and NCI Outstanding Investigator Award No. CA197695.
Clinical trial information: NCT00720109.
AUTHOR CONTRIBUTIONS
Conception and design: William B. Slayton, Kirk R. Schultz, John A. Kairalla, Meenakshi Devidas, Michael A. Pulsipher, Bill H. Chang, Michael J. Borowitz, Sherri L. Mizrahy, Thomas Merchant, Lance Sieger, Marilyn J. Siegel, Elizabeth A. Raetz, Naomi J. Winick, Mignon L. Loh, William L. Carroll, Stephen P. Hunger
Provision of study materials or patients: William B. Slayton, Valerie I. Brown
Collection and assembly of data: William B. Slayton, John A. Kairalla, Meenakshi Devidas, Charles Mullighan, Ilaria Iacobucci, Lewis B. Silverman, Michael J. Borowitz, Andrew J. Carroll, Nyla A. Heerema, Julie M. Gastier-Foster, Brent L. Wood
Data analysis and interpretation: William B. Slayton, Kirk R. Schultz, Meenakshi Devidas, Xinlei Mi, Charles Mullighan, Lewis B. Silverman, Michael J. Borowitz, Valerie I. Brown, Stephen P. Hunger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dasatinib Plus Intensive Chemotherapy in Children, Adolescents, and Young Adults With Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0622
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
William B. Slayton
No relationship to disclose
Kirk R. Schultz
Honoraria: Jazz Pharmaceuticals, Janssen Oncology
Consulting or Advisory Role: Juno Therapeutics, Mesoblast
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Janssen Oncology
John A. Kairalla
Stock or Other Ownership: Johnson & Johnson, Sophiris Bio
Meenakshi Devidas
Honoraria: Pfizer, Novartis, PSI CRO
Xinlei Mi
No relationship to disclose
Michael A. Pulsipher
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals
Speakers’ Bureau: Novartis
Research Funding: Adaptive Biotechnologies
Travel, Accommodations, Expenses: Medac, Amgen
Bill H. Chang
No relationship to disclose
Charles Mullighan
Honoraria: Amgen
Consulting or Advisory Role: TRM Oncology
Speakers’ Bureau: Amgen
Research Funding: Loxo Oncology
Travel, Accommodations, Expenses: Amgen
Ilaria Iacobucci
No relationship to disclose
Lewis B. Silverman
No relationship to disclose
Michael J. Borowitz
Honoraria: Beckman Coulter
Consulting or Advisory Role: HTG Molecular Diagnostics
Research Funding: Becton Dickinson, Bristol-Myers Squibb
Andrew J. Carroll
No relationship to disclose
Nyla A. Heerema
No relationship to disclose
Julie M. Gastier-Foster
Research Funding: Bristol-Myers Squibb (Inst), Incyte (Inst)
Brent L. Wood
Honoraria: Seattle Genetics, Amgen
Consulting or Advisory Role: Spectrum Pharmaceuticals
Research Funding: Seattle Genetics (Inst), Amgen (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Coronado Biosciences (Inst), JW Pharmaceutical (Inst), MedImmune (Inst), Stemline Therapeutics (Inst)
Travel, Accommodations, Expenses: Amgen
Sherri L. Mizrahy
No relationship to disclose
Thomas Merchant
Travel, Accommodations, Expenses: Philips Healthcare
Valerie I. Brown
No relationship to disclose
Lance Sieger
Stock or Other Ownership: Amgen, Merck, Pfizer, Celgene
Marilyn J. Siegel
Honoraria: Siemens Healthcare Diagnostics
Consulting or Advisory Role: General Electric Healthcare (I)
Speakers’ Bureau: Siemens Healthcare Diagnostics
Travel, Accommodations, Expenses: Siemens Healthcare Diagnostics
Elizabeth A. Raetz
No relationship to disclose
Naomi J. Winick
No relationship to disclose
Mignon L. Loh
No relationship to disclose
William L. Carroll
No relationship to disclose
Stephen P. Hunger
Stock or Other Ownership: Amgen, Merck (I), Amgen (I), Pfizer (I)
Honoraria: Jazz Pharmaceuticals, Spectrum Pharmaceuticals, Erytech Pharma
Consulting or Advisory Role: Novartis
REFERENCES
- 1.Conter V, Aricò M, Basso G, et al. : Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia 24:255-264, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. : Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. J Clin Oncol 30:1663-1669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, et al. : Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia 24:309-319, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Möricke A, Zimmermann M, Reiter A, et al. : Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM Study Group from 1981 to 2000. Leukemia 24:265-284, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Pei D, Sandlund JT, et al. : Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 24:371-382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aricò M, Schrappe M, Hunger SP, et al. : Clinical outcome of children with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol 28:4755-4761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aricò M, Valsecchi MG, Camitta B, et al. : Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med 342:998-1006, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Quintás-Cardama A, Cortes J: Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 113:1619-1630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerema NA, Harbott J, Galimberti S, et al. UKALL : Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia 18:693-702, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Wetzler M, Dodge RK, Mrózek K, et al. : Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: A study of the Cancer and Leukaemia Group B. Br J Haematol 124:275-288, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Martinelli G, Iacobucci I, Soverini S, et al. : New mechanisms of resistance in Philadelphia chromosome acute lymphoblastic leukemia. Expert Rev Hematol 2:297-303, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Qazi S, Uckun FM: Incidence and biological significance of IKZF1/Ikaros gene deletions in pediatric Philadelphia chromosome negative and Philadelphia chromosome positive B-cell precursor acute lymphoblastic leukemia. Haematologica 98:e151-e152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Veer A, Zaliova M, Mottadelli F, et al. : IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood 123:1691-1698, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Schultz KR, Bowman WP, Aledo A, et al. : Improved early event-free survival with imatinib in Philadelphia chromosome–positive acute lymphoblastic leukemia: A Children’s Oncology Group study. J Clin Oncol 27:5175-5181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz KR, Carroll A, Heerema NA, et al. : Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia 28:1467-1471, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biondi A, Schrappe M, De Lorenzo P, et al. : Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol 13:936-945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blatt J, Bercu BB, Gillin JC, et al. : Reduced pulsatile growth hormone secretion in children after therapy for acute lymphoblastic leukemia. J Pediatr 104:182-186, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Meadows AT, Gordon J, Massari DJ, et al. : Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet 2:1015-1018, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Pizzo PA, Poplack DG, Bleyer WA: Neurotoxicities of current leukemia therapy. Am J Pediatr Hematol Oncol 1:127-140, 1979 [PubMed] [Google Scholar]
- 20.Riccardi R, Brouwers P, Di Chiro G, et al. : Abnormal computed tomography brain scans in children with acute lymphoblastic leukemia: Serial long-term follow-up. J Clin Oncol 3:12-18, 1985 [DOI] [PubMed] [Google Scholar]
- 21.O’Hare T, Walters DK, Stoffregen EP, et al. : In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 65:4500-4505, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Carter TA, Wodicka LM, Shah NP, et al. : Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci USA 102:11011-11016, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NP, Tran C, Lee FY, et al. : Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305:399-401, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Porkka K, Koskenvesa P, Lundán T, et al. : Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 112:1005-1012, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Borowitz MJ, Wood BL, Devidas M, et al. : Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children’s Oncology Group study AALL0232. Blood 126:964-971, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts KG, Gu Z, Payne-Turner D, et al. : High frequency and poor outcome of Philadelphia chromosome–like acute lymphoblastic leukemia in adults. J Clin Oncol 35:394-401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 28.Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 29.Peto R, Peto J: Asymptotically efficient rank invariant test procedure. J R Stat Soc [Ser A] 135:185-192, 1972 [Google Scholar]
- 30.Breslow NE: Analysis of survival data under proportional hazards model. Int Stat Rev 43:45-58, 1975 [Google Scholar]
- 31.Hunger SP, Mullighan CG: Acute lymphoblastic leukemia in children. N Engl J Med 373:1541-1552, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Pui CH, Yang JJ, Hunger SP, et al. : Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol 33:2938-2948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry DA, Zhou S, Higley H, et al. : Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol 3:e170580, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borowitz MJ, Devidas M, Hunger SP, et al. : Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children’s Oncology Group study. Blood 111:5477-5485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conter V, Bartram CR, Valsecchi MG, et al. : Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115:3206-3214, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Schrappe M, Valsecchi MG, Bartram CR, et al. : Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 118:2077-2084, 2011 [DOI] [PubMed] [Google Scholar]
- 37. doi: 10.1182/blood-2006-09-045369. Zhou J, Li A, Goldwasser MA, et al: Quantitative analysis of minimal residual disease at the completion of induction therapy predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood 104, 2004 (abstr 323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen EC, Devidas M, Chen S, et al. : Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group study AALL0232. J Clin Oncol 34:2380-2388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möricke A, Zimmermann M, Valsecchi MG, et al. : Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 127:2101-2112, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Campana D, Pui CH: Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood 129:1913-1918, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckert C, von Stackelberg A, Seeger K, et al. : Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia: Long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer 49:1346-1355, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Pulsipher MA, Langholz B, Wall DA, et al. : Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: For whom and when should interventions be tested? Bone Marrow Transplant 50:1173-1179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulsipher MA, Langholz B, Wall DA, et al. : The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: A phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood 123:2017-2025, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantarjian H, Jabbour E, Topp MS: Blinatumomab for Acute Lymphoblastic Leukemia. N Engl J Med 376:e49, 2017 [DOI] [PubMed] [Google Scholar]
- 45.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]