Abstract
Purpose
To compare peripheral nervous system function and balance between adult survivors of childhood acute lymphoblastic leukemia (ALL) and matched controls and to determine associations between peripheral neuropathy (PN) and limitations in static balance, mobility, walking endurance, and quality of life (QoL) among survivors.
Patients and Methods
Three hundred sixty-five adult survivors of childhood ALL and 365 controls with no cancer history completed assessments of PN (modified Total Neuropathy Score [mTNS]), static balance (Sensory Organization Test [SOT]), mobility (Timed Up and Go), walking endurance (6-minute walk test), QoL (Medical Outcomes Study 36-Item Short Form Survey), and visual-motor processing speed (Wechsler Adult Intelligence Scale).
Results
PN, but not impairments, in performance on SOT was more common in survivors than controls (41.4% v 9.5%, respectively; P < .001). In multivariable models, higher mTNS scores were associated with longer time to complete the Timed Up and Go (β = 0.15; 95% CI, 0.06 to 0.23; P < .001), shorter distance walked in 6 minutes (β = −4.39; 95% CI, −8.63 to −0.14; P = .04), and reduced QoL (β = −1.33; 95% CI, −1.79 to −0.87; P < .001 for physical functioning; β = −1.16; 95% CI, −1.64 to −0.67; P < .001 for role physical; and β = −0.88; 95% CI, −1.34 to −0.42; P < .001 for general health). Processing speed (β = 1.69; 95% CI, 0.98 to 2.40; P < .001), but not mTNS score, was associated with anterior-posterior sway on the SOT.
Conclusion
PN in long-term ALL survivors is associated with movement, including mobility and walking endurance, but not with static standing balance. The association between processing speed and sway suggests that static balance impairment in ALL survivors may be influenced by problems with CNS function, including the processing of sensory information.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer.1 Risk-directed treatment strategies have improved 5-year survival in developed countries to > 85%,2 with approximately 70,000 survivors of childhood ALL living in the United States.1 However, improved survival does not come without cost. Survivors of pediatric ALL are at risk for developing late effects that negatively affect function, limit participation in activities, and reduce quality of life (QoL).3-5
Many adverse outcomes observed in cancer survivors are associated with specific treatment exposures, including the association between vincristine chemotherapy and peripheral neuropathy (PN).6,7 PN manifests as decreased muscle strength, particularly in the distal lower extremities, and limited ankle range of motion.8-10 Loss of ankle mobility and strength may negatively affect balance, which in turn may limit daily mobility and participation in usual activities.11,12 The extent to which these impairments influence function and QoL in survivors of ALL has been investigated13 but on a limited basis. Therefore, the primary objectives of this study were to compare PN and static balance in adult survivors of childhood ALL with sex-, race-, and age-matched controls and determine associations between PN and limitations in static balance, mobility, walking endurance, and QoL among survivors.
PATIENTS AND METHODS
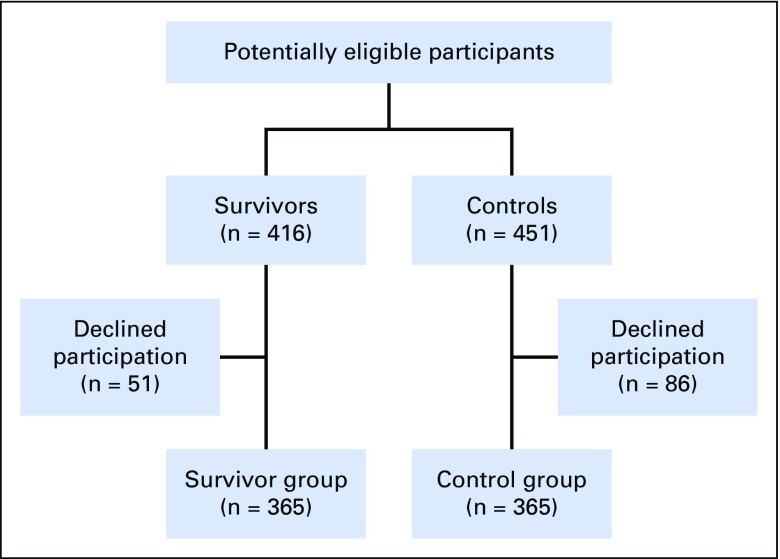
Participants were members of the St Jude Lifetime study, which examines health outcomes among aging survivors of childhood cancer. Survivors were ≥ 10 years since primary cancer diagnosis, ≥ 18 years of age, treated for childhood ALL between January 1, 1980, and December 31, 2003, and cancer free at the time of assessment. Among 416 potentially eligible ALL survivors, 365 (87.7%) agreed to and 51 declined participation. A comparison group (controls) frequency matched with survivors on sex, race, and age (within 5 years) was recruited from friends, relatives, and family members of other patients at St Jude Children’s Research Hospital. Of 451 potential controls, 365 (80.9%) agreed to and 86 declined participation (Fig 1). The protocol was approved by the St Jude Children’s Research Hospital institutional review board, and all the participants provided informed consent.
Fig 1.
Participant flow diagram.
Measures
Outcomes.
Static balance was characterized with the Sensory Organization Test (SOT), which uses computerized dynamic posturography (NeuroCom SMART EquiTest; Natus Medical, Pleasanton, CA)14,15 to evaluate postural sway (ability to maintain upright in a 12° sway envelope) under six conditions. Conditions 1 to 3 have a fixed standing surface and are done with eyes open, eyes closed, and eyes open but sway referenced (visual surround moves in reference to anterior-posterior sway). Conditions 4 to 6 are the same but with a sway-referenced standing surface (the force plate moves in reference to anterior-posterior sway). Two or three trials (20 seconds long) were collected for each condition. Scores from the six conditions were used to derive an overall SOT score. For this study, similar to a previous St Jude Lifetime study,9 participants with an SOT score 1.5 standard deviations below the control mean (< 74.43) were classified as having impaired balance (on the basis of a standard normal distribution, 6.7% of a healthy population would score < 74.43). The ability of participants to use sensory inputs to control balance was also evaluated using performance ratios calculated from various test conditions: somatosensory ratio (condition 2/condition1), vision ratio (condition 4/condition1), and vestibular ratio (condition 5/condition 1).
Mobility was assessed with the Timed Up and Go (TUG) test.16,17 Participants rose from a standard armchair, walked to a line 10 feet away, turned, returned, and sat down while encouraged to complete this task as fast as possible. Two trials were attempted; the time to complete the second trial was used for the analysis. Walking endurance was evaluated with the 6-minute walk test.18,19 Participants walked as fast as possible along a corridor for 6 minutes. Customary walking aids (canes, walkers, crutches) were allowed; distance was recorded in meters.
QoL was measured with the Medical Outcomes Study 36-Item Short Form Survey, which includes questions about health and well-being over the previous 4 weeks.20 In this study, we only used the physical functioning, role physical, and general health subscales of this survey. Results are reported as T scores, with a population mean of 50 and a standard deviation of 10. Higher scores indicate better QoL.
Independent variables.
Sensory and motor integrity were evaluated with the modified Total Neuropathy Score (mTNS).21 This test assesses sensory and motor symptoms and evaluates distal muscle strength, deep tendon reflexes, light touch sensation, and vibration threshold. Results range from 0 (no neuropathy) to 24 (severe neuropathy). Although even a score of 1 indicates the presence of neuropathy,22,23 we used a score 1.5 standard deviations above the control mean (mTNS ≥ 4) to classify neuropathy—an approach used in previous studies of PN among pediatric and adult ALL survivors.9,24
Active ankle dorsi- and plantar-flexion range of motion were measured with a goniometer25 while sitting, with the hips and knees in 90° of flexion. System 4 (Biodex, Shirley, NY)26 was used to measure peak isokinetic ankle dorsi- and plantar-flexor strength at 60° per second and endurance at 90° per second.
Visual-motor processing speed (VMPS) in survivors was evaluated with the digit symbol coding test from the Wechsler Adult Intelligence Scale–Fourth Edition.27 Scores were transformed into age-adjusted z scores with a population mean of 0 and a standard deviation of 1.
Smoking status and the presence of type 2 diabetes that requires medication were determined by self-report. Treatment data were abstracted from medical records.
Analyses
Demographics were compared between survivors and controls using the t or χ2 test. Measures of PN, balance, and QoL were compared between survivors and matched controls in linear models adjusted for height and smoking status. t tests were used to compare static balance, mobility, walking endurance, and QoL between survivors with and without PN and to compare SOT score sensory ratios between survivors with and without impaired balance. To address the increased risk of committing type I error associated with multiple comparisons, α-levels were corrected using Bonferroni’s method.28 Associations between mTNS and static balance, mobility, walking endurance, QoL, and sensory ratios in survivors, adjusting for age at assessment, sex, body mass index (BMI), history of cranial radiation treatment (CRT), and VMPS, were evaluated using linear regression models. SPSS version 22 statistical software (IBM Corporation, Chicago, IL) was used for analyses.
RESULTS
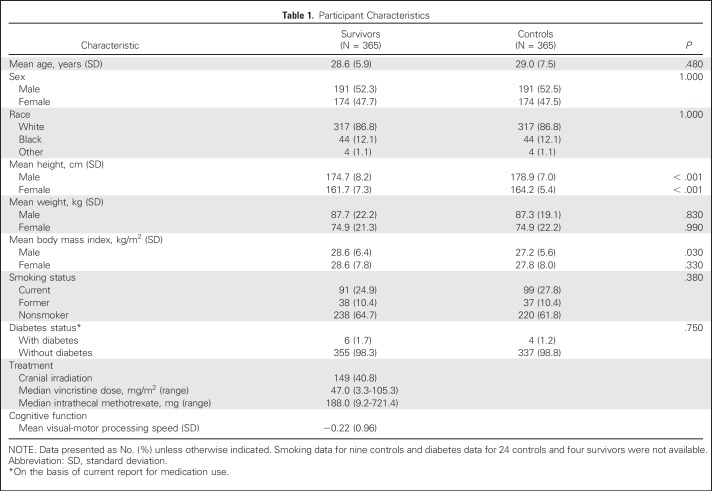
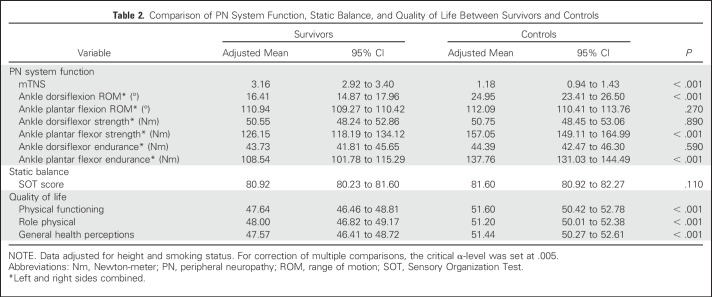
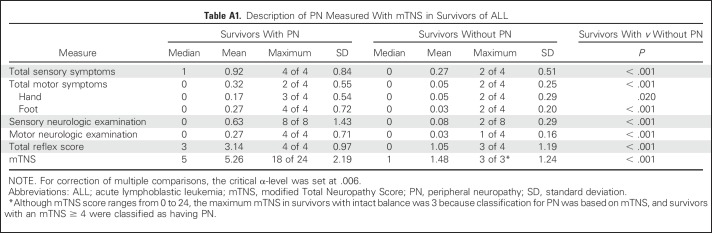
Participant characteristics are listed in Table 1. Survivors were shorter, and male survivors had a higher mean BMI than controls. All survivors were exposed to vincristine and intrathecal methotrexate; 40.8% had received CRT. Group comparisons of mTNS, SOT score, and QoL, adjusted for height and smoking status, are listed in Table 2. Mean score on the mTNS was higher among survivors, and more survivors were classified with PN than controls (41.4% v 9.5%; P < .001). In survivors with PN, mean mTNS was 5.26 (v 1.48 in survivors without PN). Motor symptoms were uncommon in survivors who scored < 4 on the mTNS (Appendix Table A1, online only). Smoking was identified as a significant risk factor (Appendix Table A2, online only) for PN. A test for interaction indicated that this association did not differ by survivor status (P = .50).
Table 1.
Participant Characteristics
Table 2.
Comparison of PN System Function, Static Balance, and Quality of Life Between Survivors and Controls
Survivors had lower mean values (Table 2) for dorsiflexion range of motion, plantar-flexor strength, and plantar-flexor endurance. Although more survivors than controls were classified as having impaired balance, the difference was not significant (11.5% v 7.5%; P = .07), and SOT mean scores between survivors and controls were not different. Survivors had lower mean scores on QoL measures than controls for physical functioning, role physical, and general health perceptions.
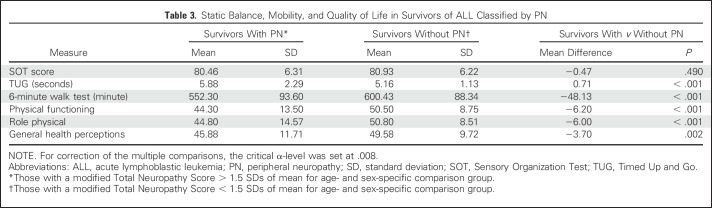
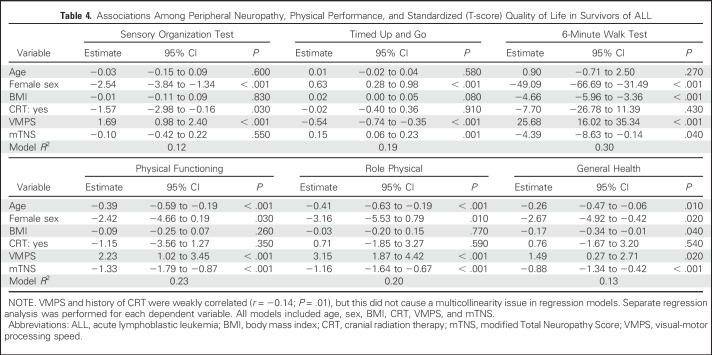
Survivors without PN had better performances on TUG, 6-minute walk test, and QoL than those with PN (Table 3). No difference was found in mean SOT scores among survivors with or without PN. These results were confirmed in multivariable analysis when controlling for age, sex, BMI, CRT, and VMPS; a lower score on the mTNS was associated with better performance on the TUG, the 6-minute walk test, and domains of QoL but not performance on SOT (Table 4). A better SOT score was associated with the absence of CRT and higher VMPS.
Table 3.
Static Balance, Mobility, and Quality of Life in Survivors of ALL Classified by PN
Table 4.
Associations Among Peripheral Neuropathy, Physical Performance, and Standardized (T-score) Quality of Life in Survivors of ALL
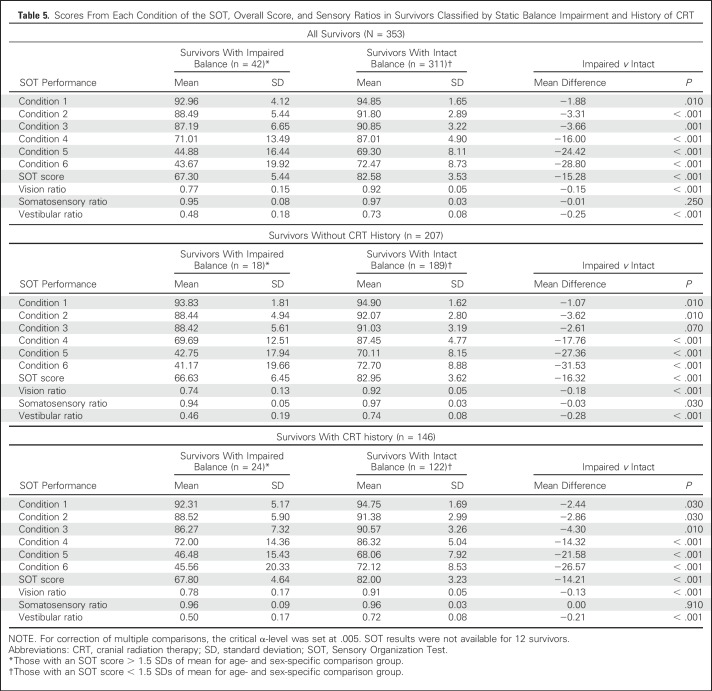
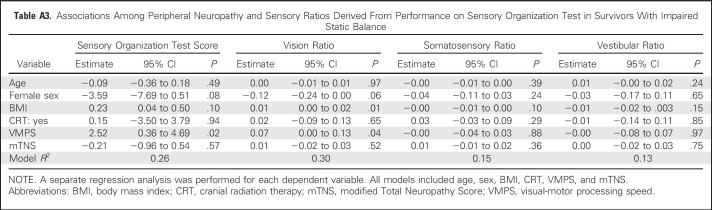
Performance on the SOT and sensory ratios are listed in Table 5 and stratified by static balance status and CRT. Survivors with impaired static balance had lower vision and vestibular ratios than those without impaired static balance. No difference was found in the mean somatosensory ratios among survivors with impaired and intact static balance. Similarly, no association was found between mTNS and any sensory ratio in survivors with impaired balance. However, VMPS was associated with vision ratio (Appendix Table A3, online only).
Table 5.
Scores From Each Condition of the SOT, Overall Score, and Sensory Ratios in Survivors Classified by Static Balance Impairment and History of CRT
DISCUSSION
To better understand the consequences of PN, we examined associations with static balance, mobility, walking endurance, and QoL in adult survivors of childhood ALL. Compared with individuals with no history of childhood cancer, ALL survivors were more likely to experience PN. PN was associated with poor mobility and QoL, albeit not with static balance impairments. Our analyses indicate that among ALL survivors, poorer VMPS is associated with static balance impairments, limited mobility, and poorer QoL and that those with impaired static balance have difficulty using both visual and vestibular inputs to maintain balance.
Forty percent of ALL survivors in this study had impaired peripheral sensation, motor function, or both. This rate is higher than previously reported in pediatric survivor populations. In a longitudinal study in pediatric survivors with non-CNS tumors, Gilchrist et al29 observed that 11.5% of ALL survivors had PN 6-months after treatment. Jain et al30 reported electrophysiologic and functional evidence of PN in 34% of childhood ALL survivors up to 3 years after chemotherapy, and Ramchandren et al13 found nerve conduction abnormalities in approximately 30% of ALL survivors a mean of 7 years after treatment. These differences may be due to the older mean age of the participants in the current study or are related to the mTNS cut point we used to classify neuropathy. Our cut point for classifying neuropathy was less conservative than that used by others,29 who used a score of ≥ 5.
In older populations, diabetes and smoking may contribute to a higher prevalence of PN. In the current sample, only six survivors and four controls had diabetes at the time of assessment, so we could not examine the effect of diabetes on PN. Smoking status was associated with PN and may have contributed to the higher prevalence of PN in this study than in studies among pediatric ALL survivors who might be less likely to smoke.
In adjusted models, we did not find an association between mTNS and amount of anterior-posterior sway during a standing balance evaluation in our young adult population (mean age, 28 years). This finding contrasts that of studies with pediatric participants. In a study of 41 children with non-CNS malignances, Gilchrist and Tanner31 reported that a higher score (more impairment) on the pediatric mTNS is associated with poorer performance on the balance subscale of the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition, which evaluates both static and dynamic balance, and includes such activities as standing on one leg and heel-toe walking. These activities require postural control during movement and may be more challenging than our test of static balance because impairments in muscle strength and general coordination are likely more important during movement than in quiet standing. It is also possible that the longer-term survivors in the current study have learned to compensate for PN over time. Participants in the Gilchrist and Tanner study were children evaluated during cancer treatment who may not have yet learned how to compensate for the effects of neuropathy on balance.
The current results are similar to some and differ from other studies of PN and balance in patients with adult-onset cancer. In a study of 29 adult patients with various cancer diagnoses, Vasquez et al32 reported no association between mTNS and balance (using the Berg Balance Scale). In contrast, in a study of 20 patients with breast cancer treated with taxane-based chemotherapy, Wampler et al21 identified an association between mTNS and performance on the SOT. One important difference between Wampler et al and our study is the severity of PN. Although the mean mTNS score in participants in the study by Wampler et al was 7, this value in the current study was 3. The survivors in the study by Wampler et al were older (mean age, 50 ± 9 v 28 ± 6 years) than our survivors and were evaluated < 1 month after therapy completion.33 Survivors in our cohort were ≥ 10 years since diagnosis, which again provided these participants with an opportunity to compensate for persistent impairment. Winter-Stone et al34 found associations between self-reported symptoms of PN and number of falls reported in the past year in 512 female cancer survivors with a mean age of 62 years. They only used the presence of sensory symptoms to classify neuropathy, whereas we included motor signs and symptoms in the classification schema. In addition, their measure of balance was previous fall history, which reflects dynamic loss of postural control, whereas our measure, standing balance, was more static.
Although PN does not seem to be associated with impaired static balance, as determined by amount of anterior-posterior sway during static standing in long-term ALL survivors, it is associated with mobility. Our finding of an association between neuropathy and performance on the TUG is similar to that reported by Wampler et al21 among patients with breast cancer in whom more severe PN was associated with longer time to complete the TUG. Conversely, Vasquez et al32 reported no association between PN and performance on the TUG in adult patients with various cancer diagnoses. We also found an association between the mTNS and distance walked in 6 minutes, which were similar to those reported by Gilchrist and Tanner35 who observed that scores on the pediatric mTNS explained 35% of the variability on the 6-minute walk test in 5- to 22-year-old patients with cancer. The associations between PN and mobility in the current study may indicate an association between PN and ability to maintain balance during movement not reflected in performance on the SOT.
Survivors with PN had lower mean scores on physical QoL measures than those without PN. The single previous study that examined this association in pediatric ALL survivors (age 8 to 18 years) found no association between PN measures and scores on the pediatric QoL inventory.13 The association between PN and QoL has been investigated extensively in adult patients with cancer and survivors, where excellent reviews indicate that more-severe neuropathy predicts worse QoL.36,37
Although PN was not associated with the overall SOT score in our population, the analyses identified a potential mechanism for poor static balance and another contributor to impaired mobility and QoL among adult survivors of childhood ALL. VMPS was associated with performance on SOT, TUG, 6-minute walk test, and QoL. In addition, survivors who had impaired static balance did not seem to make use of visual and vestibular inputs to maintain standing when perturbed. These findings are similar to those reported by Einarsson et al,38 who examined the effect of visual input on balance among adult survivors of pediatric solid tumors. These authors found that survivors had more difficulty than controls in maintaining standing when perturbed in an eyes open condition and concluded that survivors likely have difficulty with processing visual information. Although condition 2 on the SOT possibly was too easy for our sample of survivors, even those with impaired balance, these data suggest that static balance impairments in long-term survivors of ALL may be influenced by problems in CNS function, including the processing of visual and vestibular information.
The current results should be considered in the context of some study limitations. First, although the participation rate was excellent, not all eligible survivors participated. If those who agreed to participate were in better or worse health than those who did not agree to participate, study results could have been different. Furthermore, all the survivors included in this study were diagnosed with ALL between 1980 and 2003 and received their treatment at a single institution. Although the characteristics and CRT exposure for this population generally were similar to national protocols for childhood ALL during the same period, our findings may not be generalizable to individuals treated in other centers and with different treatment protocols. Finally, because not every child treated for ALL survived for 10 years after diagnosis, survival bias may have influenced the prevalence estimates and should not be extrapolated to populations whose survival is < 10 years.
Other methodological limitations also need to be considered when interpreting results. First, the medical needs of this complex study population prohibited blinding of study staff during measurement. Whenever possible, objective evaluation tools were used. Research personnel received extensive training, and intra- and interrater reliability testing was conducted before the start of the study and annually. Second, balance was measured by evaluating deviations in anterior-posterior sway during static standing. More dynamic balance assessment and gait analysis would enhance results. Third, although the validity of the mTNS has been evaluated for concordance with the total neuropathy score (a measure that includes nerve conduction velocity) in breast cancer survivors,21 we did not include nerve conduction studies. Whether impairment determined with mTNS is associated with long-lasting abnormalities in nerve conduction in adult survivors of childhood ALL remains to be examined. Finally, we used the digit symbol coding subset of the Wechsler Adult Intelligence Scale as a measure of VMPS, which is a paper-and-pen test. Poor performance could have been influenced by the presence of PN that affected hand function. However, because the prevalence of impaired hand function in our survivor sample was low, this influence was likely minimal.
In conclusion, mild or moderate PN is prevalent among adult survivors of childhood ALL and interferes with mobility and QoL but does not seem to be associated with static standing balance. The study provides evidence in favor of the application of contemporary neurorehabilitation approaches, such as cognitive-motor task training, to address functional problems in survivors. These strategies are effective in improving postural control in healthy individuals and in persons with neurologic impairment.39,40 The current finding of associations among VMPS, balance, and mobility in survivors hints that a similar approach may be useful for remediating balance and mobility impairments in survivors of childhood ALL.
Appendix
Table A1.
Description of PN Measured With mTNS in Survivors of ALL
Table A2.
The Effect of Group Assignment (survivors v controls) on Peripheral Neuropathy Score Controlling for Height and Smoking Status
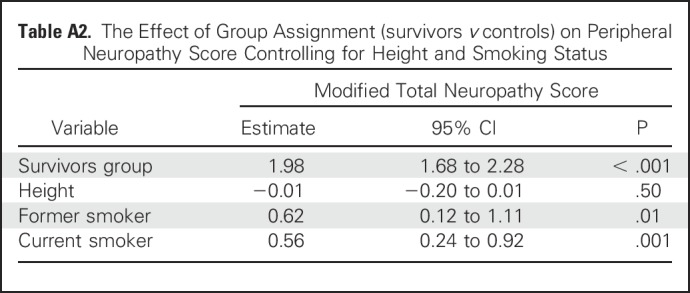
Table A3.
Associations Among Peripheral Neuropathy and Sensory Ratios Derived From Performance on Sensory Organization Test in Survivors With Impaired Static Balance
Footnotes
Supported by National Cancer Institute Grants No. CA132901, CA195547, and CA21765. Support also was provided to St Jude Children’s Research Hospital by the American Lebanese-Syrian Associated Charities.
Presented at the American Congress of Rehabilitation Medicine, Atlanta, GA, October 25-28, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Mitra Varedi, Carrie R. Howell, Melissa M. Hudson, Leslie L. Robison, Kirsten K. Ness, Raymond F. McKenna
Financial support: Leslie L. Robison
Administrative support: Robyn E. Partin, Melissa M. Hudson
Provision of study materials or patients: Ching-Hon Pui, Kevin R. Krull, Leslie L. Robison, Kirsten K. Ness
Collection and assembly of data: Robyn E. Partin, Kirsten K. Ness
Data analysis and interpretation: Lu Lu, Kevin R. Krull, Kirsten K. Ness
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Peripheral Neuropathy, Sensory Processing, and Balance in Survivors of Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Mitra Varedi
No relationship to disclose
Lu Lu
No relationship to disclose
Carrie R. Howell
No relationship to disclose
Robyn E. Partin
No relationship to disclose
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children With Cancer, Oncology Research Information Exchange Network, Pfizer Genotropin Advisory Board 2016, Princess Máxima Center
Ching-Hon Pui
Research Funding: National Cancer Institute (Inst)
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
Leslie L. Robison
No relationship to disclose
Kirsten K. Ness
No relationship to disclose
Raymond F. McKenna
No relationship to disclose
REFERENCES
- 1. https://seer.cancer.gov/archive/csr/1975_2014 Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD,
- 2.Pui CH, Yang JJ, Hunger SP, et al. : Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol 33:2938-2948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanellopoulos A, Hamre HM, Dahl AA, et al. : Factors associated with poor quality of life in survivors of childhood acute lymphoblastic leukemia and lymphoma. Pediatr Blood Cancer 60:849-855, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Järvelä LS, Niinikoski H, Lähteenmäki PM, et al. : Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J Cancer Surviv 4:339-345, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Kawashima T, Leisenring W, et al. : Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 32:1218-1227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vainionpää L, Kovala T, Tolonen U, et al. : Vincristine therapy for children with acute lymphoblastic leukemia impairs conduction in the entire peripheral nerve. Pediatr Neurol 13:314-318, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Dougherty PM, Cata JP, Burton AW, et al. : Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage 33:166-179, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Ness KK, Armenian SH, Kadan-Lottick N, et al. : Adverse effects of treatment in childhood acute lymphoblastic leukemia: General overview and implications for long-term cardiac health. Expert Rev Hematol 4:185-197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ness KK, DeLany JP, Kaste SC, et al. : Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood 125:3411-3419, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Hudson MM, Pui CH, et al. : Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: Associations with physical performance and chemotherapy doses. Cancer 118:828-838, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone M, Viret P, Bui HT, et al. : Assessment of gross motor skills and phenotype profile in children 9-11 years of age in survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer 61:46-52, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Wright MJ, Galea V, Barr RD: Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Phys Ther 85:782-790, 2005 [PubMed] [Google Scholar]
- 13.Ramchandren S, Leonard M, Mody RJ, et al. : Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst 14:184-189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nashner LM, Peters JF: Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin 8:331-349, 1990 [PubMed] [Google Scholar]
- 15.Pedalini MEB, Cruz OLM, Bittar RSM, et al. : Sensory organization test in elderly patients with and without vestibular dysfunction. Acta Otolaryngol 129:962-965, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Bohannon RW: Reference values for the Timed Up and Go test: A descriptive meta-analysis. J Geriatr Phys Ther 29:64-68, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Podsiadlo D, Richardson S: The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142-148, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Enright PL, Sherrill DL: Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158:1384-1387, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Butland RJ, Pang J, Gross ER, et al. : Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 284:1607-1608, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr: SF-36 health survey update. Spine 25:3130-3139, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Wampler MA, Miaskowski C, Hamel K, et al. : The modified total neuropathy score: A clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol 4:W9-W16, 2006 [Google Scholar]
- 22.Cavaletti G, Frigeni B, Lanzani F, et al. : The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst 12:210-215, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Cornblath DR, Chaudhry V, Carter K, et al. : Total neuropathy score: Validation and reliability study. Neurology 53:1660-1664, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lavoie Smith EM, Li L, Chiang C, et al. : Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 20:37-46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boone DC, Azen SP, Lin CM, et al. : Reliability of goniometric measurements. Phys Ther 58:1355-1360, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Harbo T, Brincks J, Andersen H: Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol 112:267-275, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Wechsler D: Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: Pearson Education, 2008. [Google Scholar]
- 28.Napierala MA: What is the Bonferroni correction. AAOS Now 6:40, 2012 [Google Scholar]
- 29.Gilchrist LS, Tanner LR, Ness KK: Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer 64:180-187, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Jain P, Gulati S, Seth R, et al. : Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: Prevalence and electrophysiological characteristics. J Child Neurol 29:932-937, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Gilchrist LS, Tanner L: The pediatric-modified total neuropathy score: A reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer 21:847-856, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Vasquez S, Guidon M, McHugh E, et al. : Chemotherapy induced peripheral neuropathy: The modified Total Neuropathy Score in clinical practice. Ir J Med Sci 183:53-58, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Grisold W, Cavaletti G, Windebank AJ: Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-oncol 14:iv45-iv54, 2012. (suppl 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winters-Stone KM, Horak F, Jacobs PG, et al. : Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol 35:2604-2612, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilchrist L, Tanner L: Gait patterns in children with cancer and vincristine neuropathy. Pediatr Phys Ther 28:16-22, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Argyriou AA, Bruna J, Marmiroli P, et al. : Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev Oncol Hematol 82:51-77, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Mols F, Beijers T, Vreugdenhil G, et al. : Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer 22:2261-2269, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Einarsson E-J, Patel M, Petersen H, et al. : Decreased postural control in adult survivors of childhood cancer treated with chemotherapy. Sci Rep 6:36784, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghai S, Ghai I, Effenberg AO: Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin Interv Aging 12:557-577, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fritz NE, Cheek FM, Nichols-Larsen DS: Motor-cognitive dual-task training in persons with neurologic disorders: A systematic review. J Neurol Phys Ther 39:142-153, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]