Abstract
Purpose
To determine whether a T2 mapping sequence could depict early changes in the composition and microstructure of cartilage overlying stable lesions of the medial femoral condyle in patients with juvenile osteochondritis dissecans (JOCD).
Materials and Methods
This retrospective study analyzed a sagittal T2 mapping sequence performed between September 1, 2015, and March 31, 2017, on 16 patients (10 boys and six girls; median age, 11.5 years) with 18 stable medial femoral condyle JOCD lesions and 18 age-, sex-, and skeletal maturation–matched control participants (11 boys and seven girls; median age, 11.5 years). Cartilage T2 values were quantitatively measured within regions of interest placed around the cartilage within and overlying the JOCD lesion in patients with JOCD and around the cartilage on the weight-bearing medial femoral condyle in patients with JOCD and controls. Wilcoxon signed rank and Wilcoxon rank sum tests were used to compare T2 values.
Results
T2 values were significantly higher (P < .001) for cartilage within the JOCD lesion than for cartilage overlying the JOCD lesion in patients with JOCD. However, there were no significant differences in T2 values between cartilage overlying the JOCD lesion and cartilage on the weight-bearing medial femoral condyle in patients with JOCD (P = .67) or in T2 values of the cartilage on the weight-bearing medial femoral condyle between patients with JOCD and controls (P = .30).
Conclusion
There were no significant quantifiable differences in T2 values of cartilage overlying stable JOCD lesions and normal cartilage on the medial femoral condyle, suggesting no substantial changes in cartilage composition and microstructure.
© RSNA, 2018
Introduction
Osteochondritis dissecans (OCD) is one of the leading causes of knee pain in children and young adults (1). The medial femoral condyle is the most commonly affected location, and an estimated 15%–30% of individuals affected with OCD have bilateral involvement (2). OCD lesions were classically divided into juvenile OCD (JOCD) and adult OCD lesions according to whether the growth plate is open or closed at the time of diagnosis. Compared with adult OCD lesions, which are often symptomatic and rarely spontaneously heal, JOCD lesions are commonly asymptomatic, and 50%–67% of lesions heal after conservative therapy (3–6). Therefore, it is uncertain whether adult OCD and JOCD lesions represent different extremes of a common abnormality or separate pathologic processes that produce lesions that appear similar. Growing evidence (7,8) suggests that JOCD lesions may represent focal endochondral ossification dysfunction of the secondary growth plate that is responsible for the growth of the femoral condyles. This may help explain why most JOCD lesions are asymptomatic, are incidentally discovered, and typically have intact overlying cartilage at both imaging and arthroscopy (3–6).
MRI can provide a noninvasive method for assessing OCD lesion stability. Unfortunately, the original MRI criteria for lesion instability lacks specificity when applied to JOCD lesions (9), leading to additional secondary criteria. O’Connor et al (10) found the combination of a T2-weighted high-signal rim and a T1-weighted cartilage cleft to be 85% accurate in identifying unstable JOCD lesions. Kijowski et al (11) described five secondary MRI criteria that were each 100% specific for identifying unstable JOCD lesions: a linear T2-weighted fluid-like high-signal rim, a T2-weighted low-signal outer rim, circumferential cysts, cyst larger than 5 mm, and multiple breaks in the subchondral bone plate on T2-weighted images. However, most JOCD lesions lack these morphologic MR features of instability (5,10,11).
Quantitative T2 mapping is commonly used to evaluate the composition and microstructure of articular cartilage. T2 mapping sequences emerged as sensitive tools for depicting early cartilage degeneration in adults (12) and children with juvenile idiopathic arthritis (13) and patellofemoral instability (14). However, a comprehensive investigation of the use of T2 mapping sequences for evaluating cartilage in patients with JOCD, to our knowledge, has not been previously described. Therefore, the purpose of our study was to determine whether a T2 mapping sequence could depict early changes in the composition and microstructure of cartilage overlying stable JOCD lesions of the medial femoral condyle.
Materials and Methods
Study Group
Our study adhered to Health Insurance Portability and Accountability Act regulations. The institutional review board approved the study and waived the need for informed consent. Participants in the study group and control group were retrospectively selected from a database of all pediatric patients who underwent a routine 3.0-T MR examination of the knee at our institution between September 1, 2015, and March 31, 2017.
The study group consisted of 16 children (median age, 11.5 years), which included 10 boys (median age, 11.5 years; interquartile range, 10.8–13.0 years) and six girls (median age, 11.0 years; interquartile range, 10.3–12.8 years), with 18 JOCD lesions of the medial femoral condyle. These patients had not undergone surgical treatment; had not sustained knee trauma within the past year; and had no past or present history of inflammatory arthritis, infectious arthritis, or malignancy. A JOCD lesion was defined as an OCD lesion present within the medial femoral condyle in a skeletally immature individual who did not have a closed distal femur growth plate. A total of 21 patients with JOCD lesions of the medial femoral condyle underwent a routine 3.0-T MR examination of the knee at our institution. Five patients with JOCD lesions were excluded because they had previously undergone surgical treatment. No patients were excluded on the basis of the severity of their JOCD lesion. The electronic medical records of all patients in the study group were reviewed to collect pertinent information that included the presence of symptoms at the time of referral to a pediatric orthopedic surgeon at our institution, symptom duration, length of follow-up, symptoms at follow-up, and, if available, subsequent arthroscopic and MR findings.
The control group consisted of 18 consecutive children (median age, 11.5 years) with knee pain, including 11 boys (median age, 12.0 years; interquartile range, 11.0–14.0 years) and seven girls (median age, 10.0 years; interquartile range, 10.0–11.0 years) who had normal findings at MR examination of the knee and whose age, sex, and skeletal maturation best matched those of the patients in the study group. The appearance of the distal femur growth plate at sagittal and coronal fluid-sensitive fast spin-echo sequences was used to determine skeletal maturity. An open growth plate, reflecting skeletal immaturity, was defined as a uniform, well-defined band of hyperintense growth plate cartilage signal. A closing growth plate, reflecting impending skeletal maturity, was defined as narrowing and indistinctness of the hyperintense cartilage signal that involved at least 25% of the growth plate. A closed growth plate, reflecting skeletal maturity, was defined as complete loss of the hyperintense growth plate cartilage signal. Both the study group and control group consisted of 13 knees with open growth plates and five knees with closing growth plates.
MR Examination
All participants underwent MRI of the knee on the same 3.0-T imager (Signa Excite HDx; GE Healthcare, Waukesha, Wis) at our institution with an eight-channel phased-array extremity coil (Precision Eight TX/TR High Resolution Knee Array; Invivo, Orlando, Fla). The MR examination consisted of multiplanar fast spin-echo sequences with and without fat suppression and a commercially available sagittal T2 mapping sequence (CartiGram; GE Healthcare). The imaging parameters of all sequences are summarized in Table 1. Sagittal T2 color maps with a color scale set between 25 msec and 75 msec were created from the source data by a technologist immediately after the MR examination by using postprocessing software (AW MR FuncTool, GE Healthcare) at the MR workstation.
Table 1:
Imaging Parameters of Sequences in MR Examination of Knee

Note.—FS = fat suppressed; FSE = fast spin echo; NA = not applicable; PDW = proton density weighted; T2W = T2-weighted.
Qualitative Review of MR Images in the Study Group
The MR images of the knee of all patients in the study group were retrospectively reviewed independently by two fellowship-trained musculoskeletal radiologists (R.W.K. and D.G.B.) with 14 and 16 years of clinical experience, respectively, with a picture archiving and communication system workstation (Horizon Medical Imaging; McKesson, San Francisco, Calif). The radiologists were blinded to the clinical history of the patients when they evaluated the MR images. Both radiologists had extensive experience in qualitative assessment of the articular cartilage of the knee on T2 color maps because of routine use of the T2 mapping sequence in clinical practice and previous cartilage research.
The radiologists first evaluated the multiplanar fast spin-echo sequences to determine the presence or absence of predefined morphologic features for each JOCD lesion. These features include perilesional marrow edema, T2-weighted non–fluid-like high-signal rim, T2-weighted fluid-like high-signal rim, T2-weighted low-signal outer rim, cyst or cysts (including the total number and size of the largest cyst), subchondral bone plate disruption on T2-weighted images, overlying cartilage degeneration (defined as cartilage irregularity or thinning), and displaced fragment. The overall stability of each JOCD lesion was determined by using previously described MRI criteria (11).
The radiologists then evaluated the T2 color maps at a second review session to determine the presence or absence of increased T2 color signal of the cartilage within each JOCD lesion and the cartilage overlying the JOCD lesion. If T2 color signal within cartilage was increased, the change was described as diffuse (involving all the cartilage on multiple image sections), heterogeneous (involving a portion of the cartilage on multiple image sections), or focal (involving a portion of the cartilage on a single image section). The second review session was separated from the first review session by a minimum of 1 month to minimize recall bias.
When the two radiologists disagreed on a particular feature of the JOCD lesion on the independent reviews, a consensus review of the MR examination was performed to make the final interpretation. During the consensus review, the radiologists also measured the largest dimensions of each JOCD lesion in the anteroposterior and mediolateral orthogonal planes by using electronic calipers at the picture archiving and communication system workstation. The two dimensions were multiplied to calculate the cross-sectional area of the JOCD lesion. The MR images of all patients were retrospectively reviewed again by one of the radiologists (R.W.K.) at a separate review session performed at least 1 month after the consensus review session to assess intrareader agreement for determining the presence or absence of the qualitative MR features of the JOCD lesions on the fast spin-echo images and T2 map color maps.
Quantitative Analysis of T2 Maps for the Study and Control Groups
Sagittal T2 maps of the knee of all participants in the study group and control group were created by using a custom-made linear least-squares fitting method developed in Matlab (Math-Works, Natick, Mass) (15). A fellowship-trained pediatric radiologist (J.C.N.) with 5 years of clinical experience segmented the cartilage of the medial femoral condyle on the T2 maps by using custom-made software developed in Matlab under the supervision of a fellowship-trained musculoskeletal radiologist (R.W.K.) with 10 years of research experience performing quantitative cartilage T2 analysis. The musculoskeletal radiologist (R.W.K.) independently segmented the cartilage of the medial femoral condyle of all participants in the study group and control group at a separate session by using the same custom-made software to access the repeatability for performing the quantitative analysis of the T2 maps.
For patients in the study group, regions of interest were placed around the JOCD lesion, the cartilage overlying the JOCD lesion, and the cartilage on the weight-bearing medial femoral condyle (Fig 1). For participants in the control group, regions of interest were placed around the cartilage on the weight-bearing medial femoral condyle. The weight-bearing medial femoral condyle was defined as the cartilage directly overlying the medial meniscus. The mean T2 values of all pixels within the regions of interest were calculated. Pixels with T2 values greater than 100 msec were considered to represent partial volume averaging with joint fluid and were excluded from the analysis. Similarly, pixels with T2 values less than 5 msec were considered to represent ossified bone and were also excluded from analysis.
Figure 1a:

MR images of juvenile osteochondritis dissecans (JOCD) cartilage segmentation. (a) Region of interest placed around the cartilage within JOCD lesion (blue contour) Overlying epiphyseal cartilage is shown with the green contour. (b) Region of interest placed around cartilage overlying JOCD lesion (dark green and blue contours). Green and red contours (b, c) define the excluded anterior and posterior portions of the oversying epiphyseal cartilage, respectively. Cartilage partitioning was performed by using the dashed lines adjusted by using the red box labeled L in b and c. (c) Region of interest placed around cartilage on weight-bearing medial femoral condyle (dark green and blue contours). Anterior boundary of weight-bearing surface was defined as anterior margin of anterior horn of medial meniscus, and posterior boundary was defined as posterior margin of posterior horn. The same region of interest was placed around the weight-bearing medial femoral condyle in control participants.
Figure 1b:

MR images of juvenile osteochondritis dissecans (JOCD) cartilage segmentation. (a) Region of interest placed around the cartilage within JOCD lesion (blue contour) Overlying epiphyseal cartilage is shown with the green contour. (b) Region of interest placed around cartilage overlying JOCD lesion (dark green and blue contours). Green and red contours (b, c) define the excluded anterior and posterior portions of the oversying epiphyseal cartilage, respectively. Cartilage partitioning was performed by using the dashed lines adjusted by using the red box labeled L in b and c. (c) Region of interest placed around cartilage on weight-bearing medial femoral condyle (dark green and blue contours). Anterior boundary of weight-bearing surface was defined as anterior margin of anterior horn of medial meniscus, and posterior boundary was defined as posterior margin of posterior horn. The same region of interest was placed around the weight-bearing medial femoral condyle in control participants.
Figure 1c:
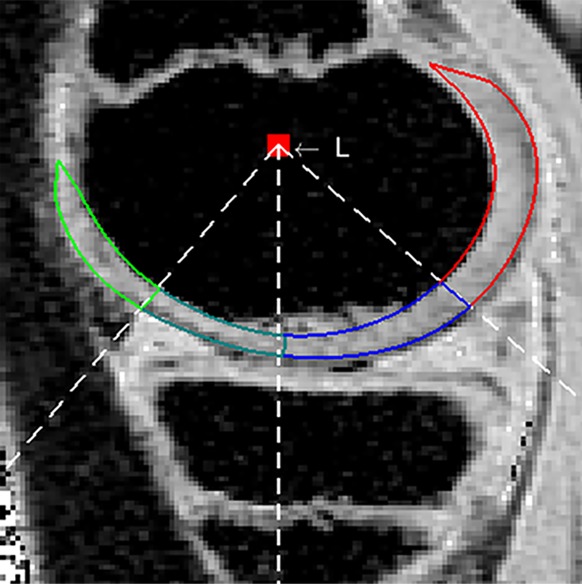
MR images of juvenile osteochondritis dissecans (JOCD) cartilage segmentation. (a) Region of interest placed around the cartilage within JOCD lesion (blue contour) Overlying epiphyseal cartilage is shown with the green contour. (b) Region of interest placed around cartilage overlying JOCD lesion (dark green and blue contours). Green and red contours (b, c) define the excluded anterior and posterior portions of the oversying epiphyseal cartilage, respectively. Cartilage partitioning was performed by using the dashed lines adjusted by using the red box labeled L in b and c. (c) Region of interest placed around cartilage on weight-bearing medial femoral condyle (dark green and blue contours). Anterior boundary of weight-bearing surface was defined as anterior margin of anterior horn of medial meniscus, and posterior boundary was defined as posterior margin of posterior horn. The same region of interest was placed around the weight-bearing medial femoral condyle in control participants.
Statistical Analysis
Statistical analysis was performed by using the statistical software (R 3.3.1; R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were two-sided and significance was indicated by a P value less than .05.
Percentage agreement was used to assess interreader and intrareader agreement for determining the presence or absence of the qualitative MR findings of the JOCD lesions on the fast spin-echo images and T2 color maps. The concordance correlation coefficient was used to access the repeatability between the two radiologists for performing the quantitative analysis of the T2 maps for participants in the study group and control group. The concordance correlation coefficient combines measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance, with values closer to 1 indicating greater concordance between the two sets of measurements.
The Wilcoxon signed-rank test was used to compare T2 values between the cartilage within the JOCD, the cartilage overlying the JOCD, and the cartilage on the weight-bearing medial femoral condyle for patients in the study group. The Wilcoxon rank sum test was used to compare cartilage T2 values on the weight-bearing medial femoral condyle between the study group and the control groups.
The Spearman rank correlation (r) and a multivariate linear regression model were used to analyze the association between T2 values of the cartilage within the JOCD lesion, the cartilage overlying the JOCD lesion, and the cartilage on the weight-bearing medial femoral condyle; patient age; and the cross-sectional area of the JOCD lesions for patients in the study group. The Wilcoxon rank-sum test and the Kruskal-Wallis test were used to determine the association between the T2 values of the same three cartilage regions of interest and the symptoms at presentation (present or absent); the duration of symptoms (absent, ≤12 months, or >12 months); and the MR findings of the JOCD lesion, including a T2-weighted non–fluid-like high-signal rim (present or absent), number of cysts (one cyst or no cysts vs two or more cysts), size of largest cyst (≤1 mm or >1 mm), and T2 color signal of cartilage overlying the JOCD lesion (normal or increased).
Results
Half of the 18 JOCD lesions were symptomatic at presentation, whereas the symptoms of the remaining JOCD lesions had resolved by the time of referral to the pediatric orthopedic surgeon. The median symptom duration was 12.0 months (interquartile range, 5.5–15.0 months). With the exception of one JOCD lesion that underwent no further clinical or imaging follow-up, the remaining 17 JOCD lesions had favorable prognosis with a median follow-up duration of 6.0 months (interquartile range, 3.8–8.1 months). Three JOCD lesions that remained symptomatic despite 6 months of conservative therapy were treated with knee arthroscopy, which showed normal intact and nonballottable cartilage overlying the lesions. The remaining 15 JOCD lesions were asymptomatic at presentation or became asymptomatic at the latest clinical follow-up evaluation. Six JOCD lesions had follow-up MR examinations, which showed unchanged appearance (n = 2) or signs of healing (n = 4) that included decreased cross-sectional area and resolving at MRI findings of marrow edema and cysts.
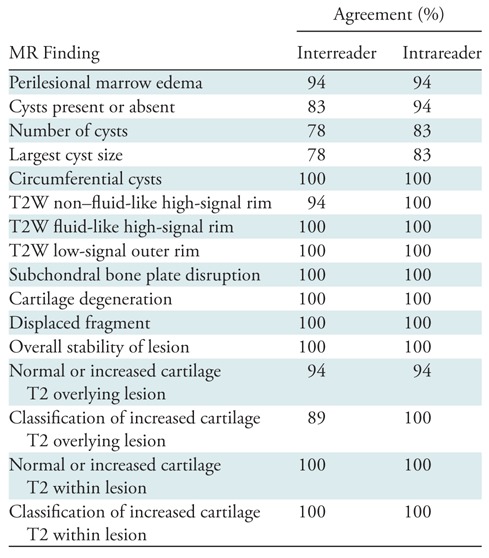
There was high interreader and intrareader agreement for determining the presence or absence of the qualitative MR features of the JOCD lesions on the fast spin-echo images and T2 color maps. Interreader percentage agreement ranged between 78% and 100%, and intrareader percentage agreement ranged between 83% and 100% (Table 2). There was also high repeatability between radiologists for quantitative analysis of the T2 maps. The concordance correlation coefficients for patients with JOCD lesions were 0.794 (95% confidence interval: 0.602, 0.899) for the cartilage within the JOCD lesion, 0.992 (95% confidence interval: 0.980, 0.997) for the cartilage overlying the JOCD lesion, and 0.998 (95% confidence interval: 0.996, 0.999) for the cartilage on the weight-bearing medial femoral condyle. The concordance correlation coefficient for the cartilage on the weight-bearing medial femoral condyle of the control participants was 0.998 (95% confidence interval: 0.995, 0.999).
Table 2:
Interreader and Intrareader Agreement for Determining Presence or Absence of Qualitative MR Findings of Juvenile Osteochondritis Dissecans Lesions
Note.—T2W = T2-weighted.
Qualitative assessment of the 18 JOCD lesions on the multiplanar fast spin-echo images showed that 17 JOCD lesions (94%) had surrounding marrow edema, three JOCD lesions (17%) had a T2-weighted non–fluid-like high-signal rim, and none of the JOCD lesions had a T2-weighted fluid-like high-signal rim. Although 15 of 18 JOCD lesions (83%) had a cyst or cysts, none of the JOCD lesions had circumferential cysts or a cyst larger than 5 mm. None of the JOCD lesions had a T2-weighted low-signal outer rim, subchondral bone plate disruption on T2-weighted images, overlying cartilage degeneration, or a displaced fragment. Therefore, none of the 18 JOCD lesions fulfilled the MR criteria for instability (Table 3).
Table 3:
MR Findings of Stable Juvenile Osteochondritis Dissecans Lesions of Medial Femoral Condyle

Note.—Data are numerator/denominator; data in parentheses are percentages. T2W = T2 weighted.
*Median number of cysts, 1.0; interquartile range, 1.0–1.8.
†MR findings that are 100% specific for identifying unstable juvenile osteochondritis dissecans lesions (11).
Figure 2 shows the appearance of normal articular cartilage on a T2 color map of a control participant without a JOCD lesion of the medial femoral condyle. Qualitative assessment of the 18 JOCD lesions with the T2 color maps showed that 14 JOCD lesions (78%) had a diffuse increased T2 color map signal of the cartilage within the JOCD lesion (Figs 3, 4), whereas the remaining four JOCD lesions (22%) had heterogeneous increased T2 color map signal with focal areas of low T2 color map signal, which corresponded to ossified bone on the fast spin-echo images. Nine of 18 JOCD lesions (50%) had focal increased T2 color map signal within the cartilage overlying the JOCD lesion (Fig 3), whereas the remaining nine JOCD lesions (50%) had normal T2 color map signal (Fig 4).
Figure 2:

MR image in a 12-year-old female control participant without juvenile osteochondritis dissecans lesion of the medial femoral condyle. T2 color map shows normal articular cartilage on weight-bearing medial femoral condyle (arrows). Red and black color map signal in deep cartilage layer corresponds to low T2, and yellow and green color map signal in superficial cartilage layer corresponds to higher T2. Black color map signal indicates pixels with T2 s below minimum color threshold of 25 msec. T2 of normal cartilage primarily reflects its inherent microstructure with low T2 in the deep layer, where there are thick, uniformly aligned bundles of collagen fibers oriented perpendicular to the subchondral bone plate, and higher T2 in the superficial layer, where there are more randomly oriented collagen fibers.
Figure 3a:
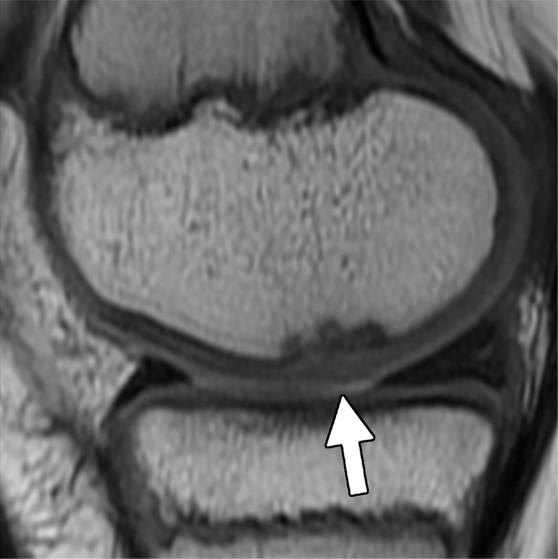
MR images in a 13-year-old boy with stable juvenile osteochondritis dissecans (JOCD) lesion of the medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of the knee show a JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has focal area of increased T2 color map signal (arrowheads in c).
Figure 4a:
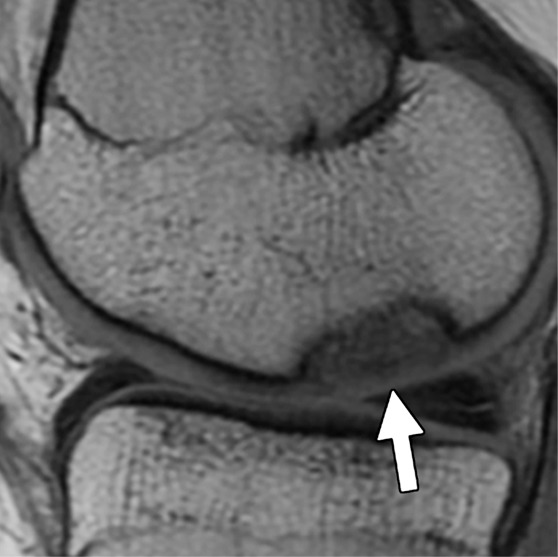
MR images in a 13-year-old girl with stable juvenile osteochondritis dissecans (JOCD) lesion of medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of knee show JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has normal T2 color map signal (arrowheads in c).
Figure 3b:
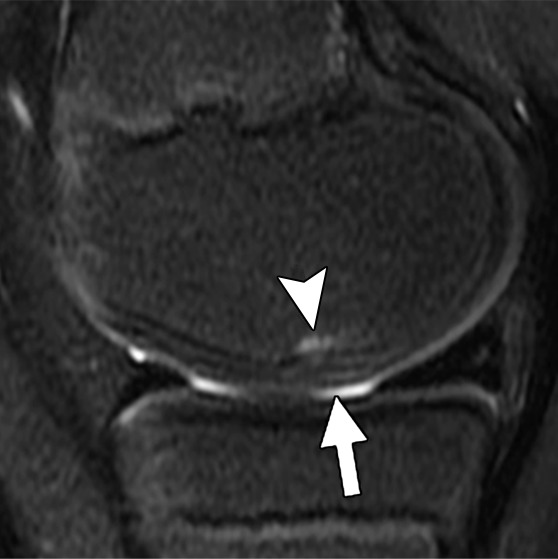
MR images in a 13-year-old boy with stable juvenile osteochondritis dissecans (JOCD) lesion of the medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of the knee show a JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has focal area of increased T2 color map signal (arrowheads in c).
Figure 3c:
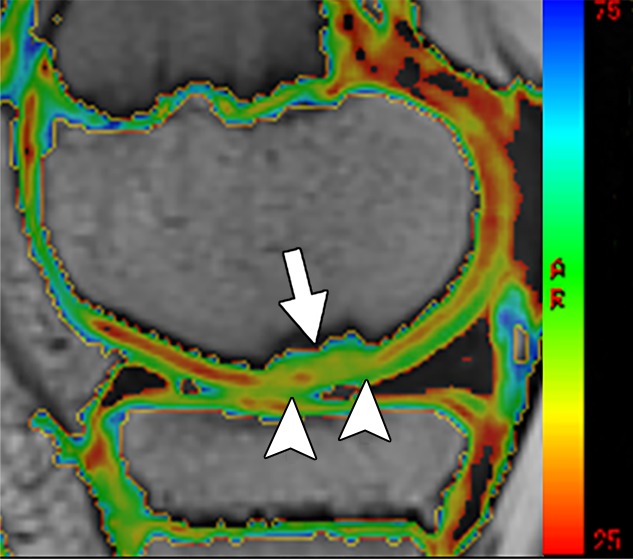
MR images in a 13-year-old boy with stable juvenile osteochondritis dissecans (JOCD) lesion of the medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of the knee show a JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has focal area of increased T2 color map signal (arrowheads in c).
Figure 4b:

MR images in a 13-year-old girl with stable juvenile osteochondritis dissecans (JOCD) lesion of medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of knee show JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has normal T2 color map signal (arrowheads in c).
Figure 4c:

MR images in a 13-year-old girl with stable juvenile osteochondritis dissecans (JOCD) lesion of medial femoral condyle. (a) Sagittal proton density–weighted fast spin-echo and (b) sagittal fat-suppressed T2-weighted fast spin-echo images of knee show JOCD lesion on medial femoral condyle (arrows) with surrounding T2-weighted non–fluid-like high-signal rim (arrowhead). (c) Corresponding T2 color map shows diffuse increased T2 color map signal within JOCD lesion (arrow). The cartilage overlying the JOCD lesion has normal T2 color map signal (arrowheads in c).
In the study group, T2 values were significantly higher (P < .001) for the cartilage within the JOCD lesion than the cartilage overlying the JOCD lesion. However, T2 values did not significantly differ (P = .67) between the cartilage overlying the JOCD lesion and the cartilage on the weight-bearing medial femoral condyle. Furthermore, T2 values of the cartilage on the weight-bearing medial femoral condyle were not significantly different (P = .30) between the study group and the control group. In the JOCD group, the median T2 values were as follows: within JOCD, 54.9 msec (interquartile range, 51.7–57.3 msec); overlying JOCD, 44.5 msec (interquartile range, 41.1–49.0 msec); and for weight-bearing medial femoral condyle, they were 43.9 msec (interquartile range, 41.8–48.3 msec). The T2 values in the control group, however, were 46.9 msec (interquartile range, 44.1–48.5 msec) for weight-bearing medial femoral condyle.
There was a strong negative correlation between age and the T2 values of the cartilage overlying the JOCD lesion (r = −0.72; 95% confidence interval: −0.89, −0.38) and the cartilage on the weight-bearing medial femoral condyle (r = −0.72; 95% confidence interval: −0.89, −0.37), and a moderate negative correlation between age and the T2 values of the cartilage within the JOCD lesion (r = −0.48; 95% confidence interval: −0.77, −0.02). There was a moderate negative correlation between the cross-sectional area of the JOCD lesion and the T2 values of the cartilage within the JOCD lesion (r = −0.58; 95% confidence interval: −0.82, −0.15), the cartilage overlying the JOCD lesion (r = −0.44; 95% confidence interval: −0.75, −0.03), and the cartilage on the weight-bearing medial femoral condyle (r = −0.44; 95% confidence interval: −0.76, −0.02). At the multivariate analysis, there was a significant association between age and the T2 values of cartilage overlying the JOCD lesion (P < .001) and the cartilage on the weight-bearing medial femoral condyle (P = .02). However, there was no significant association between age and the T2 value of the cartilage within the JOCD lesion (P = .24) and between cross-sectional area of the JOCD lesion and the T2 value of the cartilage within the JOCD (P = .40), the cartilage overlying the JOCD (P = .95), and the cartilage on the weight-bearing medial femoral condyle (P = .91). There was also no significant association between the T2 values of the cartilage within the JOCD lesion, the cartilage overlying the JOCD lesion, and the cartilage on the weight-bearing medial femoral condyle and symptom duration (P = .09–.12), symptoms at presentation (P = .20–.90), or JOCD lesion findings at MRI (P = .29–.99).
Discussion
In our study, the quantitatively measured T2 values did not significantly differ between the cartilage overlying JOCD lesions and normal cartilage on the weight-bearing medial femoral condyle. Although qualitative assessment did identify increased T2 color map signal within the cartilage overlying half of the JOCD lesions, the signal change involved only a portion of the cartilage on a single image section and thus did not affect the overall measured mean T2 values. Our results suggest that there are no substantial changes in the normal composition and microstructure of cartilage overlying stable JOCD lesions as assessed with quantitative MRI. To our knowledge, no previous study investigated changes in quantitative MR parameters in the cartilage overlying JOCD lesions. However, our results agree with those of a previously published histopathologic study, which documented relatively normal cartilage overlying JOCD lesions (16). The presence of normal or near-normal T2 of cartilage overlying a JOCD lesion is a good prognostic sign because no patients with JOCD lesions in our study had surgical or MR evidence of lesion instability and all had good clinical outcome at follow-up.
The MRI and histopathologic findings of recent studies supported the postulated theory that JOCD represents focal dysfunction of endochondral ossification of the acrophysis, a secondary growth plate responsible for the growth of the femoral condyles (7,8,16). It is widely accepted that skeletally immature children have a great potential for self-repair and healing of JOCD lesions, which may be related to the still-vascularized unossified epiphyseal cartilage and the open acrophysis (17). If a JOCD lesion remains stable and eventually heals, the overlying cartilage remains intact with normal composition and microstructure, as reflected by the essentially normal cartilage T2 values reported in our study.
Our study showed a strong negative correlation between patient age and the T2 values of the cartilage overlying the JOCD lesion and the cartilage on the weight-bearing medial femoral condyle. This negative correlation probably reflects the normal decrease in cartilage T2 with maturation because of a greater organization of collagen fibers within the macromolecular matrix (18,19). Although larger JOCD lesions were found to be associated with less favorable prognosis (20), our study showed a moderate negative correlation between the cross-sectional area of the JOCD lesion and the T2 values of the cartilage overlying the JOCD lesion and the cartilage on the weight-bearing medial femoral condyle. However, the association between the cartilage T2 values and the cross-sectional area of the JOCD lesions was not statistically significant after adjustment for patient age. Thus, the moderate negative correlation probably occurred because larger JOCD lesions were more common in older patients in our study group, and these older individuals showed normal maturation-related decreases in cartilage T2 (18,19).
Our study found that the T2 value of the cartilage within the JOCD lesion was significantly higher than the cartilage overlying the JOCD lesion and the cartilage on the weight-bearing medial femoral condyle. A recent study using a short echo time T2* mapping sequence in patients with varying stages of JOCD and adult OCD documented the epiphyseal cartilage origin of JOCD lesions, which undergo progressive ossification over time (8). However, the cartilaginous composition of the nonossified portions of JOCD lesions is different from that of normal articular cartilage, as shown by the higher T2 value in our study; this may be because of decreased collagen organization and/or increased water content. Histopathologic studies (16,21–23) have shown that JOCD lesions consist of nonossified epiphyseal cartilage and fibrovascular tissue that resemble fracture nonunion, with no evidence of bone necrosis or inflammation.
No JOCD lesions in our study had clinical, surgical, or MR findings of instability. The low frequency of unstable JOCD lesions that we found is similar to the findings of a previously published small natural history study (5) that followed 21 patients with JOCD lesions for more than 5 years and found only a single unstable lesion by using arthroscopy as the reference standard. Other studies (7,24) reported that 83%–90% of JOCD lesions evaluated at arthroscopy were stable, with intact overlying cartilage. Studies (3–6) also showed that JOCD lesions may be asymptomatic and incidentally discovered and that most JOCD lesions are located at the medial femoral condyle, where they are 16 times more likely be stable when compared with JOCD lesions in other locations within the knee, including the lateral femoral condyle and femoral trochlea (6).
Our study has several limitations. First, our study included only a small homogenous population of patients with JOCD who were age 14 years or younger and who all had stable JOCD lesions at the medial femoral condyle. However, the main objective of our study was to determine the feasibility of using a T2 mapping sequence to evaluate a clinically representative cohort of patients who typically have stable JOCD lesions with intact overlying cartilage (3–6). Another limitation was the retrospective design of our study, which did not allow us to control for the effect of joint loading on cartilage T2 values (25). However, the 25-minute routine clinical MRI protocol was always performed before the T2 mapping sequence, which allowed all patients to have at least some degree of joint unloading before the measurement of cartilage T2. Another limitation was that the radiologists were not blinded to the skeletal maturity of the patient or size of the JOCD lesion, which may have potentially biased their qualitative cartilage T2 assessment. Furthermore, qualitative evaluation of the cartilage of the knee on T2 color maps is challenging even for experienced musculoskeletal radiologists because of the influence of the so-called magic angle effect on cartilage T2 (26). Finally, although all participants in the control group had normal findings at MR examinations of the knee with no findings of joint abnormality, they cannot be considered healthy asymptomatic volunteers because all individuals had knee symptoms that warranted MRI evaluation.
In conclusion, our study has documented the feasibility of using a commercially available T2 mapping sequence to evaluate patients with stable JOCD lesions of the medial femoral condyle on a 3.0-T MR imager. Our study found no significant differences in quantitatively measured T2 values between cartilage overlying JOCD lesions and normal cartilage on the medial femoral condyle. Our results suggest that there are no substantial changes in the normal composition and microstructure of cartilage overlying stable JOCD lesions of the medial femoral condyle. Future prospective studies that include a larger number of patients with varying stages of JOCD are needed to further investigate whether baseline and longitudinal changes in T2 values of cartilage overlying JOCD lesions could help predict which lesions will spontaneously heal and which lesions will become unstable over time.
Summary
Quantitatively measured T2 values did not significantly differ between cartilage overlying juvenile osteochondritis dissecans lesions and normal cartilage on the medial femoral condyle.t
Implication for Patient Care
■ A T2 mapping sequence could not depict quantifiable differences in T2 values of cartilage overlying stable JOCD lesions and normal cartilage on the medial femoral condyle, suggesting no substantial changes in cartilage composition and microstructure in this small population.
Received August 26, 2018; revision requested October 23; revision received January 22, 2018; accepted March 26.
Study supported by Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01-AR068373-01).
Disclosures of Conflicts of Interest: J.C.N. disclosed no relevant relationships. F.L. disclosed no relevant relationships. D.G.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancy and royalties from Amirsys/Elsevier. Other relationships: disclosed no relevant relationships. K.M.W. disclosed no relevant relationships. R.W.K Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending from GE Healthcare. Other relationships: disclosed no relevant relationships.
Abbreviations:
- JOCD
- juvenile osteochondritis dissecans
- OCD
- osteochondritis dissecans
References
- 1.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg 2006;14(2):90–100. [DOI] [PubMed] [Google Scholar]
- 2.Moktassi A, Popkin CA, White LM, Murnaghan ML. Imaging of osteochondritis dissecans. Orthop Clin North Am 2012;43(2):201–211, v–vi. [DOI] [PubMed] [Google Scholar]
- 3.Eismann EA, Pettit RJ, Wall EJ, Myer GD. Management strategies for osteochondritis dissecans of the knee in the skeletally immature athlete. J Orthop Sports Phys Ther 2014;44(9):665–679. [DOI] [PubMed] [Google Scholar]
- 4.Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med 2013;41(10):2384–2391. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JA, Cook JV, Churchill MA, Warren ME. Juvenile osteochondritis dissecans: a 5-year review of the natural history using clinical and MRI evaluation. Pediatr Radiol 2003;33(6):410–417. [DOI] [PubMed] [Google Scholar]
- 6.Samora WP, Chevillet J, Adler B, Young GS, Klingele KE. Juvenile osteochondritis dissecans of the knee: predictors of lesion stability. J Pediatr Orthop 2012;32(1):1–4. [DOI] [PubMed] [Google Scholar]
- 7.Laor T, Zbojniewicz AM, Eismann EA, Wall EJ. Juvenile osteochondritis dissecans: is it a growth disturbance of the secondary physis of the epiphysis? AJR Am J Roentgenol 2012;199(5):1121–1128. [DOI] [PubMed] [Google Scholar]
- 8.Ellermann J, Johnson CP, Wang L, Macalena JA, Nelson BJ, LaPrade RF. Insights into the epiphyseal cartilage origin and subsequent osseous manifestation of juvenile osteochondritis dissecans with a modified clinical MR imaging protocol: a pilot study. Radiology 2017;282(3):798–806. [DOI] [PubMed] [Google Scholar]
- 9.De Smet AA, Fisher DR, Graf BK, Lange RH. Osteochondritis dissecans of the knee: value of MR imaging in determining lesion stability and the presence of articular cartilage defects. AJR Am J Roentgenol 1990;155(3):549–553. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor MA, Palaniappan M, Khan N, Bruce CE. Osteochondritis dissecans of the knee in children. A comparison of MRI and arthroscopic findings. J Bone Joint Surg Br 2002;84(2):258–262. [DOI] [PubMed] [Google Scholar]
- 11.Kijowski R, Blankenbaker DG, Shinki K, Fine JP, Graf BK, De Smet AA. Juvenile versus adult osteochondritis dissecans of the knee: appropriate MR imaging criteria for instability. Radiology 2008;248(2):571–578. [DOI] [PubMed] [Google Scholar]
- 12.Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 2013;267(2):503–513. [DOI] [PubMed] [Google Scholar]
- 13.Kight AC, Dardzinski BJ, Laor T, Graham TB. Magnetic resonance imaging evaluation of the effects of juvenile rheumatoid arthritis on distal femoral weight-bearing cartilage. Arthritis Rheum 2004;50(3):901–905. [DOI] [PubMed] [Google Scholar]
- 14.Kang CH, Kim HK, Shiraj S, Anton C, Kim DH, Horn PS. Patellofemoral instability in children: T2 relaxation times of the patellar cartilage in patients with and without patellofemoral instability and correlation with morphological grading of cartilage damage. Pediatr Radiol 2016;46(8):1134–1141. [DOI] [PubMed] [Google Scholar]
- 15.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology 1997;205(2):546–550. [DOI] [PubMed] [Google Scholar]
- 16.Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E. Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med 2009;37(10):2003–2008. [DOI] [PubMed] [Google Scholar]
- 17.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat 2008;190(4):305–315. [DOI] [PubMed] [Google Scholar]
- 18.Shiraj S, Kim HK, Anton C, Horn PS, Laor T. Spatial variation of T2 relaxation times of patellar cartilage and physeal patency: an in vivo study in children and young adults. AJR Am J Roentgenol 2014;202(3):W292–W297. [DOI] [PubMed] [Google Scholar]
- 19.Kim HK, Shiraj S, Anton C, Horn PS. The patellofemoral joint: do age and gender affect skeletal maturation of the osseous morphology in children? Pediatr Radiol 2014;44(2):141–148. [DOI] [PubMed] [Google Scholar]
- 20.Wall EJ, Vourazeris J, Myer GD, et al. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am 2008;90(12):2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zbojniewicz AM, Stringer KF, Laor T, Wall EJ. Juvenile osteochondritis dissecans: correlation between histopathology and MRI. AJR Am J Roentgenol 2015;205(1):W114–W123. [DOI] [PubMed] [Google Scholar]
- 22.Krause M, Lehmann D, Amling M, et al. Intact bone vitality and increased accumulation of nonmineralized bone matrix in biopsy specimens of juvenile osteochondritis dissecans: a histological analysis. Am J Sports Med 2015;43(6):1337–1347. [DOI] [PubMed] [Google Scholar]
- 23.Yonetani Y, Nakamura N, Natsuume T, Shiozaki Y, Tanaka Y, Horibe S. Histological evaluation of juvenile osteochondritis dissecans of the knee: a case series. Knee Surg Sports Traumatol Arthrosc 2010;18(6):723–730. [DOI] [PubMed] [Google Scholar]
- 24.Siegall E, Faust JR, Herzog MM, Marshall KW, Willimon SC, Busch MT. Age predicts disruption of the articular surface of the femoral condyles in knee OCD: can we reduce usage of arthroscopy and MRI? J Pediatr Orthop 2018;38(3):176–180. [DOI] [PubMed] [Google Scholar]
- 25.Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging 2008;28(1):175–180. [DOI] [PubMed] [Google Scholar]
- 26.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol 2000;35(10):602–621. [DOI] [PubMed] [Google Scholar]


