ABSTRACT
Vaccinations are an important and effective cornerstone of preventive medical care. Growing technologic capabilities and use by both patients and providers present critical opportunities to leverage these tools to improve vaccination rates and public health. We propose the Social Ecological Model as a useful theoretical framework to identify areas in which technology has been or may be leveraged to target undervaccination across the individual, interpersonal, organizational, community, and society levels and the ways in which these levels interact.
KEYWORDS: vaccinations, technology, digital, social ecological model, immunizations, influenza, SMS
Introduction
Vaccinations are an important and effective cornerstone of preventive medical care with significant health benefits. Vaccination programs and policies have greatly reduced the burden of a number of diseases. For example, polio grows closer to complete eradication, with no more new cases in India and Nigeria's last case in 2016, leaving Pakistan and Afghanistan as the sole remaining countries with circulating polio.1 Rotaviral diarrhea and pneumococcal pneumonia incidences are decreasing globally as vaccination coverage spreads.2,3 Similarly, a systematic review of HPV vaccination programs across 69 countries worldwide over the last ten years found approximate maximal reductions of 90% for HPV 6/11/16/18 strain infection, 90% for genital warts, 45% for low-grade cytological cervical abnormalities, and 85% for high-grade abnormalities.4
Yet, despite the demonstrated efficacy of vaccination for improving public health, problems still exist in translating even highly effective vaccines into vaccination coverage levels sufficient to achieve population immunity or to realize their full prevention potential. In the U.S, for most vaccines, coverage for young children is lower among non-Hispanic black children than among non-Hispanic white children, as well as for children living below the federal poverty level and those who are publically insured or uninsured. Adolescent vaccine uptake differences are fewer, except for lower HPV vaccination coverage for those not living in urban areas.5,6 National influenza vaccination rates range from 38.8% to 43.6% for adults and 51.0 to 59.3% for children since the 2010–2011 season.7 On a global level, while considerable progress in routine vaccination coverage has been made, 64 (33%) countries still have not reached the Global Vaccine Action Plan 2011–2020 (GVAP) target of ≥90% national coverage of a third dose of diphtheria and tetanus toxoids and pertussis–containing vaccine (DTP3), and 71 countries (37%) have yet to attain the 2012–2020 Global Measles and Rubella Strategic Plan target of ≥90% national measles-containing vaccine (MCV1) coverage. While countries have until 2020 to reach this goal, there has been no substantial increases in coverage rates for vaccines like MCV1 since 2010.8
Without coverage to attain population immunity, vaccine preventable disease outbreaks and significant burden of disease continue to occur. For example, measles remains a significant problem across the globe, including in the U.S. where multi-state measles outbreaks regularly arise.9,10 The WHO reported 142,512 pertussis cases globally in 2015, with an estimated 89,000 deaths.11 A recent study from the CDC and its global partners found that between 291,000 and 646,000 people worldwide die from influenza-related respiratory illnesses each year, higher than previously estimated.12
In addition to general challenges for immunization programs, there is great heterogeneity to the recommended vaccine series requiring a variety of approaches to overcome vaccine-specific barriers. These categories of barriers include target population (child, adolescent, adult), schedule (one dose, series of doses, annual vaccination) logistic (e.g. insurance status, remembering to get the vaccine) and health literacy (e.g. educational needs).
Technological innovations have greatly improved the ability to access information, facilitate communication and improve efficiency in nearly all aspects of life. Similarly, the use of technology to directly impact the health of populations, as well as to provide access to important public health information, holds great promise that is emerging at the forefront of medical care and public health industries.13 Technology can also cut across the diverse vaccines, populations, and needs, and indeed the use of health information technology (IT) interventions for vaccinations is burgeoning. The purpose of this commentary is to assess whether the Social Ecological Model14 is a useful theoretical framework to map existing interventions and identify areas in which technology may be leveraged to target undervaccination (Figure 1).
Figure 1.
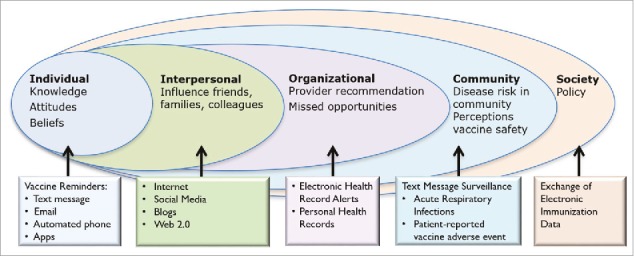
Proposed Model to Target Undervaccination Using Health Technology Interventions that Affect Levels of the Social Ecological Model.
The Social Ecological Model serves as a powerful tool to address health behaviors by attributing health outcomes to factors which exist on a number of levels expanding beyond individual level characteristics. This model has been used extensively in the field childhood obesity prevention, both as a tool for elucidating its etiology as well as developing interventions and measuring their efficacy.15-17 The CDC has recognized its use to both understand violence exposure and perpetration and as a framework for violence prevention.18 Kumar et al. demonstrated that factors on all levels of the Social Ecological Model influenced whether people received the 2009 H1N1 influenza vaccine and concluded that interventions targeting multiple levels may have greater impact than interventions that only target specific levels.19
Using the Social Ecological Model, we can assess factors and barriers of vaccine promotion at several levels including the individual (intrapersonal), interpersonal, organizational, community, and society and the ways they interact. The systematic differences between childhood (surrogate decision-making), adolescent (joint decision-making) and adult vaccines (individual decision-making), various risk-benefit profiles, and challenges health institutions face with attaining vaccine coverage goals require structured organization upon implementation. We argue that the Social Ecological Model can be a useful analytical platform to address vaccine coverage across all populations, specific barriers at each level, and how interaction occurs between levels, which may help improve the impact of interventions.
Individual
Many vaccination interventions target the individual level of the Social Ecological Model theoretical framework depicted to influence individual vaccine-related decision-making or behavior (Figure 1). Health technology tools can be used to provide information to patients and their families in a variety of ways.
Reminder-recalls are a cornerstone of vaccination interventions.20,21 However, traditional forms of reminder-recalls have historically been less effective in the United States for low-income, adolescent and rural pediatric patients.22-25 Increasing cell phone access and health technologies present unique opportunities to employ different methods of reminder-recalls than traditional forms. In the U.S., 95% of adults have a cellphone, with high levels of ownership among diverse populations differing by race/ethnicity, education, income, age, and community type.26 Currently over half of U.S. homes (52.5%) are wireless-only, meaning they have no landline telephones, and that rate is higher (62.3%) in households with children.27 Cell phone rates are also high globally, reaching an estimated 5.07 billion mobile phone users world-wide by 2019.28
Due to widespread cell phone access, large-scale text messaging has the potential to overcome the barriers of traditional reminder-recall including change in contact information, intrusiveness and scalability.29-31 The use of text message vaccine reminders is a burgeoning field; three quarters of papers studying them were published between 2015 and 2017. Overall, parents have generally been open to receiving text message vaccine reminders32-35 and it has been shown to be successful amongst various populations. Ahlers-Schmidt et al. and Hofstetter et al. demonstrated their potential for early childhood vaccinations and for keeping appointments.36,37 Likewise, a number of scholars have demonstrated their use with adolescents, particularly for HPV vaccine series completion, using both practice-based and immunization information system (registry) based reminders.35,38–45 We have also demonstrated the use of text message reminders, particularly those in which health education information is embedded into the text of the message, for influenza vaccination in children and adolescents.31,46,47 In one pediatric study (n = 9,213 patients), we demonstrated a 19% relative increase in influenza vaccination due to text message reminders.46 In an accompanying editorial, it was noted that if similar effects were observed nationally, an additional 2.5 million children would be vaccinated.48 The CDC recently published an article outlining a pilot of a text message-based vaccine reminder system for pandemics to remind people who received a first dose of pandemic influenza vaccine when to return for the second dose, highlighting the potential public health impact of influenza vaccine reminders.49
Text message vaccine reminders are starting to be used globally in both high and low-middle income countries (LMIC), primarily for pediatric populations. Regan et al. demonstrated their effect on influenza vaccination in Western Australia across ten practices with the greatest effect in children < 5 years old (relative risk 2.43; 95% CI, 1.79-3.29); also finding them to be relatively inexpensive with 1 additional high-risk patient vaccinated for every 29 messages sent, costing AUS$3.48.50 Studies have demonstrated the effectiveness of text message reminders for pediatric vaccinations in multiple LMIC including Guatemala, Bangladesh, Kenya and Zimbabwe.51-55 Finally, in Pakistan Kazi et al. are exploring how to use mobile phones both for text-message reminders as well as for geospatial mapping to monitor and visualize vaccination coverage at the household and town level.56
While most text message vaccine reminder studies have focused on pediatric and adolescent populations, these reminders have also been assessed in obstetric populations. We demonstrated in an obstetric population that text messages were effective overall, but particularly in the subgroup of women early in their third trimester at randomization showed the greatest intervention effect vs. usual care (61.9% vs. 49.0%; adjusted odds ratio 1.88; 95% CI 1.12, 3.15).57 Similarly the Text4baby program has shown that participants were more likely than non-participants to report influenza vaccinations and in one study showed a particularly strong effect for women receiving neither a provider recommendation nor offer to vaccinate (adjusted prevalence ratios 3.39, 95% CI 2.03, 5.67).58,59 However, others have not found text message vaccine reminders for pregnant women to be effective.60,61 Similarly, text message vaccine reminders generally have been more mixed in college age youth, adults, and older adults.62-66
Another technology-related intervention that can affect the individual domain are autodialer telephone reminders, which have historically been a mainstay in the United States. Autodialer telephone reminders have demonstrated effectiveness in pediatric and family medicine settings67-69 and may continue to play a role in the global setting especially for illiterate populations.70 Email vaccine reminders have also begun to be used for vaccination with differential effects.71 The greatest effects have generally been in interventions where parents can choose their reminder type between text messages, postcards or email.35,38
The interest in the use of mobile apps is also growing, and descriptive studies of their use have been published. For example, ReadyVax is a mobile smartphone app which targets not only parents and adult patients but also healthcare providers and pharmacists and has been downloaded by users in 102 different countries, most (52%) from the United States.72 In Canada, a pilot evaluation of ImmunizeCA, a Pan-Canadian immunization app, has shown promising preliminary results with 32% of parent participants reporting that they perceived that the app made them more likely to vaccinate on time. Researchers in Italy demonstrated an increase in vaccination knowledge and empowerment with mobile app use.73 However, while they found some individuals' attitudes toward vaccines improved with app use, some participants' attitudes became less positive and in others there was no change, highlighting the importance of further evaluation.74 Such apps can be used by patients and families as part of personal health records documenting vaccinations given, reminders for upcoming vaccinations, and/or a place for trusted health information about both vaccines and the diseases they prevent. For an organization or public health entity, such apps can also be used as a form of communication with patients and their communities or can be used by providers to track vaccination. One study in China, demonstrated that when village doctors used an EPI (Expanded Programme on Immunization) smartphone application, full vaccination coverage increased statistically among young children.75 Similarly, providers in Bangladesh were able to utilize an app to show that a mobile phone intervention improved vaccination coverage in rural hard-to-reach and urban street dweller communities.52
Interpersonal
On the interpersonal level, the perceptions of families and friends regarding vaccine efficacy and safety are important.76 Central to how vaccine information is exchanged between parents and their family and friends include technological resources such as the internet (web content) and social media platforms (i.e. Web 2.0: Facebook, Twitter, Instagram, YouTube). A majority of parents use the internet for pediatric health information, and most rely on websites found using general public search engines instead of starting at a known or trusted healthcare website.77 With regards to vaccine safety information, many parents use and trust various non-governmental Web sites, some of which oppose vaccination.76 Studies have shown that parents who use the internet compared to traditional resources (e.g. physicians) for reliable vaccine information are more likely to be vaccine hesitant76 or have a non-medical exemption for their child, and are less likely to believe vaccines are safe or effective.78 Betsch et al. found that parents who read online anti-vaccine messages for 10 minutes had increased perceived vaccine risk and decreased vaccine intention.79 A call for involvement by physicians, nurses, public health officials, and peer-reviewed research-driven organizations to provide accurate, easily and publicly accessible internet content focused on vaccine efficacy and safety may positively affect parents' conversations with their family and friends.
In addition to informational websites, social media has had an increasing effect on vaccine perceptions and vaccine decision-making.80 It has been shown that anti-vaccine messages through social media may increase parental worry and decrease vaccine intention.81 Blogs with online discussion boards and social media platforms (Web 2.0) enable the exchange of ideas about vaccine perceptions between the individual and their family and friends, as well as with acquaintances, groups, or strangers with a wide range of views. The interactive ability of social media can often lead to debates about or reaffirmation of a user's beliefs, whether they are true or not, or scientifically supported. As noted by Kata, the anti-vaccine movement has successfully used Web 2.0 for many years, using tactics such as “skewing science, shifting hypotheses, censorship, and attacking critics.”82 Negative vaccine perspectives portrayed on social media report that vaccines are unsafe, ineffective, and can cause serious injury including death.83,84 Compared to positive perspectives, negative vaccine content online tends to recruit more views and a larger network,83,84 and use emotionally driven content to spread their message.85 Particularly for those parents who are impressionable and searching for information, vaccine-hesitant, or even with a history of vaccine refusal, ensuring that social media vaccine content that contains an effective and evidenced-based message86,87 is readily available may be pertinent to positively influence social media vaccine-related conversations on the interpersonal level. Public health programs can also leverage well-informed parents and community members to participate in these social media conversations. Schoeppe et al. found that engaging parent volunteers as trained vaccine advocates had a positive impact on knowledge and attitudes regarding vaccination among their peers in parent communities.88
While there are websites with pro-vaccine, evidence-based information (CDC.gov, AAP.org), there seems to be a minimal presence of healthcare workers (public health officials, physicians, nurses, etc.) or medical institutions engaging in social media, as well as limited research on the topic. One study focused on analyzing content accuracy of health-related blogs found approximately 84 pediatrician bloggers in the entire United States, however 27 (32%) of these blogs were inactive since 2014 and 25 (30%) did not address vaccines. While most blogs which discussed vaccines were accurate, two pediatrician bloggers provided “extremely inaccurate, anti-vaccine information,” which the authors note may have a disproportionate negative influence on parents.89 As noted by Betsch and Sachse, while a permanent online presence by healthcare workers is needed, so is short-term, reactionary representation, such as social media events hosted by public health and medical personnel in response to current issues.81 An analysis of online interactions after a nationalized showing of an Australian documentary focused on the MMR vaccine and autism demonstrated that most people were either searching for information or vaccine critics, and very few users in the social media-based debate were health professionals.90 Given the influence of negative vaccine sentiments on parental vaccine perceptions, there is a need to engage healthcare professionals and organizations on social media with the goal of producing fact-based vaccine content and well-informed discussions among social network users on the interpersonal, organizational and community based-level.
While some studies have categorized pro- and anti-vaccine sentiments as discussed above, others have proposed or used social media as an intervention for vaccine education and uptake. Connolly and Reb proposed hierarchical vaccine decision-making interfaces, with the most involved version utilizing Web 2.0 platforms, to help create a personalized model in partnership with the individual's healthcare team (midwife, OB, pediatrician, nurses, etc.).91 In a randomized controlled trial with pregnant women, Glanz et al. found that those who experienced interactive, educational social media interventions were more likely to have their child up-to-date with vaccines at 6 months of age, compared to those who received usual care.92 Participants were able to engage with other study participants, as well as medical or public health experts, regarding any vaccine related topics they desired. Approximately 30% of the pregnant women in this interventional study arm actually used the social media discussion forum, leaving questions about how to further engage users on a larger scale, particularly vaccine-hesitant parents. The American Academy of Pediatrics has created an Immunization Social Media Toolkit suggested for use by parents and pediatric healthcare personnel which include Twitter posts, videos, YouTube links and guidance on managing social media accounts or creating videos. Social media tools (Ask the Expert on Vacunas.org93) and “ready-to-send” messages (AAP toolkit) are available. Similarly the WHO has implemented Vaccine Safety Net with the mission “to help internet users find reliable vaccine safety information tailored to their needs.”94 Lastly, the National Foundation for Infectious Diseases hosts an adolescent vaccine website where they can find vaccine information, an HPV resource center, and share their vaccine story on a blog.95 The use of social media for vaccine education among family and friends is promising and further research on its efficacy and effectiveness of content and delivery is warranted.
Organizational
On the organizational level, institutions can influence vaccination rates through the provision of clinical decision support tools, electronic health records, and vaccine registries as well as a workplace culture that emphasizes the importance of vaccination. Interventions at the organizational level help to ensure that providers recommend vaccination consistently at each opportunity, to optimize workflow for providers, and to maintain vaccine availability.
A common problem with vaccinations are missed opportunities, which occur when health care providers see a patient for a visit who is in need of vaccination but the patient leaves unvaccinated. This issue may particularly arise during acute care visits in which a provider may not be focusing on vaccinations. Clinical decision support tools have been employed to decrease missed opportunities, providing clinicians with patient-specific information at an appropriate time in their workflow coupled with a suggested course of action, such as vaccination.96 Decision support is made increasingly possible through the use of electronic health records (EHR) which are becoming more prevalent in the U.S. in large part through the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009 which encourages the meaningful use of EHRs with financial incentives. Overall, 87% of U.S. office-based physicians have adopted an EHR;97 however one recent study showed that nearly 40% of ambulatory practices were only minimally using their EHR and associated health IT functionalities.98
Fiks et al. have demonstrated the impact of EHR reminders on childhood vaccinations at 2 years of age at both sick and well visits99 as well as on HPV vaccination, including combining a clinician and family intervention.100,101 His team also assessed the impact of EHR reminders on influenza vaccination rates with only small increases.102 One concern of providers regarding an EHR vaccine alert was that the alert would be acting on incomplete vaccine information due to fragmented patient records.103 Such record fragmentation can occur particularly among low-income and minority populations who may seek care from multiple providers.104-106
Historically, electronic vaccination alerts in EHRs have acted only on local vaccination data. If a record is incomplete, then providers may be incorrectly prompted to administer a vaccination that a child does not need. “False positive” events contribute to alert fatigue and overall distrust of the alerting systems. We have demonstrated the effectiveness of an alert in the EHR that integrated individual patients' influenza vaccine data from our hospital's EHR and the New York Citywide Immunization Registry (CIR), New York City's Immunization Information system (IIS) (further described below).107,108 Interestingly, although providers were not forced to act on our influenza vaccine alert, they did so >80% of the time. Others have also highlighted the importance of capturing in alerts information about care received outside a medical center.109
Clinical decision support for vaccination has also been successfully used for adult vaccinations. A meta-analysis of 16 randomized control trials showed computer reminders in an ambulatory setting improved pneumococcal and influenza vaccination delivery to adults (aOR 3.09; 95% CI 2.39-4.00).110 More recent studies demonstrated their use for herpes zoster vaccine,111 pneumococcal and influenza vaccination in rheumatology patients on immunosupressants,112 Tdap and influenza for prenatal patients,113,114 and HPV vaccination among insured college-age male students.115
The availability of personal health records (PHR) also may influence vaccination rates if patients and their families can identify missing vaccinations or upcoming need. Parents have expressed interest in viewing vaccination records as part of a PHR.116,117 At least one study has demonstrated an increase in influenza vaccination in adults through the use of PHR-based alerts.118
Other system-level interventions can also help with the documentation and tracking of vaccinations such as the use of bar coding. The U.S. Centers for Disease Control and Prevention successfully demonstrated their use in a pilot project.119,120 Another study taking place in Canadian Public Health Immunization settings demonstrated that entering vaccine data into vaccination records through barcode scanning resulted in improved data quality.121 The WHO, its regional offices and their partners have also developed various tools for vaccine data management, reporting of district data, and cold chain equipment management in order to protect the substantial investments made in vaccination.122 More work is needed across the globe in both low and middle-income countries (LMIC) as well as high income countries to further develop and promote the widespread use of these technologies so that can better track vaccinations given as well as supply. New technologies are continuing to emerge which can aid this effort such as using blockchain as a secure way to monitor and share information.
Community
On the community level of the Social Ecological Model, perceptions of risk of vaccine preventable diseases in the community may play an important role (Figure 1). The expanding use of technology for surveillance provides the opportunity to gain new insights into the actual burden of disease due to its capacity to capture non-medically attended illnesses. This expansion of surveillance capabilities may be particularly important for influenza as people do not seek medical care in the majority of cases. While documenting the burden of medically-attended disease may be central to understanding health care utilization in cases of more serious disease, documenting the true disease burden may impact how the public perceive their own risk of disease. We have demonstrated the use of text message surveillance for acute respiratory infections including influenza and experienced rapid and sustained response rates that led to timely collection of specimens for testing.123 Of note, only 27.3% of participants with laboratory-confirmed influenza had an associated medical visit, supporting the addition of community surveillance to allow for better estimates of true disease burden than is permitted by relying on medically-attended illness alone. Other surveillance systems which may act as complements to outpatient and hospital-based surveillance systems, which have had varied effects, include crowd-source reporting like Flu Near You, the now defunct Google Flu Trends, Twitter and Wikipedia.124-126 Current lines of thinking advocates for combining multiple modalities and variables to better understand and forecast influenza activity.127-129 New interest in using Google trends to assess vaccination searches has also evolved. For example, it was found that the most searched vaccines from 2004–2015 were for 1) influenza; 2) meningitis; 3) diphtheria, pertussis (whooping cough), and tetanus; 4) yellow fever; and 5) chickenpox.130 Search data trends could be used to inform public health programming and to better understand educational needs of the public regarding vaccination.
Another form of vaccine-related surveillance is the use of text messages for vaccine adverse event surveillance. Most vaccine adverse event surveillance focuses on medically-attended illness such as the Vaccine Safety Datalink system in the U.S.131 However, text messages can also be used to collect vaccine side effects directly from the patient. This type of surveillance would provide important vaccine adverse event data not gathered in medical record reviews. Understanding the burden of vaccine adverse events (VAE), including non-medically attended ones, has the potential to impact personal and community vaccination views by providing data to support realistic anticipatory guidance to families, which in turn could instill more vaccine confidence. We have conducted four studies using text messaging for VAE surveillance through the US Centers for Disease Control and Prevention's Clinical Immunization Safety Assessment (CISA) project.132-135 Interestingly, in two separate studies, we found that 14% of pregnant women and 19% of parents reported that taking part in a text message influenza VAE surveillance study positively affected how they felt about vaccine safety; 0.8% parents and no pregnant women reported a potentially negative effect.134,135 Others have also used text message vaccine adverse event surveillance, including the AusVaxSafety surveillance team in children and Regan et al. in pregnant women.136-140 AusVaxSafety, a national collaborative initiative led by the National Centre for Immunisation Research and Surveillance and funded by the Australian Government Department of Health, uses software programs run by general practitioners and vaccination clinics that send an SMS or email to patients or parents following a vaccination to capture VAE. This initiative is an interesting model for other countries to consider, allowing the collection of more information about non-medically attended vaccine side effects to provide reassurance and guidance.
Society
On the society level of the Social Ecological Model framework (Figure 1), immunization information systems (IIS) consolidate vaccination information across a population.141,142 As of 2016, nearly all states and 6 cities have IIS, and 94% of all U.S. children <6 years old have vaccinations recorded in an IIS.143 The percentage of adolescents participating in an IIS is 74% nationally, but varies widely by state.144 A systematic review of IIS studies demonstrated their capacity to “(1) create or support effective interventions to increase vaccination rates; (2) determine client vaccination status to inform decisions by clinicians, health care systems, and schools; (3) guide public health responses to outbreaks of vaccine-preventable disease; (4) inform assessments of vaccination coverage, missed vaccination opportunities, invalid dose administration, and disparities; and (5) facilitate vaccine management and accountability.”145 Based on these findings, the Community Preventive Services Task Force recommends the use of IIS to increase vaccination rates.146
The CDC's National Center for Immunization and Respiratory Diseases has also released an IIS strategic plan which includes “strengthening connections to the health IT environment.”147 Additionally, part of the U.S. federal meaningful use of EHR financial incentive program includes data exchange between IIS and EHRs.143 Indeed automated reporting via EHRs have also been shown to improve registry use.148 Data completeness within registries continues to improve, which bolsters their utility and their capacity to provide clinical decision support through vaccine forecasting.143 Bidirectional data exchange also greatly improves the operability of IIS.149 We have demonstrated that bidirectional data exchange between a local EHR and the New York City's IIS (the Citywide Immunization Registry [CIR]) improved rates of under-vaccination, over-vaccination, and vaccination record completeness.150 While this functionality may be technologically challenging to implement, the amount of U.S. jurisdictions with the capability of an IIS that could exchange vaccination histories has increased from 45% in 2013 to 67% in 2016.143
Legislation mandating childhood vaccinations reporting supports the use of and amplifies the utility of IIS. Due to issues obtaining and documenting consent, adult records are not reported to most city and state registries. Record scatter and uncoordinated fragmented healthcare delivery contribute to poor documentation of adult vaccinations. Promoting bidirectional exchange of vaccination information between EHRs and IIS for adults is an important next step. Similarly, many adults are being vaccinated outside the health care system, including in pharmacies and urgent cares. Pharmacy and urgent care participation in IIS would bolster efforts to document and better track adult vaccinations, particularly in regards to influenza and the two pneumococcal vaccines. Additional development of the technology to support bidirectional vaccination exchange and continued support of these exchanges are needed.
The utility of IIS in various global settings is currently being explored as infrastructure and funding challenges exist. Most countries do not currently have IIS, and of those that do there is high variability in implementation.151 While IIS are rare in LMIC, the WHO and Albania's Ministry of Health successfully piloted and scaled up IIS which included a vaccine registry, VAE reporting, and vaccine stock management.121
Integration across social ecological model levels
Perhaps the greatest impact technological interventions can have is not only how they operate at each of the Social Ecological Model levels, but also how interventions can act across levels. For example, when developing and testing a vaccine educational and interactive app, it would be important to consider the overlap of introducing push notifications between the user (individual) and their affiliated healthcare center's EHR and PHR (organizational). Will the push notifications be only for vaccine reminders or for educational purposes or both? If the app provides educational information, will the user have the ability to “post” this information on their social media platforms to ask questions or share with friends (intrapersonal)? Once a user receives their vaccine it will be important for the app creators to map how this information will upload from the EHR to the local IIS to ensure maximally successful data exchange, usage and limit “false positive” vaccine alerts (society). Similarly, organizations can use their organizational data (organizational) linked to their local IIS (society) to identify those not up to date for vaccination and then send text message vaccine reminders (individual). Such reminders could be linked to interventional social media platforms (intrapersonal) or data from their own community level (community). Local IIS could be designed to have text message capability either for Departments of Health themselves or local providers to send text message reminders, linking the individual with society. For those who are vaccinated, text message follow up could occur to assess any vaccine adverse events which could be used not only as a form of surveillance but also to reassure the public that vaccines are being monitored (community). Finally, linked systems could follow a vaccine from production to the patient particularly in LMIC, first by monitoring vaccine supply (organizational) then following vaccine product through the shipping process including maintenance of the cold chain, alerting local facilities that vaccines will be arriving (organizational), allowing public health staff/community workers to alert families to come for vaccination (individual) and then electronically document vaccination, allowing ministries of health to assess population vaccination coverage (society).
Conclusion
The Social Ecological Model can be utilized to classify barriers and strategies to vaccination at the individual, interpersonal, community, organizational and society levels. In the evaluation of technologic interventions for the improvement of vaccine coverage, education, communication, or data collection, researchers and practitioners may find that using this framework is helpful to better understand implications on each level and whether the approach is comprehensive.
The advances in and increased use of technology by patients, their families, and providers have created possibilities for technology-based interventions to improve vaccination rates through novel approaches as well as expanding the utility of established methods. The use of text messages or email multiplies the reach and scalability of a reminder-recall system to additional populations otherwise difficult to impact. A next step could be to increase their use across organizations as well as increase their interactivity. Online resources and social media discussions between an individual and their family and friends can influence vaccine decision-making and behaviors and likely need to be addressed as a part of any immunization program or campaign. A coherent, effective social media presence by the medical and public health communities has potential to positively change the vaccination conversations online- those communities need to move into those spaces with clear and effective communication. Novel forms of surveillance including via text message, social media, and internet searches extend the capacity to document the full disease burden in a community, which may have implications for educational counseling, focused anticipatory guidance, or campaigns regarding disease risk. Organizations with EHRs and IIS can maximize their utility through bidirectional data exchange with city and state run registries. Using this data, they can minimize missed vaccination opportunities through clinical decision support tools. Such data exchanges and alerts should become standard in EHRs and IIS not only for pediatric but for adult patients who can at times be an afterthought in vaccination programs. Further, the creation of a national vaccine registry that includes data from all state registries is a goal that could have a real public health impact for our increasingly mobile population.
When considering employing any of these interventions to improve vaccination rates in a specific population, public health and medical organizations must consider factors at each level of the Social Ecological Model and work to address any barriers that may interact or diminish the impact of an intervention. Recognizing how the individual, their family and friends, and their larger community interacts may greatly help to address the complexities of influencing attitudes and behavior of social networks through technology. As evidence grows to support various targeted approaches, efforts which combine interventions across the Social Ecological Model's levels during implementation and expansion may have higher potential to improve vaccination rates, reduce outbreaks and burden of disease of vaccine-preventable illnesses, and provide more well-rounded data.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Polio Now Available at http://polioeradication.org/polio-today/polio-now/. Accessed on April27, 2018.
- 2.World Health Organization Estimated rotavirus deaths for children under 5 years of age: 2013, 215 000. Available at http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/. Accessed on April/27/18.
- 3.World Health Organization Pneumococcal disease. Available at http://www.who.int/immunization/diseases/pneumococcal/en/. Accessed on April27, 2018.
- 4.Garland SM, Kjaer SK, Munoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al.. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: A systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63(4):519–27. doi: 10.1093/cid/ciw354. PMID:27230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19–35 Months – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1171–7. doi: 10.15585/mmwr.mm6643a3. PMID:29095807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–82. doi: 10.15585/mmwr.mm6633a2. PMID:28837546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Flu Vaccination Coverage, United States, 2016–17 Influenza Season. Available at https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. Accessed on February26, 2018.
- 8.Feldstein LR, Mariat S, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(45):1252–5. doi: 10.15585/mmwr.mm6645a3. PMID:29145357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Measles cases and outbreaks. Available at https://www.cdc.gov/measles/cases-outbreaks.html. Accessed on February18. 2018.
- 10.O'Connor P, Jankovic D, Muscat M, Ben-Mamou M, Reef S, Papania M, Singh S, Kaloumenos T, Butler R, Datta S. Measles and rubella elimination in the WHO region for Europe: Progress and challenges. Clin Microbiol Infect. 2017;23(8):504–10. doi: 10.1016/j.cmi.2017.01.003. PMID:28111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Pertussis in Other Countries. Available at https://www.cdc.gov/pertussis/countries/index.html. Accessed on April27, 2018.
- 12.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al.. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2017. PMID:29248255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odone A, Ferrari A, Spagnoli F, Visciarelli S, Shefer A, Pasquarella C, Signorelli C. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11(1):72–82. doi: 10.4161/hv.34313. PMID:25483518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–77. doi: 10.1177/109019818801500401. PMID:3068205. [DOI] [PubMed] [Google Scholar]
- 15.Ohri-Vachaspati P, DeLia D, DeWeese RS, Crespo NC, Todd M, Yedidia MJ. The relative contribution of layers of the Social Ecological Model to childhood obesity. Public Health Nutr. 2015;18(11):2055–66. doi: 10.1017/S1368980014002365. PMID:25374257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder JP, Lytle L, Sallis JF, Young DR, Steckler A, Simons-Morton D, Stone E, Jobe JB, Stevens J, Lohman T, et al.. A description of the social-ecological framework used in the trial of activity for adolescent girls (TAAG). Health Educ Res. 2007;22(2):155–65. doi: 10.1093/her/cyl059. PMID:16855014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lytle LA. Examining the etiology of childhood obesity: The IDEA study. Am J Community Psychol. 2009;44(3-4):338–49. doi: 10.1007/s10464-009-9269-1. PMID:19838791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention The Social-Ecological Model: A Framework for Prevention. Available at https://www.cdc.gov/violenceprevention/overview/social-ecologicalmodel.html Accessed on April19, 2018.
- 19.Kumar S, Quinn SC, Kim KH, Musa D, Hilyard KM, Freimuth VS. The social ecological model as a framework for determinants of 2009 H1N1 influenza vaccine uptake in the United States. Health Educ Behav. 2012;39(2):229–43. doi: 10.1177/1090198111415105. PMID:21984692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Community Preventive Services Task Force Increasing appropriate vaccination: Client reminder and recall systems: Task force finding and rationale statement. Available at https://www.thecommunityguide.org/sites/default/files/assets/Vaccination-Client-Reminders.pdf Accessed on February18, 2018.
- 21.National Vaccine Advisory Committee Rescommendations from the National Vaccine Advisory Committee: Standards for adult immunization practice. Public Health Rep. Mar-Apr 2014;129(2):115–23. doi: 10.1177/003335491412900203. PMID:24587544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irigoyen MM, Findley S, Wang D, Chen S, Chimkin F, Pena O, Mendonca E. Challenges and successes of immunization registry reminders at inner-city practices. Ambul Pediatr. 2006;6(2):100–4. doi: 10.1016/j.ambp.2005.10.006. PMID:16530147. [DOI] [PubMed] [Google Scholar]
- 23.Hambidge SJ, Davidson AJ, Phibbs SL, Chandramouli V, Zerbe G, LeBaron CW, Steiner JF. Strategies to improve immunization rates and well-child care in a disadvantaged population: A cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2004;158(2):162–9. doi: 10.1001/archpedi.158.2.162. PMID:14757608. [DOI] [PubMed] [Google Scholar]
- 24.Kempe A, Lowery NE, Pearson KA, Renfrew BL, Jones JS, Steiner JF, Berman S. Immunization recall: Effectiveness and barriers to success in an urban teaching clinic. J Pediatr. 2001;139(5):630–5. doi: 10.1067/mpd.2001.117069. PMID:11713438. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Evaluation of vaccination recall letter system for Medicaid-enrolled children aged 19–23 months–Montana, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(40):811–5. PMID:23051611. [PubMed] [Google Scholar]
- 26.Mobile Fact Sheet, Pew Research Center Available at http://www.pewinternet.org/fact-sheet/mobile/. Accessed on February28, 2018.
- 27.Blumberg SJ, Luke JV. Wireless substitution: Early Release of Estimates From the National Health Interview Survey, January–June 2017. Available at https://www.cdc.gov/nchs/data/nhis/earlyrelease/wireless201712.pdf. Accessed on February18, 2018.
- 28.Number of mobile phone users worldwide from 2013 to 2019 (in billions). Available at https://www.statista.com/statistics/274774/forecast-of-mobile-phone-users-worldwide/. Accessed on February19, 2018.
- 29.Clark SJ, Butchart A, Kennedy A, Dombkowski KJ. Parents' experiences with and preferences for immunization reminder/recall technologies. Pediatrics. 2011;128(5):e1100–5. doi: 10.1542/peds.2011-0270. PMID:22007019. [DOI] [PubMed] [Google Scholar]
- 30.Kharbanda EO, Stockwell MS, Fox HW, Rickert VI. Text4Health: A qualitative evaluation of parental readiness for text message immunization reminders. Am J Public Health. 2009;99(12):2176–8. doi: 10.2105/AJPH.2009.161364. PMID:19833982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockwell MS, Hofstetter AM, DuRivage N, Barrett A, Fernandez N, Vargas CY, Camargo S. Text message reminders for second dose of influenza vaccine: A randomized controlled trial. Pediatrics. Jan 2015;135(1):e83–91. doi: 10.1542/peds.2014-2475. PMID:25548329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofstetter AM, Vargas CY, Kennedy A, Kitayama K, Stockwell MS. Parental and provider preferences and concerns regarding text message reminder/recall for early childhood vaccinations. Prev Med. 2013;57(2):75–80. doi: 10.1016/j.ypmed.2013.04.007. PMID:23624252. [DOI] [PubMed] [Google Scholar]
- 33.Ahlers-Schmidt CR, Chesser AK, Paschal AM, Hart TA, Williams KS, Yaghmai B, Shah-Haque S. Parent opinions about use of text messaging for immunization reminders. J Med Internet Res. 2012;14(3):e83. doi: 10.2196/jmir.1976. PMID:22683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rand CM, Blumkin A, Vincelli P, Katsetos V, Szilagyi PG. Parent Preferences for Communicating With Their Adolescent's Provider Using New Technologies. J Adolesc Health. 2015;57(3):299–304. doi: 10.1016/j.jadohealth.2015.06.006. PMID:26299557. [DOI] [PubMed] [Google Scholar]
- 35.Kempe A, O'Leary ST, Shoup JA, Stokley S, Lockhart S, Furniss A, Dickinson LM, Barnard J, Daley MF, et al.. Parental choice of recall method for HPV vaccination: A pragmatic trial. Pediatrics. 2016;137(3):e20152857. doi: 10.1542/peds.2015-2857. PMID:26921286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofstetter AM, DuRivage N, Vargas CY, Camargo S, Vawdrey DK, Fisher A, Stockwell MS. Text message reminders for timely routine MMR vaccination: A randomized controlled trial. Vaccine. 2015;33(43):5741–6. doi: 10.1016/j.vaccine.2015.09.042. PMID:26424607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlers-Schmidt CR, Chesser AK, Nguyen T, Brannon J, Hart TA, Williams KS, Wittler RR. Feasibility of a randomized controlled trial to evaluate Text Reminders for Immunization Compliance in Kids (TRICKs). Vaccine. 2012;30(36):5305–9. doi: 10.1016/j.vaccine.2012.06.058. PMID:22750044. [DOI] [PubMed] [Google Scholar]
- 38.Morris J, Wang W, Wang L, Peddecord KM, Sawyer MH. Comparison of reminder methods in selected adolescents with records in an immunization registry. J Adolesc Health. 2015;56(5 Suppl):S27–32. doi: 10.1016/j.jadohealth.2015.01.010. PMID:25863551. [DOI] [PubMed] [Google Scholar]
- 39.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541. [DOI] [PubMed] [Google Scholar]
- 40.Rand CM, Vincelli P, Goldstein NP, Blumkin A, Szilagyi PG. Effects of Phone and Text Message Reminders on Completion of the Human Papillomavirus Vaccine Series. J Adolesc Health. 2017;60(1):113–119. [DOI] [PubMed] [Google Scholar]
- 41.O'Leary ST, Lee M, Lockhart S, et al. . Effectiveness and Cost of Bidirectional Text Messaging for Adolescent Vaccines and Well Care. Pediatrics. 2015;136(5):e1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aragones A, Bruno DM, Ehrenberg M, Tonda-Salcedo J, Gany FM. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rand CM, Brill H, Albertin C, et al. . Effectiveness of centralized text message reminders on human papillomavirus immunization coverage for publicly insured adolescents. J Adolesc Health. 2015;56(5 Suppl):S17–20. [DOI] [PubMed] [Google Scholar]
- 44.Bar-Shain DS, Stager MM, Runkle AP, Leon JB, Kaelber DC. Direct messaging to parents/guardians to improve adolescent immunizations. J Adolesc Health. 2015;56(5 Suppl):S21–26. [DOI] [PubMed] [Google Scholar]
- 45.Stockwell MS, Kharbanda EO, Martinez RA, et al. . Text4Health: impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012;102(2):e15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: A randomized controlled trial. JAMA. 2012;307(16):1702–8. doi: 10.1001/jama.2012.502. PMID:22535855. [DOI] [PubMed] [Google Scholar]
- 47.Hofstetter AM, Vargas CY, Camargo S, Holleran S2, Vawdrey DK, Kharbanda EO, Stockwell MS. Impacting delayed pediatric influenza vaccination: A randomized controlled trial of text message reminders. Am J Prev Med. Apr 2015;48(4):392–401. doi: 10.1016/j.amepre.2014.10.023. PMID:25812465. [DOI] [PubMed] [Google Scholar]
- 48.Szilagyi PG, Adams WG. Text messaging: A new tool for improving preventive services. JAMA. 2012;307(16):1748–9. doi: 10.1001/jama.2012.524. PMID:22535860. [DOI] [PubMed] [Google Scholar]
- 49.Lehnert JD, Shevach A, Walker S, Wang R, Fitzgerald TJ, Graitcer SB. Development and pilot testing of a text message vaccine reminder system for use during an influenza pandemic. Hum Vaccin Immunother. 2018:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regan AK, Bloomfield L, Peters I, Effler PV. Randomized controlled trial of text message reminders for increasing influenza vaccination. Ann Fam Med. 2017;15(6):507–14. doi: 10.1370/afm.2120. PMID:29133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domek GJ, Contreras-Roldan IL, O'Leary ST, Furniss A, Kempe A, Asturias EJ. SMS text message reminders to improve infant vaccination coverage in Guatemala: A pilot randomized controlled trial. Vaccine. 2016;34(21):2437–43. doi: 10.1016/j.vaccine.2016.03.065. PMID:27026145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uddin MJ, Shamsuzzaman M, Horng L, Labrique A4, Vasudevan L, Zeller K, Chowdhury M, Larson CP, Bishai D, Alam N, et al.. Use of mobile phones for improving vaccination coverage among children living in rural hard-to-reach areas and urban streets of Bangladesh. Vaccine. 2016;34(2):276–83. doi: 10.1016/j.vaccine.2015.11.024. PMID:26647290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson DG, Ochieng B, Kagucia EW, Were J, Hayford K, Moulton LH, Levine OS, Odhiambo F, O'Brien KL, Feikin DR. Mobile phone-delivered reminders and incentives to improve childhood immunisation coverage and timeliness in Kenya (M-SIMU): A cluster randomised controlled trial. Lancet Glob Health. 2017;5(4):e428–e38. doi: 10.1016/S2214-109X(17)30072-4. PMID:28288747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangure D, Chirundu D, Gombe N, Marufu T4, Mandozana G, Tshimanga M, Takundwa L. Effectiveness of short message services reminder on childhood immunization programme in Kadoma, Zimbabwe – a randomized controlled trial, 2013. BMC Public Health. 2015;15:137. doi: 10.1186/s12889-015-1470-6. PMID:25885862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haji A, Lowther S, Ngan'ga Z, Gura Z, Tabu C, Sandhu H, Arvelo W. Reducing routine vaccination dropout rates: Evaluating two interventions in three Kenyan districts, 2014. BMC Public Health. 2016;16:152. doi: 10.1186/s12889-016-2823-5. PMID:26880141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazi AM, Ali M, Ayub K, Kalimuddin H, Zubaira K, Kazi AN, Artani A, Ali SA.. Geo-spatial reporting for monitoring of household immunization coverage through mobile phones: Findings from a feasibility study. Int J Med Inform. 2017;107:48–55. doi: 10.1016/j.ijmedinf.2017.09.004. PMID:29029691. [DOI] [PubMed] [Google Scholar]
- 57.Stockwell MS, Westhoff C, Kharbanda EO, Vargas CY, Camargo S, Vawdrey DK, Castaño PM. Influenza vaccine text message reminders for urban, low-income pregnant women: A randomized controlled trial. Am J Public Health. 2014;104 Suppl 1:e7–12. doi: 10.2105/AJPH.2013.301620. PMID:24354839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bushar JA, Kendrick JS, Ding H, Black CL, Greby SM. Text4baby influenza messaging and influenza vaccination among pregnant women. Am J Prev Med. 2017;53(6):845–53. doi: 10.1016/j.amepre.2017.06.021. PMID:28867143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan ET, Bushar JA, Kendrick JS, Johnson P, Wang J. encouraging influenza vaccination among text4baby pregnant women and mothers. Am J Prev Med. 2015;49(4):563–72. doi: 10.1016/j.amepre.2015.04.029. PMID:26232904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yudin MH, Mistry N, De Souza LR, Besel K, Patel V, Blanco Mejia S, Bernick R, Ryan V, Urquia M, Beigi RH, et al.. Text messages for influenza vaccination among pregnant women: A randomized controlled trial. Vaccine. 2017;35(5):842–8. doi: 10.1016/j.vaccine.2016.12.002. PMID:28062124. [DOI] [PubMed] [Google Scholar]
- 61.Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: A randomized controlled trial. Obstet Gynecol. 2013;121(4):734–40. doi: 10.1097/AOG.0b013e31828642b1. PMID:23635672. [DOI] [PubMed] [Google Scholar]
- 62.Herrett E, Williamson E, van Staa T, Ranopa M, Free C, Chadborn T, Goldacre B, Smeeth L. Text messaging reminders for influenza vaccine in primary care: A cluster randomised controlled trial (TXT4FLUJAB). BMJ Open. 2016;6(2):e010069. doi: 10.1136/bmjopen-2015-010069. PMID:26895984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: A randomized controlled trial. Vaccine. 2015;33(43):5868–72. doi: 10.1016/j.vaccine.2015.07.050. PMID:26232345. [DOI] [PubMed] [Google Scholar]
- 64.Vilella A, Bayas JM, Diaz MT, Guinovart C, Diez C, Simó D, Muñoz A, Cerezo J. The role of mobile phones in improving vaccination rates in travelers. Prev Med. 2004;38(4):503–9. doi: 10.1016/j.ypmed.2003.12.005. PMID:15020186. [DOI] [PubMed] [Google Scholar]
- 65.Richman AR, Maddy L, Torres E, Goldberg EJ. A randomized intervention study to evaluate whether electronic messaging can increase human papillomavirus vaccine completion and knowledge among college students. J Am Coll Health. 2016;64(4):269–78. doi: 10.1080/07448481.2015.1117466. PMID:26821923. [DOI] [PubMed] [Google Scholar]
- 66.Patel A, Stern L, Unger Z, Debevec E, Roston A, Hanover R, Morfesis J. Staying on track: A cluster randomized controlled trial of automated reminders aimed at increasing human papillomavirus vaccine completion. Vaccine. 2014;32(21):2428–33. doi: 10.1016/j.vaccine.2014.02.095. PMID:24631099. [DOI] [PubMed] [Google Scholar]
- 67.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;1:CD003941. PMID:29342498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alemi F, Alemagno SA, Goldhagen J, Ash L, Finkelstein B, Lavin A, Butts J, Ghadiri A. Computer reminders improve on-time immunization rates. Med Care. 1996;34(10 Suppl):OS45–51. doi: 10.1097/00005650-199610003-00005. PMID:8843936. [DOI] [PubMed] [Google Scholar]
- 69.Brimberry R. Vaccination of high-risk patients for influenza. A comparison of telephone and mail reminder methods. J Fam Pract. 1988;26(4):397–400. PMID:3282026. [PubMed] [Google Scholar]
- 70.Kazi AM. The role of mobile phone-based interventions to improve routine childhood immunisation coverage. Lancet Glob Health. 2017;5(4):e377–e8. doi: 10.1016/S2214-109X(17)30088-8. PMID:28288737. [DOI] [PubMed] [Google Scholar]
- 71.Dombkowski KJ, Cowan AE, Reeves SL, Foley MR, Dempsey AF. The impacts of email reminder/recall on adolescent influenza vaccination. Vaccine. 2017;35(23):3089–95. doi: 10.1016/j.vaccine.2017.04.033. PMID:28455173. [DOI] [PubMed] [Google Scholar]
- 72.Bednarczyk RA, Frew PM, Salmon DA, Whitney E, Omer SB. ReadyVax: A new mobile vaccine information app. Hum Vaccin Immunother. 2017;13(5):1149–54. doi: 10.1080/21645515.2016.1263779. PMID:28059610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fadda M, Galimberti E, Fiordelli M, Romano L, Zanetti A, Schulz PJ. Effectiveness of a smartphone app to increase parents' knowledge and empowerment in the MMR vaccination decision: A randomized controlled trial. Hum Vaccin Immunother. 2017;13(11):2512–21. doi: 10.1080/21645515.2017.1360456. PMID:29125783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atkinson KM, Westeinde J, Ducharme R, Wilson SE, Deeks SL, Crowcroft N, Hawken S, Wilson K. Can mobile technologies improve on-time vaccination? A study piloting maternal use of ImmunizeCA, a Pan-Canadian immunization app. Hum Vaccin Immunother. 2016;12(10):2654–61. doi: 10.1080/21645515.2016.1194146. PMID:27322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L, Du X, Zhang L, van Velthoven MH, Wu Q, Yang R, Cao Y, Wang W, Xie L, Rao X, et al.. Effectiveness of a smartphone app on improving immunization of children in rural Sichuan Province, China: A cluster randomized controlled trial. BMC Public Health. 2016;16:909. doi: 10.1186/s12889-016-3549-0. PMID:27581655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Sources and perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;127 Suppl 1:S107–12. doi: 10.1542/peds.2010-1722P. PMID:21502236. [DOI] [PubMed] [Google Scholar]
- 77.Pehora C, Gajaria N, Stoute M, Fracassa S, Serebale-O'Sullivan R, Matava CT. Are parents getting it right? A Survey of Parents' Internet Use for Children's Health Care Information. Interact J Med Res. 2015;4(2):e12. PMID:26099207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones AM, Omer SB, Bednarczyk RA, Halsey NA, Moulton LH, Salmon DA. Parents' source of vaccine information and impact on vaccine attitudes, beliefs, and nonmedical exemptions. Adv Prev Med. 2012;2012:932741. doi: 10.1155/2012/932741. PMID:23082253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Betsch C, Renkewitz F, Betsch T, Ulshofer C. The influence of vaccine-critical websites on perceiving vaccination risks. J Health Psychol. Apr 2010;15(3):446–55. doi: 10.1177/1359105309353647. PMID:20348365. [DOI] [PubMed] [Google Scholar]
- 80.Brunson EK. The impact of social networks on parents' vaccination decisions. Pediatrics. 2013;131(5):e1397–404. doi: 10.1542/peds.2012-2452. PMID:23589813. [DOI] [PubMed] [Google Scholar]
- 81.Betsch C, Sachse K. Dr. Jekyll or Mr. Hyde? (How) the Internet influences vaccination decisions: Recent evidence and tentative guidelines for online vaccine communication. Vaccine. 2012;30(25):3723–6. doi: 10.1016/j.vaccine.2012.03.078. PMID:22472790. [DOI] [PubMed] [Google Scholar]
- 82.Kata A. Anti-vaccine activists, Web 2.0, and the postmodern paradigm–an overview of tactics and tropes used online by the anti-vaccination movement. Vaccine. 2012;30(25):3778–89. doi: 10.1016/j.vaccine.2011.11.112. PMID:22172504. [DOI] [PubMed] [Google Scholar]
- 83.Keelan J, Pavri-Garcia V, Tomlinson G, Wilson K. YouTube as a source of information on immunization: A content analysis. JAMA. 2007;298(21):2482–4. doi: 10.1001/jama.298.21.2482. PMID:18056901. [DOI] [PubMed] [Google Scholar]
- 84.Keelan J, Pavri V, Balakrishnan R, Wilson K. An analysis of the Human Papilloma Virus vaccine debate on MySpace blogs. Vaccine. 2010;28(6):1535–40. doi: 10.1016/j.vaccine.2009.11.060. PMID:20003922. [DOI] [PubMed] [Google Scholar]
- 85.Wolfe RM, Sharp LK, Lipsky MS. Content and design attributes of antivaccination web sites. JAMA. 2002;287(24):3245–8. doi: 10.1001/jama.287.24.3245. PMID:12076221. [DOI] [PubMed] [Google Scholar]
- 86.Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33(3):459–64. doi: 10.1016/j.vaccine.2014.11.017. PMID:25499651. [DOI] [PubMed] [Google Scholar]
- 87.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: A randomized trial. Pediatrics. 2014;133(4):e835–42. doi: 10.1542/peds.2013-2365. PMID:24590751. [DOI] [PubMed] [Google Scholar]
- 88.Schoeppe J, Cheadle A, Melton M, Faubion T, Miller C, Matthys J, Hsu C. The immunity community: A community engagement strategy for reducing vaccine hesitancy. Health Promot Pract. 2017;18(5):654–61. doi: 10.1177/1524839917697303. PMID:28398837. [DOI] [PubMed] [Google Scholar]
- 89.Bryan MA, Gunningham H, Moreno MA. Content and accuracy of vaccine information on pediatrician blogs. Vaccine. 2018;36(5):765–70. doi: 10.1016/j.vaccine.2017.11.088. PMID:29305176. [DOI] [PubMed] [Google Scholar]
- 90.Nicholson MS, Leask J. Lessons from an online debate about measles-mumps-rubella (MMR) immunization. Vaccine. 2012;30(25):3806–12. doi: 10.1016/j.vaccine.2011.10.072. PMID:22063388. [DOI] [PubMed] [Google Scholar]
- 91.Connolly T, Reb J. Toward interactive, Internet-based decision aid for vaccination decisions: Better information alone is not enough. Vaccine. 2012;30(25):3813–8. doi: 10.1016/j.vaccine.2011.12.094. PMID:22234264. [DOI] [PubMed] [Google Scholar]
- 92.Glanz JM, Wagner NM, Narwaney KJ, Kraus CR, Shoup JA, Xu S, O'Leary ST, Omer SB, Gleason KS, Daley MF, et al.. Web-based Social Media Intervention to Increase Vaccine acceptance: A randomized controlled trial. Pediatrics. 2017;140(6). doi: 10.1542/peds.2017-1117. PMID:29109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Basteiro AL, Alvarez-Pasquin MJ, Mena G, Llupià A, Aldea M, Sequera VG, Sanz S, Tuells J, Navarro-Alonso JA, de Arísteguí J, et al.. A public-professional web-bridge for vaccines and vaccination: User concerns about vaccine safety. Vaccine. 2012;30(25):3798–05. doi: 10.1016/j.vaccine.2011.10.003. PMID:22027485. [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization Vaccine safety net. Available at http://www.who.int/vaccine_safety/initiative/communication/network/vaccine_safety_websites/en/ Accessed on February24, 2018.
- 95.National Foundation for Infectious Disease s. Adolescent vaccination. Available at http://adolescentvaccination.org/ Accessed on April6, 2018.
- 96.Osheroff J, Pifer E, Teich J, Sittig DRJ. Improving Outcomes with Clinical Decision Support: An Implementer's Guide: Productivity Press. 2005. [Google Scholar]
- 97.Office-based physician electronic health record adoption Office of the National Coordinator for Health Information Technology. Available at https://dashboard.healthit.gov/quickstats/pages/physician-ehr-adoption-trends.php. Accessed on February19, 2018.
- 98.Rumball-Smith J, Shekelle P, Damberg CL. Electronic health record “super-users” and “under-users” in ambulatory care practices. Am J Manag Care. 2018;24(1):26–31. PMID:29350506. [PMC free article] [PubMed] [Google Scholar]
- 99.Fiks AG, Grundmeier RW, Biggs LM, Localio AR, Alessandrini EA. Impact of clinical alerts within an electronic health record on routine childhood immunization in an urban pediatric population. Pediatrics. 2007;120(4):707–14. doi: 10.1542/peds.2007-0257. PMID:17908756. [DOI] [PubMed] [Google Scholar]
- 100.Fiks AG, Grundmeier RW, Mayne S, Song L, Feemster K, Karavite D, Hughes CC, Massey J, Keren R, Bell LM, Wasserman R, Localio AR.. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–24. doi: 10.1542/peds.2012-3122. PMID:23650297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mayne SL, duRivage NE, Feemster KA, Localio AR, Grundmeier RW, Fiks AG. Effect of decision support on missed opportunities for human papillomavirus vaccination. Am J Prev Med. 2014;47(6):734–44. doi: 10.1016/j.amepre.2014.08.010. PMID:25455116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiks AG, Hunter KF, Localio AR, Grundmeier RW, Bryant-Stephens T, Luberti AA, Bell LM, Alessandrini EA. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics. 2009;124(1):159–69. doi: 10.1542/peds.2008-2823. PMID:19564296. [DOI] [PubMed] [Google Scholar]
- 103.Birmingham E, Catallozzi M, Findley SE, Vawdrey DK, Kukafka R, Stockwell MS. FluAlert: A qualitative evaluation of providers' desired characteristics and concerns regarding computerized influenza vaccination alerts. Prev Med. 2011;52(3-4):274–7. PMID:21276811. [DOI] [PubMed] [Google Scholar]
- 104.Yusuf H, Adams M, Rodewald L, Lu P, Rosenthal J, Legum SE, Santoli J. Fragmentation of immunization history among providers and parents of children in selected underserved areas. Am J Prev Med. 2002;23(2):106–12. doi: 10.1016/S0749-3797(02)00463-4. PMID:12121798. [DOI] [PubMed] [Google Scholar]
- 105.Kolasa MS, Chilkatowsky AP, Clarke KR, Lutz JP. How complete are immunization registries? The philadelphia story. Ambul Pediatr. 2006;6(1):21–24. doi: 10.1016/j.ambp.2005.08.006. PMID:16443179. [DOI] [PubMed] [Google Scholar]
- 106.Bardenheier BH, Yusuf HR, Rosenthal J, Santoli JM, Shefer AM, Rickert DL, Chu SY. Factors associated with underimmunization at 3 months of age in four medically underserved areas. Public Health Rep. 2004;119(5):479–85. doi: 10.1016/j.phr.2004.07.005. PMID:15313111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stockwell MS, Catallozzi M, Camargo S, Ramakrishnan R, Holleran S, Findley SE, Kukafka R, Hofstetter AM, Fernandez N, Vawdrey DK. Registry-linked electronic influenza vaccine provider reminders: A cluster-crossover trial. Pediatrics. 2015;135(1):e75–82. doi: 10.1542/peds.2014-2616. PMID:25548331. [DOI] [PubMed] [Google Scholar]
- 108.Vawdrey DK, Natarajan K, Kanter AS, Hripcsak G, Kuperman GJ, Stockwell MS. Informatics lessons from using a novel immunization information system. Stud Health Technol Inform. 2013;192:589–93. PMID:23920624. [PubMed] [Google Scholar]
- 109.Bowen ME, Bhat D, Fish J, Moran B, Howell-Stampley T, Kirk L, Persell SD, Halm EA, et al.. Improving performance on preventive health quality measures using clinical decision support to capture care done elsewhere and patient exceptions. Am J Med Qual. 2017:1062860617732830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc. 1996;3(6):399–409. doi: 10.1136/jamia.1996.97084513. PMID:8930856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract. 2013;19(2):263–66. doi: 10.1111/j.1365-2753.2011.01814.x. PMID:22304668. [DOI] [PubMed] [Google Scholar]
- 112.Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505–10. doi: 10.1002/art.24873. PMID:19877088. [DOI] [PubMed] [Google Scholar]
- 113.Morgan JL, Baggari SR, Chung W, Ritch J, McIntire DD, Sheffield JS. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126(2):333–7. doi: 10.1097/AOG.0000000000000975. PMID:26241423. [DOI] [PubMed] [Google Scholar]
- 114.Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119(2 Pt 1):301–5. doi: 10.1097/AOG.0b013e318242032a. PMID:22270281. [DOI] [PubMed] [Google Scholar]
- 115.Martin S, Warner EL, Kirchhoff AC, Mooney R, Martel L, Kepka D. An electronic medical record alert intervention to improve HPV Vaccination Among Eligible Male College Students at a University Student Health Center. J Community Health. 2018. doi: 10.1007/s10900-018-0480-6. [DOI] [PubMed] [Google Scholar]
- 116.Clark SJ, Costello LE, Gebremariam A, Dombkowski KJ. A national survey of parent perspectives on use of patient portals for their children's health care. Appl Clin Inform. 2015;6(1):110–19. doi: 10.4338/ACI-2014-10-RA-0098. PMID:25848417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitayama K, Stockwell MS, Vawdrey DK, Pena O, Catallozzi M. Parent perspectives on the design of a personal online pediatric immunization record. Clin Pediatr (Phila). 2014;53(3):238–42. doi: 10.1177/0009922813506608. PMID:24137033. [DOI] [PubMed] [Google Scholar]
- 118.Wright A, Poon EG, Wald J, Feblowitz J, Pang JE, Schnipper JL, Grant RW, Gandhi TK, Volk LA, Bloom A, et al.. Randomized controlled trial of health maintenance reminders provided directly to patients through an electronic PHR. J Gen Intern Med. 2012;27(1):85–92. doi: 10.1007/s11606-011-1859-6. PMID:21904945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fierro L, Gaddis M, Kinney M, Reed J, Kirkwood B, Greene M, Robinson P, Koeppl P, Friedman D, Deloitte Consulting LLP.. Implementation pilot for two-dimensional (2D) vaccine barcode utilization: Summary report. Available at http://www.cdc.gov/vaccines/programs/iis/2d-vaccine-barcodes/downloads/pilot-summary.pdf. Accessed on February25, 2018.
- 120.Daily A, Kennedy ED, Fierro LA, Reed JH, Greene M, Williams WW, Evanson HV, Cox R, Koeppl P, Gerlach K. Evaluation of scanning 2D barcoded vaccines to improve data accuracy of vaccines administered. Vaccine. 2016;34(47):5802–7. doi: 10.1016/j.vaccine.2016.09.052. PMID:27742219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pereira JA, Quach S, Hamid JS, Quan SD, Diniz AJ, Van Exan R, Malawski J, Finkelstein M, Samanani S, Kwong JC, et al.. The integration of barcode scanning technology into Canadian public health immunization settings. Vaccine. 2014;32(23):2748–55. doi: 10.1016/j.vaccine.2013.11.015. PMID:24252700. [DOI] [PubMed] [Google Scholar]
- 122.World Health Organization Optimize Evidence Brief: A case for better immunization information systems. Available at http://www.who.int/immunization/programmes_systems/supply_chain/optimize/better_immunization_information_systems.pdf Accessed on February24, 2018.
- 123.Stockwell MS, Reed C, Vargas CY, Camargo S, Garretson AF, Alba LR, LaRussa P, Finelli L, Larson EL, Saiman L. MoSAIC: Mobile Surveillance for Acute Respiratory Infections and Influenza-Like Illness in the Community. Am J Epidemiol. 2014;180(12):1196–201. doi: 10.1093/aje/kwu303. PMID:25416593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smolinski MS, Crawley AW, Baltrusaitis K, Chunara R, Olsen JM, Wójcik O, Santillana M, Nguyen A, Brownstein JS. Flu near you: Crowdsourced symptom reporting spanning 2 influenza seasons. Am J Public Health. 2015;105(10):2124–30. doi: 10.2105/AJPH.2015.302696. PMID:26270299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharpe JD, Hopkins RS, Cook RL, Striley CW. Evaluating google, twitter, and wikipedia as tools for influenza surveillance using bayesian change point analysis: A comparative analysis. JMIR Public Health Surveill. 2016;2(2):e161. doi: 10.2196/publichealth.5901. PMID:27765731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klembczyk JJ, Jalalpour M, Levin S, Washington RE, Pines JM, Rothman RE, Dugas AF. Google flu trends spatial variability validated against emergency department influenza-related visits. J Med Internet Res. 2016;18(6):e175. doi: 10.2196/jmir.5585. PMID:27354313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simonsen L, Gog JR, Olson D, Viboud C. Infectious disease surveillance in the big data era: Towards faster and locally relevant systems. J Infect Dis. 2016;214(suppl_4):S380–5. doi: 10.1093/infdis/jiw376. PMID:28830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu FS, Hou S, Baltrusaitis K, Shah M, Leskovec J, Sosic R, Hawkins J, Brownstein J, Conidi G, Gunn J, et al.. Accurate influenza monitoring and forecasting using novel internet data streams: A case study in the boston metropolis. JMIR Public Health Surveill. 2018;4(1):e4. doi: 10.2196/publichealth.8950. PMID:29317382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kandula S, Hsu D, Shaman J. Subregional nowcasts of seasonal influenza using search trends. J Med Internet Res. 2017;19(11):e370. doi: 10.2196/jmir.7486. PMID:29109069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bragazzi NL, Barberis I, Rosselli R, Gianfredi V, Nucci D, Moretti M, Salvatori T, Martucci G, Martini M, et al.. How often people google for vaccination: Qualitative and quantitative insights from a systematic search of the web-based activities using Google Trends. Hum Vaccin Immunother. 2017;13(2):464–9. doi: 10.1080/21645515.2017.1264742. PMID:27983896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moro PL, Li R, Haber P, Weintraub E, Cano M. Surveillance systems and methods for monitoring the post-marketing safety of influenza vaccines at the Centers for Disease Control and Prevention. Expert Opin Drug Saf. 2016;15(9):1175–83. doi: 10.1080/14740338.2016.1194823. PMID:27268157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stockwell MS, Broder K, LaRussa P, Lewis P, Fernandez N, Sharma D, Barrett A, Sosa J, Vellozzi C, et al.. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr. 2014;168(3):211–9. doi: 10.1001/jamapediatrics.2013.4469. PMID:24395025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stockwell MS, Broder KR, Lewis P, Jakob K, Iqbal S, Fernandez N, Sharma D, Barrett A, LaRussa P. Assessing fever frequency after pediatric live attenuated versus inactivated influenza vaccination. J Pediatric Infect Dis Soc. 2017;6(3):e7–e14. PMID:27302328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stockwell MS, Cano M, Jakob K, Broder KR, Gyamfi-Bannerman C, Castaño PM, Lewis P, Barrett A, Museru OI, Castellanos O, et al.. Feasibility of text message influenza vaccine safety monitoring during pregnancy. Am J Prev Med. 2017;53(3):282–9. doi: 10.1016/j.amepre.2017.03.014. PMID:28495223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stockwell MS, Marchant CD, Wodi AP, et al.. A multi-site feasibility study to assess fever and wheezing in children after influenza vaccines using text messaging. Vaccine. 2017;35(50):6941–8. doi: 10.1016/j.vaccine.2017.10.073. PMID:29089191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pillsbury A, Cashman P, Leeb A, Regan A, Westphal D, Snelling T, Blyth C, Crawford N, Wood N, Macartney K, et al.. Real-time safety surveillance of seasonal influenza vaccines in children, Australia, 2015. Euro Surveill. 2015;20(43). doi: 10.2807/1560-7917.ES.2015.20.43.30050. PMID:26536867. [DOI] [PubMed] [Google Scholar]
- 137.Pillsbury A, Quinn H, Cashman P, Leeb A, Macartney K. AusVaxSafety c. Active SMS-based influenza vaccine safety surveillance in Australian children. Vaccine. 2017;35(51):7101–6. doi: 10.1016/j.vaccine.2017.10.091. PMID:29128379. [DOI] [PubMed] [Google Scholar]
- 138.Regan AK, Blyth CC, Tracey L, Mak DB, Richmond PC, Effler PV. Comparison of text-messaging to voice telephone interviews for active surveillance of adverse events following immunisation. Vaccine. 2015;33(31):3689–94. doi: 10.1016/j.vaccine.2015.06.022. PMID:26079616. [DOI] [PubMed] [Google Scholar]
- 139.Regan AK, Blyth CC, Mak DB, Richmond PC, Effler PV. Using SMS to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust N Z J Obstet Gynaecol. 2014;54(6):522–8. doi: 10.1111/ajo.12266. PMID:25306915. [DOI] [PubMed] [Google Scholar]
- 140.Cashman P, Moberley S, Dalton C, Stephenson J, Elvidge E, Butler M, Durrheim DN, et al.. Vaxtracker: Active on-line surveillance for adverse events following inactivated influenza vaccine in children. Vaccine. 2014;32(42):5503–8. doi: 10.1016/j.vaccine.2014.07.061. PMID:25077424. [DOI] [PubMed] [Google Scholar]
- 141.Centers for Disease Control and Prevention Immunization Information Systems (IIS). Available at https://www.cdc.gov/vaccines/programs/iis/index.html. Accessed on February19, 2018.
- 142.American Academy of Pediatrics Immunization Information Systems. Available at https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/immunizations/Practice-Management/Pages/immunization-information-systems.aspx. Accessed on February19, 2018.
- 143.Murthy N, Rodgers L, Pabst L, Fiebelkorn AP, Ng T. Progress in childhood vaccination data in immunization information systems – United States, 2013–2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1178–81. doi: 10.15585/mmwr.mm6643a4. PMID:29095809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Centers for Disease Control and Prevention 2016 IISAR Data Participation Rates. Available at https://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/2016-data.html#adolescent Accessed on April20, 2018.
- 145.Groom H, Hopkins DP, Pabst LJ, Murphy Morgan J, Patel M, Calonge N, Coyle R, Dombkowski K, Groom AV, Kurilo MB, et al.. Immunization information systems to increase vaccination rates: A community guide systematic review. J Public Health Manag Pract. 2015;21(3):227–48. doi: 10.1097/PHH.0000000000000069. PMID:24912082. [DOI] [PubMed] [Google Scholar]
- 146.Hopkins DP, Task CPS. Recommendation for use of immunization information systems to increase vaccination rates. Journal of Public Health Management and Practice. 2015;21(3):249–52. PMID:24912083. [DOI] [PubMed] [Google Scholar]
- 147.Centers for Disease Control and Prevention 2018–2020 Immunization Information System (IIS) Strategic plan. Available at https://www.cdc.gov/vaccines/programs/iis/strategic-plan/iis-2018-2020.html. Accessed on February19, 2018.
- 148.Merrill J, Phillips A, Keeling J, Kaushal R, Senathirajah Y. Effects of automated immunization registry reporting via an electronic health record deployed in community practice settings. Appl Clin Inform. 2013;4(2):267–75. doi: 10.4338/ACI-2013-02-CR-0009. PMID:23874363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dombkowski KJ, Clark SJ. Redefining meaningful use: Achieving interoperability with immunization registries. Am J Prev Med. 2012;42(4):e33–5. doi: 10.1016/j.amepre.2012.01.009. PMID:22424260. [DOI] [PubMed] [Google Scholar]
- 150.Stockwell MS, Natarajan K, Ramakrishnan R, Holleran S, Forney K, Aponte A, Vawdrey DK. Immunization data exchange with electronic health records. Pediatrics. 2016;137(6). doi: 10.1542/peds.2015-4335.. [DOI] [PubMed] [Google Scholar]
- 151.Tozzi AE, Gesualdo F, D'Ambrosio A, Pandolfi E, Agricola E, Lopalco P. Can digital tools be used for improving immunization programs? Front Public Health. 2016;4:36. doi: 10.3389/fpubh.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]


