ABSTRACT
Archaeosomes are liposomes comprised of ether lipids derived from various archaea. Unlike conventional ester-linked liposomes, archaeosomes exhibit high pH and thermal stability. As adjuvants, archaeosomes can induce robust, long-lasting humoral and cell-mediated immune responses and enhance protection in murine models of infectious disease and cancer. Archaeosomes constituted with total polar lipids (TPL) of various archaea are relatively complex, comprising >10 different lipid compounds. Archaeosomes can be constituted with semi-synthetic glycerolipids built on ether-linked isoprenoid phytanyl cores with varied synthetic glycol- and amino-head groups. However, such semi-synthetic archaeosomes involve many synthetic steps to arrive at the final desired glycolipid composition. We have developed a novel archaeosome formulation comprising a sulfated saccharide group covalently linked to the free sn-1 hydroxyl backbone of an archaeal core lipid (sulfated S-lactosylarchaeol, SLA) mixed with uncharged glycolipid (lactosylarchaeol, LA). This new class of adjuvants can be easily synthesized and retains strong immunostimulatory activity for induction of cell-mediated immunity following systemic immunization. Herein, we demonstrate the safety of SLA/LA archaeosomes following intramuscular injection to mice and evaluate the immunogenicity, in vivo distribution and cellular uptake of antigen (ovalbumin) encapsulated into SLA/LA archaeosomes. Overall, we have found that semi-synthetic sulfated glycolipid archaeosomes are a safe and effective novel class of adjuvants capable of inducing strong antigen-specific immune responses in mice and protection against subsequent B16 melanoma tumor challenge. A key step in their mechanism of action appears to be the recruitment of immune cells to the injection site and the subsequent trafficking of antigen to local draining lymph nodes.
KEYWORDS: Adjuvants, adjuvants, Archaeosomes, delivery, Glycolipid, immune modulators, Safety, Vaccine, vaccinology
Introduction
Vaccines represent one of the most efficacious and cost-effective means of combating mortality and morbidity worldwide. However, while traditional whole killed or attenuated vaccines are usually sufficiently immunogenic on their own, most of the recombinant or synthetic antigens used in modern day vaccines are far less immunogenic and require the addition of immunostimulatory adjuvants to induce protective immunity. While aluminum salts have been approved for many years, other adjuvants, such as Monophosphoryl Lipid A (MPL) or the squalene-based oil-in-water emulsions MF59 and ASO3, have more recently been approved for use in human vaccines and hopefully this will facilitate the approval of other adjuvants for unmet medical needs.1 The identification and development of novel adjuvants with different immunostimulatory properties would aid in the design of efficacious vaccines for existing and emerging diseases, in particular for those against chronic infectious disease or cancer for which strong cell-mediated immune responses are required. However, in addition to assessing a novel adjuvant's immunostimulatory effects, it is equally important to evaluate its safety profile. As such, the dose and type of adjuvant used must be sufficient to adequately enhance the immune response to the administered antigen, while at the same time minimizing the risk of local or systemic toxicity and injection site reactions.
One potential novel adjuvant is archaeosomes, a term used to describe liposomes composed of total polar lipids (TPL) or semi-synthetic glycerolipids derived from various archaea.2 Unlike conventional liposomes that are composed of eukaryotic lipids possessing ester linkages between their carbon chains and glycerol backbone, archaeosomes are comprised of archaeal lipids containing ether linkages which provide them with a unique set of properties. For example, archaeosomes exhibit high thermal and pH stability, low proton permeability, low fusion rates translating to cold-chain stability of formulation and strong immunostimulatory effects when compared to conventional ester-phospholipid liposomes. The immunogenicity of a broad range of antigens has been shown to be greatly enhanced when encapsulated in archaeosomes resulting in strong humoral and cell-mediated responses and protection from pathogens such as Listeria monocytogenes as well as solid and metastatic tumors.3,4
Traditional TPL archaeosome formulations are however relatively complex and have their principal components (i.e., ether glycolipids) derived from archaea. In an effort to simplify this, Sprott et al., developed a formulation composed of various semi-synthetic archaeol-based glycolipids which was shown to induce strong, robust humoral and cell-mediated immune responses.5 However, although these semi-synthetic glycolipid archaeosomes are more easily and reproducibly produced than traditional TPL archaeosomes formulations, they are still relatively complex to synthesize and typically require a combination of several glycolipids to produce a stable, uniform-sized liposome formulation. Recently, our group developed a more simplified archaeosome formulation, composed of a sulfated saccharide group covalently linked to the free sn-1hydroxyl backbone of an archaeal core lipid (sulfated S-lactosylarchaeol, SLA) either alone or mixed with uncharged glycolipid (lactosylarchaeol, LA) (see Fig. 1), which was shown to induce cell-mediated immunity to encapsulated antigens (ovalbumin or melanoma associated tyrosinase-related protein 2 [TRP-2]).6 This formulation offers advantages with regards to consistency of manufacturing and ease of synthesis while retaining the strong adjuvanticity associated with archaeosomal formulations. While several reports have shown the safety and tolerability of archaeosomes composed of TPL when administered by different routes (subcutaneous, intranasal),7,8 this has not yet been evaluated for SLA/LA archaeosomes.
Figure 1.
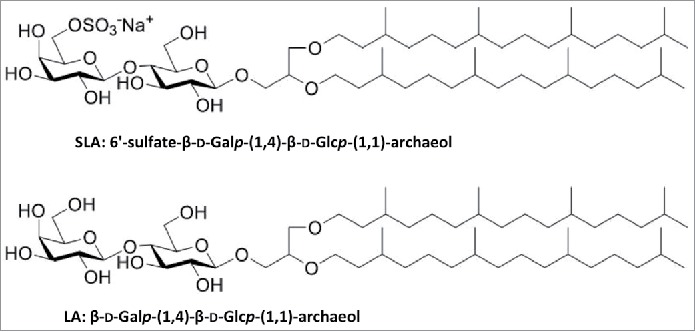
Chemical structure of sulfated S-lactosylarchaeol (SLA) and (lactosylarchaeol (LA).
Herein, we have evaluated the safety and tolerability of the novel SLA/LA archaeosome formulation after an intramuscular injection to mice. We also evaluated the ability of SLA/LA to enhance immune responses to a model antigen (ovalbumin) in a B16 tumor model as well as its effects on antigen distribution, cellular uptake and trafficking of immune cells.
Results
Safety of SLA/LA archaeosomes following intramuscular injection to mice
Mice received either a single dose of 1 or 10 mg of archaeosomes composed of sulfated archaeal glycolipids by intramuscular (i.m.) injection and their safety profile was evaluated. Dose levels of archaeosomes used for vaccination studies in mice are typically between 0.3 – 1.5 mg,5,6,9 and therefore 1 mg was selected to represent a typical vaccine dose level, whereas 10 mg of SLA/LA allowed for the evaluation of safety parameters at lipid levels approximately 10-fold higher than normally administered in vivo. No significant differences were seen between male and female mice, and as such, data from female and male mice receiving similar treatments were combined for presentation purposes.
Local reactogenicity
Mild swelling (grade 1) was observed in the tibialis anterior (T.A) of all mice injected with 10 mg of SLA/LA up to 7 days following injection, but was not observed in any of the mice that received either 1 mg SLA/LA or PBS (Table 1). Treatment did not impact the injected muscle's range of motion, with mice in all groups exhibiting a normal gait with no signs of limping or impaired movement during the observation period. In addition, no signs of erythema or skin thickening, bleeding or ulceration were observed at the injection site in either control animals or with either dose of SLA/LA (1 or 10 mg).
Table 1.
Reactogenicity in left tibialis anterior following i.m. injection of SLA/LA. Local reactions following i.m. injection were assigned a grade (0–3) based on the following criteria. No reaction (0): no swelling, normal skin; mild(1): slightly reddish and/or swollen muscle; moderate(2): red thickened skin with slightly open wound; severe(3): ulceration or abscess. N = 6–12/group.
| SLA/LA Dose (mg) |
|
|
|
|
Day |
|
|
|
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Clinical observations
Overall, SLA/LA archaeosomes were well-tolerated following a single i.m. administration to mice. There was no mortality in any of the test groups over course of the study. A slight decrease in body weight, 4.3 ± 2% and 6.2 ± 2.4%, was detected 1 day following administration of 1 and 10 mg of archaeosomes, respectively (Fig. 2A). However, this was not significantly different to that observed in control animals injected with PBS alone (weight loss of 4.3 ± 3.1%), and was transient with all animals regaining weight during the following 24 hours and throughout the course of the study. Therefore, these minor weight changes were attributed to the mice being subjected to multiple study-related procedures on Day 0 (i.e. anesthesia, bleeds). A similar trend was observed with body temperature, with decreases of 1.9 ±1.5, 1.6 ±1.3 and 1.4 ±1% measured on Day 1 in the 0, 1 and 10 mg SLA/LA dose groups, respectively (Fig. 2B). There were no adverse clinical signs (i.e. piloerection, reduced mobility/activity or altered posture, abnormal behavior, respiration, dehydration) observed in any of the study animals during the course of the study.
Figure 2.
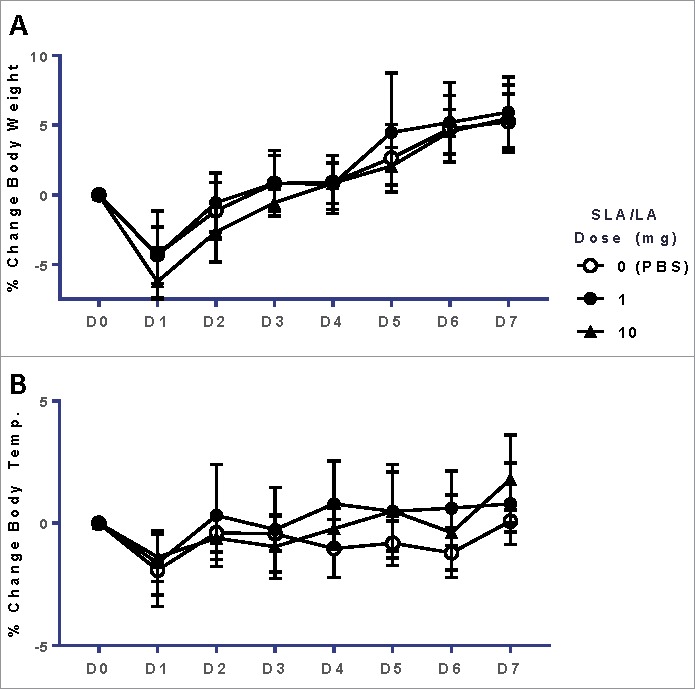
Body weight & temperature of mice after i.m. injection of SLA/LA. C57BL/6 mice were injected i.m. with 0, 1 or 10 mg SLA/LA. The percent change (mean ± standard deviation) in body weight (Panel A) and temperature (Panel B) were plotted over the course of the study (n = 12 at Days 0 and 1, at which point 6 mice were euthanized for sample collection).
Hematology and blood biochemistry
The levels of multiple chemical analytes (alkaline phosphatase, ALP; alanine aminotransferase, ALT; bilirubin; blood urea nitrogen, BUN; calcium; glucose; phosphorus; protein), hematological parameters (hematocrit; hemoglobin; mean corpuscular hemoglobin, MCH; mean corpuscular hemoglobin concentration, MCHC; mean corpuscular volume, MCV; red blood cell, RBC) and different types of white blood cell were measured in the blood of mice 1 or 7 days following administration of SLA/LA and compared to mice injected with PBS (Table 2). There were no significant differences between the 3 groups in the levels of any of the blood chemistry or hematological parameters, including those linked to hepatic/renal function and general metabolism (e.g. BUN, ALT and glucose). Significant differences were seen on Day 1 in the levels of two specific white blood cell populations, i.e. neutrophils and lymphocytes, in mice that received either dose level of SLA/LA. Average neutrophil levels in the 1 and 10 mg SLA/LA groups were measured at 0.75 × 109 cells/mL, ∼ 3-fold higher than in the PBS group (0.21 × 109 cells/mL, p < 0.05). Conversely, lymphocyte levels were significantly lower in the 10 mg SLA/LA group than in the animals injected with PBS (2.08 × 109 vs. 3.55 × 109 cells/mL, p < 0.05). This was not of a concern as all levels were still within the range reported for healthy mice,10 and the changes were transient since all mice had equivalent white blood cell differentials on day 7. There were no significant differences in the levels of other white blood cell types, i.e. monocytes, eosinophils, basophils, at either time-point.
Table 2.
Blood chemistry & hematology in SLA/LA treated mice. Multiple parameters were measured in mouse blood 1 or 7 days following i.m. injection of SLA/LA. Abbreviations: ALP (Alkaline phosphatase), ALT: (Alanine aminotransferase), BUN: (Blood urea nitrogen), WBC: (White blood cell), MCH: (Mean corpuscular hemoglobin), MCHC: (Mean corpuscular hemoglobin concentration), MCV: (Mean corpuscular volume), RBC: (Red blood cell). N = 4–6/group; *: p<0.05.
| Day 1 |
Day 7 |
|||||
|---|---|---|---|---|---|---|
| Parameter | 0 mg | 1 mg | 10 mg | 0 mg | 1 mg | 10 mg |
| Blood Chemistry | ||||||
| ALP (U/L) | 102.5 (89, 152) | 89 (71, 135) | 99.5 (69, 133) | 123 (79, 154) | 84 (75, 287) | 98 (71, 171) |
| ALT (SGPT) (U/L) | 26.5 (12, 113) | 22 (15, 31) | 25 (18, 73) | 25 (19, 32) | 29 (21, 62) | 22.5 (19, 47) |
| Bilirubin (µmol/L) | 2.1 (1.7, 14.2) | 2.7 (1.7, 7.2) | 1.7 (1.7, 2.4) | 1.9 (0.4, 2.7) | 2.7 (2, 3.5) | 2.8 (1.9, 5.3) |
| BUN(mmol/L) | 7.9 (5.1, 11.3) | 6.9 (5.1, 7.8) | 7.2 (6.3, 8.3) | 7.4 (4.8, 10.1) | 8.2 (6.1, 15.1) | 6.9 (4.9, 8.1) |
| Calcium (mmol/L) | 2.6 (2.2, 3.1) | 2.6 (2.4, 2.8) | 2.5 (2.5, 2.8) | 2.3 (2, 2.3) | 2.4 (1.8, 4.4) | 2.4 (2.1, 2.5) |
| Glucose (mmol/L) | 6.8 (3.1, 10.8) | 7.9 (5.4, 12) | 7.3 (5.3, 10.2) | 10.4 (8.8, 13.6) | 10.8 (9.1, 21.6) | 11 (7.4, 12) |
| Phosphorus (mmol/L) | 2.7 (2.2, 6.7) | 2.7 (2.1, 4.6) | 2.2 (2, 2.6) | 2 (1.8, 2.7) | 1.9 (1.4, 3.8) | 1.9 (1.6, 2) |
| Protein (g/L) | 50.5 (47, 78) | 49.5 (47, 54) | 50.5 (47, 56) | 46 (42, 46) | 49 (38, 97) | 45.5 (43, 48) |
| White Blood Cell Differential (1E9 cells/L) | ||||||
| WBC | 3.9 (2.4, 5.1) | 4.55 (2.7, 5.7) | 3.1 (1.6, 3.5) | 3.2 (2.1, 3.8) | 2.3 (1, 4.7) | 2 (1, 2.9) |
| Neutrophil | 0.21 (0.07, 0.27) | 0.75 (0.28, 1.33)* | 0.75 (0.3, 1.05)* | 0.45 (0.11, 0.64) | 0.39 (0.12, 0.94) | 0.33 (0.12, 0.61) |
| Eosinophil | 0.11 (0.05, 0.18) | 0.15 (0.11, 0.34) | 0.1 (0, 0.21) | 0.06 (0.02, 0.08) | 0.04 (0.02, 0.09) | 0.03 (0.02, 0.12) |
| Basophil | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Lymphocyte | 3.55 (2.04, 4.69) | 3.4 (2.24, 4.22) | 2.08 (1.23, 2.61)* | 2.37 (1.97, 3.34) | 1.82 (0.67, 3.57) | 1.59 (0.85, 2.38) |
| Monocyte | 0.07 (0.03, 0.1) | 0.08 (0.03, 0.11) | 0.03 (0.02, 0.04) | 0.06 (0, 0.13) | 0.07 (0.02, 0.12) | 0.02 (0, 0.05) |
| Hematology | ||||||
| Hematocrit (%) | 33 (31, 35) | 37 (32, 40) | 33 (32, 39) | 43 (37, 45) | 38 (37, 42) | 40 (31, 43) |
| Hemoglobin (g/L) | 100 (90, 101) | 110 (94, 118) | 103.5 (95, 118) | 134 (116, 138) | 128 (114, 133) | 126 (99, 134) |
| MCH (pg) | 15.4 (15.2, 15.5) | 15.35 (14.9, 15.9) | 15.5 (15.2, 15.8) | 15.4 (15.4, 15.7) | 15.7 (15.4, 16.4) | 15. 5 (14.9, 16.6) |
| MCHC (g/L) | 293.5 (291, 318) | 292 (287, 325) | 311 (296, 324) | 311 (309, 315) | 316 (312, 334) | 313.5 (304, 332) |
| MCV (fL) | 52.5 (48, 53) | 52 (49, 54) | 50.5 (47, 52) | 50 (49, 50) | 49 (48.3, 50) | 50 (48, 51) |
| RBC (1E12 cells/L) | 6.5 (5.8, 6.7) | 7.1 (6, 7.8) | 6.7 (6.1, 7.5) | 8.7 (7.5, 9) | 8 (7.3, 8.6) | 8.1 (6.2, 8.5) |
Hemolytic activity
The hemolytic potential of empty SLA/LA archaeosomes was determined on mouse and rabbit erythrocytes ex vivo. There was no hemolysis observed in either mouse or rabbit blood cells at SLA/LA concentrations of 0.25 – 2 mg/mL, with equivalent optical density (OD) readings obtained as with buffer alone (Fig. 3). As a positive control, erythrocytes were incubated with Triton-X, resulting in OD540 readings of 1.4 and 2.5 with rabbit and mouse cells, respectively.
Figure 3.
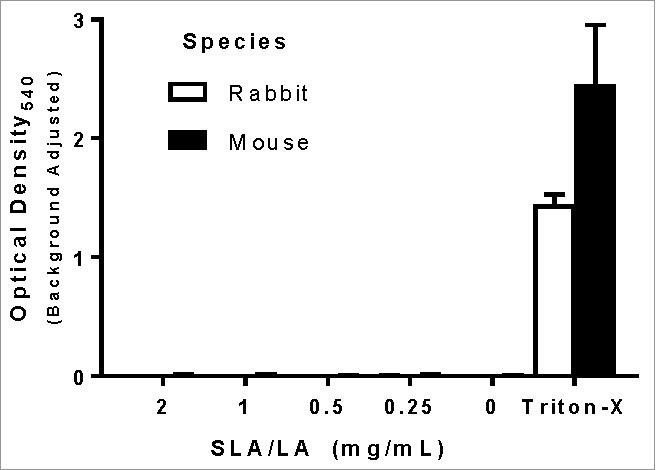
Hemolytic activity of SLA/LA. Erythrocytes from C57BL/6 mice or New Zealand white rabbits were incubated with 0.2–2 mg/mL SLA/LA, buffer alone (0 mg/mL) or Triton-X (positive control). The level of hemoglobin release indicating cell lysis was determined through the measurement of the OD540 in the cellular supernatant. Values obtained with buffer alone were subtracted from measured values (n = 3). Bars represent group means ± standard deviation.
Histological evaluation
To further evaluate the safety profile of SLA/LA, a histopathological assessment was conducted on a panel of tissues collected 1 or 7 days following i.m. administration of high dose (10 mg) SLA/LA or PBS. The bladder, brain, gonads, heart, kidney, liver, lung, spleen and thymus were collected at each time point. In addition, the injected left tibialis anterior (T.A.) muscle, draining lymph nodes (inguinal & popliteal) and one non-draining lymph node (axial) were also collected for evaluation. Haematoxylin/Eosin (H&E) stained slides were prepared and evaluated. No abnormal changes were observed in all tissues examined, except for the left T.A (injection site). Mice injected with 10 mg SLA/LA demonstrated moderate to severe interstitial edema with degeneration and necrosis of muscle fibers in the left T.A. at Day 1 (Fig. 4 & Table 3). This was accompanied by moderate inflammatory cell infiltration with mononuclear cells as the predominant cell type along with small numbers of intact or degenerated neutrophils. At Day 7, evidence of moderate tissue damage and subacute inflammation are still evident at the injection site.
Figure 4.
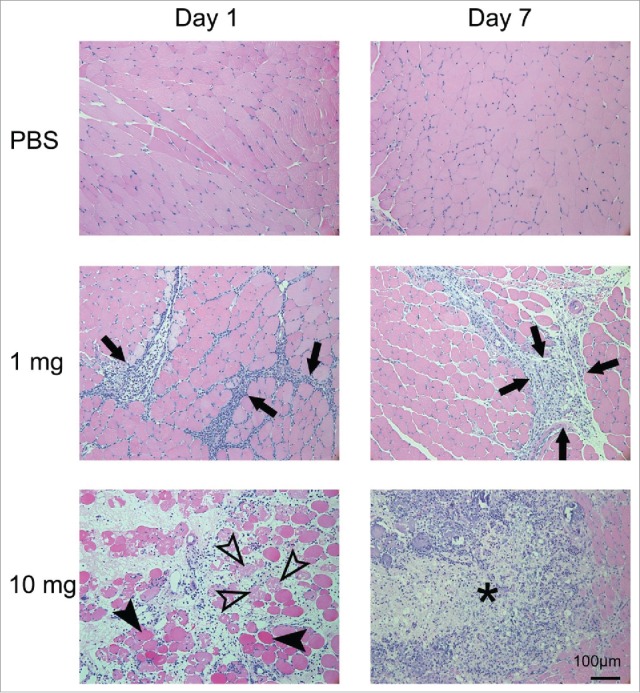
Histopathology of the left tibialis anterior muscle after injection of SLA/LA. C57BL/6 mice (n = 6/group) were injected i.m. with 0, 1 or 10 mg SLA/LA. Left T.A. muscles were collected and fixed 1 or 7 days following injection. H&E stained slides were prepared for each muscle and evaluated by a pathologist. A representative image of a single mouse per group imaged at the time points indicated is shown. Arrows: infiltration of inflammatory cells; open arrowheads: necrotic muscle cells; closed arrowheads: degenerated muscle cells; *: foci of acute to subacute inflammation.
Table 3.
Tissue damage & inflammation in left tibialis anterior following i.m. injection of SLA/LA. Degree of inflammation and immune cell infiltration were assessed on H&E stained slides of the left T.A., and assigned a grade (0–4) with 0 and 4 being the least and most severe, respectively. Standard deviation is indicated in parentheses; N = 6/group.
| Inflammation |
||||
|---|---|---|---|---|
| Time point (Day) | SLA/LA (mg) | Acute | Subacute to chronic | Tissue Damage |
| 1 | 0 | 0 (0) | 0.2 (0.4) | 0.2 (0.4) |
| 1 | 2.2 (0.8) | 0.3 (0.5) | 1.5 (0.5) | |
| 10 | 2.5 (0.5) | 0.5 (0.5) | 2.5 (0.8) | |
| 7 | 0 | 0 (0) | 0 (0) | 0 (0) |
| 1 | 2.2 (0.8) | 1.8 (0.4) | 1.3 (0.5) | |
| 10 | 3.5 (0.5) | 0.7 (0.5) | 3 (0.9) | |
To better understand the effect of lower doses (those likely to be used in a vaccine formulation) of SLA/LA at the injection site, H&E slides from the left T.A. of mice injected with 1 mg SLA/LA were also prepared and evaluated. As expected, the histopathological changes in these mice were less severe and more transient than those observed in the 10 mg SLA/LA group. At Day 1, inflammatory cell infiltration was seen at the injection site, but the degeneration and necrosis of the muscle fibers was more limited and milder with 1 mg compared to 10 mg of SLA/LA (grade 1.5 ± 0.5 vs. 2.5 ± 0.8, p < 0.05). By Day 7, a shift from an acute inflammatory response to a chronic granulomatous reaction consisting of fibroblasts, connective tissues and lymphocyte infiltration was observed. This shift was more evident with 1 mg compared to 10 mg SLA/LA as demonstrated by higher level of acute inflammation at Day 7 with the higher dose (10 mg), but a higher level of subacute to chronic inflammation at Day 7 with the lower dose (1 mg), suggesting a faster resolution of local inflammatory responses with lower dose SLA/LA. Necrotic damage to muscle fibers has also started to resolve and is significantly lower than seen in the 10 mg SLA/LA group at this point (grade 1.3 ± 0.5 vs. 3 ± 0.8, p < 0.01).
Evaluation of cytokines/chemokines/growth factors inducted by SLA/LA archaeosomes
To determine whether SLA/LA archaeosomes alone had any effects on the murine inflammatory or immune system, the circulating levels of 32 cytokines/chemokines/growth factors (see Materials & Methods) were measured at multiple time-points (3, 6, 24 and 168 hours) in the serum of mice following administration of SLA/LA archaeosomes. A time- and dose-dependent increase in the inflammatory growth factor granulocyte colony-stimulating factor (G-CSF) was observed, with significantly higher levels of G-CSF seen in the SLA/LA groups at 6 & 24 hours post-administration (p < 0.05 & p < 0.0001, respectively; Fig. 5A). Levels of the pro-inflammatory cytokine IL-6 were significantly higher with SLA/LA treatment at 6 hours post administration (p < 0.0001; Fig. 5B). Finally, at 7 days post administration, 10 mg of SLA/LA induced higher levels of IP-10 and MIP-1β (p < 0.05 & p < 0.001, respectively; Fig. 5C & Fig. 5D), both of which have chemoattractant properties. No significant differences were measured in the levels of any other cytokines/chemokines/growth factors at any of the time points tested.
Figure 5.

Cytokine/Chemokine levels in mice after i.m. injection of SLA/LA. C57BL/6 mice (n = 6/group) were injected i.m. with 0, 1 or 10 mg SLA/LA. Serum was collected at 3, 6, 24 or 168 hours post injection and the levels of 32 immune-related proteins were measured. Serum levels of G-CSF (Panel A), IL-6 (Panel B), IP-10 (Panel C) and MIP-1β (Panel D) are shown over time. Cytokine/chemokine levels were assessed in protein extracts derived from T.A. muscles 6 hours following injection of 0.25 mg SLA/LA or PBS (Panel E). *, **, ***, and **** represent p <0.05, 0.01, 0.001 and 0.0001, respectively. Bars represent group geometric means ± standard deviation.
To confirm that the cytokines/chemokines measured in the serum were indeed produced at the injection site, protein lysates were collected from muscles six hours after the injection of 0.25 mg of empty SLA/LA archaeosomes. Based on the profile seen in the serum, a panel of 11 cytokines/chemokines (G-CSF, GM-CSF, M-CSF, IL-6, IP-10, KC, MCP-1, MIP-1α, MIP-1β, MIP-2 and RANTES) was selected for measurement. Levels were compared to those in the contralateral right T.A. injected with PBS. Significant increases were observed with multiple cytokines/chemokines (Fig. 5E), confirming that the SLA/LA was mediating a local inflammatory reaction.
Efficacy of SLA/LA archaeosomes as a vaccine adjuvant
The adjuvant activity of SLA/LA archaeol lipids was confirmed through the immunization of mice with ovalbumin (OVA), either free or encapsulated in archaeosomes (OVA-SLA/LA). High levels of OVA-specific IgG were induced by OVA-SLA/LA, while only background levels were detected in animals receiving OVA alone (p < 0.0001; Fig. 6A). In addition, OVA-specific CD8+ T-cells recognizing the OVA257–264 SIINFEKL epitope were significantly increased following two immunizations with OVA-SLA/LA compared to OVA alone (p < 0.0001; Fig. 6B). The functionality of these vaccine-induced immune responses was evaluated in a tumor challenge model. At 4 weeks following last vaccination, B16 melanoma cells expressing OVA were implanted subcutaneously and tumor growth monitored over time. Animal survival was significantly improved in mice immunized with OVA-SLA/LA archaeosomes (median survival of 37 days) when compared to either control animals (median survival of 17.5 days) or to animals immunized with OVA alone (median survival of 22 days) (p < 0.05); Fig. 6C).
Figure 6.
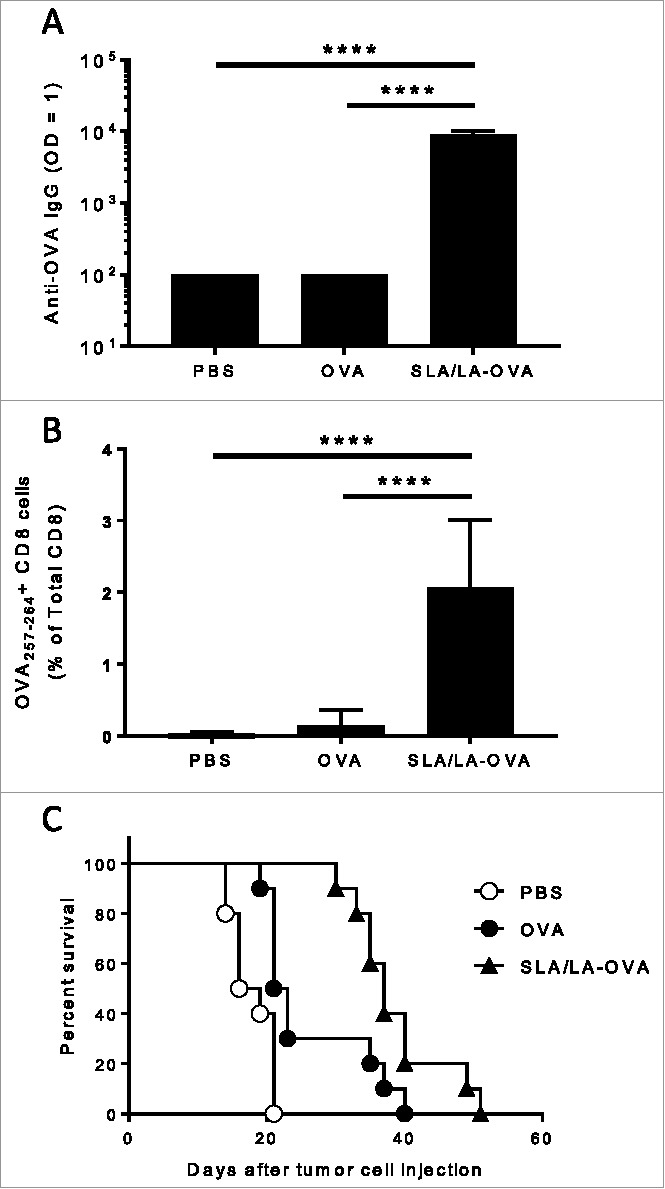
Immunogenicity of SLA/LA-OVA. C57BL/6 mice (n = 10/group) were injected i.m. on Days 0 and 21 with PBS, ovalbumin (20 µg) or OVA-SLA/LA (20 µg; based on antigen dose). Animals were bled on Day 35 and serum analyzed for anti-OVA IgG Abs by ELISA (Panel A; group geometric means ± standard deviation). In addition, the levels of OVA-specific CD8+ cells were assessed by tetramer analysis in the whole blood of mice collected on Day 28 (Panel B; group means ± standard deviation). Mouse survival was tracked after tumor challenge with of 106 B16-ova cells on Day 50 (Panel C). **** represents p <0.0001.
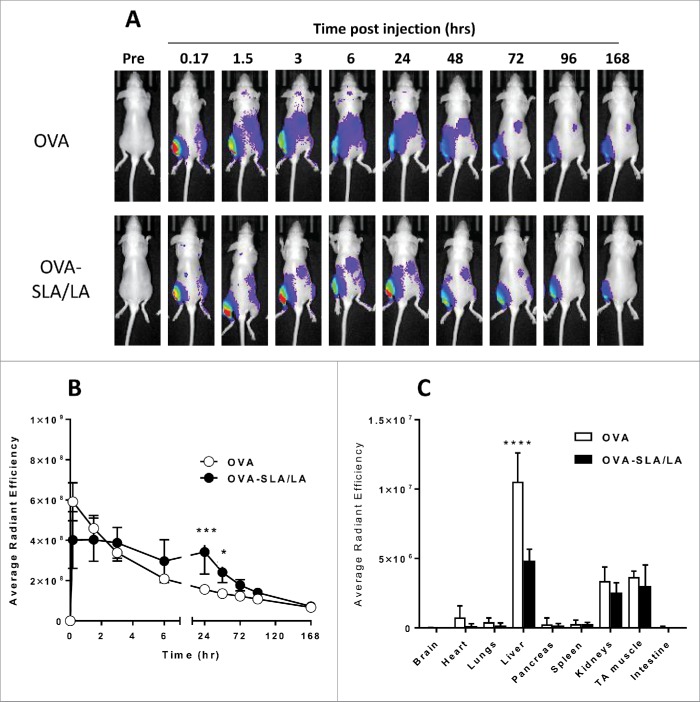
Effect of SLA/LA archaeosomes on biodistribution of encapsulated OVA
The effect of encapsulation in SLA/LA archaeosomes on the biodistribution of CF770-labeled OVA was measured at various time-points following i.m. injection. Whole body in vivo imaging was performed following vaccination with CF770-labeled OVA or OVA-SLA/LA (Fig. 7A). Encapsulation of CF770-labeled OVA in SLA/LA archaeosomes resulted in significantly higher fluorescence intensity at the injected left T.A. muscle at 24 and 48 hours post injection (Fig. 7B), suggesting that SLA/LA archaeosomes mediate a depot effect on the antigen. By 72 hours post injection, the fluorescence levels at the injection site with OVA alone or OVA-SLA/LA were no longer significantly different. At 7 days following injection of CF770-labeled OVA, various organs and tissues were collected and the levels of CF770-labeled OVA were measured ex vivo. The levels of CF770-labeled OVA were highest in the liver, kidneys and left T.A., with 2-fold higher levels of OVA measured in the liver of animals injected with free OVA (p < 0.0001; Fig. 7C). This suggests a higher degree of hepatic clearance in these animals. Fluorescence was also detected in lymph nodes, including the draining inguinal and popliteal, although no significant differences were observed between the different groups (data not shown).
Figure 7.
Biodistribution of CF770-OVA. C57BL/6 albino mice (n = 4/group) were injected i.m. on Day 0 with CF770-labeled ovalbumin (20 µg; alone or encapsulated in SLA/LA archaeosomes). Whole body imaging was conducted to localize CF770-labeled OVA at various time points. A representative image series of a single mouse per group imaged at the time points indicated is shown (Panel A). From the whole body imaging, the levels of fluorescence were measured at the injection site (left TA) over time (Panel B; group means ± standard deviation). One week following vaccination, animals were perfused with heparinized saline, and various tissues collected for ex vivo imaging. The fluorescent signals from each organ were quantified (Panel C; group means ± standard deviation). *, ***, and **** represent p <0.05, 0.001 and 0.0001, respectively.
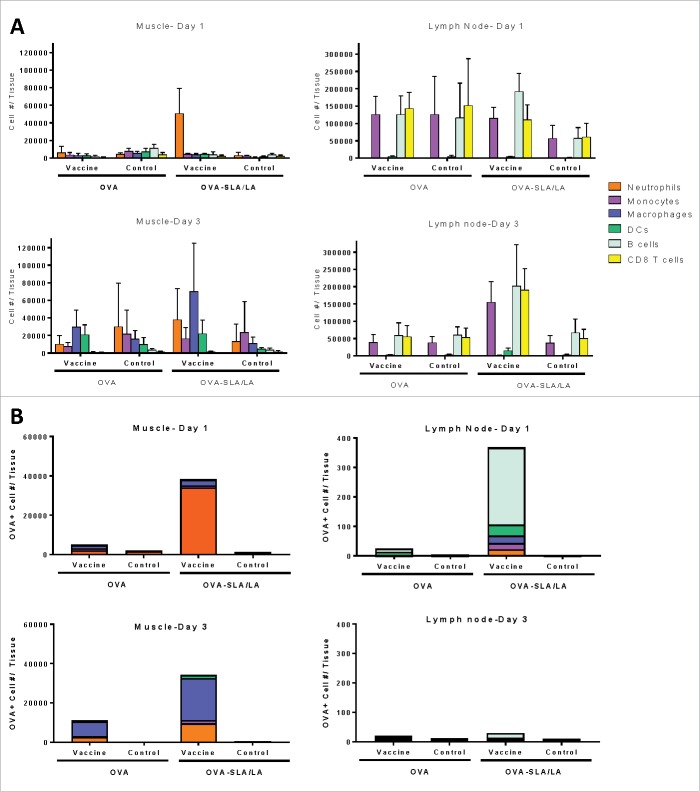
Effect of SLA/LA Archaeosomes on cellular uptake and trafficking of encapsulated OVA
To determine whether encapsulation within archaeosomes would impact antigen uptake by immune cells at the injection site and/or recruitment of immune cells into the injected muscle, we performed multi-color flow cytometry to identify the level and composition of immune cells in the muscle and draining lymph nodes. To enable identification of antigen-positive cells, a fluorescent dye was conjugated to the OVA antigen prior to encapsulation in SLA/LA. The left T.A. muscle was injected with Alexa Fluor® 647 (AF647)-labeled OVA alone or OVA-SLA/LA archaeosomes, while the right T.A. muscle of the same animal received PBS and served as a control. We also evaluated local draining lymph nodes (i.e. inguinal & popliteal) to determine whether cell trafficking would be effected by encapsulation of OVA within archaeosomes. The SLA/LA formulation induced immune cell infiltration into the muscle, with a significantly higher number of neutrophils detected one day following injection as compared to OVA alone (mean cell number ± SD of 50593 ± 28750 vs. 6134 ± 6887 cells per muscle, respectively; p < 0.01; Fig. 8A). By Day 3, increased levels of other immune cell populations, namely macrophages and dendritic cells, appear in the OVA-SLA/LA injected muscle, but the differences did not reach a level of statistical significance. In the draining inguinal lymph node, the major cell populations consisted of CD8+ T cells, B cells and monocytes. While no significant differences were observed on Day 1, the levels of all tested immune cell populations were significantly higher in the lymph node that drained from the OVA-SLA/LA injected muscle than in the control lymph nodes on Day 3 (p < 0.05 for B cells & macrophages, p < 0.01 for DCs & neutrophils and p < 0.001 for CD8 T cells and monocytes). Results in the popliteal lymph nodes were similar to those observed in the inguinal lymph nodes (data not shown).
Figure 8.
Immune cell recruitment and antigen uptake induced by SLA/LA. C57BL/6 mice (n = 4–5/group) were injected i.m. in the left tibialis anterior (TA) muscle on Day 0 with AF647-labeled OVA (20 µg; alone or encapsulated in SLA/LA archaeosomes). The right TA of all animals was injected with PBS for use as a negative control. The TAs and draining inguinal lymph nodes (right and left) were collected on Days 1 or 3 following injection. Immune cells were isolated from each tissue, counted and fluorescently stained with an antibody cocktail to differentiate different immune cell populations: B cells, CD8+ T cells, dendritic cells (DC), macrophages, monocytes and neutrophils. The number of cells from the above cell populations was determined per tissue through flow cytometry (A; group means ± standard deviation). The number and immunophenotype of antigen-positive (OVA-AF647+) cells was determined in each tissue (B; group means).
The level of antigen uptake by the different cell types in the muscle and inguinal lymph nodes was assessed through the quantification of AF647-OVA positive cells. As expected, OVA-positive cells were concentrated mainly in the vaccinated muscle and associated draining lymph node, with none or only low levels seen in tissues collected on the contralateral side injected with PBS. A higher number of antigen-positive cells was seen in the muscle 1 day following OVA-SLA/LA administration as compared to OVA alone, with a ∼15-fold increase in OVA-positive neutrophils (p < 0.001; Fig. 8B). By Day 3, the profile of OVA-positive cells had shifted from neutrophils to include professional antigen-presenting cells such as macrophages and dendritic cells in both the OVA and OVA-SLA/LA injected muscles. Interestingly, OVA-positive cells in the draining lymph node on Day 1 were mostly B cells, which along with monocytes and macrophages were significantly higher following administration of OVA-SLA/LA when compared to muscles injected with free OVA or PBS (p < 0.01). By Day 3, very low numbers of OVA-positive cells were detected in the lymph nodes.
Discussion
Archaeosomes have been previously evaluated as adjuvants in pre-clinical mice models, and have been shown to induce robust and long-lasting humoral and cell-mediated immune responses to entrapped antigen and enhance protection in murine models of infectious disease and cancer.2 While generally composed of TPLs of various genera, a number of semi-synthetic glycolipid archaeosomes have also been generated and evaluated.5 More recently, we also developed a novel class of sulfated semi-synthetic archaeosomes (i.e., SLA/LA archaeosomes) which are capable of inducing strong cell-mediated immunity following systemic immunization, yet are more easily synthesized than previous classes of archaeosomes.6 The safety of TPL archaeosomes has been evaluated previously,7,8,11 however, no such work has been conducted using SLA/LA archaeosomes and therefore any further development of this adjuvant system will require an elucidation of its local reactogenicity and tolerability in vivo. Therefore, this study was performed to better understand the safety profile of SLA/LA archaeosomes when administered intramuscularly and to gain insight into the mechanism of action of this novel adjuvant formulation.
We show that SLA/LA is well-tolerated in mice at doses 10-fold higher than generally used in a vaccine setting. In addition, we demonstrate that the administration of SLA/LA leads to enhanced antigen localization and immune cell recruitment at the injection site. Previous studies have shown archaeosomes derived from the total polar lipids of various archaea (Methanobrevibacter smithii, Halobacterium salinarum or Thermoplasma acidophilum) were well tolerated when delivered subcutaneously.7 In those studies, at doses of 1.25 mg, there were no indications of increased injection site reactions, altered body weight/temperature or clinical signs of adverse reactions. In addition, serum biochemical analyses and the macroscopic evaluation of major organs (liver, spleen, kidneys, heart and lungs) revealed no major impact on the health status of the animals indicating the relative safety of TPL archaeosomes in a vaccine setting. Herein, we delivered archaeosomes composed of sulfated glycolipids (SLA/LA) by the intramuscular route, as this is the most common route of vaccine administration in the clinical setting. Overall SLA/LA archaeosomes were well-tolerated with no observed morbidity, altered body weights/temperatures or deviations in blood biochemistry/hematology parameters compared to control mice at doses approximately ten-fold higher than typically used in vaccination studies. In addition, SLA/LA did not induce hemolysis of mouse or rabbit erythrocytes ex vivo. Small transient changes in the overall neutrophil and total white blood cell count were seen in mice that received SLA/LA compared to PBS, but the measurements were still within the normal range recorded in healthy mice. These changes may be due to the immunomodulatory effects of the SLA/LA lipids as discussed below. Immunization-associated cell degeneration, necrosis and inflammatory cell infiltration were limited to the injection site. No histopathological changes were observed in other tissues including the lymph nodes (inguinal and popliteal) draining from the injection site 1 or 7 days following archaeosome administration. The effects were more pronounced with the 10 mg dose of SLA/LA and generally mild with the more immunologically relevant 1 mg dose. This correlates with the observation of mild swelling in the injected muscle of animals that received 10 mg but not 1 mg of SLA/LA. Other adjuvants, such as aluminum salts, have also been shown to induce similar patterns of damage and inflammation at injection sites.12,13 These effects could directly contribute to their adjuvant properties and activity in vivo, as induction of inflammation in the vicinity of administered antigen would increase its uptake by infiltrating innate immune cells and consequently enhance immunogenicity.
Glycolipids have been tested previously as vaccine adjuvants in human clinical trials.14 These α-galactosylceramide-derived analogs (e.g. ABX196) activate iNKT cells through the CD1d receptor. ABX196 was shown to be an effective adjuvant in mice, with a good toxicity profile in both mice and monkeys. When tested as an adjuvant to a hepatitis B vaccine in humans, ABX196 induced strong antigen-specific antibody responses. However, some liver toxicity was observed in subjects receiving the ABX196-adjuvanted formulation, but not in individuals receiving antigen alone. While ester analogs of α-galactosylceramide strongly activate iNKT cells, ether analogs have been shown to lose this activity.15 As the constituent lipids of SLA/LA archaeosomes maintain the characteristic ether linkages of archaeal lipids, they are highly unlikely to activate CD1d and generate the type of adverse events seen with ABX196.
The unique ether lipids found in archaeal lipids are chemically more stable than ester linkages found in bacteria and eukarya and permit the archaea to thrive in extreme environmental conditions.2 When used in an adjuvant setting this may in turn result in a more stable liposomal formulation. The adjuvanticity of SLA/LA was clearly demonstrated following the administration of OVA-SLA/LA archaeosomes to mice with strong antigen-specific antibody and CD8+ T cells detected following two vaccinations. While high levels of OVA-specific IgG were induced by OVA-SLA/LA, only background levels were detected in animals receiving OVA alone even after two immunizations. The lack of response with OVA alone was not surprising since OVA is a very weak antigen and typically requires the presence of an adjuvant for the induction of strong antigen-specific responses. In addition, tumor growth was inhibited following immunization with OVA-SLA/LA, with mice demonstrating increased survival rates to a melanoma cell line challenge in comparison to PBS treated or OVA-immunized mice. Traditional archaeosomes composed of archaeal total polar lipids with archaetidylserine components have been shown to promote endocytosis and MHC class I cross-presentation of the entrapped antigen through the phosphatidylserine receptor.16 As the semi-synthetic SLA/LA formulation no longer contains the archaetidylserine moieties, it must rely on other mechanisms to enhance antigen immunogenicity. Herein, we have demonstrated that SLA/LA does have immunostimulatory effects when administered in vivo, as well as a potential depot effect, sequestering increased amounts of the encapsulated antigen at the inflammatory milieu of the injection site. An SLA/LA mediated increase in the levels of cytokines/chemokines such as G-CSF and a recruitment of immune cells to the injection site may contribute to the enhanced immunogenicity of OVA entrapped in SLA/LA. Multiple marketed adjuvants have been shown to induce similar increases in cytokine production. For example, vaccination of mice with Cervarix® (adjuvanted with AS04: aluminum hydroxide & the TLR4 agonist Monophospholipid A) or Gardasil® (adjuvanted with aluminum hydroxyphosphate sulfate alone) induces production of G-CSF and IL-6.17 The oil-in-water emulsion MF59®'s immunostimulatory effects have been well documented and include a rapid induction of multiple cytokines including G-CSF and IL-6 in the serum of mice 24 hours after immunization.18 The recruitment of immune cells by SLA/LA to the injection site was observed histologically and was confirmed through immunophenotyping of cell suspensions isolated from the muscle. While both assays showed a recruitment of neutrophils and mononuclear cells on Day 1, histology appeared to indicate a heavier recruitment of mononuclear cells, while the flow-based assay detected a major influx of neutrophils at the injection site. This difference may depend on the nature of the markers (molecular vs. gross visual characteristic). While the histological assessment is grouping all mononuclear cells together, the flow cytometric analysis is separating them into multiple subpopulations. The total number of mononuclear cells would be underrepresented by simply adding up the 5 subpopulations we focused on in our study (i.e. monocytes, macrophages, dendritic cells, B and CD8+ T lymphocytes), as other cells that did not match our marker criteria (see Materials and Methods) were not included within any of the identified populations. In addition, as this is an acute inflammatory site with a high level of cytokine/chemokine expression, it is possible that cellular differentiation is occurring and cells may be classified differently based on the assay. For example, we did observe cells that were positive for both LY-6G (neutrophil marker) and CD11c (dendritic cell marker) (data not shown). Matsushima et al. have shown the exposure of neutrophils to GM-CSF can induce their differentiation into LY-6G+CD11c+ “neutrophil-DC hybrid” cells that contain oval, instead of segmented neutrophil-like, nuclei.19 Interestingly, SLA/LA is inducing immune cell recruitment to the injection site in a manner similar to other adjuvants. Calabro et al. showed that MF59 induced an early wave (within 24 hours) of neutrophils coming to the injection site, and a later influx of antigen presenting cells such as macrophages peaking at 2–3 days following vaccination.18 They also demonstrated that depletion of neutrophils did not impact the recruitment of other cell types or the generation of Ag-specific antibodies to a flu vaccine. Whether this will be the case with SLA/LA-adjuvanted vaccine formulations would need to be confirmed in future studies. It is likely that the increased uptake of antigen by B cells (as we have shown in the lymph node) or macrophages/dendritic cells (seen on Day 3 in the muscle) is more critical to the increased humoral and cellular antigen-specific responses observed to SLA/LA-encapsulated antigens.
When we evaluated the effect of SLA/LA archaeosomes on biodistribution of encapsulated OVA, we showed increased retention of the OVA antigen at the injection site over the first few days following administration when encapsulated in SLA/LA. The formation of an antigen depot mediated by certain adjuvant systems such as the water-in-oil emulsion Montanide™ ISA 51 (Seppic, Inc., Fairfield, NJ) or aluminum salts has long been thought to enhance antigen immunogenicity through prolonged stimulation of the immune system due to slow antigen release.13 These depots can persist for multiple weeks and can result in the formation of granulomas at the injection site. In the case of aluminum salts, other mechanisms such as increased cellular uptake of antigen, immune cell recruitment and NLRP3 inflammasome activation have been shown to play a more crucial role to its adjuvant activity.20 With SLA/LA, the depot effect appears to be more transient with the sequestration coinciding with the immune cell recruitment to the injection site at Days 2 to 3 and diminishing rapidly thereafter. Whether the detection of increased antigen at the site is due to the physiochemical properties of the archaeosome particles within the muscle tissue, or is due to the uptake by immune cells residing in the tissue will need to be clarified in future studies.
In summary, we have demonstrated the in vivo safety and efficacy of SLA/LA archaeosomes in a small animal setting. In depth safety evaluations would still need to be conducted for specific vaccine formulations that contain SLA/LA prior to clinical use, but these results give a good indication that SLA/LA archaeosomes have a favorable safety profile. We have also furthered our understanding of its potential mechanism of action by characterizing its impact on multiple immune parameters and antigen biodistribution. Further study into the contribution of various immune cells and cytokines to the activity of SLA/LA may help us better understand its effects and aid in the development of new vaccine formulations.
Materials & methods
Archaeosome preparation
Halobacterium salinarum was grown and archaeol purified as previously described.5 Structural identity and purity of archaeol was confirmed by both NMR spectroscopy and negative-ion fast atom bombardment mass spectrometry. Thereafter, lactosylarchaeol (LA; β-d-Galp-(1,4)-β-d-Glcp-(1,1)-archaeol; MW = 977.46 g/mol) and sulfated lactosylarchaeol (SLA; 6′-sulfate-β-d-Galp-(1,4)-β-d-Glcp-(1,1)-archaeol; MW = 1079.49 g/mol) were synthesized as reported previously.21,22For safety studies, empty archaeosomes were formed by hydrating 20–30 mg dried lipid (1:1 w/w ratio of SLA/LA) at 40°C in 2 ml of purified water. Vesicle size was reduced to about 100 – 150 nm diameter by brief sonication in a sonic bath and the dry weight determined on an aliquot. The vesicles were concentrated by nitrogen blowdown and then diluted to the required final concentration in PBS buffer (10 mM sodium phosphate, 160 mM NaCl, pH 7.1). Archaeosomes were shown to be endotoxin free using LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific, Pittsburgh, PA, USA). For immunogenicity studies, endograde ovalbumin (Hyglos, Bernried am Starnberger See, Germany) was encapsulated within archaeosomes. Archaeosomes were formed by hydrating 20–30 mg dried lipid (1:1 ratio of SLA/LA) at 40°C in 2 ml of purified water containing ovalbumin dissolved at 10 mg/ml. Vesicle size was reduced by brief sonication as above, and the portion of OVA antigen not entrapped was removed by centrifugation (200,000 x g max for 120 minutes) from 7 ml PBS followed by 2 washes. Vesicle pellets were re-suspended in PBS and filter sterilized through 0.45 μm Millipore filters (EMD Millipore, ON, Canada). Quantification of antigen loading was conducted by separating protein(s) from lipids using SDS polyacrylamide gel electrophoresis as previously described.23 Loading of synthetic archaeosomes with antigens was also determined using SDS Lowry with standard curves prepared for the respective antigen. Loading was based on μg protein/mg salt corrected dry weight of lipid. Average diameters based on Intensity and Zeta potentials were measured using a Malvern Nano Zetasizer with a He/Ne laser (Spectra Research Corp., ON, Canada).
For the biodistribution & antigen uptake studies, succinimidyl ester (NHS ester) linked CF770 (Biotium Inc., Fremont, CA, USA) and Alexa Fluor™ 647-NHSester (Thermo Fisher Scientific, Pittsburgh, PA, USA) were conjugated to the protein antigen, respectively, prior to encapsulation in archaeosomes. OVA antigen was labelled with CF770-NHSester or Alexa Fluor 647-NHSester using methods recommended by the manufacturer. Briefly, CF770-NHSester or Alexa Fluor 647-NHSester powders were solubilized in DMSO to create a 31.38 or 10mg/ml solution, respectively. To the protein antigen in PBS (pH 7.4), 5-fold molar excess of CF770-NHSester or Alexa Fluor-647 in 10% (v/v) carbonate buffer (pH 9.3) were added in separate tubes while mixing. Reactions were then incubated at room temperature for 2 hours with slow mixing. Unreacted dye was removed using an Amicon® Ultra-4 Centrifugal Filter Unit with a 10k cutoff membrane and the labeled protein antigen was re-suspended in PBS, pH 7.4. Labeling was optimized to achieve a dye/protein ratio of approximately 1–2.
The vaccine formulations were diluted to the proper concentration with PBS (Thermo Fisher Scientific) and stored at 4 °C until administration to mice.
Animals
C57BL/6 mice (6 – 8 weeks) were obtained from Charles River Laboratories (Saint-Constant, Canada). Mice were maintained at the National Research Council Canada (NRC) in accordance with the guidelines of the Canadian Council on Animal Care. All procedures performed on animals in this study were in accordance with regulations and guidelines reviewed and approved by the NRC Human Health Therapeutics Ottawa Animal Care Committee.
Safety Assessment
The safety profile of SLA/LA archaeosomes was assessed in C57BL/6 mice (n = 6 per group; 1:1 female: male mice) following a single i.m. administration of SLA/LA lipids (1 or 10 mg) into the left T.A. muscle in a volume of 50 μL per injection. PBS was injected in the vehicle control group. The following local and systemic effects were assessed: 1) local reactogenicity, 2) clinical signs (piloerection, posture and mobility), 3) blood chemistry & hematology and 4) histological evaluation of internal organs. All safety evaluations were conducted by individuals blinded to the treatment groups. Blood and tissues were collected 1 or 7 days following administration. Blood was placed in a tube containing lithium heparin anticoagulant (Becton Dickenson, Franklin Lakes, NJ, USA) and analyzed using a standard panel of hematological and blood chemistry parameters (Antech Diagnositics, Fountain Valley, CA, USA). A tissue panel consisting of bladder, brain, heart, left gonad (testis or ovary), left kidney, left lung, liver, spleen, thymus, left T.A. (injection site), and the left axial, inguinal and popliteal lymph nodes was collected from each mouse and placed in 10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA) for 24–48 hours. Tissues were then transferred to 70% ethanol (Sigma-Aldrich) and sent for paraffin embedding and processing at the University of Ottawa Pathology and Laboratory Medicine Core Facility (Ottawa, ON, Canada). Hematoxylin and eosin (H&E)-stained slides were prepared for all tissues and were reviewed by a pathologist in a blinded manner for signs of toxicity and pathology. Serum was also collected from mice at 3, 6, 24 or 168 hours following injection and the levels of 32 different cytokines/ chemokines/ growth factors in the serum were determined by Luminex assay using the MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel – Premixed 32 Plex – Immunology Multiplex Assay (Millipore, Billerica, MA, USA) according to manufacturer's instructions. The cytokines/chemokines/growth factors analyzed in the serum were IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, IP-10, eotaxin, G-CSF, GM-CSF, IFN-γ, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNF-α and VEGF.
Cytokine/chemokine levels in muscle
0.25 mg SLA/LA was injected in a volume of 50 μL i.m. in the left T.A. of C57BL/6 female mice (n = 4). The contralateral right T.A. was injected with 50 μL of PBS. The T.A. muscles were collected 6 hours following injection and frozen on dry ice. 500 μL of tissue protein extraction buffer (T-PER; Thermo Fisher Scientific) containing protease inhibitors (Complete Protease Inhibitor Cocktail; Roche Diagnostics, Basel, Switzerland) was added to the muscle tissues prior to homogenization using a Precellys® lysing kit (Bertin Technologies, Versailles, France). Protein lysates were collected following centrifugation at 15000 rpm for 10 min at 4°C. The levels of 11 different cytokines/chemokines in the muscle were determined by Luminex assay using a custom MILLIPLEX MAP Magnetic Bead Panel (Millipore) according to manufacturer's instructions. The cytokines/chemokines/growth factors analyzed were G-CSF, GM-CSF, M-CSF, IL-6, IP-10, KC, MCP-1, MIP-1α, MIP-1β, MIP-2 and RANTES. This panel was selected based on the results obtained in the serum.
Hemolytic assay
Hemolytic assay was performed as described by Parhanam and Wetzig.24 Briefly, erythrocytes collected from heparinized rabbit (New Zealand White) and mouse (C57BL/6) blood were incubated with various concentrations of SLA/LA archaeosomes (0.25–2 mg/mL) in hemolysis assay buffer consisting of 20 mM Tris-HCl at pH7.5, 74 mM NaCl, 6 mM glucose, 147 mM sucrose. 0.2% Triton was used as a positive control for hemolysis. The cell suspension was incubated in a shaker for 1 hour at 37 °C. The supernatant was collected following centrifugation of the samples at 2,500xg for 10 minutes. The release of hemoglobin was determined through the measurement of optical density at a wavelength of 540 nm. The value obtained with the negative control (erythrocytes with assay buffer alone) was subtracted from all samples.
Immunogenicity Assessment
Antigen-specific immune responses were assessed in female C57BL/6 mice (n = 10 per group). Mice were immunized on days 0 and 21 by intramuscular (i.m.) injection into both the left and right T.A. muscles (50 μL per muscle) with a total dose of 20 μg OVA alone or entrapped in SLA/LA archaeosomes. Animals were bled on day 35, serum collected and OVA-specific IgG levels measured by ELISA as previously described using 96-well plates coated with ovalbumin (1 μg/well).25 OD = 1 titers were calculated using XLfit software (ID Business Solutions, Guildford, UK). Blood was also collected on day 28 and the number of SIINFEKL-specific CD8+ T cells determined using a tetramer assay as previously described.26 Anti-tumor responses in these mice were assessed through challenge with B-16 melanoma cell line expressing ovalbumin. Tumor cells were grown in the laboratory as per previously published methods.27 Mice were injected with 5 × 105 B16-ovalbumin tumor cells (in PBS plus 0.5% normal mouse serum) in the shaved lower dorsal region, 50 days post first vaccination. From day 5 post implantation onwards, palpable solid tumors were measured using digital calipers. Tumor size, expressed in mm2, was obtained by multiplication of diametrically perpendicular measurements. Mice were euthanized when the tumor sizes reached a maximum of 300 mm2.
Biodistribution
The in vivo biodistribution of CF770-labeled OVA or CF770-labeled OVA encapsulated in SLA/LA archaeosomes following a single i.m. administration was assessed in C57BL/6 albino female mice (n = 4 per group). A 20 µg dose of CF770-labeled OVA (alone or entrapped in SLA/LA archaeosomes) was injected into the left T.A. muscle in a volume of 50 μL per injection. Animals were subjected to in vivo imaging studies using an IVIS Kinetic small animal imager (Perkin Elmer, Waltham, Massachusetts, United States). Animals were imaged at pre-scan, 10mins, 1.5, 3, 6, 24, 48, 72, 96 and 168 hours. Total or average fluorescence intensity data was determined from select regions of interest (ROI) using the Living Image 4.1 software (Perkin Elmer, Waltham, Massachusetts, United States).
Antigen uptake & cellular trafficking
A 20 µg dose of Alexa Fluor™ 647-labeled OVA (alone or encapsulated in SLA/LA archaeosomes) was injected in a volume of 50 μL i.m. in the left T.A. of C57BL/6 female mice (n = 4 to 5 per group). The contralateral right T.A. was injected with 50 μL of PBS. The T.A. muscles, and draining inguinal lymph nodes (LN) were collected 1 or 3 days following injection in 2% FBS in PBS. Muscle tissues were first minced with sharp scissors and transferred into 2ml cellular digestion buffer containing 0.2% type 4 collagenase (Worthington Biochemical Corp., Lakewood NJ) in 2% FBS-PBS then incubated for 1 hour in a shaking incubator at 37°C. All LN were mechanically minced with the frosted ends of two glass slides. Muscle and LN cell suspensions were kept at 4°C throughout processing. Cells were passed through a 75 µm cell strainer, and rinsed with 5 mL of flow buffer (2% FBS in PBS with 3 mM EDTA). Cells were washed once with buffer and cell yields determined on a Cellometer (Nexcelom, Lawrence, MA). Cells were stained with an antibody cocktail to identify major immune cell types: α-CD45-BV786 (BioLegend, San Diego CA), α-CD11b-BV510 (Becton Dickenson), α-Ly-6G-BV421 (Becton Dickenson), α-Ly-6C-BV711 (BioLegend), α-F4/80-APC-Cy7 (BioLegend), α-CD11c-PE-CF594 (Becton Dickenson), α-B220-FITC (eBioscience, San Diego CA) and α-CD8-PerCP-Cy5.5 (Becton Dickenson). After 30 min of staining, cells were washed once with 1mL flow buffer and fixed with BD cytofix (Becton Dickenson) for 15 min. All samples were washed and resuspended in flow buffer for acquisition with a BD Fortessa flow cytometer (Becton Dickenson). Cell populations were characterized as follows: B cells: B220+; CD8 T cells: SSClow, CD8+, Macrophages: CD11b+, F4/80+, CD11c−, Ly6G−; Monocytes: Ly6G−, CD11c−, F4/80−, Ly6C+; Neutrophils: CD11b+, Ly6G+; Dendritic cells: CD11c+, Ly6G−, Ly6C−. All cell types were CD45+ and tested negative for the fixable blue dead cell stain (Thermo Fisher Scientific). Cell population numbers were determined by multiplying their frequency of Live CD45+ cells by the total cell yield.
Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA), except for the serum cytokine data which was analyzed using R.28 Statistical significance of the difference between two groups was calculated by Student's two-tailed t-test and between three or more groups by one-way analysis of variance (ANOVA) followed by post-hoc analysis using either Dunnett's (comparison with control group) or Tukey's (comparison between groups) multiple comparison tests. A Mantel-Cox test was conducted to evaluate differences in survival over time after tumor implantation. A two-way ANOVA followed by post-hoc analysis using Sidak's multiple comparison test was used to evaluate the difference in the levels of fluorescence in the injection site over time. Multi-variate analysis was conducted on the serum cytokine data. Statistical significance of the differences between groups was evaluated using ANOVA for each individual cytokine at each time point. To mitigate the impact of outliers, the logarithmic expression levels of the cytokines were compared. Reported p-values were adjusted using Benjamini & Hochberg's correction factor due to the high number of cytokines evaluated. For all analyses, differences were considered to be not significant with p > 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge John Shelvey and Perry Fleming for producing the archaeal biomass, Lise Deschatelets for isolation of archaeol, as well as Renu Dudani, Mario Mercier, Dorothy Fatehi and Greg Harris for excellent laboratory technical assistance. We would also like to thank Francis Gaudreault for assistance with statistical analysis.
References
- 1.Rappuoli R, Mandl CW, Black S, De GE. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011. November 4;11(12):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haq K, Jia Y, Krishnan L. Archaeal lipid vaccine adjuvants for induction of cell-mediated immunity. Expert Rev Vaccines. 2016. December;15(12):1557–66. doi: 10.1080/14760584.2016.1195265. [DOI] [PubMed] [Google Scholar]
- 3.Conlan JW, Krishnan L, Willick GE, Patel GB, Sprott GD. Immunization of mice with lipopeptide antigens encapsulated in novel liposomes prepared from the polar lipids of various Archaeobacteria elicits rapid and prolonged specific protective immunity against infection with the facultative intracellular pathogen, Listeria monocytogenes. Vaccine. 2001. May 14;19(25–26):3509–17. doi: 10.1016/S0264-410X(01)00041-X. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan L, Deschatelets L, Stark FC, Gurnani K, Sprott GD. Archaeosome adjuvant overcomes tolerance to tumor-associated melanoma antigens inducing protective CD8 T cell responses. Clin Dev Immunol. 2010;2010:578432. doi: 10.1155/2010/578432. PMID:21318177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprott GD, Yeung A, Dicaire CJ, Yu SH, Whitfield DM. Synthetic archaeosome vaccines containing triglycosylarchaeols can provide additive and long-lasting immune responses that are enhanced by archaetidylserine. Archaea. 2012;2012:513231. doi: 10.1155/2012/513231. PMID:23055819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCluskie MJ, Deschatelets L, Krishnan L. Sulfated archaeal glycolipid archaeosomes as a safe and effective vaccine adjuvant for induction of cell-mediated immunity. Hum Vaccin Immunother. 2017. December 2;13(12):2772–2779. doi: 10.1080/21645515.2017.1316912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel GB, Omri A, Deschatelets L, Sprott GD. Safety of archaeosome adjuvants evaluated in a mouse model. J Liposome Res. 2002. November;12(4):353–72. doi: 10.1081/LPR-120016712. [DOI] [PubMed] [Google Scholar]
- 8.Patel GB, Ponce A, Zhou H, Chen W. Safety of intranasally administered archaeal lipid mucosal vaccine adjuvant and delivery (AMVAD) vaccine in mice. Int J Toxicol. 2008. July;27(4):329–39. doi: 10.1080/10915810802352703. [DOI] [PubMed] [Google Scholar]
- 9.Stark FC, McCluskie MJ, Krishnan L. Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8(+) T Cells that Protect Against Subcutaneous B16-OVA Melanoma. Vaccines (Basel). 2016. November 17;4(4). pii:E44; doi: 10.3390/vaccines4040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everds N. Hematology of the mouse. In: Hendrich H, editor. The laboratory mouse. London, UK: Elsevier Academic Press; 2004. p. 271–284. [Google Scholar]
- 11.Omri A, Agnew BJ, Patel GB. Short-term repeated-dose toxicity profile of archaeosomes administered to mice via intravenous and oral routes. Int J Toxicol. 2003. January;22(1):9–23. doi: 10.1080/10915810305080. [DOI] [PubMed] [Google Scholar]
- 12.Goto N, Akama K. Histopathological studies of reactions in mice injected with aluminum-adsorbed tetanus toxoid. Microbiol Immunol. 1982;26(12):1121–32. doi: 10.1111/j.1348-0421.1982.tb00261.x. PMID:7169970. [DOI] [PubMed] [Google Scholar]
- 13.Petrovsky N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015. November;38(11):1059–74. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tefit JN, Crabe S, Orlandini B, Nell H, Bendelac A, Deng S. Efficacy of ABX196, a new NKT agonist, in prophylactic human vaccination. Vaccine. 2014. October 21;32(46):6138–45. doi: 10.1016/j.vaccine.2014.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiozaki M, Tashiro T, Koshino H, Nakagawa R, Inoue S, Shigeura T. Synthesis and biological activity of ester and ether analogues of alpha-galactosylceramide (KRN7000). Carbohydr Res. 2010. August 16;345(12):1663–84. doi: 10.1016/j.carres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Gurnani K, Kennedy J, Sad S, Sprott GD, Krishnan L. Phosphatidylserine receptor-mediated recognition of archaeosome adjuvant promotes endocytosis and MHC class I cross-presentation of the entrapped antigen by phagosome-to-cytosol transport and classical processing. J Immunol. 2004. July 1;173(1):566–78. doi: 10.4049/jimmunol.173.1.566. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi Y, Maeda M, Kawashima H, Nakayama T. Inflammatory responses following intramuscular and subcutaneous immunization with aluminum-adjuvanted or non-adjuvanted vaccines. Vaccine. 2014. June 5;32(27):3393–401. doi: 10.1016/j.vaccine.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011. February 17;29(9):1812–23. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013. March 7;121(10):1677–89. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghimire TR. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus. 2015;4:181. doi: 10.1186/s40064-015-0972-0. PMID:25932368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitfield DM, Yu SH, Dicaire CJ, Sprott GD. Development of new glycosylation methodologies for the synthesis of archaeal-derived glycolipid adjuvants. Carbohydr Res. 2010. January 26;345(2):214–29. doi: 10.1016/j.carres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield DM, Sprott GD, Krishnan L, inventors . Sulfated-glycolipids as adjuvants for vaccines. WO 2016/004512 A1. 2016. January 14. [Google Scholar]
- 23.Sprott GD, Patel GB, Krishnan L. Archaeobacterial ether lipid liposomes as vaccine adjuvants. Methods Enzymol. 2003;373:155–72. doi: 10.1016/S0076-6879(03)73011-0. PMID:14714403. [DOI] [PubMed] [Google Scholar]
- 24.Parnham MJ, Wetzig H. Toxicity screening of liposomes. Chem Phys Lipids. 1993. September;64(1–3):263–74. doi: 10.1016/0009-3084(93)90070-J. [DOI] [PubMed] [Google Scholar]
- 25.Chikh G, Luu R, Patel S, Davis HL, Weeratna RD. Effects of KLK Peptide on Adjuvanticity of Different ODN Sequences. Vaccines (Basel). 2016. May 4;4(2). pii:E14; doi: 10.3390/vaccines4020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan L, Gurnani K, Dicaire CJ, van FH, Zafer A, Kirschning CJ. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol. 2007. February 15;178(4):2396–406. doi: 10.4049/jimmunol.178.4.2396. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce enhanced cytotoxic T lymphocyte responses to entrapped soluble protein in the absence of interleukin 12 and protect against tumor challenge. Cancer Res. 2003. May 15;63(10):2526–34. [PubMed] [Google Scholar]
- 28.R Core Team R: A language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria: URL https://www.R-project.org/. 2015. [Google Scholar]