ABSTRACT
Background: The World Health Organization recommends that rotavirus vaccines should be included in all national immunization programs. Some currently licensed oral rotavirus vaccines contain a buffering agent (either as part of a ready-to-use liquid formulation or added during reconstitution) to reduce possible degradation of the vaccine virus in the infant gut, which poses several programmatic challenges (the large dose volume or the reconstitution requirement) during vaccine administration. Because ROTAVAC®, a WHO prequalified vaccine, was derived from the 116E neonatal strain, we evaluated the immunogenicity and safety of ROTAVAC® without buffer and ROTAVAC® with buffer in a phase 4, multicentre, single-blind, randomized clinical trial in healthy infants in India. Methods: 900 infants, approximately 6, 10 and 14 weeks of age, were assigned to 3 groups to receive ROTAVAC® (0.5 mL dose) orally: (i) 2.5 mL of citrate-bicarbonate buffer 5 minutes prior to administration of ROTAVAC® (Group I), (ii) ROTAVAC®, alone, without any buffer (Group II), or (iii) ROTAVAC®, mixed with buffer immediately before administration (Group III). Non–inferiority was compared among the groups for differences in serological responses (detected by serum anti-rotavirus IgA) and safety. Results: Geometric mean titers post vaccination at day 84 (28 days after dose 3) were 19.6 (95%CI: 17.0, 22.7), 20.7 (95%CI: 17.9, 24) and 19.2 (95%CI: 16.8, 22.1) for groups I, II and III respectively. Further, seroconversion rates and distribution of adverse events were similar among groups. Conclusions: Administration of ROTAVAC® at a 0.5 mL dose volume without buffering agent was shown to be well tolerated and immunogenic. Given the homologous nature of the strain, it is plausible that ROTAVAC® replicates well and confers immunity even without buffer administration.
KEYWORDS: ROTAVAC®, buffer, immunogenicity, oral vaccine, Rotavirus vaccine
Introduction
The introduction of rotavirus (RV) vaccines has shown a dramatic benefit to public health. Significant reductions in number of RV cases and deaths due to RV gastroenteritis have been observed.1 In 2010, the World Health Organization (WHO) recommended that RV vaccines should be included in all national immunization programs and should be given priority.2 Based on promising results of the phase 3, safety and efficacy clinical trial of the oral rotavirus human 116E strain (ORV 116E), ROTAVAC®, manufactured by Bharat Biotech International Limited, Hyderabad, India was licensed in India in 2014,3 and is now WHO prequalified (stipulations that allow for purchase by United Nations agencies).4 The Government of India has introduced the vaccine in nine States (Himachal Pradesh, Haryana, Orissa, Andhra Pradesh, Madhya Pradesh, Assam, Tamil Nadu, Rajasthan and Tripura) in preparation for a national roll-out in the near future.5
ROTAVAC® was initially approved as a 3-dose regimen to be given with routine childhood vaccines at 6, 10, and 14 weeks of age along with 2.5 mL citrate-bicarbonate buffer to facilitate passage through the acidic contents of the upper gastrointestinal tract. Other licensed, orally administered RV vaccines also contain buffering components either as part of the formulation (RotaTeq® and Rotarix®, in some markets) or reconstituted at the time of administration (Rotasiil® in India and Rotarix® as well).6-8 Of note, natural transmission of RV occurs via the faecal-oral route and occurs in the presence of un-neutralized gastric acid. In general, RVs are moderately acid labile and the acidic environment affects the viability of the virus.9,10 However, the human infant stomach with higher pH levels (approximately 3.2) compared to adults (approximately 1.0) may be more permissive for the survival of RV.11 This could also account for the fact that 60 to 90% of reported human RV disease occurs in children under the age of 3 years.12 In addition, in humans, homologous RVs (human origin RV in a human) are much more (>1000 fold) infectious than heterologous RVs (non-human origin RV in a human). Thus, very small quantities of homologous RV (10 infectious doses or less) are generally able to cause infection, illness and also confer immune response.13,14 Given its human origin and the age at immunization, it is plausible that the ORV 116E strain, which belongs to the G9 and P[11] genotypes,15 may replicate and confer immunity without a buffer administration as it was initially recovered from an asymptomatic neonate.15 In a previously conducted in-vitro study, the stability of the 116E vaccine virus strain under in various pH conditions was evaluated by acidic treatment. Virus titer loss was estimated at a specific pH as a function of time. We observed no vaccine titer loss (pH range 3–4) (supplementary Table 2). The neonatal origin and high pH stability of the 116E strain encouraged us to carry out this investigation to see if the challenge of pre-mixing the vaccine with the buffer before administration could be avoided to mitigate programmatic challenges when being administered along the other vaccines in The Expanded Program on Immunization (EPI).
Table 2.
Geometric mean titer ratios with 95 % CI at day 0 (prior to vaccination) and at day 84 (post vaccination) among treatment groups.
| Group I (n = 290) (Buffer 5 min before administration of ROTAVAC®) | Group II (n = 287) (ROTAVAC® without buffer) | Group III (n = 286) (ROTAVAC® mixed with buffer at the moment of administration) | |||||
|---|---|---|---|---|---|---|---|
| Visit |
GMT |
95% CI |
GMT |
95% CI |
GMT |
95% CI |
|
| Day 0 | 10.5 | (9.4, 11.7) | 10.8 | (9.7, 11.9) | 10.2 | (9.2, 11.3) | |
| Day 84 | 19.6 | (17.0, 22.7) | 20.7 | (17.9, 24.0) | 19.2 | (16.8, 22.1) | |
| Day 0 GMT ratio and day 84 GMT ratio* | |||||||
| Group |
GMT ratio (Day 0) |
95% CI |
GMT ratio (Day 84) |
95% CI |
|||
| II vs I | 1.0 | (0.9, 1.2) | 1.1 | (0.9, 1.3) | |||
| II vs III | 1.1 | (0.9, 1.2) | 1.1 | (0.9, 1.3) | |||
| III vs I | 0.9 | (0.8, 1.1) | 0.9 | (0.8, 1.2) | |||
*95 % CI by two-sided t-test; n= number of Infants; GMT=Geometric Mean Titer; 95 % CI (LL, UL) = Confidence Intervals (Lower Limit, Upper Limit).
Several studies have been carried out in the past to examine the role of buffer on the performance of RV vaccines.16-18 A study from a previously developed RV vaccine demonstrated higher immune responses with the inclusion of buffering components,16 a study with limitations since it did not truly mimic current usage conditions as it evaluated a single dose vaccine with low vaccine virus titre. However, licensed RV vaccines reported no difference in immune responses from buffered and un-buffered vaccines.17,18 These licensed vaccines use buffering components to minimize vaccine virus degradation in the stomach, and pose several logistic and programmatic challenges in terms of either having to separately transport and store the buffering diluent, and administer relatively large volumes (1.5 to 2.5 mL) of reconstituted or ready-to-use vaccine orally to infants. From a vaccine administration point of view for infants, low dose volumes are preferred. Immunization programs prefer vaccines that are “ready-to-use” and do not require reconstitution or administration of separate components, such as a diluent or a buffer, to minimize errors in administration.19 In fact, the WHO Guidelines for Pre-Qualification of vaccines do not permit the use of reconstitution for oral vaccines.20
ORV 116E was initially developed to be administered with buffer.3 The rationale for the inclusion of a buffer stems from the knowledge and experiences by manufacturers of RV and other oral live vaccines (cholera and typhoid).6,7,16,21-23 In order to prevent degradation of the oral vaccine by acidic conditions of the stomach, buffering components were added in all such vaccine formulations. In view of all the above discussed issues including, 116E strain characteristics, conflicting literature reports on the role of buffer,16-18 and programmatic considerations during the use of the vaccine, we have planned to specifically investigate the immunological characteristics (measured as anti-RV IgA) of the ORV 116E strain administered with and without a buffer. Infants were randomly assigned to receive ROTAVAC® (0.5 mL dose) orally: (i) 2.5 mL of citrate-bicarbonate 5 minutes prior to administration of ROTAVAC® (group I), (ii) 0.5 mL of ROTAVAC®, alone without any buffer (group II), or (iii) ROTAVAC®, mixed with buffer immediately before administration (group III).
Results
The average age of infants at enrollment was around 48 days, average weight was 4.2 kg and the proportion of males was 58.3%, 57.7% and 53.7% in group I, II and III respectively. There were no significant baseline differences among groups (Table 1). Among the infants who were randomized, 863 (96%) infants completed the trial as per protocol and 37 infants discontinued the trial either due to loss to follow-up, migration from trial area or withdrew consent.
Immunogenicity
Of the infants who were analyzed per protocol, 38.3% (95%CI: 32.8, 43.9) achieved seroresponse (serum RV IgA ≥ 20 U/mL at day 84) in group I, 42.1% (95%CI: 36.6, 47.9) in group II, and 40.6% (95%CI: 35.1, 46.3) in group III (Table 1A and Fig. 2). The proportions of infants who seroconverted (as defined in study objectives) were 30.7% (95%CI: 25.6, 36.2), 35.2% (95%CI: 29.9, 40.8), and 33.6% (95%CI: 28.3, 39.2) in group I, II and III respectively (Table 1B).
Table 1A.
Proportion of infants with serum RV IgA < 20 U/mL and ≥ 20 U/mL at day 0 (prior to vaccination) and at day 84 (post vaccine).
| Group I (N = 290) (Buffer 5 min before administration of ROTAVAC®) |
Group II (N = 287) (ROTAVAC® without buffer) |
Group III (N = 286) (ROTAVAC® mixed with buffer at the moment of administration) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titer | Visit | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI |
| <20 | Day 0 | 246 | 84.8 | (80.2, 88.5) | 239 | 83.3 | (78.0, 87.2) | 243 | 84.9 | (80.3, 88.7) |
| Day 84 | 179 | 61.7 | (56.1, 67.1) | 166 | 57.9 | (52.1, 63.4) | 170 | 59.4 | (53.7, 64.9) | |
| ≥20 | Day 0 | 44 | 15.2 | (11.5, 19.8) | 48 | 16.7 | (12.8, 21.5) | 43 | 15.1 | (11.3, 19.6) |
| Day 84 | 111 | 38.3 | (32.8, 43.9) | 121 | 42.1 | (36.6, 47.9) | 116 | 40.6 | (35.1, 46.3) | |
N = number of infants allocated to each group; n = number of infants achieving or not achieving seroresponse; (95% CI) = Confidence Intervals (Lower Limit, Upper Limit).
Figure 2.
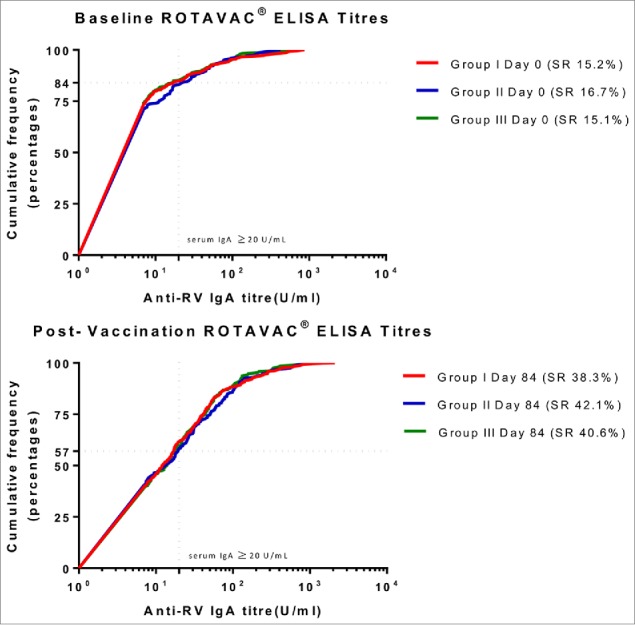
Proportion of children achieving seroresponse among groups (prior to vaccination and post-vaccine administration) *Figure 2 describes the distribution of sero-responses (measured as anti-RV IgA) in infants. The dotted line indicates a protective titer (anti-RV IgA > = 20 U/ml). Seroresponse (SR) was defined as an infant having serum Anti-RV IgA > = 20 U/ml at day 0 (prior to vaccination) or day 84 (post-vaccination). Group I (Buffer 5 min before administration of ROTAVAC®); Group II (ROTAVAC® without buffer); and Group III (ROTAVAC mixed with buffer at the moment of administration).
Table 1B.
Proportion of infants achieving seroconversion* at day 84 (post vaccination) compared to day 0 (prior to vaccination) in the cohort
| Group I (N = 290) (Buffer 5 min before administration of ROTAVAC®) |
Group II (N = 287) (ROTAVAC®without buffer) |
Group III (N = 286) (ROTAVAC® mixed with buffer at the moment of administration) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI |
| Day 0 | 44 | 15.2 | (11.5, 19.8) | 48 | 16.7 | (12.8, 21.5) | 43 | 15.0 | (11.3, 19.6) |
| Day 84 | 89 | 30.7 | (25.6, 36.2) | 101 | 35.2 | (29.9, 40.8) | 96 | 33.6 | (28.3, 39.2) |
| p-value (Fisher's exact test) | |||||||||
| Group | p-Value | ||||||||
| I vs II | 0.28 | ||||||||
| II vs III | 0.72 | ||||||||
| I vs III | 0.47 | ||||||||
As defined in methods. N = number of infants allocated to each group; n = number of infants achieving seroconversion; (95% CI) = Confidence Intervals (Lower Limit, Upper Limit).
Geometric mean titers (GMTs) were 19.6 (95%CI: 17.0, 22.7), 20.7 (95%CI: 17.9, 24.0) and 19.2 (95%CI: 16.8, 22.1) for the groups I, II and III respectively (Table 2 and Figure 3). The proportion of infants demonstrating a four-fold rise in antibody titers were 24.5% (95%CI: 19.8, 29.7), 29.2% (95%CI: 24.3, 34.8) and 25.1% (95%CI: 20.5, 30.5) in group I, II and III respectively. The three-fold rise were 27.6% (95%CI: 22.8, 33.1), 32.4% (95%CI: 24.3, 34.8) and 31.8% (95%CI: 20.5, 30.5) infants in group I, II and III respectively.
Figure 3.
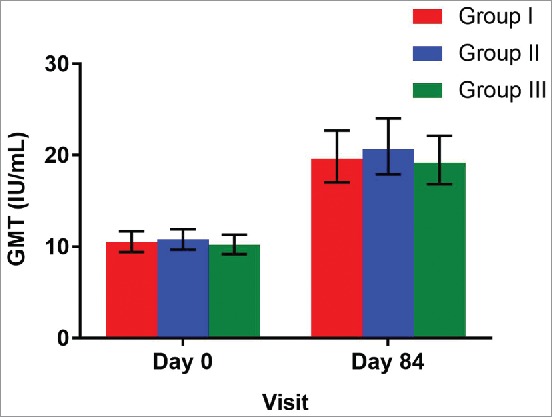
Geometric mean titer ratios with 95% CI at day 0 (prior to vaccination) and at day 84 (post vaccination) among treatment groups *GMT = Geometric Mean Titer (U/mL); Group I (Buffer 5 min before administration of ROTAVAC®); Group II (ROTAVAC® without buffer); and Group III (ROTAVAC mixed with buffer at the moment of administration).
Adverse events
The distribution of unsolicited AEs, local/general AEs and serious AEs was equal amongst all treatment groups. General solicited AEs (n = 1308) were reported across all visits in all three treatment groups (Table 3). Fever was the most common AE reported occurring in 69.3% of infants. The other solicited general AEs, refusal to feed and vomiting was similar across the three treatment groups. All AEs are mostly due to co-administration of pentavalent vaccine, along with ROTAVAC®. Diarrhea was the second most common reported AE with an overall occurrence of 12.6% among all groups.
Table 3.
General solicited adverse events by treatment group for the trial cohort
| Adverse Events* | Group I( n = 218) (Buffer 5 min before administration of ROTAVAC®) | Group II (n = 205) (ROTAVAC® without buffer) | Group III (n = 207) (ROTAVAC® mixed with buffer at the moment of administration) | Total (N = 630) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Crying | 25 (5.8) | 48 (10.9) | 39 (9.1) | 112 (8.6) |
| Fever | 304 (69.9) | 295 (66.9) | 307 (71.1) | 906 (69.3) |
| Refusal to feed | 0 (0.0) | 2 (0.5) | 1 (0.2) | 3 (0.2) |
| Diarrhea | 63 (14.5) | 55 (12.5) | 47 (10.9) | 165 (12.6) |
| Vomiting | 43 (9.9) | 41 (9.3) | 38 (8.8) | 122 (9.3) |
| Total | 435 | 441 | 432 | 1308 |
*Association of Adverse events among groups using Chi-square test showed no differences across groups with a likelihood ratio of 3.48 and a probability of 0.48.
Listing of unsolicited AEs in the study subjects reported cold and cough as the most commonly reported (58.5%, 71.2, and 68.7% in group I, II and III respectively) AEs followed by irritability (26.8%, 12.1%, and 19.4% in group I, II, and III respectively).
The trial had reported eighteen serious AEs. None were determined to be vaccine related. One case of suspected intussusception was noted but was diagnosed as infantile colic after physical examinations and ultrasonography. There was one death reported due to acute renal failure and sepsis. Both cases were not related to the vaccine.
Discussion
Administration of ROTAVAC® in the absence of bicarbonate-citrate buffer was shown to be well tolerated and immunogenic, in terms of anti RV-IgA. Even though GMT and proportion of infants showing seroconversion were apparently higher in group II (did not receive the buffer), these were found to be statistically similar in all groups. Similarly, no difference was found in the proportion of solicited/unsolicited and serious AEs among the groups.
In the current clinical trial, the results that establish the lack of requirement of buffer to confer immunity may be specific to the 116E strain, and could be due to several underlying factors: infectivity potential of homologous RV strains, unique stability of 116E strain with respect to the pH of the infant's gut, and vaccine uptake in buffered/ unbuffered conditions.
An oral live attenuated RV vaccine must pass through the acidic environment of the stomach in order to infect the villus epithelium in the infant's gut.24-26 The immune response to a live oral RV vaccine may be influenced by several factors, including those that decrease the effective titer of the vaccine virus reaching the small intestine. However, the number of infectious virions is likely to be much more critical for infection and subsequent immunogenicity with heterologous (non-human) RVs that are not capable of substantial productive replication in the human intestine.14 Hence, immunogenicity may be highly dependent on the initial dose that reaches the small bowel. On the other hand, human (and all homologous RVs in homologous hosts) undergo substantial amplification in the small bowel after the initial dose of virus passes into the duodenum. Therefore, immunogenicity may be more dependent on the replication capacity of the virus in the duodenum and not only on the amount of initial virus that reaches the small intestine. Homologous RV strains, have significantly higher replicating ability than heterologous RVs and this likely confers both mucosal and systemic immunity at low virus titers, in the case of ROTAVAC®.13,14,27 Most other RV vaccines are based on heterologous strains which require higher viral titers to confer immunity.28,29
The lack of difference in serological responses in groups with or without buffer may also be due to the unique pH stability of 116E strain. We had conducted in-vitro neutralization studies (Supplementary Table 2), which demonstrated that this vaccine (≥ 105 FFU/dose) was stable at pH 3, and at pH 2 reached a lower titer (≥ 104 FFU) after 30 minutes. Of note, in a dose escalating phase II trial, infants were vaccinated with either oral 116E 105 FFU or 116E 104 FFU. Both cohorts showed appreciable seroresponse rates.30 Hence, the 116E strain could likely afford to lose at least 90% of its infectivity during passage through the stomach and retain its immunogenicity.30
Current scientific understanding about viral stability shows that most RV strains are inactivated by a stomach pH ≤ 3 but are minimally inactivated at pH 4 or greater.10 In a study measuring gastric pH in infants with a median age of 16 weeks, pH was less than 4 for only 5% of the recorded time.31 In spite of this, it has been recommended that RV vaccines should be administered with an acid neutralizing substance or buffer; alternately, milk or formula feeding is required to negate the acid lability of the RV virus.32
Another factor which has to be considered here is the presence of breast milk which also supposedly affects the ability to infect and, therefore, confer immunity.33 However, recent studies have confirmed that withholding breastfeeding did not improve the immunogenicity of Rotatrix® in a developing country study.34 In our current clinical trial, we have kept a record of breastfeeding by mothers during the RV vaccination time window. While breastfeeding was ad lib, we observed that mothers would breastfeed the babies in a time window of 30 minutes, before and after vaccination, but never coincided with vaccine administration. In conclusion, it could be stated that the intrinsic characteristics of the RV virus determine the necessity of buffer on vaccine effectiveness, with breastfeeding playing an insignificant role.
Low immune responses to oral RV vaccines observed in developing countries may be due in part to high levels of pre-existing RV IgG antibodies transferred to the infant from the mother via the placenta.35 High titers of RV IgG have been found to diminish the immune responses of infants to ORV 116E vaccine (with initial doses).36 This trial did not examine the role of maternally transferred RV IgG on the immune response to ORV 116E.
This randomized trial was conducted in 16 sites, making our findings generalizable to population heterogeneity.37-41 With a low attrition rate of 4% (expected 20%), the power of the study improved to 96%. On day 0, the baseline sero-response (serum RV ≥ 20 U/mL) rates of infants was low (approximately 15%) in all groups which were observed with other RV vaccine studies conducted in India.42,43 The proportion of baseline and post-vaccination sero-response rates, and seroconversion rates were similar to other commercially available vaccines in low-income countries.42-49 RV-specific serum IgA antibody titers were estimated using ELISA at CMC, which serves as WHO rotavirus regional reference laboratory. To further strengthen the results obtained from CMC, a subset of paired serum samples (n = 309) of randomly selected infants distributed equally across the three groups were selected for testing at THSTI and were found to be similar (Supplementary Table 3).
The results of this study in terms of the role of the buffer on vaccine performance confirm and extend those of earlier studies that examined the role of buffer with the other licensed RV vaccine.18 If a separate or combined buffer is not required by health workers at peripheral health facilities, many important programmatic issues, such as incorrect reconstitution of a vaccine, temporary unavailability of the buffer, reduction of the cold chain footprint, waste management after each vaccination session, and finally conformance to WHO Pre-Qualification requirements, are addressed. Major health systems costs such as transport and logistics,19 can also be reduced. ROTAVAC® is the first rotavirus vaccine to be commercially adopted without buffer at a 0.5mL dose volume.
Methods
Clinical trial design and subjects
This phase 4 study was a multi-centre, randomized, single-blind, three-arm clinical trial conducted from April, 2014, to December, 2014, in 16 sites in India. The trial was approved by the National Regulatory Authority, the Drug Controller General, India, and respective Ethical Committees at all sites. The trial was conducted in compliance with the protocol, good clinical practices, schedule Y (Drugs and Cosmetics act, 2005) and ethical guidelines for biomedical research on human subjects (Indian Council of Medical Research, 2006). A data safety monitoring board reviewed safety data periodically. The trial was registered with Clinical Trials Registry of India (www.ctri.nic.in) as CTRI/2014/04/004548.
Vaccine
The live, attenuated, ROTAVAC®, contains not less than 105 Fluorescent Focus Units (FFU) of the oral human rotavirus 116E (ORV 116E) based on G9 and P[11] genotypes in a liquid dose volume of 0.5mL. The batch numbers of the vaccine vials used in the trial were 61DA13001, 61DA13002 and 61DA13003. The oral buffering agent used was citrate-bicarbonate (2.5 mL, citrate concentration was 0.032 M and bicarbonate was 0.304 M (lot number 61DB13001)). ROTAVAC®, was stored at -200 C. The citrate-bicarbonate buffer was stored at room temperature.
Infants were screened for eligibility into the study after obtaining a written informed consent from the parents/legally acceptable representative. Infants were excluded if they had received a RV vaccine, or if they had documented immunodeficiency or chronic gastroenteritis or any other disorder that was deemed necessary for exclusion by the investigator. Infants were temporarily excluded if they had any illness needing hospital referral, or diarrhea, on the day of enrolment. A total of 900 healthy infants, between 6–7 weeks of age were enrolled and were assigned randomly to a 1:1:1 ratio to one of the three treatment groups (I, II, III) based on a randomization list.
Infants were assigned to three groups to receive orally: (i) 2.5 mL of citrate-bicarbonate buffer 5 minutes prior to administration of ROTAVAC® (group I), (ii) ROTAVAC®, alone without any buffer (group II), or (iii) ROTAVAC® mixed with buffer immediately before administration (group III) (Fig. 1 & Supplementary Figure 1).
Figure 1.
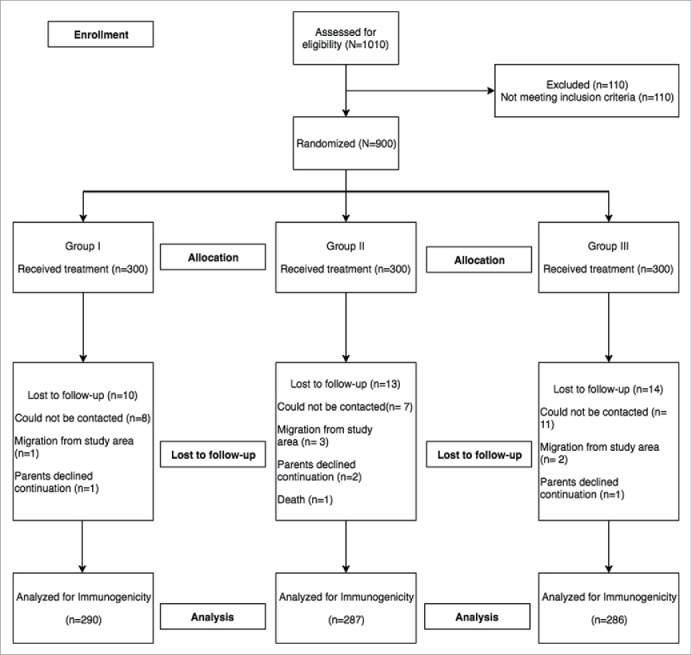
Enrollment flow chart.
Randomization and blinding
Enrolled infants were randomly assigned to the three treatment arms in a 1:1:1 ratio using block randomisation, provided by an independent agency, Croissance Clinical Research (Hyderabad, India). Vaccine and buffer vials were labeled with a combination of subject ID, a treatment code, batch code and specific dose number. A copy of computer generated randomization list of subject numbers and decode key were sent to the biostatistician at the end of the study for statistical analysis. The sponsor and site investigators were not involved in any of the analyses.
Study Endpoints
Immunogenicity was assessed by two primary outcome measures: Geometric mean titer (GMT) in each group at day 0 (prior to vaccination) and day 84 (28 days after third dose) and proportion of infants achieving 4-fold, 3-fold and 2-fold change in anti-RV IgA antibody titer at day 84 to day 0. Additionally, infants with pre-vaccination titer of <20 U/mL achieving post-vaccination titer of ≥ 20 U/mL were considered to have seroconverted, while infants with baseline titers of ≥ 20 U/mL (∼15% of in all the study groups), were also considered to have seroconverted upon achieving a 2-fold rise at day 84 titers. The endpoints selected were based on previously conducted trials on the ORV 116E vaccine.3,30
Procedures
ROTAVAC® 105 FFU, 0.5 ml, vials were distributed and stored at -200 C and allowed to thaw at 2–8°C followed by room temperature before administration. The citrate-bicarbonate buffer was stored at room temperature (25-35°C). The vaccine was administered at day 0, day 28 and day 56 concurrently with other childhood EPI vaccines (Oral Polio Vaccine (BIOPOLIO®), Diphtheria, Tetanus and Pertussis (DTP), Hepatitis B and Haemophilus influenza type b combination vaccine (ComVac5®). There were no specific instructions to mothers regarding breastfeeding before/after vaccination. Peripheral venous blood samples (5 ml) were obtained at day 0 (prior to vaccine administration) and day 84 (28 days after third dose). Clinical data management and analyses was contracted to Sensaas India, Private Limited. All laboratory assays were conducted at Wellcome Trust Research Laboratories, Christian Medical College (CMC), India and at Translational Health Science and Technology Institute (THSTI), India. GMT was calculated based on serum anti-RV specific IgA titers which were estimated using an enzyme-linked immunosorbent assay (ELISA) using 1% Blotto at CMC and a subset of paired serum samples (n = 309) of randomly selected infants distributed equally across the three groups were selected for RV IgA testing at the Translational Health Sciences and Technology (THSTI), Gurgaon, India. The quantity of serum anti-RV specific IgA titers was determined by comparison of the net optical density from sample wells to a standard curve generated by a human plasma standard.
Reactogenicity and safety
All infants were followed up for safety after vaccination by documentation of adverse events (AEs) between the administration of the first dose and 28 days after the third dose of vaccine. Infants were observed for 30 minutes post vaccination for signs of vomiting or spitting and immediate adverse reactions. Detailed safety information for solicited AEs, unsolicited AEs, and serious AEs was collected via subject diary card, telephonic contact by the clinical trial team and review at each study visit.
Vaccine reactogenicity was documented as events reported within 7 days following vaccination. Since all infants received concurrent childhood vaccines, the local solicited AEs included those related to the site of vaccine administration (pain, redness and swelling at the site of injection) and general solicited AEs included fever, crying, refusal to feed, diarrhea, and vomiting. AEs were graded for severity and relatedness by the investigators. Any cases of intussusception confirmed by the treating physician were reviewed by an independent case adjudication committee to ascertain if they met the Diagnostic Certainty Level Criteria 1 developed by Brighton Collaboration Intussusception Working Group.50 Serious AEs were reported to the Drug Controller General of India and the Ethics Committee and reviewed by a Data Safety Monitoring Board within the stipulated timeline. Medical expenses and hospital visits were covered by the sponsor.
Statistical analyses
Using 1.0 and 0.8 log10 standard deviation (IgA antibody) and 50% seroconversion rates from the Phase 3 trial,3 a total of 900 infants were enrolled in this trial. The sample size estimation was calculated using nQuery software, based on 90% power and a 20% dropout rate, with a non-inferiority margin of 10% for seroconversion and a two-sided confidence interval (CI) limit of 97.5% for GMT. An independent research organization, Sensaas India, Private Limited conducted the analyses using SAS version 9.3.
One-way ANOVA test was used to calculate differences in demographic variables among groups. GMT was calculated for both day 0 and day 84 titers and a two-sided t-test was used to calculate ratios between the three groups with 95% confidence intervals. Seroconversion and seroresponse was presented as counts and percentages with 95% confidence intervals. Chi-square test or fisher's exact test was used to calculate differences in categorical variables and proportion of adverse events reported.
Supplementary Material
Financial disclosure
Bharat Biotech International Limited was the funding source and was not involved in any stage of the conduct and analyses of this study (CTRI Number: CTRI/2014/04/004548). Bharat Biotech International Limited took charge of all the costs associated with the development and publishing of the present manuscript.
Disclosure of potential conflicts of interest
E.R., B.R., M.S., and K.M., are employees of Bharat Biotech International Limited. All authors have nothing to disclose according to the ICMJE guidelines for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgments
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Editors. The authors wish to thank the infants and their families who participated in this trial, all investigators, study coordinators and nurses, Mr. Siddharth Reddy and other staff members for contributing in many ways to this study. The authors would like to thank Dr. Iksung Cho, Former Senior Biostatistician, PATH, Washington, U.S.A for his contribution to study design/data analysis. We thank Dr. Harry Greenberg, Senior Associate Dean, Research, Stanford University, Palo Alto and Dr. Roger Glass, Director Fogarty International Center, Bethesda, U.S.A for insightful discussions and guidance on the analysis of the study results. We thank Dr. Gagandeep Kang, Executive Director, THSTI, Faridabad, India for several discussions and staff of Wellcome Trust Research Laboratories, Christian Medical College, Vellore for specimen analysis. We thank Dr. Max Ciarlet for reviewing this manuscript.
References
- 1.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. The J Infect Dis. 2017;215:1666–72. doi: 10.1093/infdis/jix186. PMID:28430997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeting of the immunization Strategic Advisory Group of Experts, April 2009–conclusions and recommendations Wkly Epidemiol Rec. 2009;84:220–36. PMID:19499606. [PubMed] [Google Scholar]
- 3.Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al.. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32 Suppl 1:A110–6. doi: 10.1016/j.vaccine.2014.04.079. PMID:25091663. [DOI] [PubMed] [Google Scholar]
- 4.http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/. accessed on 26thJanaury .
- 5.Ministry of Health & Family Welfare GoI National Health Mission. Available at http://nrhm.gov.in/nrhm-components/rmnch-a/immunization/background.html accessed December23, 2016.
- 6.GlaxoSmithKline Rotarix®, Prescribing Information. Available at https://www.gsksource.com/pharma/content/gsk/source/us/en/brands/rotarix/pi.html–section-34067-9. Accessed 23December2016. [Google Scholar]
- 7.Merck & CO. I. RotaTeq®, Prescribing Information Available at http://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf. Accessed 23December2016.
- 8.PATH PATH welcomes new promising study results for rotavirus vaccine candidate. Available at http://www.path.org/news/press-room/804/. Accessed on 8September2017.
- 9.Estes MK, Graham DY, Smith EM, Gerba CP. Rotavirus stability and inactivation. J General Virol. 1979;43:403–9. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- 10.Weiss C, Clark HF. Rapid inactivation of rotaviruses by exposure to acid buffer or acidic gastric juice. J General Virol. 1985;66:2725–30. doi: 10.1099/0022-1317-66-12-2725. [DOI] [PubMed] [Google Scholar]
- 11.Gryboski JW, ed. Gastrointestinal Problems in the Infant: W: B: Saunders and Company, 1983. [Google Scholar]
- 12.Barnett B. Viral gastroenteritis. Med Clin North Am. 1983;67:1031–58. doi: 10.1016/S0025-7125(16)31165-8. PMID:6312212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng N, Burns JW, Bracy L, Greenberg HB. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–73. PMID:7966566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DY, Dufour GR, Estes MK. Minimal infective dose of rotavirus. Arch Virol. 1987;92:261–71. doi: 10.1007/BF01317483. PMID:3028333. [DOI] [PubMed] [Google Scholar]
- 15.Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–7. doi: 10.1093/infdis/168.2.282. PMID:8393054. [DOI] [PubMed] [Google Scholar]
- 16.Ing DJ, Glass RI, Woods PA, Simonetti M, Pallansch MA, Wilcox WD, Davidson BL, Sievert AJ. Immunogenicity of tetravalent rhesus rotavirus vaccine administered with buffer and oral polio vaccine. Am J Dis Children (1960). 1991;145:892–7. [DOI] [PubMed] [Google Scholar]
- 17.Clark HF, Burke CJ, Volkin DB, Offit P, Ward RL, Bresee JS, et al.. Safety, immunogenicity and efficacy in healthy infants of G1 and G2 human reassortant rotavirus vaccine in a new stabilizer/buffer liquid formulation. Pediatric Infect Dis J. 2003;22:914–20. doi: 10.1097/01.inf.0000091887.48999.77. [DOI] [PubMed] [Google Scholar]
- 18.Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Simasathien S, Phavichitr N, Bock HL, Damaso S, Hutagalung Y, Han HH. Immunogenicity of a live-attenuated human rotavirus RIX4414 vaccine with or without buffering agent. Hum Vaccin. 2010;6:10428. doi: 10.4161/hv.6.3.10428. PMID:20220306. [DOI] [PubMed] [Google Scholar]
- 19.Mansoor OD, Kristensen D, Meek A, Zipursky S, Popovaa O, Sharma I, Miranda G, Millogo J, Lasher H. Vaccine presentation and packaging advisory group: a forum for reaching consensus on vaccine product attributes. Bulletin World Health Organization. 2013;91:75–8. doi: 10.2471/BLT.12.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Immunization VaB, World Health Organization Assessing the Programmatic Suitability of Vaccine Candidates for WHO Prequalification – Revision 2014. (WHO/IVB/14.10). Available at http://www.who.int/immunization_standards/vaccine_quality/ps_pq/en/, Accessed 24March2017 2014.
- 21.Sack DA, Shimko J, Sack RB, Gomes JG, MacLeod K, O'Sullivan D, Spriggs D. Comparison of alternative buffers for use with a new live oral cholera vaccine, Peru-15, in outpatient volunteers. Infect Immun. 1997;65:2107–11. PMID:9169739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens J, Shin S, Sur D, Nair GB, Holmgren J. New-generation vaccines against cholera. Nat Rev Gastroenterol Hepatol. 2011;8:701–10. doi: 10.1038/nrgastro.2011.174. PMID:22064524. [DOI] [PubMed] [Google Scholar]
- 23.Levine MM, Chen WH, Kaper JB, Lock M, Danzig L, Gurwith M. PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Exp Rev Vaccines. 2017;16:197–213. doi: 10.1080/14760584.2017.1291348. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–51. doi: 10.1053/j.gastro.2009.02.076. PMID:19457420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop R, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;302:1281–3. doi: 10.1016/S0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- 26.Brunet JP, Cotte-Laffitte J, Linxe C, Quero AM, Geniteau-Legendre M, Servin A. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74:2323–32. doi: 10.1128/JVI.74.5.2323-2332.2000. PMID:10666263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offit PA, Clark HF, Kornstein MJ, Plotkin SA. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984;51:233–6. PMID:6328042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medecines Sans Frontieres N , Efficacy and Safety of a Pentavalent Rotavirus Vaccine (BRV-PV) Against Severe Rotavirus Gastroenteritis in Niger In: ClinicalTrials.gov. Available at http://clinicaltrials.gov/show/NCT02145000NLMIdentifier:NCT02145000. Accessed 23December2016.
- 29.Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, Bhattacharya MK, Ghosh R, Kumar R, Sur D, et al.. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1-G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in healthy Indian adults and infants. Vaccine. 2014;32 Suppl 1:A117–23. doi: 10.1016/j.vaccine.2014.03.069. PMID:25091664. [DOI] [PubMed] [Google Scholar]
- 30.Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen-Chandola T, Appaiahgari MB, Mishra A, Singh S, Vrati S; Rotavirus Vaccine Development Group A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–9. doi: 10.1086/600104. PMID:19545211. [DOI] [PubMed] [Google Scholar]
- 31.Washington N, Spensley PJ, Smith CA, Parker M, Bush D, Jackson SJ, Kapila L, Stephenson T, Washington C. Dual pH probe monitoring versus single pH probe monitoring in infants on milk feeds: the impact on diagnosis. Arch Dis Child. 1999;81:309–12. doi: 10.1136/adc.81.4.309. PMID:10490434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vesikari T, Ruuska T, Delem A, Andre FE. Oral rotavirus vaccination in breast- and bottle-fed infants aged 6 to 12 months. Acta Paediatrica Scandinavica. 1986;75:573–8. doi: 10.1111/j.1651-2227.1986.tb10253.x. PMID:3019074. [DOI] [PubMed] [Google Scholar]
- 33.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, et al.. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatric Infect Dis J. 2010;29:919–23. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, Jiang B, McNeal MM, Steele D, Bhutta Z, et al.. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine–a randomized trial. PLoS One. 2015;10:e0127622. doi: 10.1371/journal.pone.0127622. PMID:26035743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M, Duke T, Buttery JP, Bines JE, Bishop RF, et al.. Maternal antibodies to rotavirus: Could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29:1242–7. doi: 10.1016/j.vaccine.2010.11.087. PMID:21147127. [DOI] [PubMed] [Google Scholar]
- 36.Appaiahgari MB, Glass R, Singh S, Taneja S, Rongsen-Chandola T, Bhandari N, Mishra S, Vrati S. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32:651–6. doi: 10.1016/j.vaccine.2013.12.017. PMID:24374502. [DOI] [PubMed] [Google Scholar]
- 37.Tiku VR, Sharma S, Verma A, Kumar P, Raghavendhar S, Aneja S, Paul VK, Bhan MK, Ray P. Rotavirus diversity among diarrheal children in Delhi, India during 2007–2012. Vaccine. 2014;32 Suppl 1:A62–7. doi: 10.1016/j.vaccine.2014.03.005. PMID:25091683. [DOI] [PubMed] [Google Scholar]
- 38.Mishra V, Awasthi S, Nag VL, Tandon R. Genomic diversity of group A rotavirus strains in patients aged 1–36 months admitted for acute watery diarrhoea in northern India: a hospital-based study. Clin Microbiol Infect. 2010;16:45–50. doi: 10.1111/j.1469-0691.2009.02772.x. PMID:19456832. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Ray P, Gentsch JR, Glass RI, Kalra V, Bhan MK. Emergence of G12 rotavirus strains in Delhi, India, in 2000 to 2007. J Clin Microbiol. 2008;46:1343–8. doi: 10.1128/JCM.02358-07. PMID:18272705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain V, Das BK, Bhan MK, Glass RI, Gentsch JR. Great diversity of group A rotavirus strains and high prevalence of mixed rotavirus infections in India. J Clin Microbiol. 2001;39:3524–9. doi: 10.1128/JCM.39.10.3524-3529.2001. PMID:11574567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran M, Das BK, Vij A, Kumar R, Bhambal SS, Kesari N, Rawat H, Bahl L, Thakur S, Woods PA, et al.. Unusual diversity of human rotavirus G and P genotypes in India. J Clin Microbiol. 1996;34:436–9. PMID:8789033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta SK, Suryakiran PV, Delem A, Han HH, et al.. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccines. 2009;5:414–9. doi: 10.4161/hv.5.6.8176. [DOI] [PubMed] [Google Scholar]
- 43.Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, Kang G. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine. 2014;32 Suppl 1:A129–33. doi: 10.1016/j.vaccine.2014.03.002. PMID:25091666. [DOI] [PubMed] [Google Scholar]
- 44.Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, et al.. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: A randomized, double-blind, placebo controlled trial. Vaccine. 2012;30, Supplement 1:A36–43. doi: 10.1016/j.vaccine.2011.09.120. PMID:22520135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence J, He S, Martin J, Schödel F, Ciarlet M, Murray AV. Safety and immunogenicity of pentavalent rotavirus vaccine in a randomized, double-blind, placebo-controlled study in healthy elderly subjects. Hum Vaccin Immunother. 2014;10:2247–54. doi: 10.4161/hv.29107. PMID:25424929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30 Suppl 1:A44–51. doi: 10.1016/j.vaccine.2011.08.080. PMID:22520136. [DOI] [PubMed] [Google Scholar]
- 47.Steele AD, Reynders J, Scholtz F, Bos P, de Beer MC, Tumbo J, Van der Merwe CF, Delem A, De Vos B. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202 Suppl:S93–100. doi: 10.1086/653550. PMID:20684724. [DOI] [PubMed] [Google Scholar]
- 48.Mwenda JM, Ntoto KM, Abebe A, Enweronu-Laryea C, Amina I, Mchomvu J, Kisakye A, Mpabalwani EM, Pazvakavambwa I, Armah GE, et al.. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J Infect Dis. 2010;202 Suppl:S5–S11. doi: 10.1086/653557. PMID:20684718. [DOI] [PubMed] [Google Scholar]
- 49.Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, Sow SO, Ojwando J, Ciarlet M, Steele AD. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine. 2012;30 Suppl 1:A86–93. doi: 10.1016/j.vaccine.2011.10.006. PMID:22520142. [DOI] [PubMed] [Google Scholar]
- 50.Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, Murphy TM, Music S, Niu M, Varricchio F, et al.. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:569–74. doi: 10.1016/j.vaccine.2003.09.016. PMID:14741146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


