ABSTRACT
Respiratory burst oxidase homologs (RBOHs) constitute a multigene family in plants. These reactive oxygen species (ROS)-generating enzymes participate in diverse biological processes. We previously demonstrated that PvRbohB plays an important role in lateral root (LR) development in Phaseolus vulgaris. However, little is known about the roles of other Rboh members in root development. Here, we report that PvRbohA is involved in LR emergence in P. vulgaris. PvRbohA was expressed in wild-type P. vulgaris root tissues, e.g., the radicle, inter LR zone, and LR zone, and its expression increased during LR formation. Analysis of the spatio-temporal expression patterns of a reporter construct under the control of the PvRbohA promoter (PvRbohA::GUS-GFP) in transgenic roots showed that PvRbohA was active at all three stages of LR development, but its spatial expression pattern varied at each stage. The relative expression levels of PvRbohA during LR formation correlated with the activity of PvRbohA promoter. Furthermore, upon PvRbohA transcript silencing, LR growth was significantly altered in transgenic hairy roots. These findings suggest that RbohA participates in LR initiation, emergence, and development in the legume P. vulgaris by delimiting the region for LR emergence.
KEYWORDS: common bean, lateral root emergence, NADPH oxidase, promoter RbohA, RbohA-RNAi
Root branching through lateral root (LR) formation is an important element in the adaptability of the plant root system to its environment. The degree of root branching affects the efficiency of water uptake, nutrient acquisition, and anchoring of the plant in the soil. Molecular genetics and physiological studies in Arabidopsis thaliana have demonstrated that LR formation is dependent on auxin, which regulates most steps of LR development, such as LR founder cell specification, LR initiation, LR primordium development, and LR emergence [1–4]. Reactive oxygen species (ROS) derived from the activity of NADPH oxidases are thought to function as important signals during auxin-regulated LR formation, as respiratory burst oxidase homolog (RBOH)-mediated ROS production facilitates LR emergence in Arabidopsis [5]. There is compelling evidence that ROS derived from NADPH oxidase have important roles during adventitious root formation [6] and root-to-shoot communication [7]. Among ROS, superoxide anion, hydrogen peroxide and hydroxyl radical are involved in cell wall modifications during numerous plant developmental processes, including root hair development [8,9]. ROS production in extracellular spaces depends on several classes of enzymes, including RBOH, whose activity is crucial. Treatment with the RBOH inhibitor diphenyleneiodonium (DPI) reduces the meristem cell number in Arabidopsis primary roots [10]. Accordingly, superoxide anions primarily accumulate in the meristematic region of Arabidopsis, and DPI treatment has a negative effect on ROS production and primary root growth [10]. Furthermore, loss-of-function of AtRBOHC and AtRBOHF significantly affects primary root growth [11]. Recent studies have demonstrated that disrupting (or enhancing) expression of RBOH in LR primordia and/or overlying root tissues decelerates (or accelerates) the development and emergence of LRs [5]. In Phaseolus, silencing of PvRbohB resulted in a significant reduction in ROS levels and LR density [12,13].
It is important to examine the roles of different RBOH gene family members, as each member can play distinct roles in the same biological process, ranging from synergistic to non-redundant functions. Therefore, in the current study, we downregulated PvRbohA expression via RNAi-mediated gene silencing and analyzed root growth parameters. We also assessed the spatio-temporal activity of the PvRbohA promoter during LR emergence in common bean (Phaseolus vulgaris).
PvRbohA expression in P. vulgaris roots
We previously investigated the function of PvRbohA, which is expressed at higher levels in roots than in other organs [12]. Here, we performed a more detailed analysis to examine PvRbohA expression patterns in different root zones of common bean. First, we isolated mRNA from the radicles of 2-day-old wild-type seedlings (Fig. 1A), the inter lateral root (ILR) zone, and LR zone tissues from 4-day-old wild-type seedlings (Fig. 1B). Quantitative RT-PCR analysis revealed significantly higher (∼81%) levels of PvRbohA transcript in LR zone tissues compared to the ILR zone (Fig. 1C). By contrast, PvRbohA transcript levels were significantly lower (∼111%) in radicles with respect to LR zone tissues (Fig. 1C). Together, these results indicate that PvRbohA is differentially expressed in different zones of wild-type P. vulgaris roots; however, its expression increases during LR formation.
Figure 1.
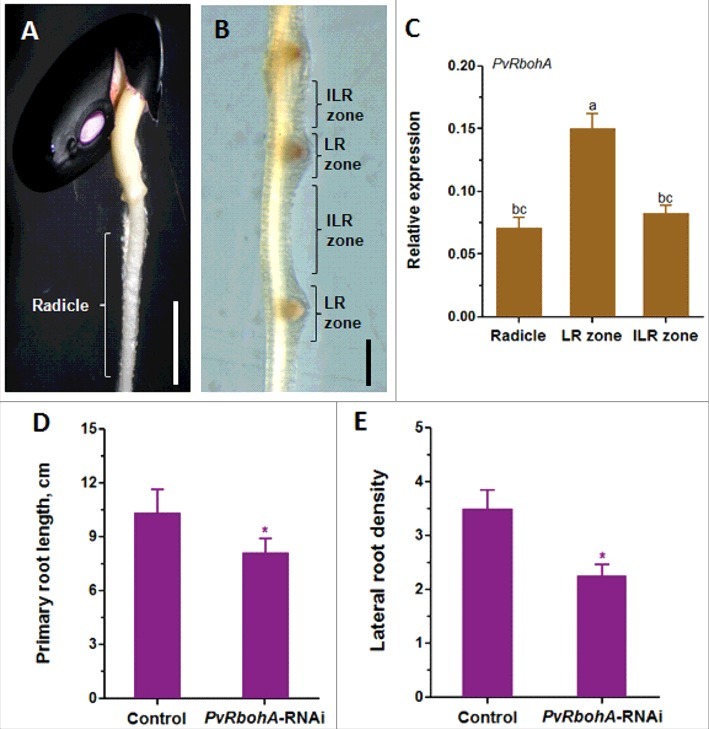
Quantitative RT-PCR analysis of PvRbohA expression and root growth parameters in Phaseolus vulgaris. A representative wild-type P. vulgaris germinating seedling showing (A) a radicle (from 2-day-old seedling) and (B) ILR and LR zones of the root (from 4-day-old seedling). (C) RT-qPCR expression analysis of PvRbohA from mRNA isolated from radicles, ILR zones, and LR zones of wild-type P. vulgaris seedlings. Transcript accumulation was normalized to the expression of the Ef1α and IDE reference genes. The statistical significance of differences between the different root zones was calculated by ANOVA and Tukey's Multiple Comparison Test, where different letters indicate significance differences (P < 0.001). P. vulgaris composite plants containing transgenic hairy roots were analyzed at 10 days post transplantation. (D) Primary root length and (E) lateral root density in control and PvRbohA-RNAi roots. The statistical significance of the differences between control and RNAi root samples was determined using an unpaired two-tailed Student's t-test (*P<0.05). The reported values represent three biological replicates (C; n > 9, D, E; n > 21). Error bars represent the means ± SEM. Scale bar: A, 5 mm; B, 2 mm. ILR zone, inter lateral root zone; LR zone, lateral root zone.
Downregulation of PvRbohA alters root growth in common bean
Next, to investigate whether downregulating PvRbohA affects root development in P. vulgaris, we generated composite plants with hairy roots expressing the PvRbohA-RNAi vector as described in our previous work [14]. We observed root growth parameters at 10 days post transplantation. PvRbohA-RNAi lines showed a significant decrease in primary root length (∼22%) and LR density (∼36%) compared to control roots (Fig. 1D-E). As evidenced in our previous studies, silencing of PvRbohB significantly affected LR density, whereas ectopic, constitutive expression of PvRbohB significantly enhanced LR density, including tertiary and quaternary root number [12,15]. Collectively, these results indicate that PvRbohA and PvRbohB play distinct roles in the same biological process, i.e., LR formation in P. vulgaris.
The RbohA promoter is active during lateral root emergence
Lateral roots originate deep within the primary root from a small number of founder cells at the periphery of the vascular tissues and must emerge through intervening layers of tissue. LR formation constitutes three major steps: pre-initiation, primordium initiation, and emergence [16]. Great progress has been made in understanding the molecular processes underlying root development in the past decade. Several genes and transcription factors have been characterized that are implicated in LR development, among which Rboh genes are especially important. We previously performed detailed analysis of PvRbohB promoter activity throughout LR development i.e., from the initial mitotic divisions in the pericycle to LR emergence [13]. Given the importance of the enhanced expression of PvRbohA in LR formation zones, we investigated the spatio-temporal expression pattern of PvRbohA by examining the expression of a promoter::GUS-GFP construct in transgenic hairy roots of common bean. Microscopy observations revealed that during the LR pre-initiation stage, PvRbohA promoter-driven GUS expression occurred in two spots close to the vascular bundles (Fig. 2A-B); subsequently, GUS activity increased in the cells flanking the primordium during the LR primordium initiation stage (Fig. 2C-D). At this stage, no GUS expression was observed in the LR primordium. During LR emergence, GUS activity gradually spread to the base of the LR primordium and the vascular bundles (Fig. 2E-G); once the LR emerged from the epidermis, the activity of the PvRbohA promoter diminished (Fig. 2H). The relative expression levels of PvRbohA during LR formation (Fig. 1C) correlate with the activity of PvRbohA promoter (Fig. 2). These observations indicate that the PvRbohA promoter is active at all developmental stages of LR formation in P. vulgaris.
Figure 2.
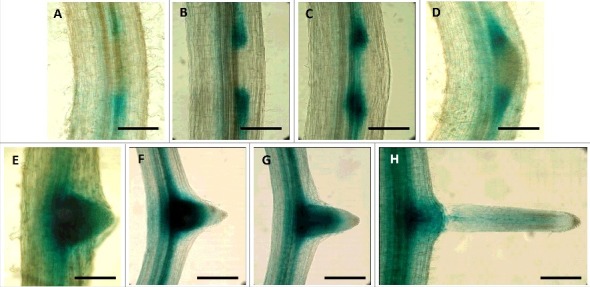
Expression analysis of the PvRbohA promoter driving the β-glucuronidase reporter gene in transgenic P. vulgaris roots. The spatio-temporal pattern of PvRbohA activity was examined in transgenic hairy roots harboring the promoter::GUS-GFP construct incubated in GUS as a substrate. (A-H). Representative images showing promoter activity detected by GUS staining during different stages of LR formation in transgenic roots. (A-B) LR pre-initiation, (C-D) LR primordium initiation, and (E-H) LR emergence. Scale bar, A-E, H: 200 µm; F-G 500 µm. LR: lateral root.
We previously showed that although the PvRbohB promoter is active at all LR developmental stages in P. vulgaris, its expression is specifically restricted to the LR primordium [13]. Likewise, the PvRbohA promoter was also active at all three stages of LR development, but interestingly, its activity varied spatially at each stage. Thus, we propose the following model for LR formation (Fig. 3): During the first two stages of LR formation (LR pre-initiation and LR primordium initiation), the PvRbohA promoter is active in cells surrounding the LR primordium, whereas the PvRbohB promoter is specifically active in the LR primordium. Nevertheless, both the PvRbohA and PvRbohB promoters are active during LR emergence. We conclude that both PvRbohA and PvRbohB contribute to LR formation in P. vulgaris.
Figure 3.
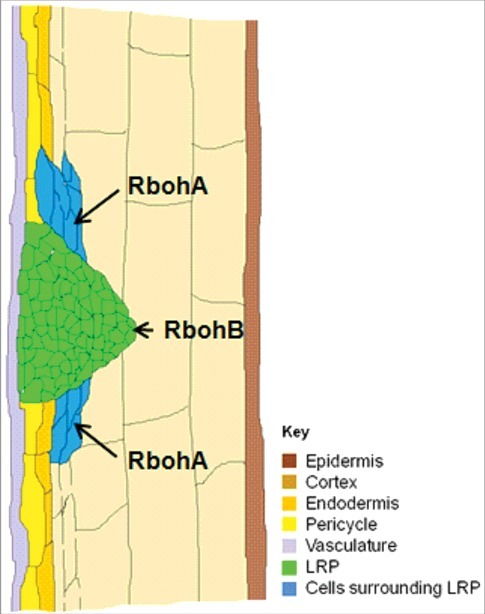
A model of lateral root formation showing a lateral root primordium and the expression of the PvRbohA (present study) and PvRbohB 13 promoters in P. vulgaris. LRP, lateral root primordium.
Materials and methods
Root growth analysis
Wild type Phaseolus vulgaris seeds were germinated on sterile moistened filter paper in Petri dishes in the dark at 28°C. Radicles (from 2-day-old seedling) and ILR and LR zones of the roots (from 4-day-old seedling) were used to isolate mRNA and measure the levels of RbohA transcripts. Composite plants grown in pots containing sterile vermiculite were subjected to root growth parameter measurements. The composite plants were irrigated daily with B&D nutrient medium [17] and maintained in a growth chamber with a 16-h photoperiod and 65% relative humidity at 27 ± 1°C. Transgenic roots expressing red fluorescent protein were selected from individual plants at 10 days post-transplantation, and root growth parameters such as root length and lateral root density were measured. Lateral root density was calculated using the following formula: D = LR/L´, where D = density of lateral roots; LR = number of lateral roots; and L´ = length of the main root between the first and last lateral root [18].
Microscopy
Transgenic hairy roots expressing the 35S:PvRbohA-RNAi vector were selected under an epifluorescence stereomicroscope (SZX7, Olympus, Japan). To analyze the spatio-temporal activity of the promoter, pPvRbohA::GUS-GFP transgenic roots were harvested at different time points and stained for GUS activity according to Jefferson [19]. The GUS-stained roots were clarified according to Arthikala et al. [20], and the expression patterns were examined under a light microscope (Axioskop microscope, Zeiss, Germany).
Funding Statement
This work was partially supported by Consejo Nacional de Ciencia y Tecnología (CB-2010-153718 to C.Q) with a postdoctoral fellowship (17656) to M-K.A., and by DGAPA IN224115 to C.Q. DGAPA/PAPIIT-UNAM grant no. IA205117 to M.-K.A.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A. 2008;105(25):8790–4. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Péret B, De Rybel B Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [3].Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol. 2009;69:437–49. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- [4].De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol. 2010;20:1697–706. doi: 10.1016/j.cub.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [5].Orman-Ligeza B, Parizot B, de Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X, et al. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development. 2016;143:3328–39. doi: 10.1242/dev.136465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liao W-B, Zhang M-L, Huang G-B, Yu J-H. Ca2+ and CaM are involved in NO− and H2O2-induced adventitious root development in marigold. J Plant Growth Regul. 2012;31:253–64. doi: 10.1007/s00344-011-9235-7. [DOI] [Google Scholar]
- [7].Passaia G, Fonini SL, Caverzan A, Jardim-Messeder D, Christoff AP, Gaeta M. L, de Araujo-Mariath JE, Margis R, Margis-Pinheiro M. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Sci. 2013;208:93–101. doi: 10.1016/j.plantsci.2013.03.017. [DOI] [PubMed] [Google Scholar]
- [8].Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- [9].Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–1001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–16. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- [11].Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Montiel J, Nava N, Cárdenas L, Sánchez-López R, Arthikala M-K, Santana O, Sánchez F, Quinto C. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by Rhizobia. Plant Cell Physiol. 2012;53:1751–67. doi:10.1093/ pcp/pcs120. [DOI] [PubMed] [Google Scholar]
- [13].Montiel J, Arthikala M-K, Quinto C. Phaseolus vulgaris RbohB functions in lateral root development. Plant Signal Behav. 2013;8:e22694. doi: 10.4161/psb.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arthikala M-K, Montiel J, Sánchez-López R, Nava N, Cárdenas L, Quinto C. Respiratory Burst Oxidase Homolog Gene A Is Crucial for Rhizobium Infection and Nodule Maturation and Function in Common Bean. Front Plant Sci. 2017;8:2003. doi: 10.3389/fpls.2017.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arthikala M-K, Sánchez-López R, Nava N, Santana O, Cárdenas L, Quinto C. RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol. 2014;202:886–900. doi: 10.1111/nph.12714. [DOI] [PubMed] [Google Scholar]
- [16].Péret B, de Rybel B, Casimiro I, Benkova I, Swarup R, Laplaze L, Beeckman T, Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [17].Broughton WJ, Dilworth MJ. Control of leghemoglobin synthesis in snake beans. Biochem J. 1971;125:1075–80. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Ann Bot. 2006;97:903–15. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jefferson RA. Assaying chimeric genes in plants, the GUS gene fusion system. Plant Mol Biol Report. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- [20].Arthikala M-K, Nanjareddy K, Lara M. In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis. Genes. 2018;9(1):11. doi: 10.3390/genes9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]


