Abstract
Adipocyte infiltration in the bone marrow follows chemotherapy or irradiation. Previous studies indicate that bone marrow fat cells inhibit hematopoietic stem cell function. Recently, Zhou et al. (2017) by using state-of-the-art techniques, including sophisticated Cre/loxP technologies, confocal microscopy, in vivo lineage-tracing, flow cytometry, and bone marrow transplantation, reveal that adipocytes promote hematopoietic recovery after irradiation. This study challenges the current view of adipocytes as negative regulators of the hematopoietic stem cells niche, and reopens the discussion about adipocytes’ roles in the bone marrow. Strikingly, genetic deletion of stem cell factor specifically from adipocytes leads to deficiency in hematopoietic stem cells, and reduces animal survival after myeloablation, The emerging knowledge from this research will be important for the treatment of multiple hematologic disorders.
Keywords: adipocytes, hematopoiesis, hematopoietic stem cells, bone marrow
Owing to the modern sedentary lifestyle, excessive fat accumulation in the body has become a worldwide epidemic, leading to immense research interest on the pathophysiological role of adipose tissue in several diseases (1,2). Fat stores energy and maintain body temperature in response to cold by generating heat (3). Furthermore, adipose tissue may act as an endocrine organ, producing an enormous repertoire of biologically active molecules, placing fat cells as key determinants for healthy metabolism and in multiple dysfunctions (4,5). Lately, our knowledge on adipose tissue biology has rapidly progressed, associating fat cells with sometimes unexpected roles throughout the organism.
Fat tissue is highly heterogeneous among distinct anatomical depots, differing in its physiological and pathological roles (6). In the bone marrow, adipocytes are smaller and appear dispersed, instead of being grouped into lobules as subcutaneous and visceral fat cells (7). Although the presence of bone marrow adipocytes histologically was detected long time ago, they have been erroneously ignored in the past, and considered merely inert cells “space fillers”. Curiously, physicians even denominate as hypocellular the adipocyte-rich bone marrow biopsy. Thus, until recently the functions of fat cells in the bone marrow were poorly explored. Only recently, growing evidence show that bone marrow adipose tissue affects insulin metabolism, energy expenditure, and bone mass (8). Fat cells secrete fatty acids, hormones, adipokines, and cytokines, which may have profound effects on the function of other neighboring cell populations in the bone marrow microenvironment (9,10).
The most illustrious residents of the bone marrow are the hematopoietic stem cells which give rise to all blood and immune cells throughout life, including platelets (11), erythrocytes, and white blood cells (12). Without hematopoietic stem cells, we would not be capable to preserve blood cell counts and would die shortly due to bleeding (as a result of platelet reduction), anemia (as a result of erythrocytes depletion), and infection (as a result of immune cells deficiency) (7). Adipocytes increase in the bone marrow microenvironment with aging, and after chemotherapy or irradiation (13). Hence, numerous studies have been carried out to explore how fat cells affect hematopoietic stem cells in the bone marrow (14–18). These works indicate that bone marrow fat cells inhibit hematopoietic stem cell function, repressing their growth and differentiation (14). However, in a recent article in Nature Cell Biology, Zhou and colleagues challenge the current view of adipocytes as negative regulators of the hematopoietic stem cells niche in the bone marrow (19). The authors demonstrated that fat cells promote increased hematopoiesis in response to myeloablation (19) (Figure 1). Zhou and colleagues investigated the role of bone marrow adipocytes as hematopoietic stem cell niche components by using state-of-the-art techniques, including sophisticated Cre/loxP technologies, confocal microscopy, in vivo lineage-tracing, and bone marrow transplantation. These experiments revealed that bone marrow fat cells from irradiated and non-irradiated mice produce high amount of stem cell factor, an essential cytokine that plays a pivotal role in hematopoietic stem cell maintenance. Strikingly, genetic deletion of stem cell factor specifically from adipocytes inhibited hematopoietic regeneration after myeloablation, leading to deficiency in hematopoietic stem cells, and reducing animal survival. Interestingly, genetic deletion of the same cytokine from other important cells present in the niche (osteoblasts, endothelial or hematopoietic cells) did not affect hematopoietic recovery after 5-fluorouracil treatment or irradiation. Additionally, Zhou and colleagues showed that in the caudal vertebrae, where adipocytes are more abundant, hematopoietic stem cell maintenance depends on stem cell factor produced in fat cells.
Figure 1. Adipocytes promotes hematopoietic regeneration in the bone marrow.
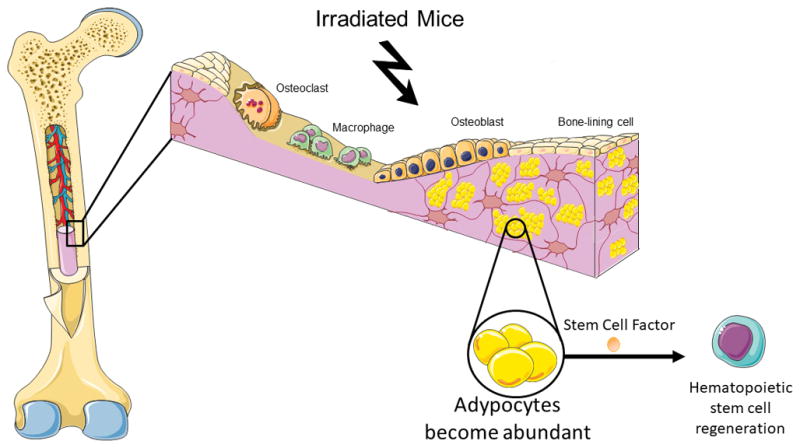
Adipocytes increase in the bone marrow microenvironment with after irradiation. The study of Zhou and colleagues now reveals a novel very important function for adipocytes in the hematopoietic stem cells recovery after myeloablation (19). Adipocyte-derived stem cell factor mediate hematopoietic regeneration in the bone marrow, and absence of stem cell factor specifically in adipocytes leads to deficiency in hematopoietic stem cells, and reduces animal survival.
Future studies will reveal other roles of adipocytes in the bone marrow niche, and whether a specific hematopoietic stem cell subpopulation is regulated by adipocytes.
Here, we discuss the findings from this work, and evaluate recent advances in our understanding of adipocyte contribution to the hematopoietic stem cell niche.
PERSPECTIVES / FUTURE DIRECTIONS
The use of traditional transgenic knockout mice models has proved to be beneficial for the discovery of the role of key molecules in physiological and pathological states. Nevertheless, these technologies produce broad changes in gene function throughout the body, affecting several tissues and cell types. Thus, they are limited in that they do little to identify the specific roles of a protein in a specific cell type from a specific tissue in a concrete moment (20). Because the molecular functions of genes may depend on a specific subset of cells in which they are expressed, restricting gene manipulation to specific cells in the organism may be useful to understand protein functions in different cell populations (21). Thus, conditional gene manipulation strategies offer a powerful tool. The main findings from this study are based on the data obtained from tamoxifen-inducible Adipoq promoter driven CreER-LoxP system (22). Adipoq encodes adiponectin, an adipokine expressed in fat cells (23). Note however that expression of Adipoq is not restricted to adipocytes. Bone-forming cells, cardiomyocytes, and skeletal muscle fibers also may express Adipoq under specific pathophysiological conditions (24–26). Additionally, in Adipoq-CreER mice, recombinase expression is not limited adipocyes in the bone marrow, as adipocytes from fat depots in other organs are marked as well. Thus, in Adipoq-CreER/stem cell factor floxed mice, stem cell factor deletion is not restricted to bone marrow adipocytes. Future studies should evaluate the contribution of other possible sources from outside the bone marrow to hematopoiesis.
The hematopoietic stem cells niche in the bone marrow that supports the homeostasis of those cells is a complex microenvironment composed, besides adipocytes, of osteoblasts, osteocytes, osteoclasts, endothelial cells, smooth muscle cells, pericytes, fibroblasts, macrophages, megakaryocytes, lymphocytes, hematopoietic progenitors, neutrophils, peripheral innervations, and Schwann cells (7,27–37). Although recent studies have revealed the contributions of distinct components of the bone marrow to hematopoietic stem cells regulation, little is known about the cross-talk between these cellular components. Future studies should explore how adipocytes affect and are affected by other hematopoietic stem cell niche constituents. Interestingly, a recent study revealed that sympathetic nerves in the bone marrow are critical for efficient hematopoietic recovery after 5-fluorouracil treatment or irradiation. Further studies will be needed to explore in depth which bone marrow cells are targeted by the sympathetic nervous system. The relationship between sympathetic nerves and adipocytes in hematopoietic recovery after genotoxic insults will need to be explored in great detail.
During development, murine hematopoiesis takes place at specific anatomical locations (12). It begins at embryonic day 7.5 in the extraembryonic yolk sac; at embryonic day 9, it progresses in the para-aortic splanchnopleura, chorioallantoic placenta (38), and aorta–gonad–mesonephros; and at embryonic day 10 it happens in the fetal liver, where a huge expansion of hematopoietic stem cells occurs. Finally, at embryonic day 15, it starts in the bone marrow, where it stably occurs throughout the whole adult life (39). Hematopoiesis can also develop under special pathological circumstances external to the bone marrow medullary spaces. Extramedullary hematopoiesis was reported in adults in the adrenal glands, periosteum, heart, spleen, liver, fatty tissue, pleural cavity, kidney, pre-sacral region, intra-spinal tissue, para-vertebral regions, nasopharyngeal region, para-nasal sinuses, and several types of benign and malign cancers (40–45). It remains to be elucidated whether adipocytes provide and adaptive niche, serving hematopoiesis at multiple developmental stages of mammalian life, or only in the bone marrow. Also, it will be interesting to explore the role of adipocytes as a niche cell in extramedullary hematopoiesis.
Zhou and colleagues revealed that adipocyte derived-stem cell factor is important for hematopoiesis (19). Stem cell factor is expressed by a number of bone marrow cell types as several other cytokines that bind to receptors on hematopoietic stem cells. The complex regulation of the multiple fates of hematopoietic stem cells, including self-renewal, quiescence, differentiation, and mobilization from the bone marrow niche, requires the cooperative orchestration of several cytokines. Future studies should explore whether other adipocyte-derived signals may be as important as stem cell factor for hematopoiesis. Multiple molecules important in hematopoietic stem cell regulation were identified, including stromal cell derived factor (CXCL12 chemokine) (46), bone morphogenic proteins (47), transforming growth factor β (48), Thrombopoietin (49), the Notch ligands Delta and Jagged (50), Angiopoietin-1 (51), Angiopoietin-like proteins (52), Insulin-like growth factor 2 (53), fibroblast growth factors (FGF) (54), VCAM-1 (55), Wnt signaling ligands (56), interleukin-10 (57), and others (58,59). Future studies will reveal whether those molecules are important in adipocytes-regulation of bone marrow hematopoiesis.
Knowledge about how hematopoietic stem cell are regulated in their normal bone marrow microenvironment has led to progress in the characterization and understanding of how leukemic stem cells interact with their niche. Recent studies have shown that alterations in the normal bone marrow microenvironment may trigger the formation of pre-leukemic niches (60). Recent studies suggest active participation of resident adipocytes in tumor biology (61–63). This growing evidence highlight the need for further investigation of the adipocyte derived signals involved in the bone marrow fat tissue influence on leukemia cell survival, progression, and spread. While Zhou and colleagues reveal that adipocyte-derived stem cell factor is an essential factor for hematopoietic stem cell regulation (19), its role in the modulation of tumor burden still needs to be explored. The mechanistic understanding of leukemic stem cells protection, through modulation of local concentrations of chemotherapy, support of tumor cells in a dormant state, or stimulation of overt anti-apoptotic signaling cascades is essential to the development of novel therapeutic strategies to blunt residual disease. Whether bone marrow adipocytes-derived signals are important in these processes, and specifically the stem cell factor, remains unknown.
Hematopoietic stem cells change functionally with physiological age (64). The alterations noted in aged hematopoietic stem cells were until recently thought to be a consequence of cell-intrinsic modifications (65,66). Nevertheless, new data the important role of multiple extrinsic factors in driving hematopoietic stem cell aging (67). Zhou and colleagues investigated the importance of adipocyte-derived stem cell factor in genotoxic insults in the bone marrow (19). It will be interesting to explore the role of adipocyte-derived stem cell factor on hematopoietic stem cells during bone marrow aging.
Hematopoietic stem cells represent a heterogeneous cell population based on their molecular markers (7), life span (68), differentiation capabilities (69), and degree of self-renewal (70). Little is known about the extrinsic regulation of hematopoietic stem cell subpopulations. The functional heterogeneity of hematopoietic stem cells points to the potential for matching heterogeneity in the niche influences that support the function of hematopoietic stem cell subsets. For instance, it is unclear whether adipocyte-derived stem cell factor affect differently distinct hematopoietic stem cell subpopulations. The anatomy of the bone marrow is complex, and the heterogeneity within bone marrow adipocytes was not explored yet. Are all adipocytes in the bone marrow equal? If not, is there a specific adipocyte subgroup more important for hematopoiesis regulation? The genetically modified mice and imaging technologies allow us to follow specific cells at a single level (71,72). These powerful tools help us to elucidate the exact roles of particular cell populations in several functions (73–78). These techniques will further reveal the complex nature of the mechanisms by which extrinsic factors regulate signal transduction and cell-fate decisions of hematopoietic stem cells. In addition to studies in genetic mouse models, transcriptomic and single cell analysis after fluorescence activated cell sorting of live cells represent fundamental tools that will help us understand the roles of adipocytes within the bone marrow microenvironment (79–82). In spite of powerful experimental mouse models providing a proof of concept for adipocytes biology in the bone marrow, we are still lacking direct demonstration of adipocytes roles in the human bone marrow. The main challenge for the future will be to translate mouse research into humans. Improving the availability of human tissue samples will be essential to reach this goal. The isolation of adipocytes by cytometry from human bone marrow biopsies may support the data provided by elegant mouse model studies.
In conclusion, the study by Zhou and colleagues reveal a novel important role of adipocytes in hematopoietic stem cells regulation in the bone marrow, adding knowledge to the progress being made in elucidating the key cells responsible for regulating the hematopoietic niche. Transgenic mouse models, powerful imaging techniques, cell sorting, and single cell gene analysis have led to a more comprehensive understanding of hematopoietic stem cell niche cellular components. Although these technological improvements have clarified certain longstanding questions, our understanding of the exact origin, identity and detailed function of the majority of bone marrow cells remained to be studied in greater detail. Thus, our understanding of hematopoietic stem cells regulation in their niche still remains limited, and the complexity and interactions of different cellular components of the bone marrow microenvironment in diverse pathophysiological conditions should be elucidated in future studies.
Supplementary Material
Acknowledgments
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD).
Footnotes
DISCLOSURES
The authors indicate no potential conflicts of interest.
LITERATURE CITED
- 1.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 5.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 6.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370:82–96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–7. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 11.Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, Santos G, Guerra D, Prazeres P, Mesquita LL, et al. Lung as a Niche for Hematopoietic Progenitors. Stem Cell Rev. 2017 doi: 10.1007/s12015-017-9747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176–80. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289:16699–710. doi: 10.1074/jbc.M114.547919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touw I, Lowenberg B. No stimulative effect of adipocytes on hematopoiesis in long-term human bone marrow cultures. Blood. 1983;61:770–4. [PubMed] [Google Scholar]
- 16.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 17.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhu RJ, Wu MQ, Li ZJ, Zhang Y, Liu KY. Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol. 2013;97:58–72. doi: 10.1007/s12185-012-1233-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A. Pericytes Make Spinal Cord Breathless after Injury. Neuroscientist. 2017 doi: 10.1177/1073858417731522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O. How Plastic Are Pericytes? Stem Cells Dev. 2017;26:1013–1019. doi: 10.1089/scd.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sassmann A, Offermanns S, Wettschureck N. Tamoxifen-inducible Cre-mediated recombination in adipocytes. Genesis. 2010;48:618–25. doi: 10.1002/dvg.20665. [DOI] [PubMed] [Google Scholar]
- 23.Hivert MF, Manning AK, McAteer JB, Florez JC, Dupuis J, Fox CS, O’Donnell CJ, Cupples LA, Meigs JB. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57:3353–9. doi: 10.2337/db08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 26.Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589–97. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- 27.Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, et al. Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-017-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis. 2017;8:e3072. doi: 10.1038/cddis.2017.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, Sena IFG, Prazeres P, Borges IT, Azevedo V, et al. Endothelial Cells as Precursors for Osteoblasts in the Metastatic Prostate Cancer Bone. Neoplasia. 2017;19:928–931. doi: 10.1016/j.neo.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, Prazeres P, Filev R, Mintz A, Birbrair A. Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience. 2017;363:62–65. doi: 10.1016/j.neuroscience.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 32.Andreotti JP, Lousado L, Magno LAV, Birbrair A. Hypothalamic Neurons Take Center Stage in the Neural Stem Cell Niche. Cell Stem Cell. 2017;21:293–294. doi: 10.1016/j.stem.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017;427:6–11. doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245. doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Guerra DAP, Lousado L, et al. Identity of Gli1+ cells in the bone marrow. Exp Hematol. 2017;54:12–16. doi: 10.1016/j.exphem.2017.06.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Santos GSP, Guerra DAP, Prazeres P et al. LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle. 2017:1–5. doi: 10.1080/15384101.2017.1367072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–63. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–31. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 40.Sohawon D, Lau KK, Lau T, Bowden DK. Extra-medullary haematopoiesis: a pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol. 2012;56:538–44. doi: 10.1111/j.1754-9485.2012.02397.x. [DOI] [PubMed] [Google Scholar]
- 41.Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49:508–23. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- 42.Tsamandas AC, Jain AB, Raikow RB, Demetris AJ, Nalesnik MA, Randhawa PS. Extramedullary hematopoiesis in the allograft liver. Mod Pathol. 1995;8:671–4. [PubMed] [Google Scholar]
- 43.Vassiliou V, Papamichael D, Lutz S, Eracleous E, Kountourakis P, Polyviou P, Michaelides I, Shoukris M, Andreopoulos D. Presacral Extramedullary Hematopoiesis in a Patient with Rectal Adenocarcinoma: Report of a Case and Literature Review. J Gastrointest Cancer. 2012;43(Suppl 1):S131–5. doi: 10.1007/s12029-012-9370-9. [DOI] [PubMed] [Google Scholar]
- 44.Macki M, Bydon M, Papademetriou K, Gokaslan Z, Bydon A. Presacral extramedullary hematopoiesis: an alternative hypothesis. J Clin Neurosci. 2013;20:1664–8. doi: 10.1016/j.jocn.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, Morrison SJ. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015;527:466–471. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19:214–223. doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 48.Ross J, Li L. Recent advances in understanding extrinsic control of hematopoietic stem cell fate. Curr Opin Hematol. 2006;13:237–42. doi: 10.1097/01.moh.0000231420.92782.8f. [DOI] [PubMed] [Google Scholar]
- 49.Matsunaga T, Kato T, Miyazaki H, Ogawa M. Thrombopoietin promotes the survival of murine hematopoietic long-term reconstituting cells: comparison with the effects of FLT3/FLK-2 ligand and interleukin-6. Blood. 1998;92:452–61. [PubMed] [Google Scholar]
- 50.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–47. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 51.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–21. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 54.de Haan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E, Miller G. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–51. doi: 10.1016/s1534-5807(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 55.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 56.Nemeth MJ, Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17:746–58. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- 57.Kang YJ, Yang SJ, Park G, Cho B, Min CK, Kim TY, Lee JS, Oh IH. A novel function of interleukin-10 promoting self-renewal of hematopoietic stem cells. Stem Cells. 2007;25:1814–22. doi: 10.1634/stemcells.2007-0002. [DOI] [PubMed] [Google Scholar]
- 58.Ueno H, Sakita-Ishikawa M, Morikawa Y, Nakano T, Kitamura T, Saito M. A stromal cell-derived membrane protein that supports hematopoietic stem cells. Nat Immunol. 2003;4:457–63. doi: 10.1038/ni916. [DOI] [PubMed] [Google Scholar]
- 59.Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316:590–3. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- 60.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–9. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner M, Dudley AC. A three-party alliance in solid tumors: Adipocytes, macrophages and vascular endothelial cells. Adipocyte. 2013;2:67–73. doi: 10.4161/adip.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25–38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, et al. Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. Stem Cells Transl Med. 2017;6:471–481. doi: 10.5966/sctm.2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–46. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–89. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 66.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C1098–113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura-Ishizu A, Suda T. Aging of the hematopoietic stem cells niche. Int J Hematol. 2014;100:317–25. doi: 10.1007/s12185-014-1641-8. [DOI] [PubMed] [Google Scholar]
- 68.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–23. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 69.Muller-Sieburg CE, Sieburg HB, Bernitz JM, Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–7. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–14. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014;5:122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res. 2013;319:45–63. doi: 10.1016/j.yexcr.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birbrair A, Delbono O. Pericytes are Essential for Skeletal Muscle Formation. Stem Cell Rev. 2015;11:547–8. doi: 10.1007/s12015-015-9588-6. [DOI] [PubMed] [Google Scholar]
- 76.Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, Assoni AF, Pelatti MV, Birbrair A, de Lima ACP, et al. Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient. Stem Cell Rev. 2017 doi: 10.1007/s12015-017-9752-2. [DOI] [PubMed] [Google Scholar]
- 77.Pereira LX, Viana CTR, Orellano LAA, Almeida SA, Vasconcelos AC, Goes AM, Birbrair A, Andrade SP, Campos PP. Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life Sci. 2017;176:67–74. doi: 10.1016/j.lfs.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 78.Santos GSP, Prazeres P, Mintz A, Birbrair A. Role of pericytes in the retina. Eye (Lond) 2017 doi: 10.1038/eye.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, Klemm DJ. Analysis and isolation of adipocytes by flow cytometry. Methods Enzymol. 2014;537:281–96. doi: 10.1016/B978-0-12-411619-1.00015-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doan M. Zooming in on adipocytes: High and deep. Cytometry A. 2017;91:1051–1054. doi: 10.1002/cyto.a.23269. [DOI] [PubMed] [Google Scholar]
- 81.Bombrun M, Gao H, Ranefall P, Mejhert N, Arner P, Wahlby C. Quantitative high-content/high-throughput microscopy analysis of lipid droplets in subject-specific adipogenesis models. Cytometry A. 2017;91:1068–1077. doi: 10.1002/cyto.a.23265. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell A, Ashton L, Yang XB, Goodacre R, Smith A, Kirkham J. Detection of early stage changes associated with adipogenesis using Raman spectroscopy under aseptic conditions. Cytometry A. 2015;87:1012–9. doi: 10.1002/cyto.a.22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


