Abstract
The genetic basis of familial primary pulmonary hypertension (FPPH) is unknown, but the clinical and pathologic features are the same as in sporadically occurring primary pulmonary hypertension (PPH). Because few families with this disease have been reported, the mode of inheritance and genetic features have not been clearly established. We previously reported a tendency for decreasing age of onset in subsequent generations of affected families. The purpose of this study was to examine the pattern of inheritance in a large number of families in an attempt to find clues to pathogenesis. From 24 families we studied 429 members, 124 of whom were known to carry the gene for disease. We constructed cumulative mortality curves for each gender of the 99 affected individuals. We analyzed gender ratios of progeny of affected members and carriers and compared age at death of affected members by generation. More females (160) than males (122) were born to persons carrying the gene, p < 0.01, suggesting selective wastage of male fetuses or an abnormal primary sex ratio. Genetic anticipation was confirmed; the age at death was 45.6 ± 14.5 versus 36.3 ± 12.6 versus 24.2 ± 11 standard deviation (SD) years in successive generations, p < 0.05. Five cases of male-to-male transmission were observed, excluding X-linkage. Age at death was the same for males and females. More females had the gene (84 females, 40 males) and more females with the gene developed disease (72 of 84 females [86%] versus 27 of 40 males [68%]). The disease has highly variable penetrance among families. Genetic anticipation suggests that trinucleotide repeat amplification is the mechanism for the molecular basis for disease, similar to fragile X syndrome, myotonic dystrophy, and Huntington’s disease. Abnormal gender ratio of progeny raises the possibility that the gene is involved in embryologic development. These clues provide direction for a search for the gene responsible for FPPH.
Primary pulmonary hypertension (PPH) is a progressive, fatal disease in which the singular abnormality is proliferative occlusion of small pulmonary arteries (1). Women, usually of child-bearing age, are affected twice as frequently as men. Interest in this illness has increased because of the advent of effective vasodilator therapy and the success of lung transplantation. PPH occurs in families (2–19). It occasionally affects most or all members in a sibship, but more often affects only a few individuals among several members at risk and skips generations. Twelve (6%) of the 187 patients enrolled in the National Institutes of Health (NIH)-sponsored Primary Pulmonary Hypertension Registry (20) had two or more family members with the disease. The clinical and pathologic features of these 12 patients (20) and 24 other patients with familial primary pulmonary hypertension (FPPH) (21) were identical to those of patients with sporadic PPH. Because of the rarity of the familial form of this disease, and because of the incomplete expression of the gene in families, the mode of inheritance and genetic features are not fully understood. It has been assumed that the familial form of PPH is transmitted as an autosomal dominant trait. In earlier studies, we and others (21, 22) found a tendency for disease to develop in younger members of subsequent generations in affected families. This phenomenon, genetic anticipation, was questioned as a real entity until the recent discovery that trinucleotide repeat amplification forms the basis for several diseases, including fragile X syndrome, myotonic dystrophy, Huntington’s disease, and X-linked spinal and bulbar muscular atrophy (Kennedy’s disease).
We have collected and analyzed information from more than 500 members of 32 families with familial PPH, 142 of whom are affected with the disease or who carry the gene. We found an abnormal gender ratio at birth in offspring of persons with the gene, suggesting selective male fetal loss, as well as the clear presence of genetic anticipation. Our results suggest that familial PPH is inherited by autosomal dominant transmission with variable penetrance. The presence of genetic anticipation suggests the presence of trinucleotide repeats in the genome, and the abnormal gender ratio suggests that the gene responsible for the vasculopathy may have a role in embryologic development.
METHODS
Between 1954 and 1984, 14 American families with two or more cases of PPH were reported in the medical literature (2–19). To study the genetic transmission of this disease, we attempted to locate all reported families and found eight new cases of PPH in the nine families we were able to contact (21). We have continued to collect biographic and clinical information on other American families with PPH, and we currently maintain records of 32 families within the United States who have had two or more members with FPPH. Medical information and records were collected from published reports, patients, family members, and hospitals. Many of these PPH patients died in the 1950s to 1970s before our studies began, so most patients were not examined by the investigators. All patients had the diagnosis of PPH made by findings at cardiac catheterization or autopsy, with compatible signs and symptoms of PPH, and the absence of secondary causes. Probable PPH was defined by premature death after signs and symptoms compatible with PPH, usually substantiated by electrocardiographic findings of right ventricular hypertrophy, and the absence of secondary causes.
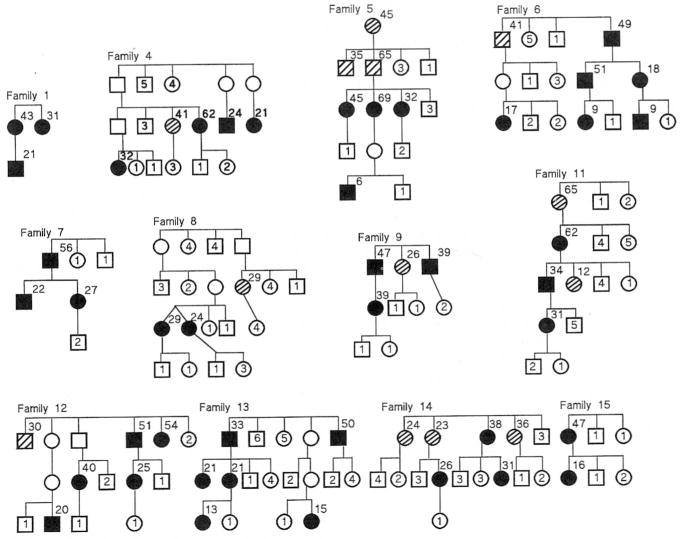
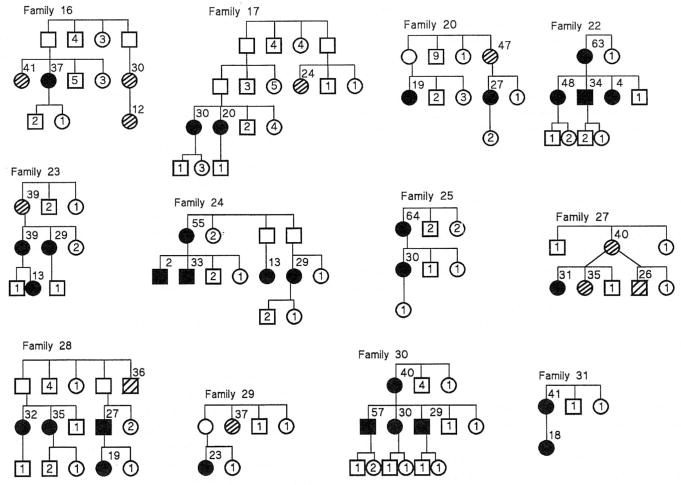
Abbreviated pedigrees of the 24 families which could be analyzed for transmission (affected members in more than one sibship) are shown in Figures 1 and 2. For brevity, the sibships are shown in condensed form without regard for order of birth and include only the individuals at risk (first degree relatives). Seventeen families (Families 1–13, 18, 19, 21, 26) have been previously reported in the medical literature. To determine if bias existed to report families with numerous cases (compared with families with fewer cases) we reviewed all reports to identify the total number of proven cases at the time of the original publication. Of the reported families, 13 (76%) of 17 families had only two proven cases at the time of the first report, 3 of 17 families (Families 7, 12, 13) had three proven cases, and 1 of 17 families (Family 10) had four proven cases. We conclude that there was no appreciable bias to report families with multiple affected members, because 76% of the PPH families were originally reported when only two cases had occurred in those families. Nine families (Families 14, 15, 16, 17, 27, 28, 29, 31, 32) were identified via direct patient referral to us, and six families were identified by colleagues who supplied clinical and biographic information to us (see acknowledgment).
Figure 1.
Abbreviated pedigrees of Families 1–15 with familial primary pulmonary hypertension which were analyzed for transmission of disease. Solid symbols represent individuals with disease. Circles represent females, squares represent males. Crosshatch symbols represent individuals who had probable PPH (see Methods). Open symbols without numbers represent carriers. Numbers inside symbols represent numbers of unaffected siblings of each gender. Numbers to upper right of symbols indicate age at PPH death.
Figure 2.
Abbreviated pedigrees of Families 16–31 with familial primary pulmonary hypertension which were analyzed for transmission of disease. Solid symbols represent individuals with disease. Circles represent females, squares represent males. Crosshatch symbols represent individuals who had probable PPH (see Methods). Open symbols without numbers represent carriers. Numbers inside symbols represent numbers of unaffected siblings of each gender. Numbers to upper right of symbols indicate age at PPH death.
Eight families including 18 PPH patients were excluded from analysis for the following reasons: five families (Families 3, 10, 18, 19, 32) with 12 patients were excluded because PPH was limited to a single sibship so transmission could not be analyzed; three families (Families 2, 21, 26) with six patients were excluded because the report included only an affected mother and one child, without other information about the family. We analyzed transmission in the other 24 families. Pedigrees of the 24 families, abbreviated to depict only individuals at risk, are presented in Figures 1 and 2.
Thirteen of the 32 FPPH families reside in Tennessee and the others are distributed throughout the United States.
Statistics
The data are reported as mean values ± 1 standard deviation. Significance testing was performed using Student’s t test and chi square with Yates correction. Significance for the gender distribution of progeny was calculated from the binomial distribution calculated under the null hypothesis that the normal proportion of males is 51% at the time of birth (23). For the age at death by generation, we performed nonparametric analysis of variance by the Kruskal-Wallis test, followed by group comparisons using the Mann-Whitney test. Significance was assumed when the p value was less than 0.05.
RESULTS
A total of 429 individuals (233 females = 54%, 196 males = 46%) were identified to be at risk in the 24 FPPH families (Figures 1 and 2). Individuals known to have the gene (they have disease or progeny with disease) had 282 children (160 females = 57%, 122 males = 43%, p < 0.01). If a normal gender ratio had occurred in the children of individuals with the gene, for the 160 females born, 167 males (rather than the observed 122) should have been born. We have no information on the rate or gender of miscarried pregnancies in these families. If the number of males calculated to be missing at birth (n = 45) were hypothesized to be PPH-affected, then the number of affected males and females would be the same, 72 versus 72.
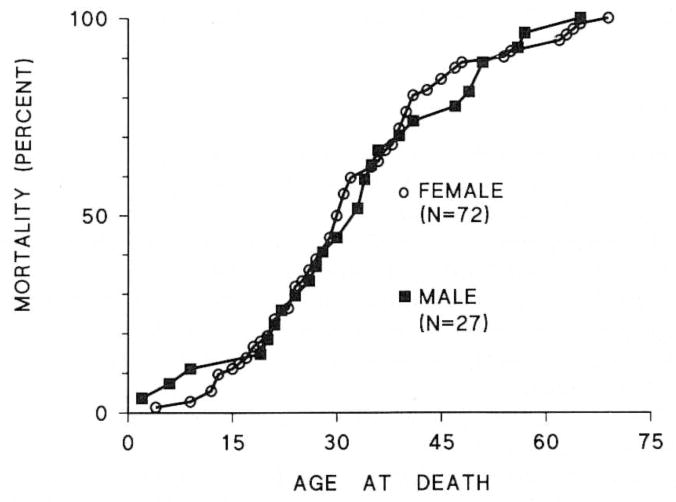
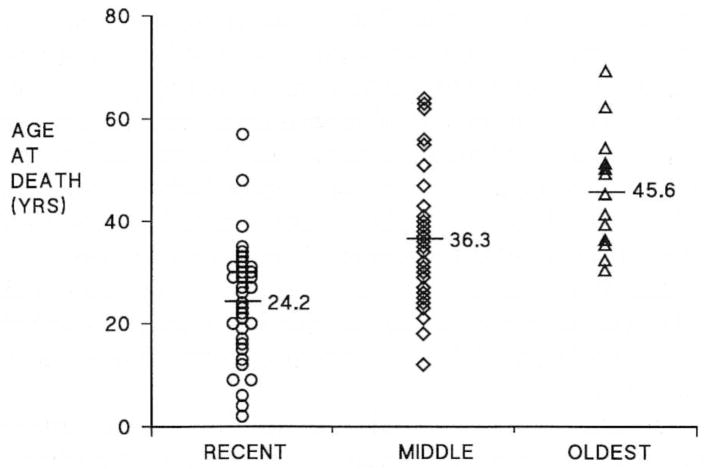
The age at death of patients with FPPH ranged from age 5 to 67 yr (Figure 3). The mean age at death between females (32.4 ± 14.5 yr) and males (34.3 ± 15.8 yr) did not differ. The gender ratio of affected patients was 72 females:27 males (2.7:1). The mean age at death in each generation (Figure 4) decreased from 45.6 ± 11.2 yr(n = 13) in the earliest generation to 36.3 ± 12.6 yr (n = 41) in the next generation, to 24.2 ± 11.0 yr (n = 42) in the most recent generation, p < 0.05 between each group. Ten patients who acquired disease from an asymptomatic heterozygous mother died at a mean of 19.8 ± 5.9 yr, whereas 16 PPH patients who acquired disease from an asymptomatic heterozygous father died at 32.6 ± 10.5 yr, p < 0.05; thus, the age at death of familial PPH patients was younger when disease was acquired from an asymptomatic heterozygous mother than from an asymptomatic heterozygous father.
Figure 3.
Cumulative mortality curves of 72 female and 27 male patients with familial primary pulmonary hypertension. There is no difference between the curves.
Figure 4.
Age at death versus generation in familial primary pulmonary hypertension. The mean age at death was significantly different for each generation, p < 0.05.
Of the total 429 individuals in the 24 families that could be analyzed for transmission, 124 had the gene for PPH, of whom 99 had PPH and 25 were asymptomatic heterozygotes (have progeny with PPH but were not themselves symptomatic). Of the 99 patients affected with PPH, 72 (73%) were female and 27 (27%) were male. Of the 25 asymptomatic heterozygotes, 12 (48%) were female and 13 (52%) were male. Therefore, there were 84 females with the gene, 72 (86%) of whom had PPH, and 12 (14%) of whom were asymptomatic heterozygotes. There were 40 males who had the gene, of whom 27 (68%) had PPH and 13 (32%) were asymptomatic heterozygotes. Thus, the absolute number of asymptomatic heterozygotes was similar between genders (12 versus 13).
The age correction for penetrance shows that by age 10, 10% of individuals with a familial PPH gene developed disease. At age 70, 92% of people with familial PPH gene had disease. Penetrance calculations for females demonstrate that 72 of the expected 116 (50% of 233 at risk) developed PPH for a penetrance of 62%. Disease was present in 27 males of the expected 98 (50% of 196) for a penetrance of 28%.
DISCUSSION
Primary pulmonary hypertension, a progressive disease of small pulmonary arteries, usually leads to death within 3 yr after diagnosis (24). The sporadic and familial forms of PPH have similar clinical and pathologic features (20, 21). Although it is possible that PPH is a syndrome with more than one pathogenesis (1), pathologic analysis of lung specimens in FPPH suggests that disease is the result of the same basic process (25). Indirect evidence suggests that PPH may be associated with autoimmune disease. Recently, other investigators (22, 26) have reported an association between the major histocompatibility complex (MHC) and PPH.
In the NIH PPH patient registry (20), 12 (6%) of the 187 patients had a positive family history of PPH. The only difference identified was a shorter time from symptom onset to diagnosis in familial PPH, as would be expected from earlier recognition of subsequent cases in affected families. In our 99 patients, the cumulative mortality curves show that there is no difference in age at death between affected men 34.3 ± 17 yr and women 32.4 ± 16 yr. The mean age at death of patients from the NIH PPH registry was not reported, but the mean age at the time of enrollment was 36.4 yr (20), similar to our patients with familial PPH. We found deaths in familial PPH at both extremes of age (Figure 3). Twenty percent died from PPH after age 50.
This is the first investigation to report an abnormal gender ratio at birth in offspring of persons carrying the gene for familial PPH. Individuals with the gene had children with a gender ratio of 57% female and 43% male. One possible explanation for the abnormal gender ratio at birth is an increased loss of males in utero. Increased male fetal wastage suggests the possibility of an X-linked dominant mode of inheritance, causing increased death in utero of hemizygous males, but this possibility is excluded by the observation of five instances of father-to-son transmission (Families 4, 6, 7, 17, 28). Another possible explanation for the abnormal gender ratio at birth is an abnormality of the gender ratio at fertilization, which suggests the possibility that the FPPH gene might be related to the developmental process, such as the capability of X- or Y-bearing sperm to reach or fertilize the ovum.
The molecular basis of genetic anticipation was recently elucidated in the fragile X syndrome, where the phenomenon has been referred to as the Sherman paradox (27, 28). A trinucleotide (CGG) in the 5′ region of the Fragile X gene (FMR-1) is repeated in tandem 6 to 54 times in normal individuals, but repeats more than 200 times in patients with fragile X syndrome. The trinucleotide repeat becomes unstable, particularly during oocyte meiosis, after the tandem repeats exceed 52 in number. Methylation of certain critical CpG dinucleotides that accompanies the expansion silences transcription of the abnormal allele.
Myotonic dystrophy is a common autosomal dominant myopathy whose severity increases over multiple generations, suggesting a mutational mechanism similar to that found in the fragile X syndrome. A highly polymorphic unstable CTG repeat has been identified at locus (19 q 13.2–13.3) in myotonic dystrophy patients (29, 30). An increase in the severity of the disease in successive generations is accompanied by an increase in the number of trinucleotide CTG repeats. Nearly all affected individuals (98% of 258 patients) display expansion of the CTG repeat region. These results suggest that myotonic dystrophy is caused by mutations that generate an amplification of a specific CTG repeat. They also illustrate a tendency for the variable length polymorphism alleles to increase in size in successive generations. The presence of genetic anticipation in familial PPH suggests a molecular mechanism (amplification of GC-rich repeat sequences) similar to those recently discovered in other diseases which manifest genetic anticipation.
Thirteen of the 32 families with PPH that we have studied reside in Tennessee. Families with PPH may cluster in Tennessee, or our interest in the disease may have brought families to our attention. We have been unable to identify any common ancestry or consanguinity among the PPH families in Tennessee. If the incidence of familial PPH in Tennessee (population = 4.8 million) is representative of the entire United States (population = 248 million), there may be more than 650 PPH families in the U.S. We know several patients who were initially diagnosed as sporadic PPH, but then later had a relative develop PPH. It seems possible that many patients who appear to have sporadic PPH may actually have familial PPH in a family with low penetrance or with inadequate information about the health of their relatives.
In summary, familial primary pulmonary hypertension has two unusual genetic manifestations, an abnormal gender ratio and genetic anticipation. These features are clues to the molecular basis of familial PPH which is probably a disease of trinucleotide repeat expansion.
Acknowledgments
Funded by NHLBI SCOR 19153, Saint Thomas Foundation, CRC 5M01RR0095.
The authors express their appreciation and acknowledge that these studies would not have been possible without the extensive information about PPH families which was contributed by the following individuals: Dr. F. Tremaine Billings, Jr., Dr. Robert Burtch, Dr. Frank Carter, Dr. Richard Childress, Dr. George H. Christ, Dr. W. C. Crowder, Dr. James Dauber, Dr. Joseph H. Davis, Dr. C. Gregory Elliott, Dr. William H. Frist, Dr. Fred Flaley, Dr. Gary F. Haverty, Dr. Grady H. Hendrix, Dr. Thomas V. Inglesby, Dr. Herbert D. Ladley, Dr. Carl V. Leier, Paul S. Levy, Sc.D., Dr. Michael D. McGoon, Dr. Kenneth Melmon, Dr. Ernest B. Page, Jr., Dr. Robert H. Peter, Dr. Walter Puckett, III, Dr. Stuart Rich, Dr. James D. Rogge, Dr. Harvey M. Rosenbaum, Dr. Lisa E. Schriver, Dr. Dan Sedmak, Dr. Theofilos Tsagaris.
References
- 1.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Levy PS, Reid LM, Vreim CE, Williams GW. Histopathology of primary pulmonary hypertension. Circulation. 1989;80:1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 2.Dresdale DT, Michtom RJ, Schultz M. Recent studies in primary pulmonary hypertension, including pharmacodynamic observations on pulmonary vascular resistance. Bull N Y Acad Med. 1954;30:195. (Family 1) [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffner F. Clinico-Pathological Conference. Mt Sinai J. 1958:479–494. (Family 2) [Google Scholar]
- 4.Husson GS, Wyatt TC. Primary pulmonary obliterative vascular disease in infants and young children. Pediatrics. 1959;23:493–506. Family 3. [PubMed] [Google Scholar]
- 5.Sleeper JC, Orgain ES, McIntosh HD. Primary pulmonary hypertension: review of clinical features and pathologic physiology with a report of pulmonary hemodynamics derived from repeated catheterization. Circulation. 1962;26:1358–1369. doi: 10.1161/01.cir.26.6.1358. Family 4. [DOI] [PubMed] [Google Scholar]
- 6.Boiteau GM, Libanoff AJ. Primary pulmonary hypertension: familial incidence. Angiology. 1963;14:260–264. doi: 10.1177/000331976301400506. Family 5. [DOI] [PubMed] [Google Scholar]
- 7.Melmon KL, Braunwald E. Familial pulmonary hypertension. N Engl J Med. 1963;269:770–775. doi: 10.1056/NEJM196310102691502. Family 5. [DOI] [PubMed] [Google Scholar]
- 8.Rogge JD, Mishkin ME, Genovese PD. The familial occurrence of primary pulmonary hypertension. Ann Intern Med. 1966;65:672–684. doi: 10.7326/0003-4819-65-4-672. (Family 6) [DOI] [PubMed] [Google Scholar]
- 9.Kingdon HS, Cohen LS, Roberts WC, Braunwald E. Familial occurrence of primary pulmonary hypertension. Arch Intern Med. 1966;118:422–426. Family 7. [PubMed] [Google Scholar]
- 10.Porter CM, Creech BJ, Billings FT. Primary pulmonary hypertension occurring in twins. Arch Intern Med. 1967;120:224–229. Family 8. [PubMed] [Google Scholar]
- 11.Czarnecki SW, Rosenbaum HM, Wachtel HL. The occurrence of primary pulmonary hypertension in twins with a review of etiological considerations. Am Heart J. 1968;75:240–246. doi: 10.1016/s0002-8703(68)90069-0. Family 8. [DOI] [PubMed] [Google Scholar]
- 12.Tsagaris TJ, Tikoff G. Familial primary pulmonary hypertension. Am Rev Respir Dis. 1968;97:127–130. Family 9. [Google Scholar]
- 13.Massoud H, Puckett W, Auerbach SH. Primary pulmonary hypertension: a study of the disease in four young siblings. J Tenn Med Assoc. 1970;63:299–305. Family 10. [PubMed] [Google Scholar]
- 14.Inglesby TV, Singer JW, Gordon DS. Abnormal fibrinolysis in familial pulmonary hypertension. Am J Med. 1973;55:5–14. doi: 10.1016/0002-9343(73)90144-7. Family 11. [DOI] [PubMed] [Google Scholar]
- 15.Hendrix GH. Familial primary pulmonary hypertension. S Med J. 1974;67:981. doi: 10.1097/00007611-197408000-00022. (Family 12) [DOI] [PubMed] [Google Scholar]
- 16.Tubbs RR, Levin RD, Shirey EK, Hoffman GC. Fibrinolysis in familial pulmonary hypertension. Am J Clin Pathol. 1979;71:384–387. doi: 10.1093/ajcp/71.4.384. Family 13. [DOI] [PubMed] [Google Scholar]
- 17.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis. 1984;129:194–197. doi: 10.1164/arrd.1984.129.1.194. (Family 14) [DOI] [PubMed] [Google Scholar]
- 18.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–587. doi: 10.1161/01.cir.70.4.580. Families 18 and 19. [DOI] [PubMed] [Google Scholar]
- 19.Brown DL, Wetli CV, Davis JH. Sudden unexpected death from primary pulmonary hypertension. J Forensic Sci. 1981;26:381–386. (Family 21-not located, Family 26) [PubMed] [Google Scholar]
- 20.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 21.Loyd JE, Primm RK, Newman JH. Transmission of familial primary pulmonary hypertension. Am Rev Respir Dis. 1984;129:194–197. doi: 10.1164/arrd.1984.129.1.194. [DOI] [PubMed] [Google Scholar]
- 22.Morse JH, Barst RJ, Fotino M. Familial pulmonary hypertension: immunogenetic findings in four caucasian kindreds. Am Rev Respir Dis. 1992;145:787–792. doi: 10.1164/ajrccm/145.4_Pt_1.787. [DOI] [PubMed] [Google Scholar]
- 23.Stern C. The Sex Ratio. 3. Chapter 21. WH Freeman & Co; San Francisco: 1973. Principles in Human Genetics. [Google Scholar]
- 24.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 25.Loyd JE, Atkinson JE, Virmani R, Peitra GG, Newman JH. Heterogeneity of pathologic lesions in familial PPH. Am Rev Respir Dis. 1988;138:952–957. doi: 10.1164/ajrccm/138.4.952. [DOI] [PubMed] [Google Scholar]
- 26.Barst RJ, Flaster EK, Menon A, Fotino M, Morse JH. Evidence for the association of unexplained pulmonary hypertension in children with the major histocompatibility complex. Circulation. 1992;85:249–258. doi: 10.1161/01.cir.85.1.249. [DOI] [PubMed] [Google Scholar]
- 27.Fu YH, Kuhl DPA, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJMH, Holden JJA, Fenwick RG, Jr, Warren ST, Oostra BA, Nelson DL, Caskey CT. Variation of the CGG repeat at the Fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 28.Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang F, Eussen BE, van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in Fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 29.Fu YH, Pizzuti A, Fenwick RG, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, DeJong P, Wieringa B, Korneluk R, Perryman MB, Epstein HF, Caskey CT. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 30.Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, Neville C, Narang M, Barcelo J, O’Hoy K, Leblond S, Earle-Macdonald J, DeJong PJ, Wieringa B, Korneluk RG. Myotonic dystrophy mutation: an ustable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]