Pazopanib is an oral tyrosine‐kinase inhibitor that is approved by the FDA for treatment of metastatic renal cell carcinoma. This article focuses on the concurrent treatment of metastatic renal cell carcinoma with pazopanib and a proton pump inhibitor or H2‐receptor antagonist in daily practice.
Keywords: Clear‐cell metastatic renal cell carcinoma, Pazopanib, Proton pump inhibitors, Histamine H2 antagonists
Abstract
Background.
Pazopanib is an oral tyrosine‐kinase inhibitor that is approved by the U.S. Food and Drug Administration for treatment of metastatic renal cell carcinoma (mRCC). Pharmacokinetic data have shown that concomitant administration of pazopanib and esomeprazole, a proton pump inhibitor (PPI), leads to decreased area under the curve and thus decreased exposure of pazopanib by 40%. Despite the pharmacokinetic data published to date, the clinical significance and impact on patient outcomes resulting from decreased pazopanib exposure remains unknown.
Materials and Methods.
In this retrospective, observational, cohort study, 90 patients with mRCC who either received pazopanib in combination with a PPI or histamine 2 receptor antagonist (H2RA; concurrent PPI/H2RA group) or who did not take concurrent pH‐elevating medications (no PPI/H2RA group) were compared to determine if there was an impact on progression‐free survival (PFS), the primary endpoint, and secondary endpoints, overall survival (OS) and safety.
Results.
The differences between the PFS of 9.0 months and OS of 28.0 months for the concomitant PPI/H2RA group versus 11.0 months and 30.1 months, respectively, for the no PPI/H2RA group were not statistically significant. Rates of adverse events were similar between the concomitant PPI/H2RA and no PPI/H2RA groups.
Conclusion.
Concomitant PPI or H2RA usage was not shown to be associated with a reduction in PFS or OS for patients receiving pazopanib for mRCC, with a similar toxicity profile in each group. Based on the results of this retrospective cohort study and the palliative nature of the treatment of patients with mRCC, clinicians should consider allowing patients to remain on concomitant pazopanib and acid‐reducing therapy.
Implications for Practice.
Pazopanib is a preferred category‐one first‐line treatment for predominant clear cell metastatic renal cell carcinoma (mRCC). However, because of an aging demographic, coupled with patients with mRCC presenting with multiple comorbidities, including symptomatic dyspepsia or gastroesophageal reflux disease, patients are commonly required to take pazopanib concomitantly with a proton pump inhibitor (PPI) or a histamine 2 receptor antagonist (H2RA). Despite earlier pharmacokinetic reports suggesting that an alkaline pH may result in poorer absorption, this institutional retrospective study found no effect on clinical outcomes. These data suggest that concurrent treatment of mRCC with pazopanib and a PPI or H2RA may be safe in everyday practice.
Background
Pazopanib is an oral vascular endothelial growth factor tyrosine‐kinase inhibitor (TKI) that is approved by the U.S. Food and Drug Administration (FDA) for treatment of mRCC [1]. FDA approval of pazopanib was based on the results of a phase III, randomized, double‐blind, placebo‐controlled, multicenter trial [2]. This trial was initially designed to enroll patients only in the second‐line setting but was modified to include treatment‐naïve patients after new evidence showed the benefit of angiogenesis inhibitors in the first‐line setting. The study met its primary endpoint of progression‐free survival (PFS) in both treatment‐naïve as well as treatment‐experienced patients (PFS 11.1 vs. 2.8 months for treatment naïve, p < .0001; 7.4 vs. 4.2 months for treatment experienced, p < .001). Finalized data for the secondary endpoint, overall survival (OS), did not show a statistically significant difference between pazopanib and placebo [3]. However, the primary investigators attributed this lack of statistical significance to confounded data as a result of patient crossover from the placebo arm to the pazopanib arm.
Pazopanib is currently listed in several professional practice guidelines, both in the U.S. and in Europe, for the first‐line treatment of mRCC. Pazopanib is a preferred, category‐one level recommendation by the National Comprehensive Cancer Network in the U.S. for first‐line treatment of predominant clear cell mRCC and is also indicated as subsequent therapy [4]. The European Society for Medical Oncology guidelines for treatment of renal cell carcinoma (RCC) similarly include pazopanib as a [I, A] recommendation for predominant clear cell mRCC in the first‐line setting and also list treatment with pazopanib as a second‐line option after cytokine‐based therapy [5]. The European Association of Urology clinical practice guidelines also include pazopanib as first‐line therapy for patients with clear cell mRCC [6]. These recommendations are based not only on the previously mentioned study but also on the COMPARZ and PISCES studies [7], [8]. The COMPARZ study proved similar efficacy of pazopanib compared with sunitinib and also showed that pazopanib was better tolerated from an adverse‐effect and quality‐of‐life standpoint [7]. The PISCES study, which was designed to evaluate patient preference for pazopanib or sunitinib from an adverse‐effect and quality‐of‐life perspective, showed similar results [8].
Earlier in vitro studies leading toward pazopanib's clinical application have shown that it is slightly soluble at a pH of 1 and practically insoluble at a pH of 4, which suggests the that extent of absorption is pH dependent [9], [10]. Not surprisingly, pharmacokinetic data have shown that concomitant administration of pazopanib and esomeprazole, a proton pump inhibitor (PPI) that increases gastric pH, leads to decreased area under the curve (AUC) of pazopanib and thus decreases exposure of pazopanib by 40% [9]. No formal pharmacokinetic studies have been performed to review concomitant administration of pazopanib and histamine 2 receptor antagonists (H2RA). However, the package insert for pazopanib suggests short‐acting antacids be considered concomitantly rather than acid‐reducing PPIs and H2Ras [1]. In an attempt to minimize this interaction, the practice at our institution is to instruct patients to administer the acid‐reducing medication in the morning and pazopanib in the evening when concomitant use in unavoidable, although no data exist suggesting that separation of doses affects the extent of absorption. It is known that esomeprazole maintains gastric pH above 4 for at least 14 hours, whereas H2RA agents as a class raise gastric pH for less than 14 hours [11], [12]. Therefore, separation of a PPI from pazopanib is unlikely to truly affect absorption. Many patients receiving pazopanib have an indication for an acid‐reducing drug, such as medication‐induced dyspepsia or a concomitant corticosteroid. In addition, pazopanib itself caused dyspepsia in up to 14% of patients in clinical trials [9]. In addition, patients often require dose reductions for toxicity, making it unclear whether decreased exposure in this setting would impact efficacy or safety. Motzer et al. reported that 44% of patients receiving pazopanib required a dose interruption of 7 days or more and that 44% required a dose reduction [7]. Despite the pharmacokinetic data published to date, the clinical significance and impact on patient outcomes resulting from decreased pazopanib exposure remain unknown.
Several other potential drug interactions with oral TKIs and acid‐reducing medications have been described in the literature. Erlotinib, a small molecule TKI used for non‐small cell lung cancer (NSCLC) and pancreatic cancer, also requires an acidic environment for absorption [13], [14]. Erlotinib concentrations were not found to be reduced in a study investigating erlotinib in combination with intravenous and oral pantoprazole; however, bioavailability was reduced when cola and esomeprazole were administered concomitantly with erlotinib [15], [16]. Furthermore, a study evaluating the effect of concomitant PPI or H2RA with erlotinib showed no significant effect on PFS or OS in patients with NSCLC [13]. Nilotinib, a TKI used for chronic myeloid leukemia (CML), has the same acidic environment requirement, and concomitant nilotinib and esomeprazole was shown to decrease the AUC of nilotinib by 34% [17], [18]. Similar reductions in the AUC of dasatinib, an oral TKI used for CML and acute lymphoblastic leukemia, were noted when dasatinib was given concomitantly with famotidine [19], [20].
Materials and Methods
This was a retrospective, observational, single‐center cohort study using an institutional review board‐approved protocol designed to review patients with metastatic RCC who received pazopanib in combination with a PPI or H2RA to determine if there was an effect on PFS, OS, or safety compared with patients who did not take concomitant pH‐elevating medications. Eligible patients were at least 18 years of age with biopsy‐proven RCC (both clear cell and non‐clear cell histology) who received pazopanib therapy from January 1, 2010, to December 31, 2015. Patients were excluded if they received pazopanib therapy for fewer than 90 days, had unknown PPI or H2RA status as determined by electronic medical record (EMR) chart review, or had missing documentation for date of pazopanib initiation, disease progression, or death.
A list of potential study patients was obtained from the EMR at Vanderbilt University Medical Center (VUMC), Vanderbilt‐Ingram Cancer Center (VICC), and Vanderbilt Specialty Pharmacy (VSP). Demographic information collected included age, race, gender, height, weight, comorbidities, tumor histology, stage of malignancy at initial diagnosis, location of metastases, Memorial Sloan‐Kettering Cancer Center (MSKCC)/Motzer risk category, Eastern Cooperative Oncology Group (ECOG) performance status (PS) at the time of pazopanib initiation, and prior nephrectomy and/or systemic treatment, if applicable [21].
The primary endpoint was progression‐free survival, defined as the time interval between the date of pazopanib initiation and the date of progression or death. One of the secondary endpoints was overall survival, defined as the time interval between the date of pazopanib initiation and date of death. Safety was also assessed as a secondary endpoint as determined by documented adverse events and dose reductions in the EMR during pazopanib therapy.
Specialty pharmacy refill data were obtained in order to assess compliance with pazopanib, along with medication possession ratio (MPR) and proportion of days covered (PDC). MPR was defined as the sum of days’ supply for all fills in a given time period divided by the number of days in that time period [22], [23], [24], [25]. PDC was defined as the number of days in a given time period “covered” with medication divided by the number of days in that time period.
This cohort study set out with the goal to collect data for at least 150 patients who received pazopanib for RCC from January 1, 2010, through December 31, 2015. Baseline patient characteristics of those who received pazopanib with or without a PPI or H2RA were assessed with the Wilcoxon rank‐sum test (continuous) and chi‐squared test (categorical). PFS and OS were evaluated using the Kaplan‐Meier method and log‐rank test and also modeled in Cox proportional‐hazard regression models. MSKCC risk category as well as the line of therapy (first‐line or second‐line and beyond) were included in the model. Other baseline patient characteristics were used to model the propensity of receiving pazopanib with a PPI or H2RA, and the propensity score was an additional covariate in the Cox models. We expected at least 120 PFS events (70 OS events), which would be sufficient to detect the treatment effect with 90% power (5% type I error rate) if the hazard ratio (HR) was 1.6. In this computation, we assumed that the treatment assignment and the other variables in the model had a weak association (R2 = 0.10). We further assumed that the proportion of the patients receiving a PPI or H2RA was 50% and noted that different values of this proportion would not be expected to affect the power. Adverse effects were compared using Fisher's exact test.
Study data were collected and managed using research electronic data capture (REDCap) tools hosted at Vanderbilt University Medical Center [26]. REDCap is a secure, Web‐based application designed to support data capture for research studies, providing (a) an intuitive interface for validated data entry, (b) audit trails for tracking data manipulation and export procedures, (c) automated export procedures for seamless data downloads to common statistical packages, and (d) procedures for importing data from external sources.
Results
One hundred forty‐six patients were identified from the VUMC/VICC EMR and VSP records as having received pazopanib therapy for RCC from January 1, 2010, to December 31, 2015. Fifty‐six patients (38%) were excluded from the analysis: 48 (86%) received fewer than 90 days of pazopanib therapy, 2 (4%) were missing the date of pazopanib initiation, 3 (5%) were missing the date of death, and 3 (5%) were missing the date of disease progression. Ninety patients were ultimately included in the final analysis, with 66 (73%) having received a concomitant PPI and/or H2RA and 24 (27%) having not received concomitant therapy.
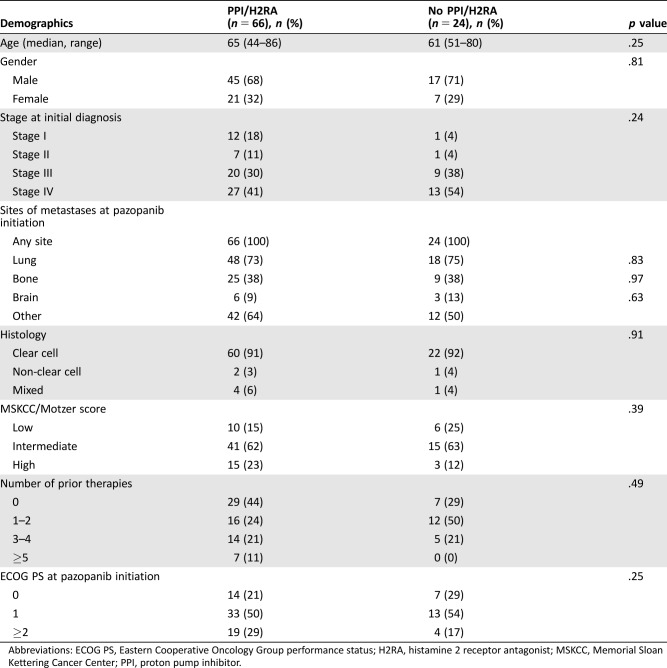
Baseline characteristics were similar between groups (Table 1). The majority of patients were male and 60–65 years of age, with clear cell histology, an intermediate MSKCC score, and an ECOG PS of 1, and had zero to two prior systemic therapies. The most common site of metastasis was the lung in both groups (73% in the concomitant PPI/H2RA group and 75% in the no PPI/H2RA group). Fifty‐nine percent of the concomitant PPI/H2RA group and 46% of the no PPI/H2RA group had recurrent disease.
Table 1. Patient characteristics.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; H2RA, histamine 2 receptor antagonist; MSKCC, Memorial Sloan Kettering Cancer Center; PPI, proton pump inhibitor.
Of the 66 patients receiving concomitant acid‐reduction therapy, the majority of patients received a PPI (Table 2). Of those receiving a concomitant PPI and/or H2RA, 45 (68%) were on a PPI and/or H2RA at the time of pazopanib initiation, whereas 21 (32%) were placed on therapy after pazopanib initiation because of toxicity. Six patients (9%) received dual PPI and H2RA therapy, and 3 patients (5%) required escalation from an H2RA to a PPI for symptom control. Indications for acid‐reduction therapy included gastroesophageal reflux disease (GERD) or dyspepsia (61%), concurrent corticosteroid therapy (14%), nausea (6%), and upper gastrointestinal bleed (2%), as described in Table 3.
Table 2. Concomitant PPI and/or H2RA usage.
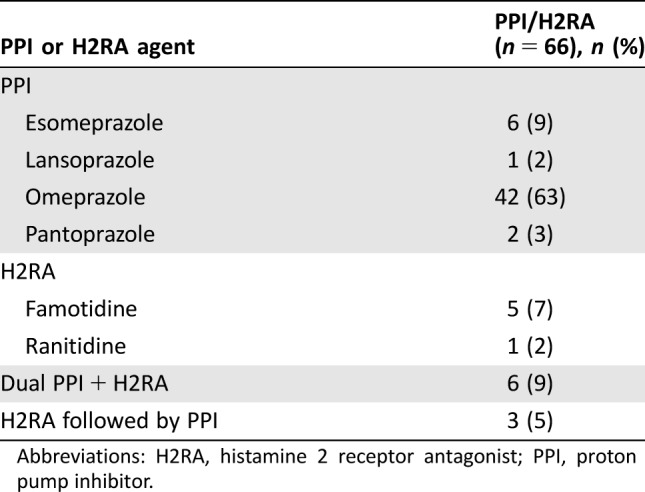
Abbreviations: H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
Table 3. Indication for PPI and/or H2RA (n = 66).
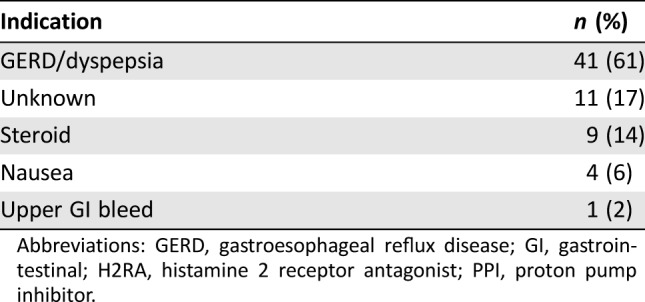
Abbreviations: GERD, gastroesophageal reflux disease; GI, gastrointestinal; H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
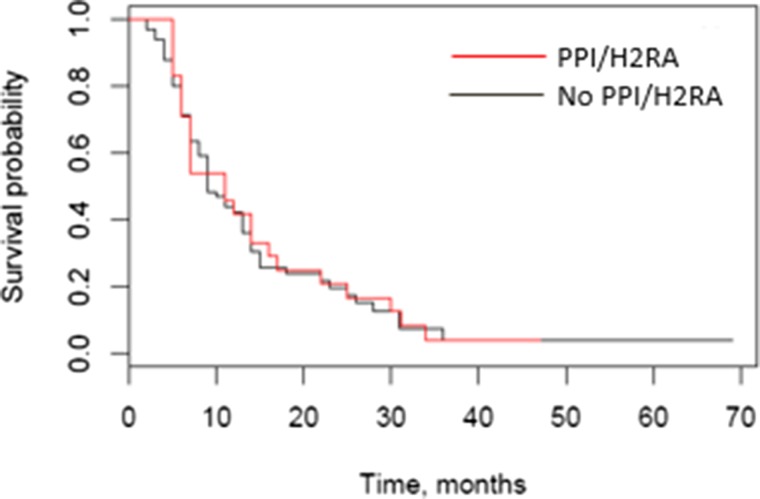
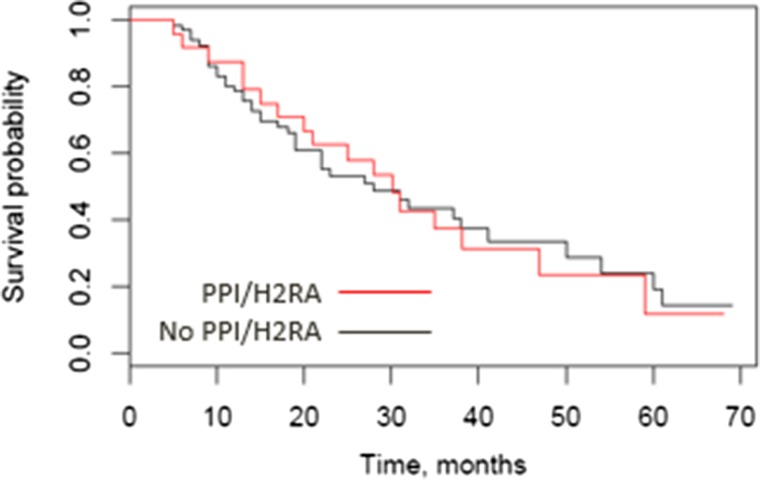
The primary endpoint of PFS (Fig. 1) was not statistically significantly different for the concomitant PPI/H2RA and no PPI/H2RA groups (median 9.0 months [95% confidence interval (CI) 8.0–13.0] vs. 11.0 months [95% CI 6.0–16.0], p = .85 by log‐rank test, adjusted HR 1.25 [95% CI 0.76–2.07] by multivariable Cox regression). From the multivariable regression, MSKCC/Motzer score was statistically significantly associated with PFS. Median overall survival (Fig. 2) in the concomitant PPI/H2RA group was 28.0 months (95% CI 19.0–41.0) and 30.1 months (95% CI 17.0–47.0) in the no PPI/H2RA group (p = .92 by log‐rank test, adjusted HR 0.99 [95% CI 0.51–1.93] by multivariable Cox regression). From the multivariable regression, MSKCC/Motzer score (p = .0375) and number of prior therapies (p = .0234) were statistically significantly associated with OS.
Figure 1.
Progression‐free survival (PFS) by concomitant pH‐elevating medication. Median PFS was 9.0 months in the PPI/H2RA group (95% confidence interval [CI] 8.0–13.0) versus 11.0 months in the no PPI/H2RA group (95% CI 6.0–16.0); p = .85 by log‐rank test, adjusted hazard ratio 1.25 (95% CI 0.76–2.07) by Cox regression.
Abbreviations: H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
Figure 2.
Overall survival (OS) by concomitant pH‐elevating medication. OS was 28 months in the PPI/H2RA group (95% confidence interval [CI] 19.0–41.0) versus 30.1 months in the no PPI/H2RA group (95% CI 17.0–47.0); p = .92 by log‐rank test, adjusted hazard ratio 0.99 (95% CI 0.51–1.93) by Cox regression.
Abbreviations: H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
We also compared patients who received a PPI versus an H2RA to determine if one or the other had an impact on PFS or OS, given the differences between these classes of medications in regard to severity and duration of pH elevation. There were 57 patients who received a PPI and 9 who received an H2RA. PFS between the PPI and H2RA groups (median 11.0 months vs. 8.0 months, respectively; p = .632 by log‐rank test) and OS between these groups (median 27.0 months vs. 31.0 months, respectively; p = .64) were not significantly different.
The median duration of pazopanib treatment was 266 days (range 91–2,098) in the concomitant PPI/H2RA group and 357 days (range 133–999) in the no PPI/H2RA group. The median duration of concomitant acid‐reducing therapy with pazopanib was 234 days (range 31–1,079) or 88% of days with concomitant pazopanib use. The median starting dose in both groups was 400 mg once daily, and the median maximum dose in both groups was 800 mg once daily. The most common adverse events that were observed are listed in Table 4. Rates of adverse events were similar between the concomitant PPI/H2RA and no PPI/H2RA groups, with the exception of dyspepsia/GERD (33% vs. 4%, respectively; p = .005). Thirty‐five out of 66 patients (53%) in the concomitant PPI/H2RA group and 14 out of 24 patients (58%) in the no PPI/H2RA group required dose reductions because of adverse events.
Table 4. Adverse effects.
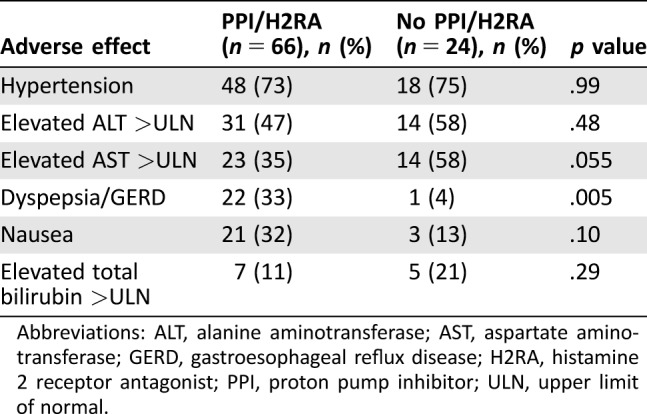
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GERD, gastroesophageal reflux disease; H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor; ULN, upper limit of normal.
Compliance data obtained through VSP records were available for 32 patients (48%) in the concomitant PPI/H2RA group and 7 patients (29%) in the no PPI/H2RA group (Table 5). Median MPR was 90% in the concomitant PPI/H2RA group versus 84% in the no PPI/H2RA group, and PDC was 100% in the concomitant PPI/H2RA group versus 98.2% in the no PPI/H2RA group.
Table 5. Compliance data.
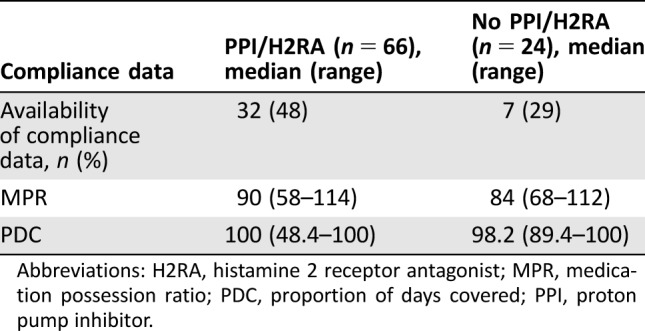
Abbreviations: H2RA, histamine 2 receptor antagonist; MPR, medication possession ratio; PDC, proportion of days covered; PPI, proton pump inhibitor.
Discussion
Our analysis demonstrated that PFS and OS were not significantly reduced in patients taking a PPI or an H2RA concomitantly with pazopanib. Unsurprisingly, the multivariable regressions showed that MSKCC/Motzer score was statistically significantly associated with PFS and OS, and number of prior therapies was statistically significantly associated with OS. This allows us to confirm our current practice of allowing patients on pazopanib to remain on concomitant acid‐reducing therapy for symptomatic relief, particularly given the palliative intent of treatment for mRCC. Given the patient population included in this study, however, these data may not be applicable to other settings in which pazopanib therapy is used, such as soft tissue sarcoma, for which pazopanib is also FDA approved [1].
After the primary analysis was completed, it was decided to also compare PFS and OS within the concomitant PPI/H2RA group based on receipt of PPI versus H2RA. In this analysis, neither PPI nor H2RA was statistically significantly associated with a reduction in PFS or OS. This was an important secondary question to answer, given the extent to which PPIs affect gastric pH compared with H2RAs and that most patients with pazopanib‐induced dyspepsia in our study were placed on a PPI for symptomatic relief. This fact, however, does limit this analysis, as the PPI and H2RA groups were unbalanced (57 patients vs. 9 patients, respectively).
Specialty pharmacies use certain measures in calculating patient compliance for specialty medications. One of the most commonly used measures of compliance is MPR, which may overestimate adherence because it can be inflated by early refills and as a result may be greater than 100% [22], [23], [24], [25]. PDC, which is more conservative because it adjusts for early refills and therefore cannot be greater than 100%, is becoming the preferred adherence measurement, as it is used by Pharmacy Quality Alliance and U.S. Centers for Medicare and Medicaid Services. Compliance data were obtained for this study to ensure that lack of compliance could not be considered a contributing factor if a PFS or OS reduction had been seen. Compliance data were only collected for 43% of patients in our analysis because of the fact that a new specialty pharmacy system was implemented at VSP during the period in which data were collected. Additionally, several patients were required to fill at specialty pharmacies other than VSP for reimbursement reasons. Despite the limited number of patients, the data we collected demonstrated that patients in both groups were compliant with pazopanib therapy, which suggests that adherence differences did not affect our findings.
Although this study was conducted solely in patients with mRCC, there are over 240 clinical trials registered to ClinicalTrials.gov (either ongoing or completed) that investigate pazopanib as monotherapy or in combination in both solid tumors and hematological malignancies [27]. If concomitant administration of a PPI or H2RA with pazopanib has no clinical impact on outcomes, as our results suggest, the inclusion criteria for these and future trials with pazopanib may require modification. The results of this institutional trial may therefore prove particularly relevant by both fostering a wider discussion on the use of a PPI or H2RA with pazopanib and by allowing for larger multicenter, retrospective studies.
One limitation of this study was its design as a retrospective chart review, which forced us to rely on patient‐reported as well as chart‐reported PPI or H2RA use. It is certainly plausible that patients were receiving concomitant acid‐reducing therapy without reporting it to their physicians or that they were not taking prescribed acid‐reducing medications. However, this study design is typical for assessing drug interactions in which outcomes are largely based on pharmacokinetic data, case reports, or retrospective outcomes data. It is also possible that despite our efforts to include several baseline patient characteristics in our Cox proportional‐hazard regression model, the PPI/H2RA and no PPI/H2RA groups were still different and unbalanced because of other confounding factors as a result of our retrospective study design. However, given the palliative nature of treatment for patients with mRCC, we feel that the results are still useful for everyday practice despite the lack of statistical significance. An additional limitation is the fact that patients who received fewer than 90 days of therapy were excluded. This may have led to selection bias, as patients who experienced early toxicity and were subsequently placed on a PPI and/or H2RA may have progressed within the 90‐day window as a result of reduced pazopanib exposure. As previously mentioned, another limitation was our lack of compliance data for all patients, although we would expect similar rates of compliance between the subset for whom we captured data and the entire study population. Finally, statistical power was not met for this study, which increases the possibility of type II statistical error. As this was a single‐center retrospective review and patients were included from a specific period that began near the time of initial FDA approval of pazopanib for mRCC, it was not possible to include 150 patients. Despite the small patient population, this analysis is based on more patients than any other published study evaluating this drug interaction to date. Additional studies with larger populations may be warranted to confirm our results.
Conclusion
Receipt of concomitant pazopanib with an acid‐reducing PPI or H2RA did not significantly affect PFS or OS in patients with metastatic RCC. Adverse effects did not differ greatly between groups. Based on the results of this retrospective cohort study and the nature of the treatment of patients with mRCC, clinicians should consider allowing patients to remain on concomitant pazopanib and acid‐reducing therapy for symptomatic control of dyspepsia, GERD, or any other clinically appropriate indication.
Footnotes
For Further Reading: Aristotle Bamias, Bernard Escudier, Cora N. Sternberg et al. Current Clinical Practice Guidelines for the Treatment of Renal Cell Carcinoma: A Systematic Review and Critical Evaluation. The Oncologist 2017;22:667–679.
Implications for Practice: Currently, there is uncertainity on the role of surgery in MRCC and on the choice of available guidelines in relapsed RCC. The best practice is individualization of targeted therapies. Systematic review of guidelines can help to identify unmet medical needs and areas of future research.
Author Contributions
Conception/design: Renee K. McAlister, Jonathan Aston, Megan Pollack
Provision of study material or patients: Renee K. McAlister
Collection and/or assembly of data: Renee K. McAlister
Data analysis and interpretation: Renee K. McAlister, Liping Du, Tatsuki Koyama
Manuscript writing: Renee K. McAlister, Jonathan Aston, Megan Pollack, Liping Du, Tatsuki Koyama, David D. Chism
Final approval of manuscript: Renee K. McAlister, Jonathan Aston, Megan Pollack, Liping Du, Tatsuki Koyama, David Chism
Disclosures
David D. Chism: Exelixis, Inc., Karyopharm Therapeutics, Inc. (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Votrient (R) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 2. Sternberg CN, Davis ID, Mardiak J et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2010;28:1061–1068. [DOI] [PubMed] [Google Scholar]
- 3. Sternberg CN, Hawkins RE, Wagstaff J et al. A randomised, double‐blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013;49:1287–1296. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. Version 2.2018. November 30, 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed December 4, 2017.
- 5. Escudier B, Porta C, Schmidinger M et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27(suppl 5):v58–v68. [DOI] [PubMed] [Google Scholar]
- 6. Ljungberg B, Albiges L, Bensalah K et al. Renal cell carcinoma. European Association of Urology Web site. Updated 2017. Available at http://uroweb.org/guideline/renal-cell-carcinoma/. Accessed December 6, 2017.
- 7. Motzer RJ, Hutson TE, Cella D et al. Pazopanib versus sunitinib in metastatic renal‐cell carcinoma. New Engl J Med 2013;369:722–731. [DOI] [PubMed] [Google Scholar]
- 8. Escudier B, Porta C, Bono P et al. Randomized, controlled, double‐blind, cross‐over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol 2014;32:1412–1418. [DOI] [PubMed] [Google Scholar]
- 9. Tan AR, Gibbon DG, Stein MN et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol 2013;71:1635–1643. [DOI] [PubMed] [Google Scholar]
- 10. Di Gion P, Kanefendt F, Lindauer A et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: Focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet 2011;50:551–603. [DOI] [PubMed] [Google Scholar]
- 11. Miner P Jr, Katz PO, Chen Y et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: A five‐way crossover study. Am J Gastroenterol 2003;98:2616–2620. [DOI] [PubMed] [Google Scholar]
- 12. Huang JQ, Hunt RH. Pharmacological and pharmacodynamic essentials of H(2)‐receptor antagonists and proton pump inhibitors for the practising physician. Best Pract Res Clin Gastroenterol 2001;15:355–370. [DOI] [PubMed] [Google Scholar]
- 13. Hilton JF, Tu D, Seymour L et al. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer 2013;82:136–142. [DOI] [PubMed] [Google Scholar]
- 14.Tarceva (R) [package insert]. South San Francisco, CA: Genentech USA, Incorporated; 2016. [Google Scholar]
- 15. Ter Heine R, Fanggiday JC, Lankheet NA et al. Erlotinib and pantoprazole: A relevant interaction or not? Br J Clin Pharmacol 2010;70:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Leeuwen RW, Peric R, Hussaarts KG et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non‐small‐cell lung cancer. J Clin Oncol 2016;34:1309–1314. [DOI] [PubMed] [Google Scholar]
- 17.Tasigna (R) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 18. Yin OQ, Gallagher N, Fischer D et al. Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol 2010;50:960–967. [DOI] [PubMed] [Google Scholar]
- 19.Sprycel (R) [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company; 2017. [Google Scholar]
- 20. Eley T, Luo FR, Agrawal S et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol 2009;49:700–709. [DOI] [PubMed] [Google Scholar]
- 21. Motzer RJ, Mazumdar M, Bacik J et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–2540. [DOI] [PubMed] [Google Scholar]
- 22. Fairman KA, Motheral B. Evaluating medication adherence: Which measure is right for your program? J Manag Care Spec Pharm 2000;6:499–506. [Google Scholar]
- 23. Crowe M. Do you know the difference between these adherence measures? Pharmacy Times. July 5, 2015. Available at http://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Accessed June 1, 2017.
- 24. Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharmacy Quality Alliance Web site. Available at https://pqaalliance.org/resources/adherence.asp. Accessed October 23, 2017.
- 25.Centers for Medicare and Medicaid Services, U.S. Department of Health and Human Services. Part C and D performance data. Centers for Medicare and Medicaid Services Web site. Available at https://www.cms.gov/Medicare/Prescription-Drug-coverage/PrescriptionDrugCovGenIn/PerformanceData.html. Accessed October 23, 2017.
- 26. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)–A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. National Library of Medicine , National Institutes of Health. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/home. Accessed October 27, 2017.