Information on the role of Ki67 as a prognostic tool in residual disease after neoadjuvant chemotherapy is scarce. This article evaluates changes in Ki67 as a prognostic factor for disease‐free survival and overall survival in patients who do not achieve a pathological complete response after neoadjuvant chemotherapy.
Keywords: Ki67, Luminal B‐like, Breast cancer, Pathological response, Cell proliferation
Abstract
Background.
Several breast cancer (BC) trials have adopted pathological complete response (pCR) as a surrogate marker of long‐term treatment efficacy. In patients with luminal subtype, pCR seems less important for outcome prediction. BC is a heterogeneous disease, which is evident in residual tumors after neoadjuvant‐chemotherapy (NAC). This study evaluates changes in Ki67 in relation to disease‐free survival (DFS) and overall survival (OS) in patients without pCR.
Subjects, Materials, and Methods.
Four hundred thirty‐five patients with stage IIA–IIIC BC without pCR after standard NAC with anthracycline and paclitaxel were analyzed. We analyzed the decrease or lack of decrease in the percentage of Ki67‐positive cells between core biopsy samples and surgical specimens and correlated this value with outcome.
Results.
Twenty‐five percent of patients presented with luminal A‐like tumors, 45% had luminal B‐like tumors, 14% had triple‐negative BC, 5% had HER2‐positive BC, and 11% had triple‐positive BC. Patients were predominantly diagnosed with stage III disease (52%) and high‐grade tumors (46%). Median Ki67 level was 20% before NAC, which decreased to a median of 10% after NAC. Fifty‐seven percent of patients had a decrease in Ki67 percentage. Ki67 decrease significantly correlated with better DFS and OS compared with no decrease, particularly in the luminal B subgroup. Multivariate analysis showed that nonreduction of Ki67 significantly increased the hazard ratio of recurrence and death by 3.39 (95% confidence interval [CI] 1.8–6.37) and 7.03 (95% CI 2.6–18.7), respectively.
Conclusion.
Patients without a decrease in Ki67 in residual tumors after NAC have poor prognosis. This warrants the introduction of new therapeutic strategies in this setting.
Implications for Practice.
This study evaluates the change in Ki67 percentage before and after neoadjuvant chemotherapy (NAC) and its relationship with survival outcomes in patients with breast cancer who did not achieve complete pathological response (pCR). These patients, a heterogeneous group with diverse prognoses that cannot be treated using a single algorithm, pose a challenge to clinicians. This study identified a subgroup of these patients with a poor prognosis, those with luminal B‐like tumors without a Ki67 decrease after NAC, thus justifying the introduction of new therapeutic strategies for patients who already present a favorable prognosis (luminal B‐like with Ki67 decrease).
摘要
背景.若干乳腺癌(BC)试验采用病理学完全缓解(pCR)作为长期治疗疗效的替代指标。对于Luminal亚型患者, pCR对于结果预测似乎不太重要。BC是一种异质性疾病, 在新辅助化疗(NAC)后的残余肿瘤中较为明显。此项研究评价了Ki67的变化与无pCR患者的无病生存期(DFS)和总生存期(OS)的关系。
受试者、材料和方法.对435例经过蒽环类药物和紫杉醇标准NAC后无pCR的IIA‐IIIC期BC患者进行了分析。我们分析了核心活检样本与手术标本之间Ki67阳性细胞百分比减少或未减少的情况, 并将此值与预后相关联。
结果.25%的患者为Luminal A样肿瘤, 45%为Luminal B样肿瘤, 14%为三阴性BC, 5%为HER2阳性BC, 11%为三阳性BC。患者主要被诊断出III期疾病(52%)和高度恶性肿瘤(46%)。NAC前中位Ki67水平为20%, NAC后中位水平降至10%。57%的患者Ki67百分比降低。Ki67降低与更好的DFS和OS显著相关(与Ki67未降低相比), 尤其是在Luminal B亚组中。多因素分析显示Ki67未降低分别导致复发和死亡风险比分别增加3.39 [95%可信区间(CI)1.8‐6.37]和7.03(95% CI 2.6–18.7)。
结论.NAC后残余肿瘤中Ki67未降低的患者预后不佳。需要在此情况下采用新的治疗策略。
对临床实践的提示:本研究评价了新辅助化疗(NAC)前后Ki67百分比的变化及其在未实现完全病理学缓解(pCR)的乳腺癌患者中与生存结果之间的关系。这些患者组成了具有不同的预后的异质组, 不能使用单一方案进行治疗, 对临床医生构成了一大挑战。此项研究确定了这些预后不佳患者(这些患者存在Luminal B样肿瘤且经过NAC后Ki67未降低)亚组, 因此证明针对已经呈现有利预后的患者(存在Luminal B样肿瘤且Ki67降低)使用新治疗策略是合理的。
Introduction
Breast cancer (BC) is a heterogeneous disease, and during the last decade, gene expression studies have identified distinct molecular subtypes with markedly different behaviors and prognoses [1], [2]. One proliferation marker that has been considered is Ki67, the routine use of which remains controversial in clinical practice. Ki67 is a protein expressed in the nucleus of cells during different phases of the cell cycle but not in the G0 quiescent state [3]. Cells that are not cycling through S‐phase exhibit a lower Ki67 score [4]. The role of Ki67 as a predictive factor of the response to neoadjuvant hormone therapy has been well established [5]. The majority of studies have identified a high Ki67 proliferation rate as a predictive factor for a higher rate of pathological complete response (pCR) after neoadjuvant chemotherapy (NAC) [5], [6], [7], [8].
Several trials have adopted pCR as a surrogate marker of long‐term treatment efficacy [5]. However, in patients who do not achieve a pCR, other biologic markers should be taken into account in order to establish further prognostic groups and to distinguish patients who might benefit from further adjuvant treatment. In addition, pCR seems less important in terms of outcome in patients with the luminal subtype of BC [2], because this subtype has been associated with a better outcome, even in patients who do not achieve a pCR after NAC [7].
Information regarding the role of Ki67 as a prognostic tool in residual disease after NAC is scarce. The aim of our study was to evaluate changes in Ki67 as a prognostic factor for disease‐free survival (DFS) and overall survival (OS) in patients who do not achieve a pCR after NAC.
Materials and Methods
All patients were selected from a database of patients with BC who were treated with NAC at the Instituto Nacional de Cancerología, Mexico (INCan) from January 2007 to December 2015. Patients in this database had been treated within the social security program “Seguro Popular.” Patients who had undergone surgery after NAC and had no pCR (defined as evidence of invasive residual tumor in the breast or axilla [9]) were included. Clinical stage, tumor size, axillary lymph node status, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, and grade were recorded. The documentation of the percentage of Ki67 positivity before and after NAC was mandatory. NAC therapy consisted of anthracycline‐based treatment (four cycles) followed by weekly treatment with paclitaxel (12 doses) and with trastuzumab for HER2‐positive patients. The case selection process is shown in Figure 1.
Figure 1.
STROBE flow diagram.
Abbreviations: NAC, neoadjuvant chemotherapy; pCR, pathological complete response.
The protocol was registered at the INCan Ethics Committee. Two highly specialized breast pathologists at INCan performed all histologic assessments. Each sample was analyzed using standardized procedures and assays. According to immunohistochemistry (IHC), we defined five BC subgroups as follows: luminal A‐like (ER‐positive and PR‐positive >20%, HER2‐negative, and Ki67‐low), luminal B‐like (ER‐positive or ‐negative, PR‐low, HER2‐negative, and Ki67‐high), triple‐negative (ER‐negative, PR‐negative, and HER2‐negative), HER2‐positive (ER‐negative, PR‐negative, and HER2‐positive), and luminal B‐like/HER2‐positive, also known as triple positive (ER‐positive, any PR, any Ki67, and HER2‐positive) [10], [11], [12], [13].
HER2 status was defined according to the American Society of Clinical Oncology and College of American Pathologists guidelines [14]. All HER2‐positive patients received trastuzumab in the neoadjuvant setting and completed 1 year of trastuzumab therapy independently of the pathological response. All patients with positive expression of ER and/or PR received adjuvant endocrine therapy. Premenopausal women received tamoxifen for 5 years, and postmenopausal women received aromatase inhibitor for 5 years [15].
The immunohistochemistry assessment of Ki67 was consistent during the study period. Ki67 was quantified using a visual scoring system, which included an external control for validation. Tissue was fixed in neutral buffered formalin [16]. The antibody used was a mouse antihuman Ki67 monoclonal antibody clone MIB‐1 (Biocare Medical, Pacheco, CA), which is recommended as the gold standard for IHC. Stained cells were counted and expressed as a percentage. Only nuclear staining was incorporated into the Ki67 score, which was defined as the percentage of positively stained cells among the total number of malignant cells scored. If staining was homogeneous, at least 500 cells within ten randomly selected high‐power fields (×400) were counted. In the presence of hot spots, which were defined as areas with highly prevalent Ki67 staining, the overall average score was recorded [17].
Patients were divided according changes in Ki67 after NAC, as follows: the decrease group included patients who demonstrated a Ki67 score at least 1% less in residual tumor than in the initial core biopsy, and the no‐decrease group included patients who demonstrated any increase or no change in Ki67 positivity between the breast core biopsy and residual disease after NAC.
Statistical Analysis
DFS and OS were defined as the time from the date of BC surgery to the date of evidence of local or distant recurrence and as the time from the date of BC diagnosis to the date of death from any cause or loss to follow‐up, respectively. DFS and OS were estimated with the Kaplan‐Meier method and were compared between groups using the log‐rank test. Univariate and multivariate Cox proportional hazards analyses were performed to evaluate the effects of age; hormonal status; American Joint Committee on Cancer (AJCC) stage; histological type; ER, PR, and HER2 status; baseline Ki67; and change in Ki67 and subgroups. The level of significance was set at p ≤ .05. Statistical analyses were performed using SPSS 23.0 for Windows (IBM, Chicago, IL). Multiple Cox proportional hazard models were used to obtain hazard ratios (HRs).
Results
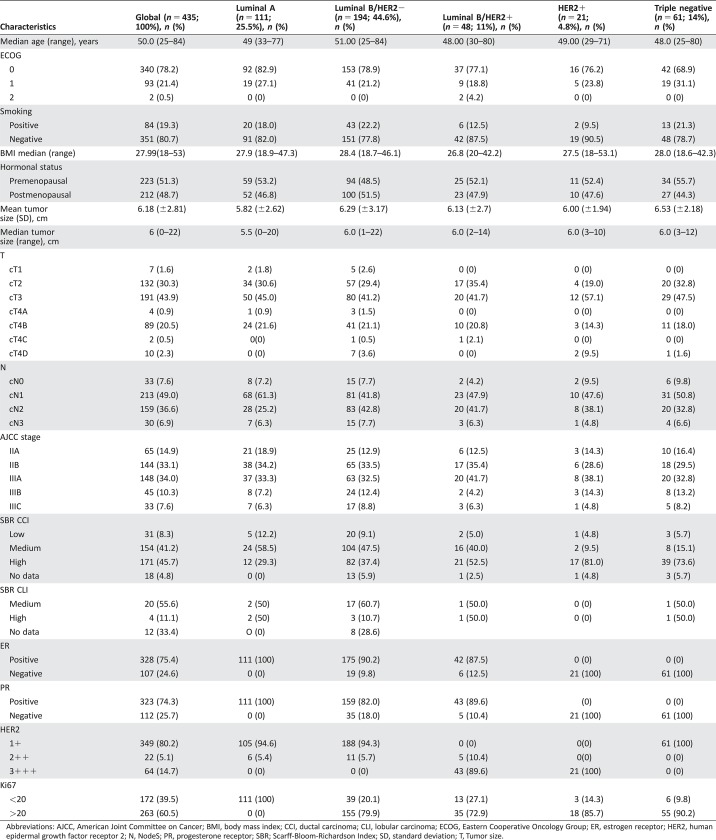
Four hundred thirty‐five patients met the inclusion criteria for analyses. The median age was 50 years (range 25–84 years), and 52% of patients were premenopausal. Before NAC, the mean tumor size was 6.2 cm; 68% of patients had T3–T4 tumors and 92% had clinically node‐positive disease. Fifty‐two percent of patients were in clinical stage IIIA–IIIC, 46% had high histological grade tumors, and 59% were diagnosed with the luminal B‐like/HER2‐negative BC subtype. Patient and tumor characteristics are listed in Table 1.
Table 1. Baseline characteristics of study population.
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index; CCI, ductal carcinoma; CLI, lobular carcinoma; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; N, NodeS; PR, progesterone receptor; SBR; Scarff‐Bloom‐Richardson Index; SD, standard deviation; T, Tumor size.
After NAC, 44 (10%) patients underwent breast‐conserving surgery. The median residual tumor size was 2.5 cm (range 0–5 cm), and the pathological nodal status was ypN0 in 137 (32%), ypN1 in 160 (37%), ypN2 in 97 (22%), and ypN3 in 38 (9%) patients. The median percentage of Ki67 positivity before NAC was 20%, which decreased to 10% after NAC. Fifty‐seven percent of residual tumors exhibited a decrease in Ki67 positivity by at least 1%. The distribution of patients according to phenotype and Ki67 changes after NAC is described in Table 2.
Table 2. Patient distribution by phenotype and Ki67 changes after neoadjuvant chemotherapy.

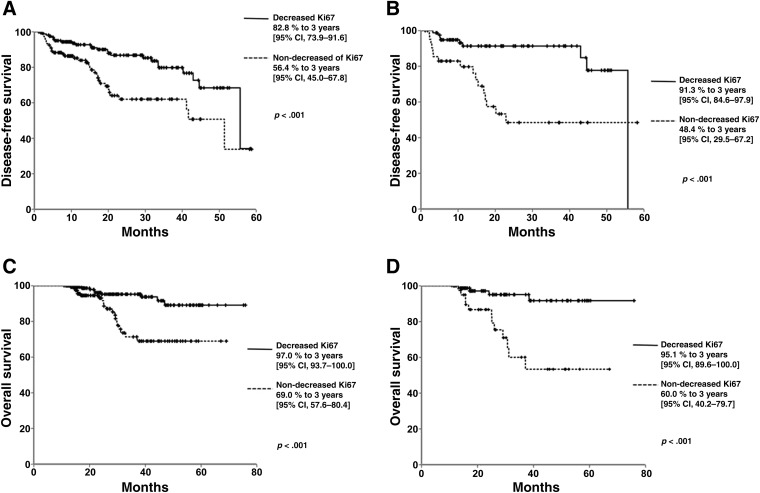
Median follow‐up was 27.4 months (±14.9 months). The global median DFS was 43.9 months (95% confidence interval [CI] 40.8–47.1). Patients who had decreased Ki67 levels had a longer median DFS compared with those with no decrease in Ki67: 47.6 months (95% CI 44.1–51.3) versus 38 months (32.7–43.3), respectively (p < .001). Additionally, 3‐year DFS in patients with a decrease in Ki67 levels was 82.8% (95% CI 79.3–91.6), compared with 56.4% (95% CI 45.0–67.8) in patients without decreased Ki67 (Fig. 2A). Other factors that affected DFS in the univariate analysis included AJCC stage (stage II, 78.6 [95% CI 70.5–86.6], vs. stage III, 59.2 [45.5–72.9]; p < .001); ER status (positive, 76.1 [95% CI 68.2–83.9], vs. negative, 57.3 [42.6–72.1]; p < .001); PR status (positive, 73.0 [95% CI 63.9–82.0], vs. negative, 67.4 [55.2–79.5]; p < .001). In the luminal A‐like, triple‐negative, HER2‐positive, and luminal B/HER2‐positive subtypes, no statistically significant differences were found in DFS and OS. In contrast, when DFS was compared in patients with luminal B‐like tumors, patients with a decrease in Ki67 had a longer DFS compared with those without a decrease (47 months [95% CI 39.7–47.6] vs. 36.2 months [29.2–43.3] respectively; p = .001). Patients with luminal B‐like tumors had a 3‐year DFS of 91.3% (95% CI 84.6–97.9) vs. 48.4% (29.5–67.2; Fig. 2B). The multivariate analysis showed that the difference in Ki67 was a highly significant independent predictor of DFS with an HR of 3.39 (95% CI 1.8–6.37, p < .001) in all patients (Table 3).
Figure 2.
Patient outcomes according to Ki67 decrease vs. Ki67 non‐decrease. Disease‐free survival of patients with decreased Ki67 and those with no decrease in Ki67 after neoadjuvant chemotherapy (NAC), in patients with all tumor types (A) and in patients with luminal B‐like phenotype tumors (B). Overall survival of patients with decreased Ki67 and those with no decrease in Ki67 after NAC, in patients with all tumor types (C) and in patients with luminal B‐like phenotype tumors (D).
Abbreviation: CI, confidence interval.
Table 3. Survival and DFS: Univariate and multivariate analysis of all neoadjuvant chemotherapy‐treated patients with residual tumor biopsy.
Abbreviations: Δ, change; AJCC, American Joint Committee on Cancer; CI, confidence interval; DFS, disease‐free survival; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; BMI: body mass index; OS, overall survival; ER: estrogen receptor; PR: progesterone receptor; HR: hazard ratio; ypTNM: pathological stage post neoadjuvance.
Values in bold reached a statistical significance of <0.05
The global OS was 67.2 months (95% CI 64.3–70.2). When patients with a decrease in Ki67 were compared with those without a decrease, the median OS was 71.2 months (95% CI 68.3–74.2) months versus 55.9 months (50.9–60.9), respectively (p < .0001), with a 3‐year OS of 97.0% (95% CI 93.7–100) for patients with a decrease in Ki67 compared with 69.0% (57.6–80.4) for patients without a decrease (Fig. 2C). In patients with the luminal B‐like subtype, the OS was 70.7 months (95% CI 66.7–74.8) for patients with a decrease in Ki67 compared with 52.9 months (46.2–59.7) for those without a decrease (p < .0001), whereas the 3‐year OS was 95.1% (95% CI 89.6–100) in the first group and 60% (40.2–79.7) for the latter (Fig. 2D). Factors that affected OS in the univariate analysis were as follows: clinical stage (II, 90.8 [95% CI85.0–90.0], vs. III, 76.0 [64.4–87.5]; p < .001), ER status (positive, 89.8 [95% CI 84.7–94.8], vs. negative, 69.0 [51.7–86.2]; p < .006), PR status (positive, 88.9 [95% CI 83.2–94.5], vs. negative, 78.1 [66.3–89.8]; p = .040), and initial Ki67 level (higher than 20%, 88.4 [95% CI 80.7–96.0], lower or equal to 20%, 84.3 [77.2–91.3]; p = .048). The multivariate analysis showed that the difference in Ki67 was a significant independent predictor of OS with an HR of 7.03 (95% CI 2.6–18.7, p < .001) (Table 3).
Discussion
A pCR after NAC is strongly associated with favorable long‐term outcomes [18], [19]; however, patients who do not achieve pCR are a heterogeneous group with diverse prognoses, and unfortunately, until now, no definite biomarker has served as a prognostic discriminator. An even more difficult issue presents in patients with ER‐positive BC, who tend to have low pCR rates and among whom it is highly challenging to distinguish patients with a good prognosis from those with a poor prognosis [20]. Such cases make it fundamental to acquire further tools that are urgently needed to assess potential outcomes.
In this study, we found that changes in Ki67 in residual disease after NAC can be used to separate a subgroup of patients with better outcomes from the general BC population and specifically, from those in the ER‐positive subgroup. It is quite common that, in the presence of a significant disease burden after NAC, clinicians expect a high rate of recurrence even after completion of standard treatment (adjuvant chemotherapy and endocrine management, as well as radiotherapy), which leads to an increased use of additional non‐evidence‐based therapy [21], [22]. A better selection of patients at high risk after NAC is important for the tailoring of new therapeutic strategies [23]. We found that patients without a decrease in Ki67 after NAC had worse outcomes in terms of DFS and OS, which allows the possibility of the identification of these high‐risk populations and the customization of treatment schemes.
Since 1999, it has been reported that a decrease in the cell proliferation fraction has a predictive value with respect to the recurrence rate [24], [25]. Ki67 has been used as a marker of such proliferation. Thus, routine assessment has not been recommended when patients receive primary chemotherapy because most data were derived from retrospective studies, and the cutoff points used were selected empirically or were arbitrarily established [5], [26]. It has also been found that patients who experienced progression during NAC had a higher proliferation rate than those who responded to chemotherapy [6]. It is also known that patients with high Ki67 expression at diagnosis have a higher risk of recurrence and death [27], [28]. All of the above suggests that Ki67 may be used to define prognosis.
High Ki67 expression at baseline is significantly associated with improved pCR rates [6], [29], [30], primarily in the triple‐negative and HER2‐positive BC subtypes [5], [31]. The potential prognostic value of Ki67 after NAC is less well known [32]. Our findings suggest that the reduction of at least 1% of the absolute value of the Ki67 score after NAC compared with the baseline level is associated with a favorable prognosis, as previously demonstrated by other research groups [32], [33], [34].
Billgren et al. demonstrated that a decrease of more than 25% in the proliferation fraction (measured as Ki67 percent) after the first course of chemotherapy significantly predicted a reduced risk of recurrence. Further studies added information on the role of Ki67 in predicting a pathological response [24], [35], [36]. In our study, we found that a decrease of at least one point of the percentage of Ki67‐positive cells between the core biopsy sample and the surgical specimen after the completion of NAC (anthracycline‐taxane‐based regimens) was related to better DFS and OS compared with no decrease in the percentage of Ki67‐positive cells, mostly in the luminal B‐like/HER2‐negative subgroup. These data are consistent with the study of Diaz‐Botero et al., who previously reported that patients whose tumors had low Ki67 expression after NAC had better OS and DFS compared with those whose tumors maintained high Ki67 expression [34]. However, it is important to indicate that the evaluation methods were different in both studies.
This study provides evidence that patients without a decrease in Ki67 expression after NAC had worse outcomes with respect to DFS and OS. In addition, Sheri et al. found that in patients who did not achieve pCR after NAC. An increase in Ki67 expression was a significant negative prognostic factor for both DFS and OS [23]. Ingolf and Yoshioka reported that high Ki67 expression in post‐treatment tumors was strongly correlated with poor DFS and OS, regardless of tumor subtype. Other studies corroborate that patients with high Ki67 values in the residual tumor after chemotherapy had worse outcomes in terms of recurrence and mortality [20], [30], [37], [38], [39].
In this regard, Ki67 might serve as a valuable prognostic marker for patients who do not achieve a pCR, but no clear evidence shows the optimal way to measure the changes in Ki67 after chemotherapy. We agree that Ki67 reflects the percentage of proliferating cells in the tumor [40] and that it is possible that the best way to measure this proliferation is as a continuous variable [41]. In this sense, our work presents an easier method to evaluate these changes, which involves the dichotomization of the decrease or nondecrease in the percentage of Ki67‐positive cells in residual disease after NAC, which is based on its own control (i.e., the initial biopsy). We propose that patients without a pCR after NAC are heterogeneous and can be subdivided according to changes in Ki67 into good and poor prognostic groups, as others have previously suggested [37], [42]. An appropriate identification of patients who are at high risk of relapse after NAC could help design treatment strategies among high‐risk populations [23], as has been previously established in the HER2‐positive and triple‐negative phenotype subgroups. We, as do others, believe that patients with high post‐treatment Ki67 levels are candidates for innovative postneoadjuvant treatment concepts [42].
It is interesting that our work showed a higher prevalence of luminal B‐like tumors with non‐pCR, contrasting with other reports in which patients with luminal B tumors achieved higher pCR rates than those with the luminal A subtype [43]. However, because of the retrospective nature of this study, we cannot currently draw any conclusions. Luminal B tumors have been associated with an increased frequency of p53 mutations, and previous studies have reported that patients with this subtype reap a relatively lower benefit from endocrine therapy than do patients with luminal A tumors [43]. Therefore, the value of Ki67 as a prognostic tool in this phenotype could direct future approaches.
Our data suggest that the evaluation of Ki67 after neoadjuvant chemotherapy could act as a clinically available tool that might allow clinicians to stratify patients into those who could benefit from “complementary” treatment. Clinicians must therefore be made aware that there are data available that incline us to believe that patients with a poor prognosis can be timely identified, and therefore more therapeutic options be made available for them.
As a retrospective study, our results require further validation. The lack of a validated method to measure the Ki67 cutoff points for prognosis, prediction, and monitoring hinders the correlation between basal and post‐treatment results. Thus, its use should only be applied in local practice [44]. In many occasions, concerns have been raised about Ki67 reproducibility, because interobserver and interlaboratory variations may produce different results [45]. Efforts to improve the concordance have been performed following the international recommendations for the assessment [46]. It is well recognized that adherence to these guidelines improves reproducibility and concordance [47], so assuring quality procedures and standardized analyses is highly recommended. It is important to mention that all studies that have evaluated the difference between initial Ki67 level and the Ki67 level in the surgical specimen after NAC have measured Ki67 using different methodologies, and thus a prospective study is essential to verify these results.
Conclusion
The decrease in Ki67 in residual BC tumors after NAC is a profoundly significant prognostic factor for DFS and OS. Change in Ki67 accurately identifies patients with high risk for recurrence and death; this marker is particularly relevant in patients who present with the luminal B‐like phenotype, in whom Ki67 decrease appears to have a more important predictive role than even some of the quintessential cancer prognosis factors, such as clinical stage and hormone receptor status. This study warrants further trials that may bring about new treatment strategies to improve the prognosis in patients with BC.
Acknowledgments
We acknowledge the work of Laura Marysol Alvarez Guadarrama, for her assistance organizing the histology material.
Footnotes
Editor's Note: See the related commentary, “Can We Hang Our Hats on One Percent?” by Nathalie LeVasseur and Karen A. Gelmon, on page 642 of this issue.
Author Contributions
Conception/design: Paula Cabrera‐Galeana, Wendy Muñoz‐Montaño, Fernando Lara‐Medina, Alberto Alvarado‐Miranda, Victor Pérez‐Sánchez, Cynthia Villarreal‐Garza, R. Marisol Quintero, Fany Porras‐Reyes, Enrique Bargallo‐Rocha, Ignacio Del Carmen, Alejandro Mohar, Oscar Arrieta
Provision of study material or patients: Paula Cabrera‐Galeana, Wendy Muñoz‐Montaño, Fernando Lara‐Medina, Alberto Alvarado‐Miranda, Victor Pérez‐Sánchez, Cynthia Villarreal‐Garza, R. Marisol Quintero, Fany Porras‐Reyes, Enrique Bargallo‐Rocha, Ignacio Del Carmen, Alejandro Mohar, Oscar Arrieta
Collection and/or assembly of data: Victor Pérez‐Sanchéz, Fany Porras‐Reyes
Data analysis and interpretation: Wendy Muñoz‐Montaño, Oscar Arrieta
Manuscript writing: Paula Cabrera‐Galeana, Wendy Muñoz‐Montaño, Fernando Lara‐Medina, Alberto Alvarado‐Miranda, Victor Pérez‐Sánchez, Cynthia Villarreal‐Garza, R. Marisol Quintero, Fany Porras‐Reyes, Enrique Bargallo‐Rocha, Ignacio Del Carmen, Alejandro Mohar, Oscar Arrieta
Final approval of manuscript: Paula Cabrera‐Galeana, Wendy Muñoz‐Montaño, Fernando Lara‐Medina, Alberto Alvarado‐Miranda, Victor Pérez‐Sánchez, Cynthia Villarreal‐Garza, R. Marisol Quintero, Fany Porras‐Reyes, Enrique Bargallo‐Rocha, Ignacio Del Carmen, Alejandro Mohar, Oscar Arrieta
Disclosures
The authors indicated no financial relationships.
References
- 1. Knutsvik G, Stefansson IM, Aziz S et al. Evaluation of Ki67 expression across distinct categories of breast cancer specimens: A population‐based study of matched surgical specimens, core needle biopsies and tissue microarrays. PLoS One 2014;9:e112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lara‐Medina F, Pérez‐Sánchez V, Saavedra‐Pérez D et al. Triple‐negative breast cancer in Hispanic patients: High prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer 2011;117:3658–3669. [DOI] [PubMed] [Google Scholar]
- 3. Andre F, Arnedos M, Goubar A et al. Ki67–No evidence for its use in node‐positive breast cancer. Nat Rev Clin Oncol 2015;12:296–301. [DOI] [PubMed] [Google Scholar]
- 4. Johnston SR, MacLennan KA, Sacks NP et al. Modulation of Bcl‐2 and Ki‐67 expression in oestrogen receptor‐positive human breast cancer by tamoxifen. Eur J Cancer 1994;30A:1663–1669. [DOI] [PubMed] [Google Scholar]
- 5. Alba E, Lluch A, Ribelles N et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. The Oncologist 2016;21:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fasching PA, Heusinger K, Haeberle L et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011;11:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colleoni M, Bagnardi V, Rotmensz N et al. A nomogram based on the expression of Ki‐67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer 2010;46:2216–2224. [DOI] [PubMed] [Google Scholar]
- 8. Fernández‐Sánchez M, Gamboa‐Dominguez A, Uribe N et al. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol 2006;23:171–183. [DOI] [PubMed] [Google Scholar]
- 9. Baron P, Beitsch P, Boselli D et al. Impact of tumor size on probability of pathologic complete response after neoadjuvant chemotherapy. Ann Surg Oncol 2016;23:1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viale G, Bottiglieri L. Pathological definition of triple negative breast cancer. Eur J Cancer 2009;45(suppl 1):5–10. [DOI] [PubMed] [Google Scholar]
- 11. Bhargava R, Striebel J, Beriwal S et al. Prevalence, morphologic features and proliferation indices of breast carcinoma molecular classes using immunohistochemical surrogate markers. Int J Clin Exp Pathol 2009;2:444–455. [PMC free article] [PubMed] [Google Scholar]
- 12. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies–Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Y, Zhang S, Zang L et al. Combination of shear wave elastography and Ki‐67 index as a novel predictive modality for the pathological response to neoadjuvant chemotherapy in patients with invasive breast cancer. Eur J Cancer 2016;69:86–101. [DOI] [PubMed] [Google Scholar]
- 14. Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 15. Senkus E, Kyriakides S, Penault‐Llorca F et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi7–vi23. [DOI] [PubMed] [Google Scholar]
- 16. Dowsett M, Nielsen TO, A'Hern R et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeCensi A, Guerrieri‐Gonzaga A, Gandini S et al. Prognostic significance of Ki‐67 labeling index after short‐term presurgical tamoxifen in women with ER‐positive breast cancer. Ann Oncol 2011;22:582–587. [DOI] [PubMed] [Google Scholar]
- 18. Wang‐Lopez Q, Chalabi N, Abrial C et al. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol 2015;95:88–104. [DOI] [PubMed] [Google Scholar]
- 19. Pennisi A, Kieber‐Emmons T, Makhoul I et al. Relevance of pathological complete response after neoadjuvant therapy for breast cancer. Breast Cancer (Auckl) 2016;10:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingolf JB, Russalina M, Simona M et al. Can ki‐67 play a role in prediction of breast cancer patients' response to neoadjuvant chemotherapy? Biomed Res Int 2014;628217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Promberger R, Dubsky P, Mittlböck M et al. Postoperative CMF does not ameliorate poor outcomes in women with residual invasive breast cancer after neoadjuvant epirubicin/docetaxel chemotherapy. Clin Breast Cancer 2015;15:505–511. [DOI] [PubMed] [Google Scholar]
- 22. Lebeau M, Mathoulin‐Pélissier S, Bellera C et al. Breast cancer care compared with clinical guidelines: An observational study in France. BMC Public Health 2011;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheri A, Smith IE, Johnston SR et al. Residual proliferative cancer burden to predict long‐term outcome following neoadjuvant chemotherapy. Ann Oncol 2015;26:75–80. [DOI] [PubMed] [Google Scholar]
- 24. Billgren AM, Rutqvist LE, Tani E et al. Proliferating fraction during neoadjuvant chemotherapy of primary breast cancer in relation to objective local response and relapse‐free survival. Acta Oncol 1999;38:597–601. [DOI] [PubMed] [Google Scholar]
- 25. Arrieta O, Villarreal‐Garza C, Vizcaíno G et al. Association between AT1 and AT2 angiotensin II receptor expression with cell proliferation and angiogenesis in operable breast cancer. Tumour Biol 2015;36:5627–5634. [DOI] [PubMed] [Google Scholar]
- 26. Lagios MD. The impact of Ki‐67 on immunostaining in classification of luminal subtypes of breast cancer. Breast J 2015;21:463–464. [DOI] [PubMed] [Google Scholar]
- 27. de Azambuja E, Cardoso F, de Castro G Jr et al. Ki‐67 as prognostic marker in early breast cancer: A meta‐analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrelli F, Viale G, Cabiddu M et al. Prognostic value of different cut‐off levels of Ki‐67 in breast cancer: A systematic review and meta‐analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–491. [DOI] [PubMed] [Google Scholar]
- 29. Horimoto Y, Arakawa A, Tanabe M et al. Ki67 expression and the effect of neo‐adjuvant chemotherapy on luminal HER2‐negative breast cancer. BMC Cancer 2014;14:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshioka T, Hosoda M, Yamamoto M et al. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer 2015;22:185–191. [DOI] [PubMed] [Google Scholar]
- 31. Matsubara N, Mukai H, Fujii S et al. Different prognostic significance of Ki‐67 change between pre‐ and post‐neoadjuvant chemotherapy in various subtypes of breast cancer. Breast Cancer Res Treat 2013;137:203–212. [DOI] [PubMed] [Google Scholar]
- 32. Romero Q, Bendahl PO, Klintman M et al. Ki67 proliferation in core biopsies versus surgical samples ‐ A model for neo‐adjuvant breast cancer studies. BMC Cancer 2011;11:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsubara N, Mukai H, Masumoto M et al. Survival outcome and reduction rate of Ki‐67 between pre‐ and post‐neoadjuvant chemotherapy in breast cancer patients with non‐pCR. Breast Cancer Res Treat 2014;147:95–102. [DOI] [PubMed] [Google Scholar]
- 34. Diaz‐Botero S, Espinosa‐Bravo M, Gonçalves VR et al. Different prognostic implications of residual disease after neoadjuvant treatment: Impact of Ki 67 and site of response. Ann Surg Oncol 2016;23:3831–3837. [DOI] [PubMed] [Google Scholar]
- 35. Burcombe R, Wilson GD, Dowsett M et al. Evaluation of Ki‐67 proliferation and apoptotic index before, during and after neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Res 2006;8:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dowsett M, A'Hern R, Salter J et al. Who would have thought a single Ki67 measurement would predict long‐term outcome? Breast Cancer Res 2009;11(suppl 3):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones RL, Salter J, A'Hern R et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009;116:53–68. [DOI] [PubMed] [Google Scholar]
- 38. Montagna E, Bagnardi V, Viale G et al. Changes in PgR and Ki‐67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol 2015;26:307–313. [DOI] [PubMed] [Google Scholar]
- 39. Yamazaki N, Wada N, Yamauchi C et al. High expression of post‐treatment Ki‐67 status is a risk factor for locoregional recurrence following breast‐conserving surgery after neoadjuvant chemotherapy. Eur J Surg Oncol 2015;41:617–624. [DOI] [PubMed] [Google Scholar]
- 40. Rossi L, Laas E, Mallon P et al. Prognostic impact of discrepant Ki67 and mitotic index on hormone receptor‐positive, HER2‐negative breast carcinoma. Br J Cancer 2015;113:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denkert C, Loibl S, Müller BM et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 2013;24:2786–2793. [DOI] [PubMed] [Google Scholar]
- 42. von Minckwitz G, Schmitt WD, Loibl S et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res 2013;19:4521–4531. [DOI] [PubMed] [Google Scholar]
- 43. Ades F, Zardavas D, Bozovic‐Spasojevic I et al. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J Clin Oncol 2014;32:2794–2803. [DOI] [PubMed] [Google Scholar]
- 44. Polley MY, Leung SC, McShane LM et al. An international Ki67 reproducibility study. J Natl Cancer Inst 2013;105:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knutsvik G, Stefansson IM, Aziz S et al. Evaluation of Ki67 expression across distinct categories of breast cancer specimens: A population‐based study of matched surgical specimens, core needle biopsies and tissue microarrays. PLoS One 2014;9:e112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dowsett M, Nielsen TO, A'Hern R et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103(22):1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ekholm M, Grabau D, Bendahl PO et al. Highly reproducible results of breast cancer biomarkers when analysed in accordance with national guidelines ‐ A Swedish survey with central re‐assessment. Acta Oncol 2015;54:1040–1048. [DOI] [PubMed] [Google Scholar]