Diffuse large B‐cell lymphoma (DLBCL) is one of the most frequent types of malignant lymphoma in elderly people; thus, the management of elderly DLBCL patients is important. This study aimed to validate the ACA index in Chinese elderly DLBCL patients and to refine the ACA index to propose a more effective comprehensive geriatric assessment method that could serve as a guide for optimal personalized therapy for elderly DLBCL patients treated with R‐CHOP.
Keywords: Albumin, Comorbidities, Diffuse large B‐cell lymphoma, Instrumental activities of daily living, Elderly patients
Abstract
Background.
We aimed to validate and refine the Age, Comorbidities, and Albumin (ACA) index in elderly Chinese patients with diffuse large B‐cell lymphoma (DLBCL) and propose a more effective method for comprehensive geriatric assessment (CGA).
Materials and Methods.
Patients ≥65 years of age who had been diagnosed with de novo DLBCL in the Institute of Hematology, Beijing Hospital, were screened for eligibility (n = 99).
Results.
Based on the ACA index, 39, 31, 26, and 3 patients were categorized into the “excellent,” “good,” “moderate,” and “poor” groups, respectively. The 2‐year treatment‐related mortality rate was significantly higher and the survival rates poorer in the ACA “moderate to poor” group compared with those of the ACA “good” and “excellent” groups. Multivariable model analysis identified two independent predictors of overall survival: the instrumental activities of daily living (IADL) scale and the ACA index. IADL scores of 6 to 7 and the ACA “good” group were assigned 1 point; IADL scores ≤5 and the ACA “moderate to poor” group were assigned 2 points. Based on these data, we created a three‐category system (IADL ACA index [IACA index]): low risk, score 0; intermediate risk, score 1 to 2; and high risk, score 3 to 4. The IACA index could effectively discriminate the response rates, overall survival, and progression‐free survival rates in elderly patients with DLBCL.
Conclusion.
We observed that the ACA index could partially predict the clinical outcomes of elderly DLBCL patients in China. Based on this index, we proposed the IACA index as an effective tool for CGA in DLBCL.
Implications for Practice.
Diffuse large B‐cell lymphoma (DLBCL) is one of the most frequent types of malignant lymphoma in elderly people, and identifying patients suitable for curative therapy is critical in the improvement of clinical outcomes. Recently, some authors proposed the Age, Comorbidities, and Albumin (ACA) index. Combining the use of the instrumental activities of daily living (IADL) scale and the ACA index, this article describes the IADL ACA index (IACA index), which is an effective tool for comprehensive geriatric assessment in DLBCL.
摘要
背景.我们旨在验证和细化研究中国弥漫性大B细胞淋巴瘤(DLBCL)老年患者的年龄、合并症和白蛋白(ACA)指数, 并提出一种更有效的综合老年评估(CGA)方法。
材料与方法.对北京医院血液病研究所诊断为原发DLBCL的已满65岁的患者进行合格性筛查(n=99例)。
结果.根据ACA指数, 分别有39、31、26和3名患者被划分至“极好”、“好”、“中等”和“差”各组中。与ACA“好”和“极好”组相比, ACA“中等至差”组患者的2年治疗相关死亡率显著更高并且生存率更差。多变量模型分析确定了总生存期的两个独立预测因子:工具性日常生活活动(IADL)量表和ACA指数。IADL得分6到7且ACA“好”的组给予1分;IADL得分≤5且ACA“中等至差”的组给予2分。基于这些数据, 创建一个三级系统[IADL ACA指数(IACA指数)]:低风险, 0分;中等风险, 1到2分;和高风险, 3到4分。IACA指数可有效区分老年DLBCL患者的反应率、总生存期和无进展生存率。
结论.我们观察到, ACA指数可以部分预测中国老年DLBCL患者的临床结局。基于这一指数, 我们提出, IACA指数可用作DLBCL的有效CGA工具。
对临床实践的启示:弥漫性大B细胞淋巴瘤(DLBCL)是老年人中最常见的恶性淋巴瘤之一, 确定适于治愈性治疗的患者对于改善临床结局至关重要。最近, 一些作者提出了年龄、合并症和白蛋白(ACA)指数。综合运用工具性日常生活活动(IADL)量表和ACA指数, 本文阐述了IADL ACA指数(IACA指数), 它是DLBCL综合老年评估的有效工具。
Introduction
Diffuse large B‐cell lymphoma (DLBCL) is one of the most frequent types of malignant lymphoma in elderly people [1]. In China, in elderly patients (older than 64 years), DLBCL made up approximately half of all lymphomas [2]. Thus, the management of elderly patients with DLBCL is very important.
The wide use of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) has significantly improved the clinical outcome of DLBCL even in older patients [3], [4]. However, some elderly patients cannot bear the toxicities related to this therapy, and identifying patients suitable for curative therapy is critical in the improvement of clinical outcomes. Over the last few decades, geriatricians have developed comprehensive geriatric assessment (CGA) methods for elderly patients, which are useful for the evaluation of patients with cancer [5], [6]. Many authors have suggested that CGA is more effective than clinical judgment in identifying elderly patients with DLBCL who would benefit from aggressive therapy, but the tools used for CGA are varied and not uniform [7]. Recently, Miura et al. [8] proposed the Age, Comorbidities, and Albumin (ACA) index, which is simple to use and has the ability to stratify the prognosis, tolerability to cytotoxic drugs, and adherence to treatment of elderly patients with DLBCL treated with R‐CHOP. However, the ACA index does not include evaluation for functional status, which may influence its efficacy.
Thus, we aimed to validate the ACA index in elderly Chinese patients with DLBCL. In addition, we wanted to refine the ACA index and propose a more effective CGA method that could serve as a guide for optimal personalized therapy for elderly patients with DLBCL treated with R‐CHOP.
Materials and Methods
Patients
In this retrospective study, patients ≥65 years of age who had been diagnosed with de novo DLBCL and had received R‐CHOP‐based treatments between January 1, 2003, and December 31, 2016, in the Department of Hematology, Beijing Hospital, People's Republic of China, were screened for eligibility. Fifteen patients were excluded because no information on treatments (n = 4) or follow‐ups (n = 11) was available. The final follow‐up visits for survival analysis were conducted on June 1, 2017. Informed consent was obtained from all patients, and the study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Beijing Hospital.
Chemotherapy Regimens
The decision to treat a patient and the choice of the type and intensity of treatment were always left to the clinical judgment of the attending physician, who was blinded to the results of the ACA index. According to the intensity of chemotherapy, the regimen included (a) high intensity:R‐CHOP‐21 (375 mg/m2 rituximab on day 0; 750 mg/m2 cyclophosphamide on day 1; 50 mg/m2 doxorubicin, 70 mg/m2 epirubicin, or 40 mg liposomal doxorubicin, 40 mg on day 1; 1.4 mg/m2 vincristine [capped at 2 mg per day] or 4 mg vindesine on day 1; and 100 mg prednisolone per day on days 1 to 5) every 3 weeks, (b) intermediate intensity:R‐reduced intensity CHOP (R‐riCHOP; 375 mg/m2 rituximab on day 0; 750 mg/m2 cyclophosphamide on day 1; 25–35 mg/m2 doxorubicin, 35–50 mg/m2 epirubicin, or 20–30 mg liposomal doxorubicin on day 1; 1.4 mg/m2 vincristine [capped at 2 mg per day] or 4 mg vindesine on day 1; and 100 mg prednisolone per day on days 1 to 5) every 3 weeks, or (c) low intensity:R‐miniCHOP (375 mg/m2 rituximab on day 0; 400 mg/m2 cyclophosphamide on day 1; 25 mg/m2 doxorubicin, 35 mg/m2 epirubicin, or 20 mg liposomal doxorubicin on day 1; 2 mg vindesine on day 1; and 40 mg/m2 prednisolone per day on days 1 to 5) every 3 weeks or R‐COP (375 mg/m2 rituximab on day 0, 750 mg/m2 cyclophosphamide on day 1, 1.4 mg/m2 vincristine [capped at 2 mg per day] or 4 mg vindesine on day 1, and 100 mg prednisolone per day on days 1 to 5) every 3 weeks.
ACA Index
According to the research of Miura et al. [8], the ACA index comprises three risk factors: advanced age (>75 years), hypoalbuminemia (<3.7g/dL), and high burden of clinical comorbidities (Charlson Comorbidity Index [CCI] score ≥3). Based on this ACA index score, patients were categorized into “excellent” (0 points), “good” (1 point), “moderate” (2 points), and “poor” (3 points) groups. In order to verify the efficacy of ACA index in our cohort, we used the same cutoff values for age, hypoalbuminemia, and CCI scores in the present study.
Definitions and Assessments
The comorbidities at diagnosis were assessed based on the CCI scores [9]. The performance status was assessed based on the Eastern Cooperative Oncology Group (ECOG) and Karnofsky scale scores. The patients’ activity ability was assessed based on activities of daily living (ADL) [10] and instrumental activities of daily living (IADL) scales [11]. Toxicities of therapy were rated according to the World Health Organization criteria [12]. Overall response rate (ORR) included complete remission (CR), complete remission unconfirmed (CRu), and partial remission (PR). Responses were assessed by the treating physicians after the end of treatment [13], [14]. After the fourth and eighth cycles, patients underwent disease reassessment by fluorodeoxyglucose positron emission tomography or computed tomography (CT), the choice of which depended on the economic status of the patients. During the first 2 years, clinical and biochemical follow‐up was conducted every 3 months and thereafter every 6 months. A CT scan or ultrasonography was carried out every 3 months for the first 2 years of the follow‐up period. Relapse or disease progression was defined according to the criteria of Cheson et al. [14]. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Progression‐free survival (PFS) was defined as the time from diagnosis until progression, relapse, or death from any cause. Patients without PFS or OS events were censored to the last date with valid information for that end‐point. Treatment‐related mortality (TRM) was defined as death without evidence of disease relapse or progression.
Statistical Analysis
Continuous variables were compared using the Mann‐Whitney U test; categorical variables were compared using chi‐square and Fisher's exact tests. Survival probabilities were estimated by means of the Kaplan‐Meier method. Competing risk analyses were used to calculate the cumulative incidence of relapse and progression and TRM, using Gray's test to test for differences between the groups [15].
To refine the ACA index, hazard ratios for OS were estimated from univariate and multivariate Cox regression analyses. All factors with p < .1 in the univariate analysis were included in a multivariate regression, and p < .05 was considered to be statistically significant. The level of significance was set at p < .05. All reported p values were based on two‐sided tests. Data analyses were primarily conducted with SPSS software (SPSS Inc., Chicago, IL), and the R software package (version 2.6.1; R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org) was used for competing risk analysis.
Results
Patient Characteristics
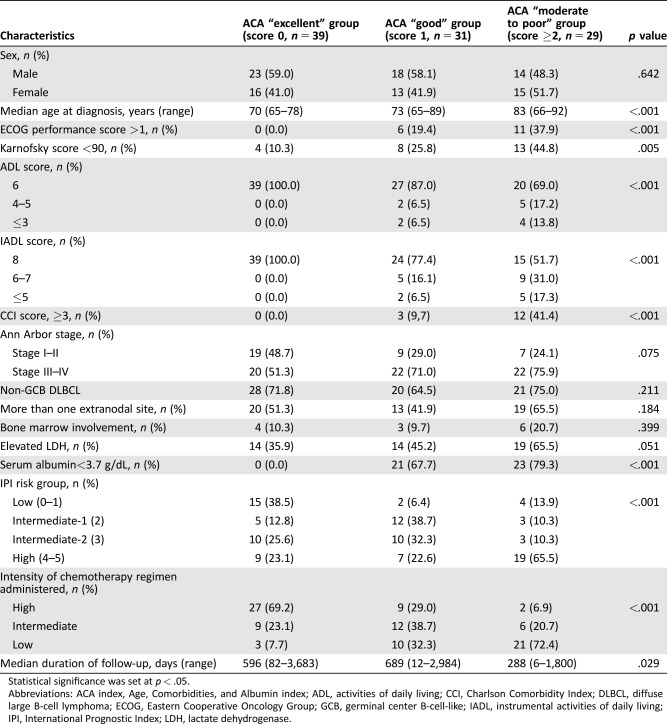
Characteristics of the 99 patients are summarized in Table 1. Thirty‐nine, 31, 26, and 3 patients were categorized into the ACA “excellent,” “good,” “moderate,” and “poor” groups, respectively. Because there were only 3 patients in the “poor” group, we combined the “moderate” and “poor” groups to form the “moderate to poor” group. In the “moderate to poor” group, more patients showed high ECOG and low Karnofsky scores, were low on the ADL and IADL scales, and showed elevated lactate dehydrogenase levels and high‐risk international prognostic index DLBCL. Most patients in the “excellent” group received high‐intensity chemotherapy regimens, while most patients in the “moderate to poor” group received low‐intensity chemotherapy regimens.
Table 1. Patient characteristics.
Statistical significance was set at p < .05.
Abbreviations: ACA index, Age, Comorbidities, and Albumin index; ADL, activities of daily living; CCI, Charlson Comorbidity Index; DLBCL, diffuse large B‐cell lymphoma; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B‐cell‐like; IADL, instrumental activities of daily living; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
Toxicities and TRM
The grade ≥3 toxicities observed are summarized in supplemental online Table 1. Hematologic toxicities were the most common toxicity noted after chemotherapy, followed by infectious, cardiovascular, and gastrointestinal toxicities. The ratios of grade ≥3 infectious toxicities were significantly higher in the ACA “moderate to poor” group, and the other toxicities were comparable among the three groups.
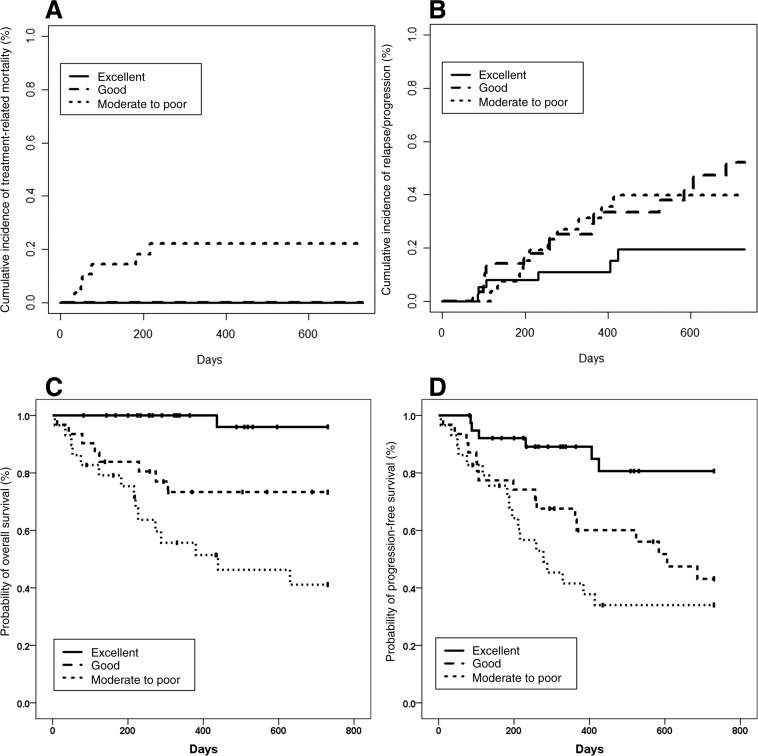
Eight patients died of TRM. The most common cause of TRM was infection (n = 4), followed by myocardial infarction (n = 1), malignant ventricular arrhythmia (n = 1), stroke (n = 1), and second malignancy (n = 1). The 2‐year cumulative incidence of TRM was significantly higher in the ACA “moderate to poor” group (22.1%) compared with that of the ACA “good” (0.0%) and “excellent” groups (0.0%; Fig. 1A).
Figure 1.
Outcomes for patients grouped according to the Age, Comorbidities, and Albumin index. (A): Treatment‐related mortality. (B): Relapse or progression. (C): Overall survival. (D): Progression‐free survival.
Response and Relapse
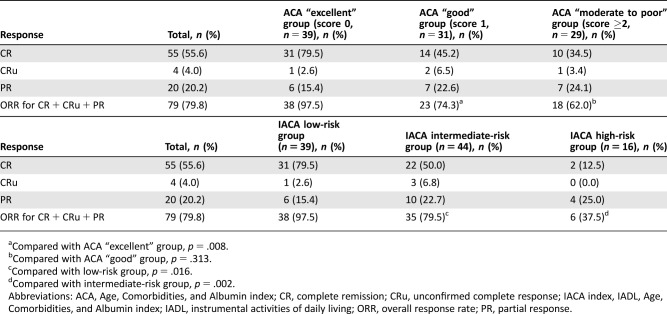
The rates of CR, CRu, PR, and ORR were 55.6%, 4.0%, 20.2%, and 79.8%, respectively, in the total population. The ORR in the ACA “excellent” group was the highest (97.5%), but the ORRs were comparable between the ACA “good” and “moderate to poor” groups (Table 2). The 2‐year cumulative incidence of relapse was lowest in the ACA “excellent” group (Fig. 1B).
Table 2. Response to treatment.
Compared with ACA “excellent” group, p = .008.
Compared with ACA “good” group, p = .313.
Compared with low‐risk group, p = .016.
Compared with intermediate‐risk group, p = .002.
Abbreviations: ACA, Age, Comorbidities, and Albumin index; CR, complete remission; CRu, unconfirmed complete response; IACA index, IADL, Age, Comorbidities, and Albumin index; IADL, instrumental activities of daily living; ORR, overall response rate; PR, partial response.
OS and PFS
The 2‐year probabilities of OS were 96.0% and 73.3% in the ACA “excellent and “good” groups, respectively (p = .005), which were both significantly higher than that of the ACA “moderate to poor” group (41.1%, Fig. 1C). The 2‐year probability of PFS was the highest in the ACA “excellent” group (80.6%), but the 2‐year probabilities of PFS was comparable between the ACA “good” group (43.1%) and “moderate to poor” group (34.0%, p = .264; Fig. 1D).
Refinement of the ACA Index
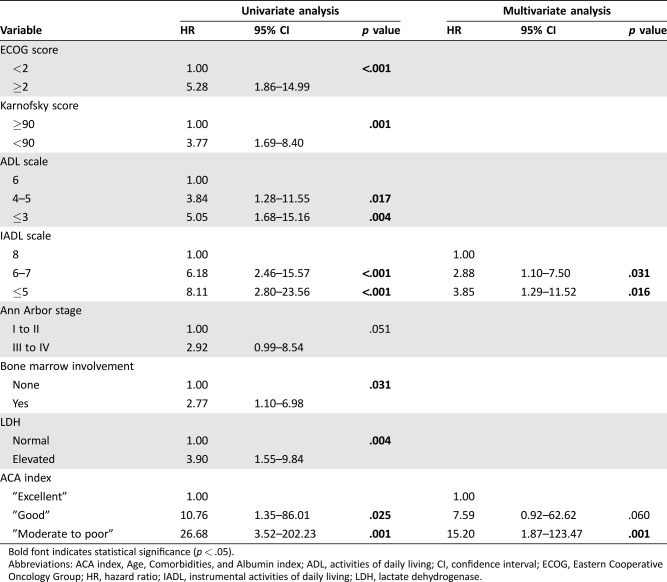
We constructed a Cox proportional hazards model with the following variables: ECOG score (<2 vs. ≥2), Karnofsky performance status (90–100 vs. <90), ADL scale (6 vs. 5–4 vs. 3), IADL scale (8 vs. 6–7 vs. ≤5), Ann Arbor stage (grades I–II vs. grades III–IV), bone marrow involvement (yes vs. no), lactate dehydrogenase levels (normal vs. elevated), extranodal involvement (>1 vs. 0–1), and ACA index (“excellent” vs. “good” vs. “moderate to poor”).
In the univariate analysis, ECOG score, Karnofsky performance status, ADL scale, IADL scale, Ann Arbor stage, bone marrow involvement, lactate dehydrogenase levels, and ACA index were the potential predictors of OS. Finally, the multivariable model identified two independent predictors of OS: IADL scale and ACA index (Table 3). IADL scale score 6 to 7 and the ACA “good” group were assigned 1 point and IADL scale ≤5 and the ACA “moderate to poor” group were assigned 2 points. Thus, we created a three‐category system, the IADL ACA index (IACA index): low risk, score 0 (n = 39); intermediate risk, score 1 to 2 (n = 44); and high risk, score 3 to 4 (n = 16; supplemental online Table 2).
Table 3. Univariate and multivariate analyses of variables for overall survival.
Bold font indicates statistical significance (p < .05).
Abbreviations: ACA index, Age, Comorbidities, and Albumin index; ADL, activities of daily living; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IADL, instrumental activities of daily living; LDH, lactate dehydrogenase.
Validation of the IACA Index in the Present Cohort
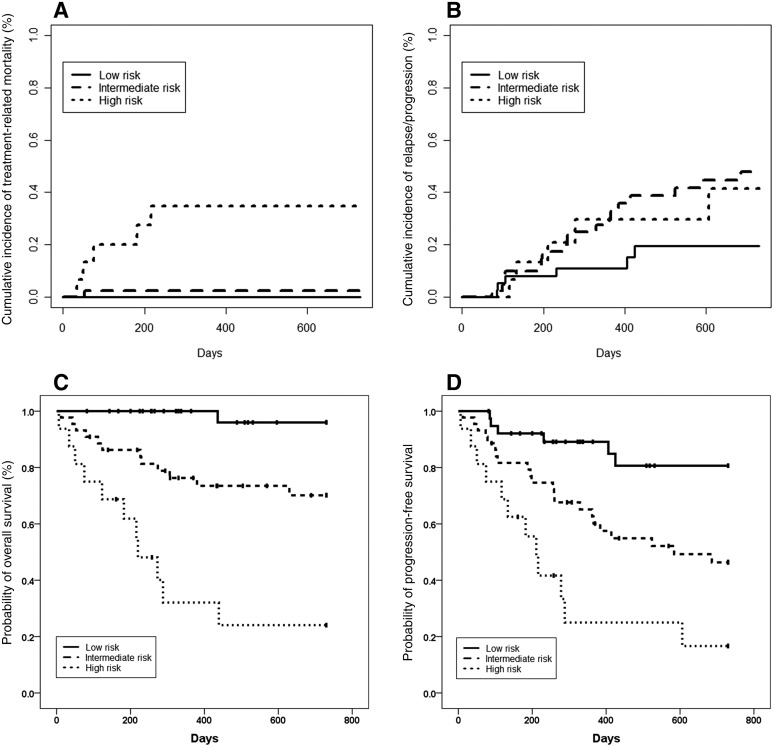
The ORR of the low‐risk group was higher than that of the intermediate‐risk group (97.4% vs. 79.5%, p = .016), and the ORR of the intermediate‐risk group was higher than that of the high‐risk group (37.5%, p = .002; Table 2). The 2‐year cumulative incidence of TRM was the highest in the high‐risk group (Fig. 2A), and the 2‐year cumulative incidence of relapse was the lowest in the low‐risk group (Fig. 2B).
Figure 2.
Outcomes for patients grouped according to the instrumental activities of daily living, Age, Comorbidities, and Albumin index. (A): Treatment‐related mortality. (B): Relapse or progression. (C): Overall survival. (D): Progression‐free survival.
The 2‐year probabilities of OS were 96.0% and 70.1% in the low‐risk and intermediate‐risk groups, respectively (p = .003), which were both significantly higher than that of the high‐risk group (24.1%, Fig. 2C). The 2‐year probabilities of PFS were 80.6% and 46.4% in the low‐risk and intermediate‐risk groups, respectively (p = .005), which were both significantly higher than that of the high‐risk group (16.7%, Fig. 2D).
Clinical Outcomes According to the Other CGA System
According to the CGA system of Tucci et al. [16], patients were classified as “fit” (n = 49), “unfit” (n = 14), and “frail” (n = 36) based on age, ADL/IADL, and the Cumulative Illness Rating Score for Geriatrics (CIRS‐G). The 2‐year probabilities of OS (90.8%) and PFS (72.9%) were the highest in the “fit” group, but survival was comparable between the “unfit” (OS: 43.0%; PFS: 32.5%) and “frail” groups (OS: 58.5%; PFS: 37.3%; supplemental online Fig. 1A and 1B). We found that the IACA index resulted in the reclassification of patients in the “fit,” “unfit,” and “frail” classifications (supplemental online Fig. 2).
Discussion
In our study, we first observed that the ACA index could partially predict the clinical outcomes in an independent Chinese cohort. Combining the features of ACA index and IADL scale, we propose a new and more effective index (IACA index) for elderly patients with DLBCL that can predict both ORR and survival.
The ORR was 79.8% in the present study, which was similar to the results of Coiffier et al. (82%) [17], Pfreundschuh et al. (81%–84%) [18], and Habermann et al. (77%) [19]. These results supported that chemotherapy plus rituximab is useful for elderly patients with DLBCL.
IADL skills are those required to maintain independence in the community [11]. The evaluation of IADL skills, in particular, adds substantially to the functional information provided by ECOG and Karnofsky performance status values [20], and patients over the age of 80 years are more likely to require assistance in IADL skills [21]. The importance of the IADL scale has been observed in the CGA of patients with acute myeloid leukemia [22], multiple myeloma [23], and chronic lymphoblastic leukemia [24], and it is also one of the important components of the CGA in patients with DLBCL [25].
CGA is an effective method to identify elderly patients with DLBCL who can benefit from a curative approach with anthracycline‐containing immunochemotherapy. In the study of Tucci et al. [25], patients were classified as “fit” and “unfit.” The “fit” patients received curative treatment, and their response rate and median survival were significantly better than those of “unfit” patients. Other authors have also reported that CGA (including age, ADL/IADL, and CIRS‐G) is a valid tool to prospectively identify frail subjects among elderly patients with DLBCL [26] and also to identify patients who can benefit from a curative approach [16]. In addition, the chemoimmunotherapy adjustments based on a CGA are associated with manageable toxicity and excellent outcomes [27]. Combining the use of the IADL and ACA indexes (including age and comorbidity score according to CCI), our IACA index is a relative integral CGA system [20]. We observed that the IACA index could predict response rate, OS, and PFS in elderly patients with DLBCL. Particularly, ORR and survival were both significantly better in the intermediate‐risk group than in the high‐risk group. Thus, it is suggested that IACA index is an effective method for CGA in DLBCL.
The critical difference between the IACA index and the previous CGA system [16], [25], [26], [27] was the scores for comorbidity evaluation. CCI in the IACA index, which may be easier and quicker to apply than CIRS‐G [28], was also suggested to be useful in CGA for patients with other hematologic malignances [29], [30]. Some authors suggested that CIRS‐G provides a more complete assessment of comorbidity and, therefore, may be more suitable to measure comorbidity in older people [31], [32]. However, when we used the CGA system of Tucci et al. [16] in the present study, we observed that although survival was best in the “fit” group, it was comparable between the “unfit” and “frail” groups, and this result is in accordance with previous studies [16], [27]. Thus, the IACA index may be more suitable for elderly patients with DLBCL. However, as only a few studies have compared the efficacy of CCI and CIRS‐G directly in CGA, we could not derive substantial conclusions regarding the superiority of CCI over CIRS‐G in CGA for DLBCL.
There were several limitations in this study. First, this is a retrospective study with a relatively small number of elderly patients with DLBCL, which could have influenced the accuracy of our findings. In addition, only three patients were categorized as being “poor” in the ACA index, which could have influenced our validation of the index. Lastly, CCI is not as extensively used in the U.S. as in other countries, which may limit the applicability of IACA index in elderly patients with DLBCL. Thus, we suggest that elderly patients with DLBCL are worthy of receiving CCI evaluation.
Conclusion
We observed that that ACA index can partially predict clinical outcomes of elderly patients with DLBCL in China. Based on this index, we propose the use of the IACA index as an effective tool for CGA in DLBCL. Additional large, prospective studies can further identify and compare the validity of the IACA index and other CGA methods among elderly patients with DLBCL. In addition, we should identify the efficacy and safety of IACA‐index‐directed therapy in elderly patients with DLBCL.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Editage and Dr. Xiao‐Dong Mo for their assistance in editing this manuscript. This work was supported by the Beijing Committee of Science and Technology (grants Z171100001017200 and Z171100001017084).
Contributed equally
Author Contributions
Conception/design: Hui Liu, Chun‐Li Zhang
Provision of study material or patients: Ru Feng, Jiang‐Tao Li, Yuan Tian, Ting Wang
Collection and/or assembly of data: Chun‐Li Zhang, Jiang‐Tao Li
Data analysis and interpretation: Chun‐Li Zhang
Manuscript writing: Hui Liu, Chun‐Li Zhang
Final approval of manuscript: Hui Liu, Chun‐Li Zhang, Ru Feng, Jiang‐Tao Li, Yuan Tian, Ting Wang
Disclosures
The authors indicated no financial relationships.
References
- 1. Morton LM, Wang SS, Devesa SS et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang QP, Zhang WY, Yu JB et al. Subtype distribution of lymphomas in Southwest China: Analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol 2011;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison VA. Evolution of R‐CHOP therapy for older patients with diffuse large B‐cell lymphoma. Expert Rev Anticancer Ther 2008;8:1651–1658. [DOI] [PubMed] [Google Scholar]
- 4. Morrison VA, Hamlin P, Soubeyran P et al. Approach to therapy of diffuse large B‐cell lymphoma in the elderly: The International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol 2015;26:1058–1068. [DOI] [PubMed] [Google Scholar]
- 5. Repetto L, Fratino L, Audisio RA et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 6. Monfardini S, Ferrucci L, Fratino L et al. Validation of a multidimensional evaluation scale for use in elderly cancer patients. Cancer 1996;77:395–401. [DOI] [PubMed] [Google Scholar]
- 7. Morrison VA, Hamlin P, Soubeyran P et al. Diffuse large B‐cell lymphoma in the elderly: Impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J Geriatr Oncol 2015;6:141–152. [DOI] [PubMed] [Google Scholar]
- 8. Miura K, Konishi J, Miyake T et al. A host‐dependent prognostic model for elderly patients with diffuse large B‐cell lymphoma. The Oncologist 2017;22:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 10. Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 11. Lawton MP, Brody EM. Assessment of older people: Self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 12. Miller AB, Hoogstraten B, Staquet M et al. Reporting results of cancer treatment. Cancer 1981;47:207–214. [DOI] [PubMed] [Google Scholar]
- 13.Chinese Society of Hematology, Chinese Medical Association ; Chinese Society of Lymphoma, Chinese Anti‐Cancer Association. Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma (2013) [in Chinese]. Zhonghua Xue Ye Xue Za Zhi 2013;34:816–819. [DOI] [PubMed] [Google Scholar]
- 14. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 15. Gooley TA, Leisenring W, Crowley J et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 16. Tucci A, Martelli M, Rigacci L et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B‐cell lymphoma: A prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921–926. [DOI] [PubMed] [Google Scholar]
- 17. Coiffier B, Lepage E, Briere J et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002;346:235–242. [DOI] [PubMed] [Google Scholar]
- 18. Pfreundschuh M, Schubert J, Ziepert M et al. Six versus eight cycles of bi‐weekly CHOP‐14 with or without rituximab in elderly patients with aggressive CD20+ B‐cell lymphomas: A randomised controlled trial (RICOVER‐60). Lancet Oncol 2008;9:105–116. [DOI] [PubMed] [Google Scholar]
- 19. Habermann TM, Weller EA, Morrison VA et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol 2006;24:3121–3127. [DOI] [PubMed] [Google Scholar]
- 20. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 21. Repetto L, Fratino L, Audisio RA et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 22. Wedding U, Röhrig B, Klippstein A et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol 2006;132:665–671. [DOI] [PubMed] [Google Scholar]
- 23. Bila J, Jelicic J, Djurasinovic V et al. Prognostic effect of comorbidity indices in elderly patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2015;15:416–419. [DOI] [PubMed] [Google Scholar]
- 24. Goede V, Bahlo J, Chataline V et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk Lymphoma 2016;57:789–796. [DOI] [PubMed] [Google Scholar]
- 25. Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 26. Merli F, Luminari S, Rossi G et al. Outcome of frail elderly patients with diffuse large B‐cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: Results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 2014;55:38–43. [DOI] [PubMed] [Google Scholar]
- 27. Spina M, Balzarotti M, Uziel L et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B‐cell lymphoma. The Oncologist 2012;17:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dias A, Teixeira‐Lopes F, Miranda A et al. Comorbidity burden assessment in older people admitted to a Portuguese University Hospital. Aging Clin Exp Res 2015;27:323–328. [DOI] [PubMed] [Google Scholar]
- 29. Rao AV. Fitness in the elderly: How to make decisions regarding acute myeloid leukemia induction. Hematology Am Soc Hematol Educ Program 2016;2016:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Artz AS. Biologic vs physiologic age in the transplant candidate. Hematology Am Soc Hematol Educ Program. 2016;2016:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harboun M, Ankri J. Comorbidity indexes: Review of the literature and application to studies of elderly population [in French]. Rev Epidemiol Sante Publique 2001;49:287–298. [PubMed] [Google Scholar]
- 32. Abizanda Soler P, Paterna Mellinas G, Martínez Sánchez E et al. Comorbidity in the elderly: Utility and validity of assessment tools [in Spanish]. Rev Esp Geriatr Gerontol 2010;45:219–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.