This article provides a summary of the U.S. Food and Drug Administration (FDA) review of the marketing application for lenalidomide as maintenance therapy for patients with newly diagnosed multiple myeloma after autologous hematopoietic stem cell transplantation.
Keywords: Multiple myeloma, Lenalidomide, Revlimid
Abstract
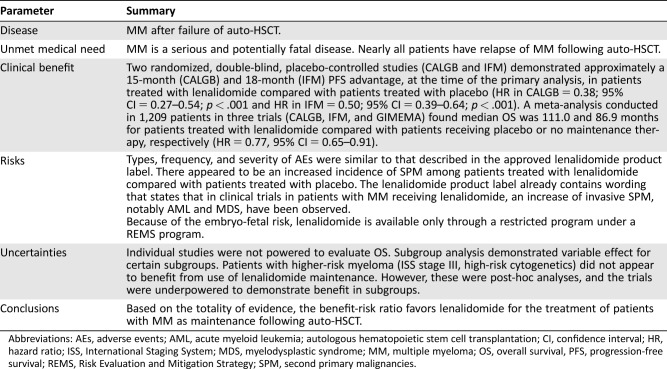
On February 22, 2017, the U.S. Food and Drug Administration (FDA) granted approval for the use of lenalidomide as maintenance therapy after autologous hematopoietic stem cell transplantation (auto‐HSCT) for patients with multiple myeloma. The approval was based on evidence from two randomized, blinded trials of maintenance lenalidomide versus placebo in patients with myeloma who had undergone auto‐HSCT along with a third trial of lenalidomide versus no therapy. Each of the trials demonstrated superior progression‐free survival for the patients treated with lenalidomide. The effect on overall survival was mixed, with one trial showing longer overall survival and another showing no effect. Subgroup analysis suggested better results for patients with International Staging System stage I or II disease compared with stage III disease. Safety evaluation did not reveal any new safety concerns. More second primary malignancies were observed in the lenalidomide arm compared with the placebo arm. The FDA concluded that lenalidomide maintenance showed a favorable benefit‐to‐risk ratio when used as maintenance therapy after auto‐HSCT.
Implications for Practice.
Prior to this approval, there were no U.S. Food and Drug Administration‐approved maintenance therapies for patients with multiple myeloma (MM) who have undergone autologous hematopoietic stem cell transplantation (auto‐HSCT). Maintenance therapy with lenalidomide after auto‐HSCT in patients with MM demonstrated an approximately 15‐ to 18‐month advantage in progression‐free survival compared with placebo at the time of the primary analysis. Patients treated with lenalidomide also appeared to have a survival advantage compared with patients treated with placebo. Because of the high rate of relapse of MM in patients following auto‐HSCT and because MM is a serious and often fatal disease, these results appear to be clinically meaningful.
Introduction
Multiple myeloma (MM) is a highly treatable, but incurable, hematologic malignancy. For younger and fit patients with symptomatic newly diagnosed multiple myeloma (NDMM), high‐dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auto‐HSCT) is the standard of care and leads to longer progression‐free survival (PFS) and overall survival (OS) compared with other treatment options [1], [2]. In addition, a recent trial comparing PFS and OS for patients undergoing consolidation with auto‐HSCT versus chemotherapy alone followed by maintenance with lenalidomide demonstrated longer PFS in the auto‐HSCT group, although OS was not different between the two groups, perhaps due to salvage with auto‐HSCT at relapse in the chemotherapy group [3]. Unfortunately, relapse after auto‐HSCT is nearly universal.
Maintenance therapy after auto‐HSCT has been proposed as a method of increasing PFS and potentially OS in patients who have undergone auto‐HSCT [4], [5]. Lenalidomide has been examined as maintenance therapy as a single agent in several clinical trials [6], [7], [8].
Here, we provide a summary of the U.S. Food and Drug Administration (FDA) review of the marketing application for lenalidomide as maintenance therapy for patients with NDMM after auto‐HSCT.
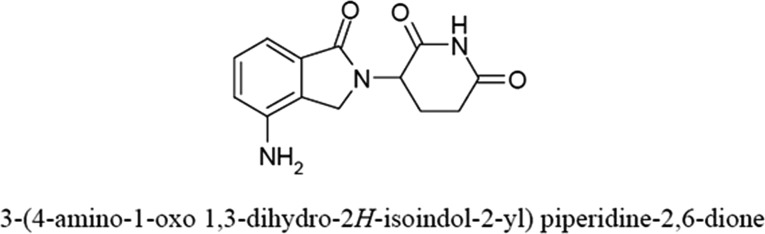
Lenalidomide (Revlimid; Celgene Corporation, Summit, NJ) is an immunomodulatory drug and analogue of thalidomide. The molecular structure of lenalidomide is shown in Figure 1 [9]. The FDA initially approved lenalidomide for use in myelodysplastic syndromes with a 5q deletion on December 27, 2005. It was approved June 29, 2006, for use with dexamethasone in MM patients who had been treated with at least one prior line of therapy. Use in mantle cell lymphoma was added on June 5, 2013. On February 17, 2015, the approval was extended to include newly diagnosed patients with multiple myeloma who are not candidates for transplant [9].
Figure 1.
Chemical structure of lenalidomide.
Lenalidomide is an oral medication and is supplied as capsules in 2.5, 5, 10, 15, 20, and 25 mg strengths. Lenalidomide is subject to a Risk Evaluation and Mitigation Strategy program because of concern about the risk of embryo‐fetal toxicity given its similarity to thalidomide. Other known potential serious adverse reactions include venous and arterial thrombosis, hematologic toxicity, and risk of second primary malignancies [9].
Recent work elucidating the mechanism of action of lenalidomide has demonstrated that cereblon, a protein previously identified as the primary target of thalidomide‐related teratogenicity [10], is necessary for the antimyeloma effect of lenalidomide and other related drugs. Cereblon forms an E3 ubiquitin ligase complex with damaged DNA binding protein 1. Cereblon down‐regulation results in marked resistance to lenalidomide [11].
Clinical Trials
Three clinical trials of maintenance lenalidomide versus placebo or no treatment in patients with NDMM who had undergone auto‐HSCT were used to assess the efficacy. These trials included the CALGB 100104 trial (ClinicalTrials.gov identifier NCT00114101), the IFM 2005‐02 trial (NCT00430365), and the RV‐MM‐PI‐209 (GIMEMA) trial (NCT00551928). Each of these trials was a randomized phase 3 trial of maintenance lenalidomide versus placebo or no treatment after auto‐HSCT with treatment until progression, unacceptable adverse events, or withdrawal of consent.
Patients aged 18–70 years who had active NDMM (at least stage I) with Eastern Cooperative Oncology Group performance status of 0–1 and at least stable disease after auto‐HSCT were eligible for enrollment in the CALGB trial. Patients were stratified by β‐2‐microglobulin, prior therapy with thalidomide, and prior therapy with lenalidomide. A total of 568 patients were registered and 460 included in the intent‐to‐treat (ITT) population.
Patients aged 18–65 years with symptomatic NDMM (defined as at least stage II or stage I with bony disease) who had no signs of progressive disease (PD) within 6 months after auto‐HSCT and a good performance status were eligible for enrollment in the IFM trial. Patients were stratified by β‐2‐microglobulin, presence of del 13 at diagnosis, and response to last auto‐HSCT. A total of 614 patients were included in the ITT population.
Patients with symptomatic NDMM, Karnofsky performance status of at least 60 and aged 65 years or younger were eligible for enrollment in the GIMEMA trial. Patients were stratified by International Staging System (ISS) stage at diagnosis and age. A total of 135 patients were included in the ITT population of the arm receiving auto‐HSCT.
CALGB and IFM were double‐blinded, placebo controlled trials of maintenance lenalidomide at 10 mg daily with increase to 15 mg daily if tolerated. The GIMEMA trial had two randomizations, both performed at the time of enrollment: patients were randomized initially to receive auto‐HSCT versus melphalan, prednisone, and lenalidomide, with a second randomization to maintenance lenalidomide, 10 mg daily on 21 days of a 28‐day cycle, versus no treatment after completion of induction therapy. Only patients on the auto‐HSCT arm were included in this analysis.
Differences in treatment between the trials included the difference in dosing regimen between IFM and CALGB versus GIMEMA as described above. In addition, crossover to lenalidomide was allowed in the CALGB trial after an interim analysis revealed a PFS advantage in one arm and the study was unblinded. The trial is ongoing with patients who have not progressed continuing to receive treatment and being followed for progression, survival, second‐line therapy, and second primary malignancies (SPM). Crossover was not allowed in IFM and lenalidomide treatment was stopped in January 2011, when an increase in SPM was observed in the lenalidomide arm. Two cycles of consolidation therapy with 25 mg daily for 21 days of a 28‐day cycle were added to both arms of IFM in an amendment early in the study, with 32 patients being enrolled and treated prior to this amendment. Crossover was not allowed in GIMEMA and treatment is ongoing for patients without PD. Finally, time between transplant and start of maintenance varied, with patients in CALGB starting 90–100 days after transplant, patients in IFM starting within 6 months of transplant, and patients on GIMEMA starting within 2–3 months of transplant.
Endpoints
PFS was the primary endpoint in each trial. PFS was defined as the time from transplant until PD or death from any cause for CALGB and as the time from randomization to PD or death for IFM. In CALGB, PFS calculated as the time from randomization to PD or death was also prespecified as a sensitivity analysis. The individual trials were not powered to detect a difference in OS, but OS was examined as a secondary endpoint. The timing of OS analysis was not prespecified. In addition, PFS from diagnosis, response duration, complete response duration, time to progression, and time to best response were examined.
Results
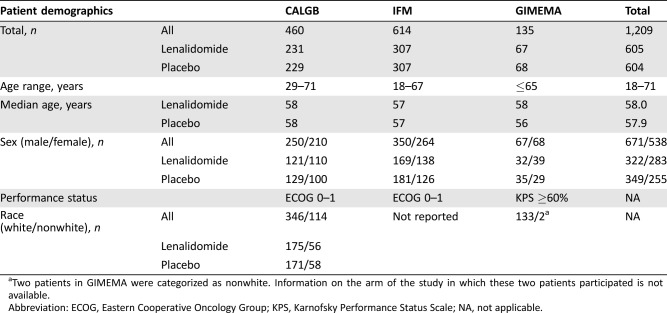
Baseline demographic characteristics of patients included in the trials are presented in Table 1. A total of 1,209 patients, 605 in the lenalidomide arm and 604 in the placebo/no therapy arm, were included in the ITT population. Patients’ ages ranged from 23 to 71, with a median age of 58.0 and 57.9, respectively, for lenalidomide and placebo/no therapy. There was a male predominance, with 671 patients (55.5%) being male and 538 (44.5%) female. Race was not recorded for the IFM trial due to French law, and only two patients on the GIMEMA trial were recorded as nonwhite. On the CALGB trial, 346 patients were white and 114 were nonwhite.
Table 1. Patient demographics for patients in the intent‐to‐treat populations of the included clinical trials.
Two patients in GIMEMA were categorized as nonwhite. Information on the arm of the study in which these two patients participated is not available.
Abbreviation: ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status Scale; NA, not applicable.
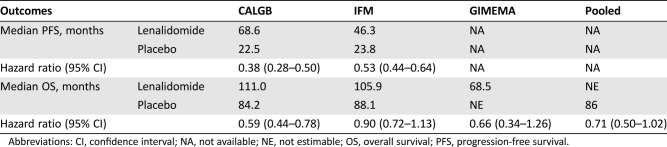
PFS was prolonged for patients receiving lenalidomide in CALGB and IFM, as has been previously reported [5], [6], [7]. Hazard ratios for PFS, calculated as the time from randomization to PD or death, for lenalidomide versus placebo were 0.38 for CALGB and 0.53 for IFM (Table 2). However, results for OS were mixed, with a statistically significant improvement in OS observed in CALGB, but not in IFM or GIMEMA. The differences in outcomes between these studies could not be explained by patient demographics, second‐line treatment, disease characteristics, use of consolidation therapy, or any other identifiable factor. It was noted that survival in the placebo group of the CALGB trial was lower than for the IFM, whereas the opposite was true of the treatment arm.
Table 2. Outcomes analysis for included clinical trials.
Abbreviations: CI, confidence interval; NA, not available; NE, not estimable; OS, overall survival; PFS, progression‐free survival.
Meta‐Analysis
Because the individual trials were not powered to determine OS, a meta‐analysis of the three trials was performed. For the meta‐analysis, patients were stratified by trial only. The treatment effect across studies was considered heterogeneous and thus a random effect model was preferred for the analysis. When a random effect model was used to compensate for the heterogeneity, a hazard ratio of 0.71 was observed for the OS comparison, with a 95% confidence interval of 0.50–1.02.
The FDA review team performed additional subgroup analyses on both individual studies and pooled data from the three studies. Results were derived from the most updated data provided for each study. Missing data were an issue for a number of characteristics. In particular, adverse risk cytogenetics were not reported for the CALGB study. In addition, information on stage and creatinine clearance at diagnosis was missing in 31.2% and 69.3% of patients on the lenalidomide arm and 27.5% and 68.1% on the placebo arm, respectively, in the CALGB trial. Race was not reported for the IFM trial, and only two patients on the GIMEMA trial were nonwhite. Thus, virtually all the information on race is derived from data from the CALGB trial.
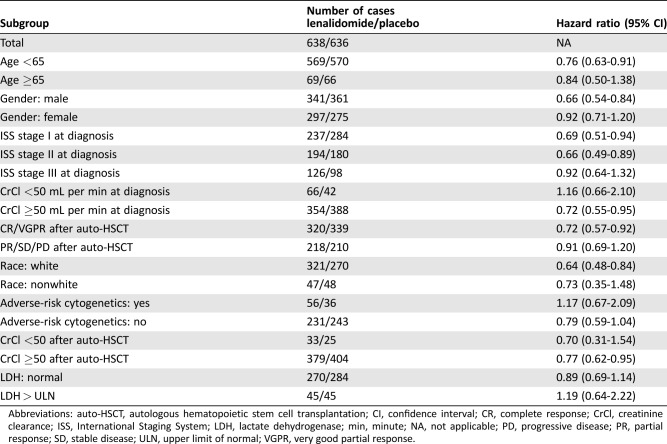
Point estimates for the hazard ratios by subgroup were greater than 1 (favored placebo) for creatinine clearance less than 50 mL per minute at diagnosis, adverse risk cytogenetics at diagnosis, and LDH greater than the upper limit of normal (Table 3). Although point estimates for survival favored lenalidomide in all other subgroups analyzed, the 95% confidence interval crossed one for patients aged 65+ years, women, less than very good partial response (VGPR) after transplant, ISS stage III at diagnosis, creatinine clearance of less than 50 mL per minute after transplant, and nonwhite race. Case numbers were small for many subgroups and, as noted above, missing data were an issue.
Table 3. Hazard ratios (lenalidomide vs. placebo) for overall survival for selected subgroups using pooled data for intent‐to‐treat population, updated through February 2016.
Abbreviations: auto‐HSCT, autologous hematopoietic stem cell transplantation; CI, confidence interval; CR, complete response; CrCl, creatinine clearance; ISS, International Staging System; LDH, lactate dehydrogenase; min, minute; NA, not applicable; PD, progressive disease; PR, partial response; SD, stable disease; ULN, upper limit of normal; VGPR, very good partial response.
Safety Assessment
Adverse Reactions
The acute adverse reaction profile of lenalidomide is well characterized. No new adverse events were identified in the trials examined. Frequent adverse events included cytopenias, fatigue, diarrhea, constipation, muscle pain, and infectious complications. Deep venous thromboses and arterial thromboses were observed, although at a low rate.
Second Primary Malignancies
There has been concern for some time that the use of lenalidomide may increase the risk of SPM. Hematologic malignancies, especially myelodysplastic syndrome and acute myeloblastic leukemia, are of particular interest, but the increase in risk is not limited to these conditions. Data from the safety populations in CALGB and IFM showed that 22 patients (4.2%) in the lenalidomide arm and 10 in the placebo arm (1.9%) died due to SPM or complications of SPM. In addition, an increase in noninvasive skin cancers was observed in the lenalidomide arms of IFM and CALGB. No specific histology predominated within the invasive solid tumor cases.
FDA analysis of risks associated with the development of SPM demonstrated that ISS stage III, male gender, and high body mass index were risk factors for hematologic SPM. Risk factors identified for all SPM included age >55 years and ISS stage III only.
Discussion
Maintenance therapy for patients with NDMM who have undergone auto‐HSCT has been studied in multiple clinical trials. Most trials, including the three trials used to evaluate the application for an indication for lenalidomide as maintenance therapy in NDMM patients undergoing transplant, have demonstrated improvements in PFS with use of maintenance therapy. However, the effect of maintenance therapy and, specifically, of lenalidomide maintenance therapy on OS has been mixed. A clear benefit in OS was observed in the CALGB trial, whereas no statistically significant benefit to OS was observed in IFM or GIMEMA. In addition, subgroup analysis demonstrating possibly less benefit in some subgroups and the competing risk of SPM further complicate the issue.
Although the overall results demonstrate an improved PFS and a trend toward improved OS, justifying the approval of lenalidomide maintenance in NDMM after auto‐HSCT (Table 4), several caveats should be considered when making a decision concerning the use of maintenance therapy in an individual patient.
Table 4. U.S. Food and Drug Administration benefit‐risk assessment.
Abbreviations: AEs, adverse events; AML, acute myeloid leukemia; autologous hematopoietic stem cell transplantation; CI, confidence interval; HR, hazard ratio; ISS, International Staging System; MDS, myelodysplastic syndrome; MM, multiple myeloma; OS, overall survival, PFS, progression‐free survival; REMS, Risk Evaluation and Mitigation Strategy; SPM, second primary malignancies.
First, the FDA review team's exploratory subgroup analyses showed that the trend toward improved survival was not observed in patients with high‐risk cytogenetics, a creatinine clearance of less than 50 mL per minute at diagnosis, or LDH greater than upper limit of normal at diagnosis, with point estimates for these subgroups favoring placebo, although the confidence interval crossed 1 in each case; therefore, no definite conclusions can be drawn with respect to benefit or lack thereof in these subgroups. The point estimate for survival for ISS stage III was 0.92, considerably higher than the point estimates for ISS stage I and II disease, and the 95% confidence interval included 1. Creatinine clearance may be of significance, as lenalidomide is renally excreted. Patients who have suboptimal renal function may be less able to tolerate the drug and therefore have difficulty consistently complying with the medication, leading to worse outcomes. Taken together, these data suggest that the risks and benefits of lenalidomide maintenance should be carefully considered in certain subgroups. However, the subgroup analyses are post‐hoc analyses in small numbers of cases, and missing information may confound the results in some cases. Thus, care should be taken to avoid over‐interpretation of the data. Finally, the results of the FDA analysis vary slightly compared with those from a recently published analysis performed by the sponsor and investigators [12] due to differences in cutoff dates used and grouping for subgroup analyses.
Second, the demographic information for trials included in this evaluation was incomplete. In particular, the IFM trial, the largest of the three examined, did not include information concerning patients’ race. This lack of information, along with the relative lack of minority participants in the examined clinical trials, makes it hard to fully evaluate whether the medication is as effective in people of minority ethnic and racial background as it is for white patients.
Third, the optimum dose of lenalidomide is uncertain. Two of the three trials evaluated used 10 mg daily with an increase to 15 mg daily if tolerated as the preferred dose, whereas the third trial used 10 mg 21/28 days. There was no clear difference in survival based on dose, although the case numbers for the 10 mg/21‐day dose are small.
Finally, heterogeneity among the trials leaves open the issue of the optimal treatment and which patients benefit the most from maintenance. The differences in OS between the three trials remains unexplained and, therefore, it is difficult to definitively state whether the OS benefit observed in CALGB or the lack of benefit in IFM is more likely to be representative of typical results in practice. Of note, a recent publication of long‐term results in the CALGB trial, with a median follow‐up time of 91 months, showed a continued difference in PFS and OS between the two arms, with a median PFS and OS of 57.3 and 113.8 months in the lenalidomide arm and 28.9 and 84.1 months in the placebo arm, respectively. Increased differences in the rate of secondary malignancies were also demonstrated, with total SPM rates of 14% in the lenalidomide arm and 5% in the placebo arm, with most of the difference being due to hematologic malignancies (8% vs. 1%). Of note, all hematologic malignancies observed in the placebo group occurred in patients who crossed over to lenalidomide maintenance [13].
Although no other medications for maintenance therapy after auto‐HSCT in newly diagnosed multiple myeloma are FDA approved, several other drugs and drug combinations have been used for maintenance therapy, including bortezomib and combination therapies using a lenalidomide or bortezomib backbone [14], [15]. Expert opinion varies on the best treatment option in a variety of clinical settings, and all options should be considered when deciding on the best regimen for a given patient. Furthermore, participation in clinical trials should be considered an option for eligible patients.
Conclusion
There are a number of unanswered questions concerning the optimal use of maintenance therapy in patients with NDMM undergoing auto‐HSCT, and further trials are needed to optimize treatment for these patients. Lenalidomide maintenance demonstrates improved PFS and a trend toward improved OS in patients with NDMM after transplant.
Acknowledgment
This is a U.S. Government work. There are no restrictions on its use.
Author Contributions
Conception/design: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Provision of study material or patients: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Collection and/or assembly of data: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Data analysis and interpretation: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Manuscript writing: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Final approval of manuscript: E. Dianne Pulte, Andrew Dmytrijuk, Lei Nie, Kirsten B. Goldberg, Amy E. McKee, Ann T. Farrell, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Child JA, Morgan GJ, Davies FE et al. High‐dose chemotherapy with hematopoietic stem‐cell rescue for multiple myeloma. N Engl J Med 2003;348:1875–1883. [DOI] [PubMed] [Google Scholar]
- 2. Moreau P, San Miguel J, Ludwig H et al. Mulitple myeloma: ESMO clinical practice guidelines for diagnosis, treatment, and follow up. Ann Oncol 2013;24(suppl 6):vi133–vi137. [DOI] [PubMed] [Google Scholar]
- 3. Attal M, Lauwers‐Cances V, Hulin C et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017;376:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajikumar SV. Multiple myeloma: 2016 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2016;91:719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gentile M, Vigna E, Recchia AG et al. Role of new drugs incorporated into consolidation and maintenance therapy in transplant‐eligible multiple myeloma. Expert Opin Pharmacother 2014;15:1315–1320. [DOI] [PubMed] [Google Scholar]
- 6. Attal M, Lawers‐Cances V, Marit G et al. Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. N Engl J Med 2012;366:1782–1791. [DOI] [PubMed] [Google Scholar]
- 7. McCarthy PL, Owzar K, Hofmeister CC et al. Lenalidomide after stem cell transplantation for multiple myeloma. N Engl J Med 2012;366:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palumbo A, Cavallo F, Gay F et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014;371:895–905. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Revlimid, Full Prescribing Information. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021880s041lbl.pdf. Accessed January 3, 2017.
- 10. Ito T, Ando H, Suzuki T et al. Identification of a primary target of thalidomide teratogenicity. Science 2010;327:1345–1350. [DOI] [PubMed] [Google Scholar]
- 11. Zhu XY, Braggio E, Shi CX et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011;118:4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy PL, Holstein SA, Petrucci MT et al. Lenalidomide maintenance after autologous stem‐cell transplantation in newly diagnosed multiple myeloma: A meta‐analysis. J Clin Oncol 2017;35:3279–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holstein SA, Jung SH, Richardson PG et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem‐cell transplantation for multiple myeloma: A randomized, double‐blind, phase 3 trial. Lancet Haematol. 2017;4:e431–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Center Network. Multiple Myeloma (Version 3.2.2017). Available at https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 3, 2017.
- 15. Al‐Mansour Z, Ramanathan M. Post‐autologous (ASCT) stem cell transplantation therapy in multiple myeloma. Adv Hematol 2014;2014:652395. [DOI] [PMC free article] [PubMed] [Google Scholar]