Abstract
Environmental factors profoundly affect the addictive potential of drugs of abuse and may also modulate the neuro-anatomical/neuro-chemical impacts of uncontrolled drug use and relapse propensity. This study examined the impact of environmental enrichment on heroin self-administration, addiction-related behaviors, and molecular processes proposed to underlie these behaviors. Male Sprague-Dawley rats in standard and enriched housing conditions intravenously self-administered similar amounts of heroin over 14 days. However, environmental enrichment attenuated progressive ratio, extinction, and reinstatement session responding after 14 days of enforced abstinence. Molecular mechanisms, namely DNA methylation and gene expression, are proposed to underlie abstinence-persistent behaviors. A global reduction in methylation is reported to coincide with addiction, but no differences in total genomic methylation or repeat element methylation were observed in CpG or non-CpG (CH) contexts across the mesolimbic circuitry as assessed by multiple methods including whole genome bisulfite sequencing. Immediate early gene expression associated with drug seeking, taking, and abstinence also were examined. EGR1 and EGR2 were suppressed in mesolimbic regions with heroin-taking and environmental enrichment. Site-specific methylation analysis of EGR1 and EGR2 promoter regions using bisulfite amplicon sequencing (BSAS) revealed hypo-methylation in the EGR2 promoter region and EGR1 intragenic CpG sites with heroin-taking and environmental enrichment that was associated with decreased mRNA expression. Taken together, these findings illuminate the impact of drug taking and environment on the epigenome in a locus and gene-specific manner and highlight the need for positive, alternative rewards in the treatment and prevention of drug addiction.
Keywords: Heroin, relapse, epigenetic, self-administration
1. Introduction
Heroin addiction is characterized by chronic relapse to uncontrolled use, even after extended abstinence. Heroin use has increased, as individuals dependent upon prescription opioids turn to cheaper and more accessible heroin (Cicero et al., 2014; Diamond et al., 1972; Lankenau et al., 2012). Half of heroin users will devolve into substance dependence (U.S. Dept of Health and Human Services, 2012), while less than half of people with heroin use disorder will achieve long-term abstinence (>5 years) (Hser, 2007). With relapse comes greater risk for accidental overdose. Over a half million people died of drug overdose between the year 2000 and 2016 (Centers for Disease Control and Prevention, 2017) and opioid overdose deaths are estimated to cost the nation $51.2 billion annually (Jiang et al., 2017). This trend has continued unabated, with 64,000 people losing their lives to drug overdose in 2016 alone (Hedegaard et al., 2017). Of those deaths, 42,200 were attributed to opioids (Hedegaard et al., 2017). These losses of life exceed those sustained at the height of the AIDS epidemic (Katz, 2017) and have surpassed those due to automobile accidents annually (Warner et al., 2011). Understanding the neurobiological mechanisms that drive addiction and relapse could aid in preventing heroin addiction, in maintaining abstinence during recovery and, thereby, in reducing needless loss of life.
1.1 The Effect of Environment on Drug Self-Administration
Genetic and environmental factors contribute to addiction susceptibility (Nielsen et al., 2012). While negative or aversive experiences can cause a predisposition to substance use disorders and relapse, positive experiences can be protective (Eitan et al., 2017; Stairs and Bardo, 2009). Environmental enrichment in the laboratory is normally considered a positive experience, consisting of engaging stimuli and social interactions (Diamond et al., 1972; van Praag et al., 2000). Enrichment during adolescence decreases cocaine-associated addiction-like behaviors later in life (Solinas et al., 2008; Solinas et al., 2010). Adulthood environmental enrichment during enforced abstinence from methamphetamine, heroin, nicotine (Sikora et al., 2018) and cocaine (Chauvet et al., 2009) diminishes cue-induced relapse-like behaviors and these effects disappear with removal of environmental enrichment (Nader et al., 2012). Additionally, enrichment in adulthood before and during acquisition of cocaine self-administration, significantly diminishes acquisition of cocaine taking (Puhl et al., 2012). The effectiveness of environmental enrichment on addiction-like behaviors for heroin self-administration is largely unknown. Reports suggest that environmental enrichment decreases heroin reinstatement (Galaj et al., 2016) and promotes abstinence (Peck et al., 2015). Given the ongoing opioid epidemic, modifying environmental factors and examining their impacts on opiate addiction is highly relevant. The present study examined the effects of environmental enrichment (novel objects, a running wheel, and group housing) on heroin-induced addiction-like behaviors and on the vulnerability to relapse following abstinence. We hypothesized that rats housed in an enriched environment during acquisition and testing would exhibit fewer addiction-like behaviors for heroin and would be more resistant to drug-induced relapse than standard-housed rats.
1.2 Molecular Effects of Drugs and Environment
Neurobiologically, drugs of abuse have profound structural and functional effects (Koob and Volkow, 2016) that can endure long after drug-use has ceased and may be driven by molecular and epigenetic mechanisms (Nestler, 2013). Previously, we reported mRNA changes that were associated with incubation of heroin seeking during enforced abstinence (Kuntz-Melcavage et al., 2009; Kuntz et al., 2008a). Here, we extend these findings by also exploring the hypotheses that environmental enrichment will alter genes associated with drug-self administration and abstinence through epigenetic regulation.
Although cells within an organism share the same genetic code, epigenetic mechanisms (DNA and chromatin modifications, and transcription factor expression/activation) orchestrate genomic structure and availability, creating cell- and state-specific transcriptomes. In the central nervous system (CNS), epigenetic mechanisms are not only essential for development and cell differentiation (Keverne et al., 2015), but also for responsivity to the environment throughout adolescence and adulthood (Cholewa-Waclaw et al., 2016). DNA modifications, specifically cytosine methylation (mC) (the most common covalent DNA modification), have received special attention as an epigenetic mechanism in drug addiction (Nielsen et al., 2012). Traditionally, methylation at CG dinucleotide motifs has been viewed as repressive of gene expression. It is now recognized that non-CG methylation (termed CH, where H is an A, C, or T) also is common in the CNS (Lister et al., 2013) and that methylation can be associated with suppression or induction of gene expression, depending on the genomic context (He and Ecker, 2015; Kinde et al., 2015). As cytosine methylation appears to play important roles in memory and synapse regulation (Lister and Mukamel, 2015), processes that are central to addiction, altered DNA methylation may be a molecular regulator of addiction. In people who heavily use heroin, altered methylation of the μ opioid receptor has been found in blood samples (Nielsen et al., 2010; Nielsen et al., 2009) and across a number of genomic regions in prefrontal cortex (Kozlenkov et al., 2017). In vitro morphine treatment causes genome-wide hypomethylation (Trivedi et al., 2014). However, to our knowledge, no studies have examined DNA methylation genome-wide, in repeat regions, or in specific gene promoter in controlled animal self-administration models of heroin addiction using the state-of-the-art methodologies, especially those that provide base-specific absolute quantitation and discriminate between CG and CH cytosine contexts (Masser et al., 2018).
2. Materials and Methods
2.1 Behavioral studies
2.1.1 Subjects and housing conditions
The animal treatment paradigm is summarized in Figure 1A. Rats (31, 3 month old male Sprague-Dawley, Charles River) were randomly assigned to Standard and Enriched conditions. Standard condition rats (n=16) were singly housed in standard wire mesh cages (38cm × 21.5cm × 20cm). Enriched Condition rats (n=15) were housed in groups (2–3 subjects per 38cm × 46cm × 20cm cage) with novel objects presented daily and continuous access to a 12″ exercise wheel. Standard and enriched conditions were continued throughout the duration of the study. Food and water were available ad libitum unless stated otherwise. All studies were approved by the Penn State College of Medicine, Institutional Animal Care and Use Committee.
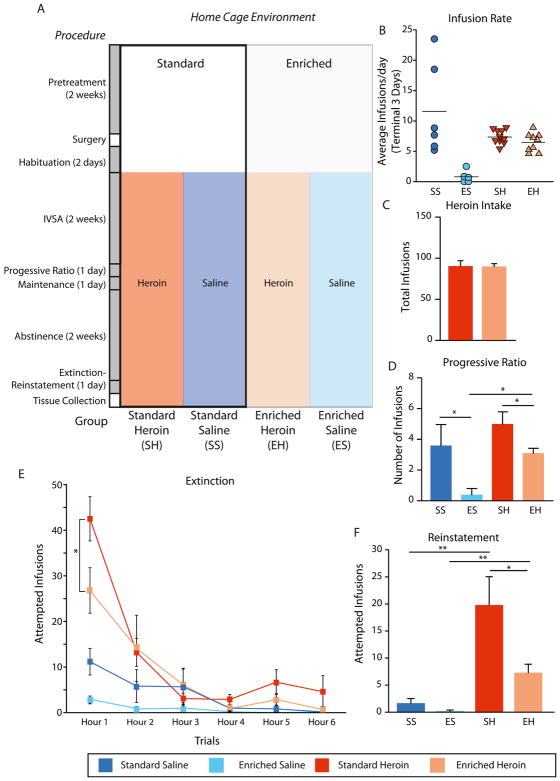
Figure 1. Experimental design and Addiction-like behavior.
Rats were split between a standard and enriched environment for the duration of the study (A). After two weeks, catheter surgery was performed followed by habituation to the test chambers and recovery for two days. Self-administration lasted for two weeks, at the end of which the progressive ratio test was performed. Following two weeks of abstinence, extinction reinstatement testing was performed for a day. Euthanasia was the next day, to ensure no drug was remaining at the time of tissue collection. (B) Rate of infusions (saline or heroin) per day over the last three days of acquisition demonstrated some variability in the standard saline group but a high rate of infusions in the heroin groups as compared to the enriched saline condition. (C) Sum of heroin (0.06 mg/0.2ml of heroin) infusions/3 h for standard (SE) heroin and enriched (EE) heroin across 12 trials. (C) Number of saline or heroin infusions earned during progressive ratio testing for standard saline, enriched saline, standard heroin, and enriched heroin. (E) Number of responses/1 h exhibited during the 6 h extinction session and (F) during the 1 h after heroin administration reinstatement session for standard saline, enriched saline, standard heroin, and enriched heroin. *p<0.05, ANOVA n=5–10/group, data are presented as mean (+/− SEM).
2.1.2 Surgeries
Each rat was implanted with an intravenous jugular catheter as described previously (Colechio et al., 2018; Grigson and Twining, 2002). Rats recovered 1 week prior to the start of procedures. Catheter patency was maintained by daily examination and flushing of catheters using heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified when necessary using 0.2 ml of propofol (Diprivan 1%).
2.1.3 Apparatus
Testing was conducted as previously described (Puhl et al., 2013) in 24 self-administration chambers (MED Associates, St. Albans, VT). Each chamber measures 30.5 cm in length × 24.0 cm in width × 29.0 cm in height, and is individually housed in a light- and sound-attenuated cubicle. The chambers consist of a clear Plexiglas top, front, and back wall. The side walls are made of aluminum. Grid floors consist of nineteen 4.8-mm stainless steel rods, spaced 1.6 cm apart (center to center). Each chamber was equipped with two retractable sipper spouts that enter through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). Rat were given the opportunity to self-administer saline or heroin by completing a ratio requirement via contacts on the rightmost empty active spout. The center empty spout served as the inactive spout. Contacts with the active spout, measured via a contact relay circuit, operating a syringe pump (Model PHM-100VS, Razel Scientific Instruments, Stamford, CT) to deliver saline or heroin iv. A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from the rat. The tubing was attached to a 2-channel counterbalanced swivel assembly (Instech, Plymouth Meeting, PA). A stimulus light was located 6.0 cm above each tube. Each chamber was also equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Spout contacts were recorded using a contact relay circuit. Events in the chamber and collection of the data were controlled on-line with a PC computer that used programs written Medstate notation language (MED Associates).
2.1.4 Acquisition
Rats were habituated to the self-administration chambers 5 min per day for 2 days prior to the start of acquisition. During habituation, the rats were water restricted, with 5 min access to water through one of the two spouts, alternated across days, and 25 ml of water in the home cage overnight. Thereafter, ad lib water was returned and acquisition of drug self-administration began. During acquisition, the 2 empty spouts advanced and the drug session started. Completion of ten contacts (fixed ratio 10, FR10) on the rightmost empty active spout resulted in a 6 sec i.v. infusion of either saline (n=11) or 0.06 mg/infusion heroin (n=20) delivered in 0.2 ml of solution over 6 sec as previously described (Imperio and Grigson, 2015). Drug or saline delivery was signaled by offset of the stimulus light and onset of the tone and house light, which remained on for a total of 20 sec, as well as the retraction of both spouts. Further responding during this time was not reinforced. The access period for heroin was 3 h/day for 12 days of acquisition.
2.1.5 Progressive Ratio
After trial 12 of the drug acquisition phase, a single progressive ratio (PR) test was conducted on the following day to assess motivation as previously described (Imperio and Grigson, 2015). The test began with the FR 10 requirement on the active spout for the 1st infusion, with subsequent infusions requiring the completion of an increasing number of operant responses (i.e., 10, 12, 16, 22, 30, 40, 52, 66, 82, 100, 120, 142, 166, ect.,). Breakpoint was defined as the last ratio completed. The trial terminated when 30 min elapsed without having earned an infusion.
2.1.6 Maintenance trial
After the single PR test, all rats were given one additional FR10 drug access trial to reestablish drug-taking behavior prior to the extinction and reinstatement session. Self-administration was profiled as it was during acquisition.
2.1.7 Drug Abstinence
Following the single maintenance trial, all rats underwent 14 d of enforced home cage abstinence. All rats were left undisturbed in their enriched or standard home cages, except for typical maintenance such as weighing, flushing of the catheters, and daily changing of the novel objects for the enriched condition.
2.1.8 Extinction & Reinstatement Session
Extinction. Upon completion of the abstinence phase, all rats participated in a one-day extinction and reinstatement session as detailed previously (Imperio and Grigson, 2015). During extinction, rats underwent 6 h where responding on the active empty spout was not rewarded with an infusion, but all cues previously paired with the drug were presented each time 10 responses were made. Reinstatement. Immediately following extinction, rats received a single, non-contingent i.v. infusion of 0.06 mg/0.2ml of heroin for 1 hour and responding on the active and inactive spouts was recorded with no drug available.
2.1.9 Drugs
Heroin HCl was generously provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, N.C., USA). Heroin was dissolved in sterile physiological saline to a concentration of 0.3 mg/ml.
2.1.10 Behavioral Statistics
Behavioral data were analyzed using a mixed factorial analysis of variance (ANOVA). Factors were housing condition (standard or enriched) and drug (saline or heroin). Drug intake over the acquisition phase was measured using a 2 × 2 × 12 (housing × drug × trials) mixed factorial ANOVA. Drug-seeking behavior during the extinction session across the 6 hours was measured using a 2 × 2 × 6 mixed factorial ANOVA. Goal directed behavior were compared using a 2 × 2 ANOVA at Trial 1 and Trial 12 independently. Progressive ratio and the reinstatement session responding data were analyzed by a 2 × 2 ANOVA at the relevant time points. Post hoc tests were conducted, when appropriate, using Fisher’s least significant difference (LSD) tests, α< 0.05. Planned comparisons using standard t-test were conducted between the standard environment heroin vs. enriched environment heroin groups for all behavioral tests.
2.2 Molecular Studies
2.2.1 Euthanasia and Tissue Collection
Rats were decapitated 24 hours after the reinstatement session to ensure that all drug was metabolized. Brains were rapidly removed and the nucleus accumbens (NAc), including both core and shell, ventral tegmental area (VTA), and medial prefrontal cortex (mPFC) were dissected manually (Freeman et al., 2001; Kuntz et al., 2008a). Brain samples were immediately frozen in liquid nitrogen and stored at −80°C.
2.2.3 RNA & DNA Isolation
DNA and RNA were co-isolated from brain tissues using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) (Mangold et al., 2017a). Quantity and quality of DNA and RNA was assessed spectrophotometrically and by capillary electrophoresis (Agilent Bioanalyzer).
2.2.4 qRT-PCR for Gene Expression
Quantitative PCR (qRT-PCR) analyses of genes of interest were performed using primers on separate exons with exon junction-spanning fluorgenic probes as previously described (Mangold et al., 2017b) with β-actin as the endogenous control. For full methodological details, see Supplementary Methods.
2.2.5 Genomic Repeat Element Pyrosequencing
Genomic DNA (n=5–10/group) was bisulfite-converted (EZ DNA methylation-lightening, Zymo Research, Irvine, CA) and then PCR-amplified using bisulfite-specific converted primers for LINE-1 and ID elements (EpigenDx, Hopkinton, MA). Amplicons were analyzed for CG methylation via pyrosequencing (EpigenDx, Hopkinton, MA) (Hadad et al., 2016).
2.2.5 Low Coverage Whole-Genome Bisulfite Sequencing
Whole-genome bisulfite sequencing was performed using a Swift accel-NGS methyl-seq library reagents (Swift Bioscience, Ann Arbor, MI) as previously described (Hadad et al., 2016). Whole genome CG and CH levels, as well as methylation of repetitive elements, and patterns of promoter and CpG island methylation were derived from whole genome bisulfite sequencing data. For full experimental details, see Supplementary Methods
2.2.6 Bisulfite Amplicon Sequencing (BSAS)
To analyze DNA methylation of EGR1, EGR2, and OPRM1 with base resolution for both CG and CH contexts, BSAS was performed as previously described (Mangold et al., 2017a; Masser et al., 2013; Masser et al., 2015). For full methodological details, see Supplementary Methods.
3. Results
3.1 Behavioral Analysis
3.1.1 Drug Intake
Standard and enriched conditions were continued over the entire duration of the study (Figure 1A). Terminal saline and heroin infusions (i.e., the average number of infusions administered/3 h across terminal trials 10, 11 and 12) are shown in Figure 1B for rats maintained in standard or enriched housing conditions. As is evident, the terminal number of heroin infusions/3 h did not differ as a function of housing condition. There was, however, individual variability in the saline self-administering rats housed in standard conditions, with two rats self-administering a high number of saline infusions. In our experience, this is an anomaly. However, our laboratory recently observed that some rats, in the absence of alternative rewards (e.g., saccharin, heroin and/or environmental enrichment) show higher responding for saline (Coffey et al., 2016), an effect that may be driven by the novelty of manipulating the cue light and spout. That said, enriched animals in the saline condition engaged little with the operandi. This may be a real effect, possibly related to their, otherwise, enriched housing environment. Further support for the conclusion that environmental enrichment did not impact self-administration of this dose of heroin is shown in Figure 1C. Thus, no differences were observed in total heroin intake averaged across the 12 trials of acquisition between standard housed and enriched rats. To determine if the lack of difference in drug-taking behavior between the groups could be attributed to a difference in preference for the active vs. inactive spouts, goal-directed behavior was calculated using criteria described (Imperio and Grigson, 2015). Specifically, goal-directed behavior was calculated as the total number of licks on the active spout divided by the total number of licks on the active and inactive spouts combined. Trial 1 revealed no significant difference in goal directed behavior among the 4 groups (Figure S1). However, by trial 12 (Figure S1), a significant interaction between environment and drug was seen (p<0.05). Post hoc tests revealed greater goal-directed behavior in standard and enriched heroin groups (and standard saline) as compared the enriched saline condition (ps<0.05). No significant difference in goal-directed behavior was seen between standard heroin and enriched heroin groups (p>0.05).
3.1.2 Motivation
When challenged with a single PR test, significant main effects of environment (p<0.01) and drug (p<0.01) were observed (Figure 1C). Although the number of heroin infusions was somewhat low for the standard housed rats (previously we have observed rats self-administering about 9 infusions of heroin in 3 h (Imperio and Grigson, 2015)), rats in the enriched condition were significantly less willing to work for heroin (p<0.05). Environmental enrichment also decreased saline responding, while greater work was performed for heroin than saline in the enriched environment, as expected (p<0.05). Non-enriched rats may exhibit greater responding for saline than enriched rats during the progressive ratio test because there is, otherwise, nothing to engage with when standard housed.
3.1.3 Extinction and reinstatement session
The extinction/reinstatement session was conducted after 14 days of enforced abstinence. Significant main effects of drug (p<0.001) and time (p<0.001) indicated that rats made more infusion attempts for heroin, and/or heroin-related cues, than for saline and/or saline-related cues, that the number of infusion attempts was greater in Hour 1 than in Hours 2 – 6, overall, ps < 0.05 (Figure 1E). Planned comparisons on the Hour 1 data revealed that enriched environment heroin-experienced rats engaged in significantly less seeking behavior than the standard housed heroin-experienced rats (p<0.05). Group differences were not evident across hours 2 – 6, ps > 0.05.
When primed with a single non-contingent infusion of heroin, responding resumed (Figure 1F). In heroin primed groups, responding increased during the last hour of the extinction reinstatement session for both standard (p<0.0001) and enriched groups (p<0.01). Comparing responses during the reinstatement session, the main effect of drug was significant, (p<0.001) showing greater drug-induced reinstatement session responding by rats with a history of heroin vs. saline self-administration, overall. The main effect of environment (p<0.05) demonstrated that enriched rats exhibited fewer infusion attempts than standard housed rats overall. While the housing × drug interaction did not attain statistical significance (p=0.16), planned t-tests confirmed that heroin-experienced rats housed in an enriched environment demonstrated less reinstatement session seeking behavior compared to heroin-experienced rats housed in the standard environment. Additionally, greater reinstatement session responding was evident in heroin groups as compared to their matched environment saline controls (ps < 0.05).
3.2 Global DNA methylation
3.2.1 Total genomic methylation does not change with heroin self-administration
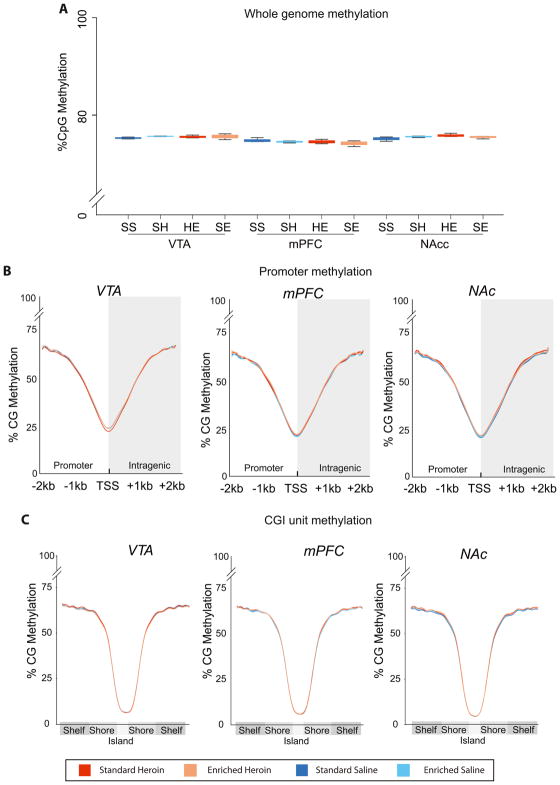
Both cocaine (Novikova et al., 2008; Tian et al., 2012) and morphine (Trivedi et al., 2014) have been reported to decrease total genome methylation levels. Low coverage whole genome bisulfite sequencing was used to assess whether in vivo heroin self-administration decreased total DNA methylation (Hadad et al., 2016). This approach combines the quantitative accuracy of bisulfite sequencing and discriminates between CG and CH contexts (Masser et al., 2018). An average of 26.7×106±5.5×106 CG and 5.5×109±1.2×109 CH counts were collected across the genome per sample. Average levels of CG methylation were 74.3–75.8% across all treatment groups and brain regions. No differences in CG methylation (Figure 2A) were evident, nor were differences observed when counts were restricted to promoters (±2kb the transcription start site of every annotated rat gene) (Figure 2B) or across all annotated CG island/shores/shelves (Figure 2C). Consistent methylation patterns – lowest at transcription start sites and CG islands with higher methylation in flanking regions – were observed across treatments and between brain regions.
Figure 2. CG methylation levels across whole genome, and promoter and CG island regions.
Low coverage whole genome bisulfite sequencing was performed across VTA, NAc, and mPFC brain regions. (A) Total methylation counts / total counts for CG was used to determine the average genomic methylation per sample (n=3/group). (B) Profiles of the average CG methylation across all annotated promoters were generate for +/− 2kb of the transcription start site (TSS). (D) Similarly, average CG methylation profiles for all annotated CG islands, shores and shelves were calculated per animal. No differences across the whole genome, promoters or CG island unites were observed.
Previously reported whole genome methylation differences did not differentiate between CG and CH motifs as ELISA and HPLC methods do not distinguish between the base contexts of methylation. CH methylation is highest in the CNS and could be the source of genome-wide differences. Therefore, CH methylation levels were extracted from the whole genome sequencing date. CH methylation averages were lower than CG across the genome, as would be expected (Figure S2A) and no differences were evident at the whole genome level or when restricted to genic regions (Figure S2B).
3.2.2 Repeat element methylation does not change with heroin self-administration
Previous studies have reported hypomethylation of LINE (long interspersed nuclear elements) elements with opiate exposure (Trivedi et al., 2014). LINE, SINE (short interspersed nuclear elements), and LTR (long terminal repeat retroposons) were extracted from the whole genome bisulfite sequencing data. mCG levels were high (>80%) and extremely consistent between treatments and across brain regions (Figure S3A–C). mCH levels in repeat elements were lower, but did not differ between brain regions or treatments (Figure S3D–F). Repeat elements (LINE-1 and ID) were examined here by pyrosequencing, as in previous reports (Trivedi et al., 2014). Again, no differences were observed between the four treatment groups, either as individual positions or as averages (Figure S4). As previous experiments also used ELISA-based approaches, these were also performed for mPFC and NAc with no differences evident (data not shown). Across independent methods no changes in total methylation with environmental enrichment or heroin self-administration were evident across the mesolimbic pathway. Data were in fact quite consistent and nothing approaching the >50% decrease reported in vitro was observed (Trivedi et al., 2014).
3.3 Gene expression
3.3.1 Immediate early gene expression is suppressed with heroin self-administration and environmental enrichment
While total genomic and repeat element methylation were not altered with environmental enrichment or heroin self-administration, the potential remained for methylation differences at specific loci. Gene expression responses to opiates have also been reported across the mesolimbic pathway (Cooper et al., 2017; Sanchez et al., 2016). Targeted gene expression analysis was conducted on the VTA, mPFC, and NAc using genes of interest that have been shown to be altered with heroin administration or abstinence (Kuntz-Melcavage et al., 2009; Kuntz et al., 2008a) to determine both their responsiveness to environmental enrichment and to identify specific genomic loci that may be differentially methylated.
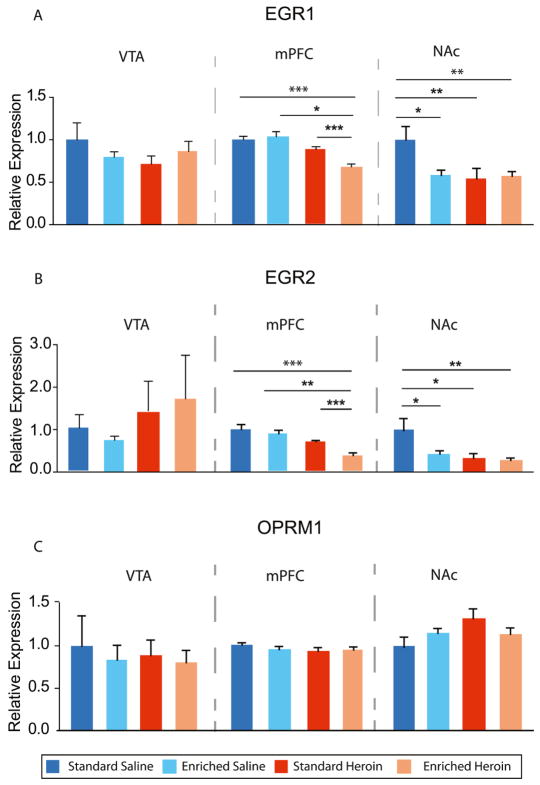
EGR1, an early response gene sensitive to heavy substance use (El Rawas et al., 2009; Kuntz et al., 2008a), demonstrated a significant drug × environment interaction (p<0.05) in the mPFC (Figure 3A). Enriched heroin subjects displayed significantly lower amounts of EGR1 expression as compared to the other groups. In the NAc, EGR1 expression demonstrated an interaction effect (p<0.05) of drug × environment. EGR1 expression was higher in the standard saline group as compared to all other groups. No differences in EGR1 in the VTA were observed. Significant relationships were seen between mPFC qPCR and drug seeking during reinstatement session responding (r=0.54; p<0.05) and between NAc EGR1 expression and first hour extinction session responding (r=0.51; p<0.05).
Figure 3. EGR1, EGR2 and OPRM1 gene expression.
Relative gene expression was assessed via RT-qPCR on genes EGR1, EGR2 and OPRM1 in the VTA, mPFC and NAc. (A) Differential expression of EGR1 was observed between SS and EH, ES and EH, and SH and EH in the mPFC. Higher expression of EGR1 was observed in the SS group relative to ES, SH and EH. (B) Differential EGR2 expression was observed between SS and SH, ES and EH, and SH and EH in the mPFC. In the NAc, SS had higher expression of EGR2 relative to EH and SH. (C) No differences in OPRM1 expression were observed in the VTA, mPFC or NAc *p<0.05 **p<0.01, ***p<0.001, (B) ANOVA n=5–10/group (standard saline = SS, enriched saline = ES, standard heroin = SH enriched heroin = EH)
EGR2 is also an early response gene that increases in expression following heroin abstinence (Kuntz et al., 2008a) and has been reported to be responsive to other drugs of abuse (Cadet et al., 2013). In the mPFC, EGR2 expression demonstrated main effects of environment (p<0.001) and drug (p <0.05) but no interaction effect (Figure 3B). Expression was lower in the enriched heroin group compared to all other groups. In the NAc, main effects of environment (p <0.01) and drug (p <0.05), but no interaction effect were observed. EGR2 expression was higher in the standard saline group compared to all other groups. EGR2 expression did not vary between treatment groups in the VTA. OPRM1 was also examined across all of the treatment groups and brain regions, but no differences were found between groups (Figure 3C). Expression of Drd2, Crh, Pdyn, Npy, and Bdnf was also examined and did not differ among the four conditions in any of the brain regions examined (data not shown).
3.4 Site-specific DNA methylation via BSAS
3.4.1 Site-specific EGR1 cytosine methylation is altered with heroin self-administration
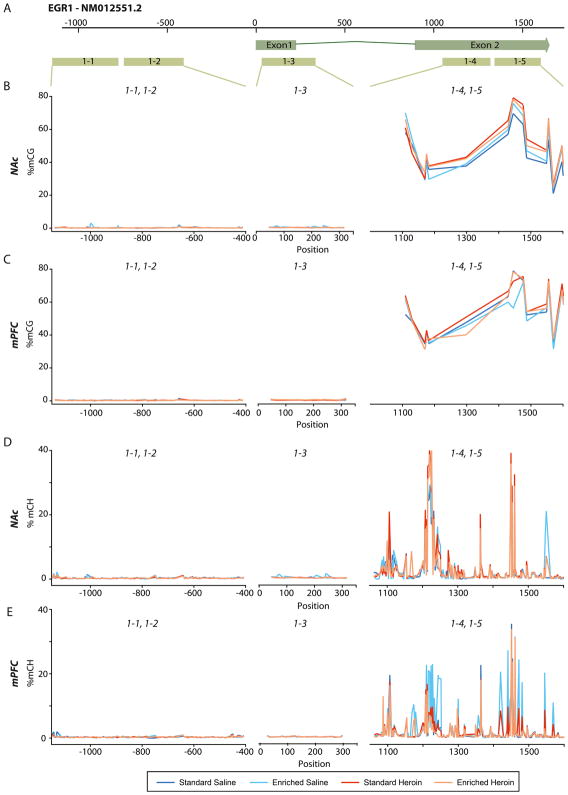
The lack of significant differences in global DNA methylation status is unsurprising as such changes would be indicative of a widespread rearrangement of genome availability and thereby a change in cell status. However, given that expression of EGR1 and EGR2 genes changed with environment and heroin self-administration in the mPFC and NAc, DNA methylation at these specific genes was examined in a base-specific manner in promoter and intragenic regions. Regions of interest for EGR1 (Figure 4A) were selected based on reports of altered methylation with reward learning (Day et al., 2013). mCG levels were low in the promoter and first exon (Figure 4B&C). Within the second exon, mCG levels were higher with a consistent pattern between the NAc and mPFC. Mean CG methylation did not differ between treatment groups. Utilizing the base-by-base resolution of BSAS, in exon 2 (EGR1-4, EGR1-5) of the NAc, a two-way ANOVA revealed a significant main effect of drug. Planned comparisons conducted at sites 1429, 1445, 1476, 1487, and 1555 found significant differences among the four groups (p<0.05) (Figure 4B and Table 1). In the second exon region, no sites were found to be differentially methylated among the four groups for the mPFC (Figure 4C and Table 1).
Figure 4. CpG and CH methylation of EGR1 in the mPFC and NAc.
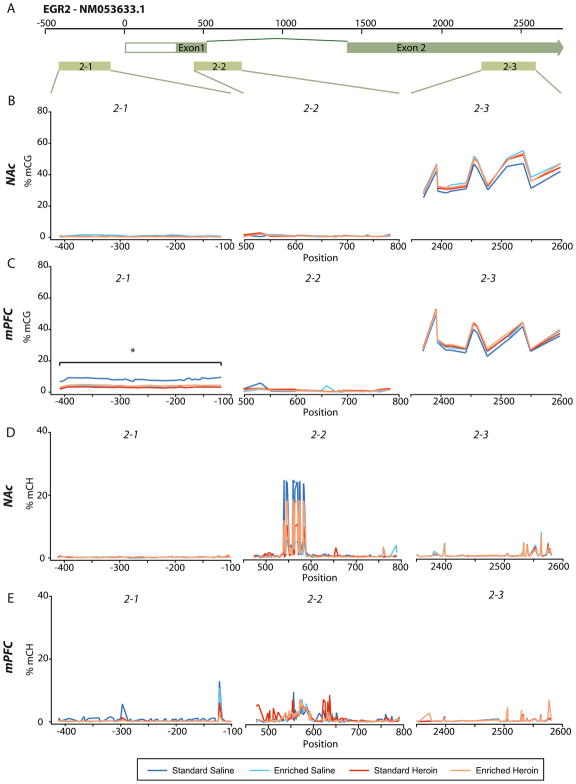
Bisulfite amplicon sequencing (BSAS) was used to assess site-specific methylation in EGR2. (A) Representation of EGR2 gene (promoter and gene body) and BSAS primer placement (1–1, 1–2, 1–3, 1–4, 1–5). (B) Site-specific differences in methylation were observed in within the gene body of EGR1 in the NAc. (C) No site-specific methylation differences were observed in the EGR2 in the mPFC. (D) Higher CH methylation at site 1461 was observed in the NAc of the enriched saline group. (E) No differences in CH methylation were observed in the mPFC. 2-way ANOVA, n=5–10/group.
Table 1.
Base-specific differences in CG methylation.
| NAc | mPFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EGR1 CpG site | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value |
| 1429 | 0.57 | 0.60 | 0.65 | 0.62 | 0.03 | 0.49 | 0.68 | 0.58 | 0.64 | ns |
| 1445 | 0.70 | 0.76 | 0.79 | 0.78 | 0.03 | 0.65 | 0.84 | 0.72 | 0.79 | ns |
| 1476 | 0.63 | 0.68 | 0.75 | 0.72 | 0.01 | 0.58 | 0.78 | 0.67 | 0.74 | ns |
| 1487 | 0.43 | 0.47 | 0.54 | 0.50 | 0.01 | 0.40 | 0.58 | 0.48 | 0.53 | ns |
| 1555 | 0.54 | 0.62 | 0.67 | 0.66 | 0.03 | 0.56 | 0.76 | 0.65 | 0.73 | ns |
| EGR1 CH site | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value | standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value |
| 1461 | 0.25 | 0.27 | 0.33 | 0.29 | 0.02 | 0.32 | 0.21 | 0.28 | 0.31 | ns |
| EGR2 CpG site | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value |
| −394 | 0.01 | 0.01 | 0.01 | 0.00 | ns | 0.09 | 0.04 | 0.03 | 0.04 | 0.01 |
| −346 | 0.01 | 0.02 | 0.01 | 0.00 | ns | 0.09 | 0.04 | 0.03 | 0.05 | 0.05 |
| −265 | 0.00 | 0.01 | 0.00 | 0.00 | ns | 0.08 | 0.03 | 0.03 | 0.04 | 0.02 |
| −221 | 0.00 | 0.01 | 0.00 | 0.00 | ns | 0.07 | 0.03 | 0.03 | 0.04 | 0.03 |
| −202 | 0.00 | 0.01 | 0.01 | 0.00 | ns | 0.08 | 0.03 | 0.03 | 0.04 | 0.05 |
| −180 | 0.00 | 0.01 | 0.00 | 0.00 | ns | 0.09 | 0.03 | 0.03 | 0.04 | 0.02 |
| −175 | 0.00 | 0.01 | 0.00 | 0.00 | ns | 0.09 | 0.03 | 0.03 | 0.04 | 0.01 |
| −118 | 0.00 | 0.01 | 0.00 | 0.00 | ns | 0.10 | 0.03 | 0.03 | 0.04 | 0.03 |
| 2477 | 0.31 | 0.33 | 0.33 | 0.35 | ns | 0.23 | 0.26 | 0.27 | 0.28 | 0.02 |
| EGR2 CH site | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value | Standard Saline mean | Enriched Saline mean | Standard Heroin mean | Enriched Heroin mean | p value |
| 569 | 0.24 | 0.05 | 0.10 | 0.18 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 | ns |
As BSAS quantifies methylation at both CG and CH, methylation at CH sites of EGR1 promoter and intragenic regions was also examined. mCH was extremely low across the promoter and first exon, with higher levels in the second exon. Reproducible, site-specific mCH patterns were observed in NAc and mPFC (Figure 4D&E). No differences in mean mCH were observed. Analysis of CH methylation using a 2-way ANOVA revealed increased methylation at site 1461 in the standard heroin group in the NAc (Figure 4D and Table 1). No differences in mCH were observed in the mPFC.
3.4.2 EGR2 cytosine methylation changes with environmental enrichment and heroin self-administration
Promoter, intronic, and exonic regions were analyzed for EGR2 (Figure 5A). CG methylation was low in the promoter and across the first exon with higher methylation in the second exon in both the NAc and mPFC (Figure 5B&C). Methylation of CG sites was higher in standard saline than other groups across the EGR2 promoter in the mPFC (Figure 5C), (EGR2-1) (p<0.05). At specific CG sites in the EGR2 promoter region of the mPFC greater methylation was observed in the standard saline group relative to the standard heroin and enriched heroin groups (p<0.05) (Figure 5C and Table 1). Site 2477 (exon 2) of EGR2 in the mPFC was also found to be differentially methylated between groups (Figure 5C). No differences were observed in the NAc. mCH was low across EGR2 with specific sites demonstrating higher levels of CH methylation (Figure 5D&E), but with some inter-animal variability. Differential methylation analysis by 2-way ANOVA revealed only one site - 569 - within the NAc (Table 1). No differences in mCH were found in the mPFC.
Figure 5. Site-specific CpG and CH methylation of EGR2 in the mPFC and NAc.
Bisulfite amplicon sequencing (BSAS) was used to assess site-specific methylation in EGR2. (A) Representation of EGR2 gene (promoter and gene body) and BSAS primer placement (2–1, 2–2, 2–3). (B) No site-specific differences in methylation were observed in EGR2 within the NAc. (C) Site-specific methylation differences were observed in the promoter region and gene body of EGR2 in the mPFC. (D) Higher CH methylation at site 569 was observed in the NAc of the standard saline group. (E) No differences in CH methylation were observed in the mPFC. 2-way ANOVA, n=5–10/group.
3.4.3 OPRM1 cytosine methylation is unchanged with environmental enrichment and heroin self-administration
Even through different expression of OPRM1 was not observed, the methylation of OPRM1 was also analyzed given reported differences in DNA methylation in non-using patients with opioid use disorder (Nielsen et al., 2009). Analysis of the OPRM1 gene promoter region (data not shown) revealed no significant differences among the 4 conditions as either regions or individual sites (p>0.05)
4. Discussion
With the current epidemic of opiate addiction, environmental factors that could prevent misuse or aid in treatment are needed. Insight into the molecular mechanisms of opiate addiction may provide novel targets for pharmacological intervention to prevent addiction or aid abstinence. We found that environmental enrichment did not affect heroin self-administration in acquisition, but significantly reduced motivational measures of drug seeking and drug taking. At the molecular level, expression in specific immediate early genes, EGR1 (also known as Zif268 or NGFI-A) and EGR2 (also known as Krox20), is evident with heroin self-administration. Epigenetically, heroin self-administration and abstinence is not accompanied by widespread changes in genomic methylation. Site-specific responses in methylation within the EGR1 and EGR2 promoters and intragenic regions, however, are responsive to environment and drug variables.
4.1 The Effect of Enrichment on Addiction-Like Behaviors
Contrary to previous studies using cocaine from our laboratory (Puhl et al., 2012), the present data demonstrate that environmental enrichment in adult rats does not have an effect on acquisition of heroin self-administration. However, environmental enrichment decreased willingness to work for heroin, lessened drug and/or cue seeking behavior. Thus, even though all rats took the same amount of heroin during acquisition, environmental enrichment reduced addiction-like behavior in heroin-experienced adult rats. This finding parallels previous reports that environmental enrichment did not reduce methamphetamine self-administration, but did reduce cue-induced reinstatement of methamphetamine seeking behavior (Hofford et al., 2014).
Reductions in stress and anxiety by environmental enrichment have been proposed as protective mechanisms to counteract the deleterious effects of drugs of abuse (Solinas et al., 2010). Indeed, alternative rewards, such as a sweet or exercise, show promise at the clinical and preclinical levels (Brown et al., 2009; Lenoir et al., 2013b; Liu and Grigson, 2005; Lynch et al., 2010). Yet, in the present study, both enriched and standard housed subjects infused equal drug amounts. This discrepancy may be due to the low cocaine doses used previously, while this study employed a standard to high heroin dose. An effect on acquisition may be observed at lower heroin doses. The short-access (3 h) procedure used here also produces fairly stable (i.e., less escalation) drug-taking (Ahmed and Koob, 1998) but longer access paradigms or longer acquisition periods may allow for differences in intake or escalation to become evident. Additionally, heroin may have a higher abuse liability than cocaine thus limiting the ability of enriched environment to reduce intake (Madsen and Ahmed, 2015).
A central finding was that environmental enrichment reduced seeking for heroin and/or heroin-related cues during extinction and heroin-induced reinstatement. Similarly, environmental enrichment can prevent relapse after abstinence from sucrose self-administration, an effect that was attributed to enhanced learning ability (i.e., regarding the discontinued availability of a reward) (Grimm et al., 2008). Indeed, environmental enrichment can have a powerful effect on learning and memory, as well as increasing neurite branching and synapse formation (Diamond et al., 1976; Diamond et al., 1966; Walsh et al., 1969). It is possible that our study reflects the beneficial effect of environmental enrichment on neuronal and behavioral plasticity.
The relationship between environmental enrichment and addiction may be bi-directional. Although loss of interest in rewards and personal responsibilities is a hallmark symptom of addiction, natural rewards can serve as a means to treat and protect the individual (Grigson, 2008). The availability of alternative rewards has profound effects on drug-taking behavior, as demonstrated by the finding that a brief exposure to a sweet solution can reduce resumption of cocaine-seeking behaviors following abstinence (Liu and Grigson, 2005). Additionally, concurrent choices of an alternative reward along with drug access can protect against drug taking (Carroll et al., 2008; Lenoir et al., 2013a). The importance of alternative rewards can further be shown with the loss of enrichment status before drug exposure. Mice that had previously been housed in an enriched environment prior to drug exposure had increased sensitivity to the rewarding properties of cocaine when the enrichment was removed (Nader et al., 2012).
These findings are bolstered by clinical studies using alternative rewards to combat addiction. Inclusion of aerobic exercise during treatment can help promote drug abstinence in recovering alcoholics (Brown et al., 2009). Availability of monetary rewards can attenuate the choice of heroin self-administration (Comer et al., 1997). Therefore, in addition to the neurobiological benefits of environmental enrichment, the existence of an alternative reward can reduce drug-taking behaviors and aid in the treatment of drug addiction. This was seen here when the rats were challenged on a PR test, during extinction testing, and during drug-induced reinstatement. Exposure to an enriched environment significantly reduced the willingness to work for heroin, reduced seeking during the first hour of extinction, and reduced heroin-induced reinstatement of seeking behavior relative to standard housed controls with an identical history of heroin self-administration.
4.2 Global Methylation
One of the most intractable aspects of drug addiction is relapse to drug taking, even after prolonged periods of drug abstinence. Stressors and environmental factors can contribute to relapse, but a neurobiological basis of relapse has proven difficult to identify and modulate. Epigenetic factors have emerged as a potential mechanism for addiction’s persistence due to their perceived stability and thus there is a growing interest in the interaction of epigenetics and drug abuse (Nestler, 2014). In studies of non-contingent cocaine administration, decreased methylation in the PFC, but not NAc (Tian et al., 2012), have been described. However, others have reported no changes in genome-wide methylation with non-contingent cocaine administration (Chao et al., 2014; Fragou et al., 2013). In contrast, global methylation has been reported to decrease in the NAc, but not mPFC, with cocaine self-administration (Wright et al., 2015). Opiates have been reported to cause a profound decrease in total genomic and repeat region DNA methylation in vitro (Knothe et al., 2016; Trivedi et al., 2014) and to a lesser extent in leukocytes from opiate-treated subjects (Doehring et al., 2013). Here we extend these findings to heroin self-administration and DNA methylation levels across the mesolimbic circuit using multiple analytical methods. We observed neither global nor repeat element changes in methylation as a function of heroin self-administration or environmental enrichment. Pyrosequencing of DNA repeat elements, whole genome bisulfite sequencing, and whole-genome ELISA all provided highly consistent data across the groups. While conceptually seductive, it is unlikely that large changes in genome-wide methylation would occur given that, as a prime regulation of gene-expression, de-methylation of the genome would cause dramatic dysregulation of gene expression. Rather than functioning as a blunt instrument, epigenetic changes are more likely to be locus- and temporally-specific, with some sites gaining methylation and others losing methylation. Further, it is well known that different regions of a given promoter region can increase or decrease the expression of a given gene depending on the transcriptional environment (Lemon and Tjian, 2000; Tropepe et al., 2006; Whitfield et al., 2012). These findings demonstrate the need for site-specific methylation analyses that can be tied to gene expression analyses.
4.3 Gene-Expression
We have previously shown that enforced abstinence after heroin self-administration is accompanied by incubation of drug seeking during extinction testing and changes in mRNA expression in the brain (Kuntz-Melcavage et al., 2009; Kuntz et al., 2008a). Here, we extend these findings to show that environmental enrichment can attenuate relapse-like behaviors following a period of abstinence. Further, environmental enrichment appears to reduce the expression of genes previously found to be increased following drug abstinence, a phenomenon that appears to be independent of drug-taking history since both standard heroin and enriched heroin rats self-administered the same amount of drug. For example, basal levels of EGR1 in the mPFC of the enriched heroin group were significantly lower than the other conditions. Environmental enrichment has been previously shown to decrease basal levels of EGR1 due to the repeated exposure to novel objects and stimulating experiences (El Rawas et al., 2009). Previous studies have also shown that inhibition of EGR1 expression prevents cue-induced cocaine seeking (Lee et al., 2006). EGR2 follows a similar pattern of lower expression in the mPFC in the enriched heroin subjects compared to the other conditions. Decreased EGR2 expression has been linked with vaccine-induced attenuation of oxycodone self-administration in the brain (Pravetoni et al., 2014). These findings suggest that decreases in mPFC EGR1 and EGR2 may contribute to the protective effect of enrichment on addiction-like behaviors such as motivation to work for drug, drug-seeking, and relapse-like behaviors (e.g., drug-induced reinstatement of heroin seeking).
Interestingly, EGR1 and EGR2 both show decreases in the NAc in the standard heroin, enriched saline, and enriched heroin conditions compared to standard saline subjects. This decrease across the three conditions may result from the fact that subjects were exposed to rewards either in the form of heroin or in environmental enrichment. EGR1 expression can be induced by mitogens, injury and neuronal excitation (Gashler and Sukhatme, 1995). Given the known stress-reducing effects of environmental enrichment (Bahi, 2017; Ragu Varman and Rajan, 2015), as well as the fact that our rats were sacrificed at the time that they would have been placed into the self-administration chamber, it is may be that the differences in basal IEG levels reflect increased basal excitability of the mPFC and NAc of rats in the standard condition or rats with no access to reward (heroin). However, more testing is needed to evaluate the merits of this hypothesis. Of note, not all of the genes previously found (Kuntz-Melcavage et al., 2009; Kuntz et al., 2008a) to have been changed with abstinence were found to be altered here. Although the present study utilized a similar procedure as Kuntz et al. (2008) such as the same drug access period (3 h) and the length of drug abstinence (14 d)(Kuntz et al., 2008b), the present procedure also included a progressive ratio test and a more robust extinction and drug-primed reinstatement phase. These different parameters, along with the fact that sacrifice occurred without anesthesia and 24 h later than in the previous studies, could account for the inability to replicate earlier findings regarding changes in the expression of some genes.
4.4 Site-Specific Differences in Cytosine Methylation
DNA methylation was once perceived as a stable modification that persists for long periods of time. Recent advances in the field have shown that methylation status is more dynamic (Schubeler, 2015), and can be altered with cocaine experience and reward learning (Day et al., 2013; Feng et al., 2015; Watson et al., 2015). Here, we extended these results and report that the availability of a reward can alter the methylation profile at specific regions of the genome. The EGR2 gene promoter region was found to be significantly hypomethylated in the mPFC in subjects that received either access to heroin or environmental enrichment compared with standard housed subjects that possessed no access to a reward. Closer inspection at the base resolution further showed a decrease in methylation status at promoter CG sites in the subjects exposed to heroin or enrichment, while CG sites in exon 2 exhibited the opposite pattern: standard saline subjects have lower levels of CG methylation compared to the other three groups. Additionally, in the NAc, we found lower levels of methylation at specific CG sites in the EGR1 gene in our standard saline group which is associated with higher expression of this gene.
While there is much yet to be elucidated regarding the relationship between DNA methylation and gene-expression, methylation of the promoter region is generally associated with gene repression (Razin and Cedar, 1991). Here, we find that increased methylation of the EGR2 gene promoter is associated with increased gene-expression, whereas methylation of the gene body, instead, is associated with increased gene-expression for both EGR2 and EGR1. Our findings add to the growing body of literature that suggests that DNA methylation is not strictly a repressive mechanism, but plays multiple roles in transcription regulation (for review, see (Jones, 2012)). Moreover, the present data demonstrate the impact that the availability of a reward, either a drug of abuse or environmental enrichment, can have on the methylation landscape in an individual.
To our knowledge, this is the first paper to quantify site-specific mCH in the context of addiction liability and environment. While mCH occurs at lower levels (most are 2–20% methylated) than mCG, it is enriched in neuronal cell types (occurring in approximately 2–6 % of CNS cells) (Lister et al., 2013). Interestingly, mCH accumulates in the neuronal genome concomitantly with synaptogenesis in the post-natal development of mouse and human. In this study, we examined CH methylation in EGR1 and EGR2 in the mPFC and NAc and found two sites to be differentially methylated between our 4 populations: site 1461 of EGR1 in the NAc and site 569 of EGR2 in the NAc. There remains much to be learned in the field regarding the relationship of CH methylation and fluctuations in gene expression; however, thus far it has been shown to be a repressive mechanism whether in the 5′region, gene body or 3′ region (Guo et al., 2014). Here, we show that in the gene body of EGR1, decreased levels of CH methylation are associated with increased gene expression in our standard saline group. Conversely, increased CH methylation in the gene body of EGR2 was associated with increased gene expression in the standard saline group. Just as CG methylation is not solely repressive, and may site-specifically enhance gene-expression (Jones, 2012), CH methylation may augment gene-expression in a locus specific fashion. Taken together, these findings highlight the complex relationship of CH methylation and gene-expression.
We also show that basal methylation levels (as exemplified by our standard housed saline group) in the NAc and mPFC have remarkably similar CG methylation profiles in EGR1 and EGR2 promoters and gene bodies. This provides evidence for a common set point, regulated by undetermined mechanisms. Additionally, we observed extremely low levels of methylation in regions previously reported to dynamically change with reward learning (Day et al., 2013). Important distinctions between the Day et al. study and the present report are two: Day and colleagues examined a cue-food association and at early (not late) acquisition. Findings like those reported by Day et al., then, might be obtained were we to examine gene and epigenetic changes early (i.e., within the first 3 – 5 trials), rather than late, in acquisition. Also, previous studies only used relative quantitation of methylation, so the differences observed could potentially be shifts in background levels of methylation.
These findings also provide an example of how much more work is required to understand methylation patterns and their potential role in addiction. With the extensive efforts to understand the genetics of opiate addiction (Kreek et al., 2005; Levran et al., 2008), clearly a commensurate effort is justified to understand the associated epigenetic changes and their role in addiction and relapse. Future studies will need to examine the epigenome in a cell-type specific manner to gain the clearest understanding and additional genomic annotation (e.g., chromatin status, enhancer elements) are needed for the rat genome to place the findings of this and future studies in a fuller genomic context).
5. Conclusion
The present study examined the effect of constant exposure to environmental enrichment in adulthood on addiction-like behavior following heroin self-administration at the behavioral and molecular levels. Constant exposure to environmental enrichment attenuated the motivation to work for heroin and reduced relapse-like behavior following enforced abstinence. Global fluctuations in DNA methylation have been postulated to underlie addiction-like behavior, but we found no supporting evidence. Instead, we maintain that the relationship between DNA methylation and behavior is most likely a function of locus-specific methylation in relevant genes. Environmental enrichment appeared to selectively decrease the expression of some genes found to be altered due to heroin abstinence (e.g., EGR1) in some brain regions (e.g., mPFC and NAc, but not VTA). Additionally, heroin exposure and enrichment status appeared to influence the epigenetic landscape of specific sites in the genome. Taken together, the data support the premise that environmental enrichment can protect against addiction-like behaviors and provides further support for the use of alternative rewards to help protect and treat humans with substance use disorders. These findings also demonstrate the need for detailed mapping of DNA methylation changes in addiction with the goal of modulating DNA methylation patterns to prevent drug relapse.
Supplementary Material
Highlights.
Environmental enrichment decreases the reinforcement efficacy of heroin
Genome-wide methylation is not altered in mesolimbic structures with heroin self-administration or environmental enrichment
Gene expression and base-specific methylation responses to heroin are modified by environmental enrichment
Acknowledgments
The authors thank Robert Brucklacher in the Genome Sciences Facility at the Penn State Hershey College of Medicine for quantitative PCR assistance, the Oklahoma Medical Research Foundation Next Generation Sequencing Core for RNA-seq assistance, and E.M Bishop for assistance with figure preparation.
Footnotes
Funding and Disclosure
The authors declare no financial conflicts of interest. This project was funded by a grant from the Pennsylvania Department of Health using Tobacco CURE Funds to P.S.G, K.E.V and W.M.F. SAP# 4100055576 (the Commonwealth of Pennsylvania specifically disclaims responsibility for any analyses, interpretations, or conclusions), the Donald W. Reynolds Foundation to W.M.F., the National Institute on Aging (P30AG050911) to W.M.F and (T32AG052363) to D.R.M., the National Eye Institute (T32EY023202) to D.R.M. and the National Institute on Drug Abuse F31DA036322 to C.G.I. and R37DA009815 to P.S.G
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Bahi A. Environmental enrichment reduces chronic psychosocial stress-induced anxiety and ethanol-related behaviors in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:65–74. doi: 10.1016/j.pnpbp.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart G, Dubreuil ME, Gordon AA. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33:220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Ladenheim B, Saint-Preux F, Lehrmann E, De S, Becker KG, Brannock C. Genome-wide profiling identifies a subset of methamphetamine (METH)-induced genes associated with METH-induced increased H4K5Ac binding in the rat striatum. BMC Genomics. 2013;14:545. doi: 10.1186/1471-2164-14-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Opioid Overdose: Understanding the Epidemic. 2017. [Google Scholar]
- Chao MR, Fragou D, Zanos P, Hu CW, Bailey A, Kouidou S, Kovatsi L. Epigenetically modified nucleotides in chronic heroin and cocaine treated mice. Toxicol Lett. 2014;229:451–457. doi: 10.1016/j.toxlet.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewa-Waclaw J, Bird A, von Schimmelmann M, Schaefer A, Yu H, Song H, Madabhushi R, Tsai LH. The Role of Epigenetic Mechanisms in the Regulation of Gene Expression in the Nervous System. J Neurosci. 2016;36:11427–11434. doi: 10.1523/JNEUROSCI.2492-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Coffey AA, Guan Z, Grigson PS, Fang J. Reversal of the sleep-wake cycle by heroin self-administration in rats. Brain Res Bull. 2016;123:33–46. doi: 10.1016/j.brainresbull.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Colechio EM, Alexander DN, Imperio CG, Jackson K, Grigson PS. Once is too much: Early development of the opponent process in taste reactivity behavior is associated with later escalation of cocaine self-administration in rats. Brain Res Bull. 2018;138:88–95. doi: 10.1016/j.brainresbull.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Kechner M, Caraballo-Perez D, Kaska S, Robison AJ, Mazei-Robison MS. Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Sci Rep. 2017;7:8445. doi: 10.1038/s41598-017-09106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E, Tahir A, Sweatt JD. DNA methylation regulates associative reward learning. Nat Neurosci. 2013;16:1445–1452. doi: 10.1038/nn.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of environment on morphology of rat cerebral cortex and hippocampus. J Neurobiol. 1976;7:75–85. doi: 10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J Comp Neurol. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J Neurobiol. 1972;3:47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- Doehring A, Oertel BG, Sittl R, Lotsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154:15–23. doi: 10.1016/j.pain.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Eitan S, Emery MA, Bates MLS, Horrax C. Opioid addiction: Who are your real friends? Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.05.017. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Feng J, Shao N, Szulwach KE, Vialou V, Huynh J, Zhong C, Le T, Ferguson D, Cahill ME, Li Y, Koo JW, Ribeiro E, Labonte B, Laitman BM, Estey D, Stockman V, Kennedy P, Courousse T, Mensah I, Turecki G, Faull KF, Ming GL, Song H, Fan G, Casaccia P, Shen L, Jin P, Nestler EJ. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat Neurosci. 2015;18:536–544. doi: 10.1038/nn.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragou D, Zanos P, Kouidou S, Njau S, Kitchen I, Bailey A, Kovatsi L. Effect of chronic heroin and cocaine administration on global DNA methylation in brain and liver. Toxicol Lett. 2013;218:260–265. doi: 10.1016/j.toxlet.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Robertson DJ, Roberts DC, Vrana KE. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Galaj E, Manuszak M, Ranaldi R. Environmental enrichment as a potential intervention for heroin seeking. Drug Alcohol Depend. 2016;163:195–201. doi: 10.1016/j.drugalcdep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Reward Comparison: The Achilles’ heel and hope for addiction. Drug Discov Today Dis Models. 2008;5:227–233. doi: 10.1016/j.ddmod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Shin JH, Shin J, Li H, Xie B, Zhong C, Hu S, Le T, Fan G, Zhu H, Chang Q, Gao Y, Ming GL, Song H. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci. 2014;17:215–222. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad N, Masser DR, Logan S, Wronowski B, Mangold CA, Clark N, Otalora L, Unnikrishnan A, Ford MM, Giles CB, Wren JD, Richardson A, Sonntag WE, Stanford DR, Freeman W. Absence of genomic hypomethylation or regulation of cytosine-modifying enzymes with aging in male and female mice. Epigenetics Chromatin. 2016;9:30. doi: 10.1186/s13072-016-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ecker JR. Non-CG Methylation in the Human Genome. Annu Rev Genomics Hum Genet. 2015;16:55–77. doi: 10.1146/annurev-genom-090413-025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Minino AM. NCHS Data Brief. National Center for Health Statistics; Hyattsville, MD: 2017. Drug overdose deaths in the United States 1999–2016. [Google Scholar]
- Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT. Environmental enrichment reduces methamphetamine cue-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav Brain Res. 2014;270:151–158. doi: 10.1016/j.bbr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI. Predicting long-term stable recovery from heroin addiction: findings from a 33-year follow-up study. J Addict Dis. 2007;26:51–60. doi: 10.1300/J069v26n01_07. [DOI] [PubMed] [Google Scholar]
- Imperio CG, Grigson PS. Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats. Behav Neurosci. 2015;129:380–388. doi: 10.1037/bne0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lee I, Lee TA, Pickard AS. The societal cost of heroin use disorder in the United States. PLoS One. 2017;12:e0177323. doi: 10.1371/journal.pone.0177323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Katz J. The New York Times. The New York Times Company; New York: 2017. The First Count of Fentanyl Deaths in 2016: up 540% in Three Years. [Google Scholar]
- Keverne EB, Pfaff DW, Tabansky I. Epigenetic changes in the developing brain: Effects on behavior. Proc Natl Acad Sci U S A. 2015;112:6789–6795. doi: 10.1073/pnas.1501482112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde B, Gabel HW, Gilbert CS, Griffith EC, Greenberg ME. Reading the unique DNA methylation landscape of the brain: Non-CpG methylation, hydroxymethylation, and MeCP2. Proc Natl Acad Sci U S A. 2015;112:6800–6806. doi: 10.1073/pnas.1411269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe C, Doehring A, Ultsch A, Lotsch J. Methadone induces hypermethylation of human DNA. Epigenomics. 2016;8:167–179. doi: 10.2217/epi.15.78. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlenkov A, Jaffe AE, Timashpolsky A, Apontes P, Rudchenko S, Barbu M, Byne W, Hurd YL, Horvath S, Dracheva S. DNA Methylation Profiling of Human Prefrontal Cortex Neurons in Heroin Users Shows Significant Difference between Genomic Contexts of Hyper- and Hypomethylation and a Younger Epigenetic Age. Genes (Basel) 2017:8. doi: 10.3390/genes8060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kuntz-Melcavage KL, Brucklacher RM, Grigson PS, Freeman WM, Vrana KE. Gene expression changes following extinction testing in a heroin behavioral incubation model. BMC Neurosci. 2009;10:95. doi: 10.1186/1471-2202-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav. 2008a;90:349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacol Biochem Behav. 2008b;90:344–348. doi: 10.1016/j.pbb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23:37–44. doi: 10.1016/j.drugpo.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Augier E, Vouillac C, Ahmed SH. A choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci. 2013a;Chapter 9(Unit 9):44. doi: 10.1002/0471142301.ns0944s64. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013b;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA. Turning over DNA methylation in the mind. Front Neurosci. 2015;9:252. doi: 10.3389/fnins.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Grigson PS. Brief access to sweets protect against relapse to cocaine-seeking. Brain Res. 2005;1049:128–131. doi: 10.1016/j.brainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2015;20:433–444. doi: 10.1111/adb.12134. [DOI] [PubMed] [Google Scholar]
- Mangold CA, Masser DR, Stanford DR, Bixler GV, Pisupati A, Giles CB, Wren JD, Ford MM, Sonntag WE, Freeman WM. CNS-wide Sexually Dimorphic Induction of the Major Histocompatibility Complex 1 Pathway With Aging. J Gerontol A Biol Sci Med Sci. 2017a;72:16–29. doi: 10.1093/gerona/glv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017b;14:141. doi: 10.1186/s12974-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser DR, Berg AS, Freeman WM. Focused, high accuracy 5-methylcytosine quantitation with base resolution by benchtop next-generation sequencing. Epigenetics Chromatin. 2013;6:33. doi: 10.1186/1756-8935-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser DR, Hadad N, Porter H, Stout MB, Unnikrishnan A, Stanford DR, Freeman WM. Analysis of DNA modifications in aging research. Geroscience. 2018;40:11–29. doi: 10.1007/s11357-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser DR, Stanford DR, Freeman WM. Targeted DNA methylation analysis by next-generation sequencing. J Vis Exp. 2015 doi: 10.3791/52488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Chauvet C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–1587. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–443. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Hamon S, Yuferov V, Jackson C, Ho A, Ott J, Kreek MJ. Ethnic diversity of DNA methylation in the OPRM1 promoter region in lymphocytes of heroin addicts. Hum Genet. 2010;127:639–649. doi: 10.1007/s00439-010-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Utrankar A, Reyes JA, Simons DD, Kosten TR. Epigenetics of drug abuse: predisposition or response. Pharmacogenomics. 2012;13:1149–1160. doi: 10.2217/pgs.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, Kreek MJ. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3:e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JA, Galaj E, Eshak S, Newman KL, Ranaldi R. Environmental enrichment induces early heroin abstinence in an animal conflict model. Pharmacol Biochem Behav. 2015;138:20–25. doi: 10.1016/j.pbb.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One. 2014;9:e101807. doi: 10.1371/journal.pone.0101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacol Biochem Behav. 2013;109:8–15. doi: 10.1016/j.pbb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragu Varman D, Rajan KE. Environmental Enrichment Reduces Anxiety by Differentially Activating Serotonergic and Neuropeptide Y (NPY)-Ergic System in Indian Field Mouse (Mus booduga): An Animal Model of Post-Traumatic Stress Disorder. PLoS One. 2015;10:e0127945. doi: 10.1371/journal.pone.0127945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A, Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991;55:451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Carpenter MD, Yohn NL, Blendy JA. Long-lasting effects of adolescent oxycodone exposure on reward-related behavior and gene expression in mice. Psychopharmacology (Berl) 2016;233:3991–4002. doi: 10.1007/s00213-016-4425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- Sikora M, Nicolas C, Istin M, Jaafari N, Thiriet N, Solinas M. Generalization of effects of environmental enrichment on seeking for different classes of drugs of abuse. Behav Brain Res. 2018;341:109–113. doi: 10.1016/j.bbr.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Zhao M, Li M, Song T, Zhang M, Quan L, Li S, Sun ZS. Reversal of cocaine-conditioned place preference through methyl supplementation in mice: altering global DNA methylation in the prefrontal cortex. PLoS One. 2012;7:e33435. doi: 10.1371/journal.pone.0033435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi M, Shah J, Hodgson N, Byun HM, Deth R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3-mediated cysteine uptake. Mol Pharmacol. 2014;85:747–757. doi: 10.1124/mol.114.091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Li S, Dickinson A, Gamse JT, Sive HL. Identification of a BMP inhibitor-responsive promoter module required for expression of the early neural gene zic1. Dev Biol. 2006;289:517–529. doi: 10.1016/j.ydbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- U.S. Dept of Health and Human Services, S. A. a. M. H. S. A., Center for Behavioral Health Statistics and Quality. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. 2012. [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Budtz-Olsen OE, Penny JE, Cummins RA. The effects of environmental complexity on the histology of the rat hippocampus. J Comp Neurol. 1969;137:361–366. doi: 10.1002/cne.901370309. [DOI] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug Poisoning Deaths in the United States, 1990–2008. NCHS Data Brief. 2011 [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, Hurd YL. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology. 2015;40:2993–3005. doi: 10.1038/npp.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Wang J, Collins PJ, Partridge EC, Aldred SF, Trinklein ND, Myers RM, Weng Z. Functional analysis of transcription factor binding sites in human promoters. Genome Biol. 2012;13:R50. doi: 10.1186/gb-2012-13-9-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ, Kabbaj M. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci. 2015;35:8948–8958. doi: 10.1523/JNEUROSCI.5227-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.