Abstract
Introduction
Barrett’s esophagus (BE) may be present in patients with esophageal adenocarcinoma (EAC) after bimodality therapy (BMT). There is no specific guidance for follow-up of these patients with regard to the presence of BE or dysplasia. In this study, we assessed the outcomes of patients who, after BMT, had BE and those who did not.
Method
Patients with EAC who had BMT were identified and analyzed retrospectively in two groups, with and without BE. We compared patient characteristics and outcome variables (local, distant and no recurrence).
Results
Of 228 patients with EAC, 68 (29.8%) had BE before BMT. 98 (42.9%) had BE after BMT and endoscopic intervention was done in 11 (11.2%). With a median follow-up of 37 months, the presence of post-BMT BE was not significantly associated with overall survival (OS) and local recurrence-free survival (LRFS). Similarly, endoscopic intervention was not significantly associated with OS and LRFS. 50 (73.5%) patients with BE before BMT had BE after BMT (P<0.0001).
Conclusion
The presence of BE after BMT was not associated with increased risk of local recurrence. The local recurrence rate was not influenced by endoscopic intervention. Prospective studies are warranted to generate guidance for intervention, if necessary, for this group of EAC patients
Keywords: Esophageal adenocarcinoma, Barrett’s esophagus, endoscopy, recurrence, survival
Introduction
Esophageal adenocarcinoma (EAC) is the 6th common cause of cancer death worldwide (1). In 2017, it is estimated that there will be 16,940 new cases and an estimated 15,960 people will die of this disease in the US. The overall 5-year survival rate is 18.8% but it is 42.9% for localized EAC and 23.4% for regional EAC (2). Obesity, GERD, and the presence of Barrett’s esophagus (BE) are the major risk factors for developing EAC. Patients with BE have 30 to 125 times greater risk of developing EAC than the general population (3, 4). Annual risk of BE progression to EAC is reported to be between 0.5%-1% (5–9).
Per current American College of Gastroenterology (ACG) guideline, suspected BE in normal population needs an endoscopy to document BE and four-quadrant biopsies are recommended every 1-2 years. For patients with low-grade dysplasia (LGD) and high-grade dysplasia (HGD) without severe comorbidities, endoscopic therapy is recommended. Endoscopic resection (ER) is recommended for T1a lesions and diagnostic/therapeutic ER for T1b lesions. Following ER, ablation of the remaining high-risk tissue is also recommended. However, for the primary that is ≥T2, patients are best treated with multimodality therapy. (10). Similarly, in the NCCN guideline 2017, it is recommended that after ER and ablation, patients need to be surveyed periodically based on the severity of the lesion at baseline.(11) However, no specific recommendations exist for how to tackle the persistence of BE with or without dysplasia in patients who have bimodality therapy (BMT). The recommendations have been left out mainly due to the lack of data. For patients who get surgery for EAC where high-risk esophagus is often removed, endoscopic surveillance is not recommended (11–14).
Patients who receive BMT are of special interest because they tend to develop more local events than those after trimodality (12, 15, 16). We wanted to review the outcome of patients after BMT and focused mainly on the presence of BE/dysplasia vs. no BE/dysplasia and patient outcomes. Such analysis has not been published, to our knowledge.
Methods
Patients
We identified patients from our prospectively maintained database in the department of GI Medical Oncology at the University of Texas MD Anderson Cancer Center between 2002 and 2015. We selected patients with EAC (Esophagus, adenocarcinoma of esophagogastric junction (AEG) type I or II)(17) who received BMT. We selected patients who had at least two or more biopsies at different time points during the follow-up period. EAC staging was based on the American Joint Committee on Cancer staging manual (7th edition). We extracted demographics and clinical data, clinical staging, endoscopy and pathology reports, recurrence and survival data. The institutional review board approved this analysis. Patient characteristics were compared in the two groups of patients with and without BE.
Treatment and follow-up
All patients had a radiation dose range of 40-66.3 Gy (Intensity-modulated radiation therapy or proton). 81 patients (35.5%) received induction chemotherapy prior to chemoradiation. Chemotherapy included a fluropyrimidine with either a platinum compound, taxanes or rarely irinotecan. 23 patients (10%) started with stage IV disease but were dispositioned to receive local consolidation chemoradiation after a favorable response to systemic chemotherapy; these decisions were made in the Esophageal Multidisciplinary Conference. Local and distant recurrences were defined by the imaging studies or endoscopy. Outcomes were categorized in 3 groups: 1-Local recurrence, 2-Distant recurrence, and 3-None.
Follow-up time was calculated from the starting date of BMT to death or last visit.
Statistical analysis
Patient characteristics are summarized using frequency (%) for categorical variables and median (range) for continuous variables. Wilcoxon rank sum tests or Kruskal-Wallis tests were performed to assess the differences in continuous variables between subgroups of patients. Chi-squared tests or Fisher’s exact tests were performed to assess the differences in categorical variables among subgroups of patients. Overall survival (OS) is defined as the time interval between diagnosis and the date of death due to any cause. Local recurrence-free survival (LRFS) is defined as the time interval between CTRT date and the date of local recurrence or death due to any cause. The probabilities of OS and LRFS were estimated using the method of Kaplan and Meier (18). Cox proportional hazards regression models were fit to assess the association between OS or LRFS and patient characteristics(19). The effectiveness of the endoscopic interventions as well as the outcomes were assessed including it in the Cox model as a time-dependent covariate. All statistical analyses were conducted in SAS and Splus.
Results
Characteristics of the entire population
Of the 228 patients, 208 (91.2%) patients were men. 185 (81.1%) of all patients were diagnosed with stage II-III. 68 patients (29.8%) had BE before BMT and 98 patients (42.9%) had BE after BMT. BE was first diagnosed within the median of 5 months (range: 2-51) after BMT. Endoscopic interventions were performed in 11 patients with BE/dysplasia (Figure 1).
Figure 1.
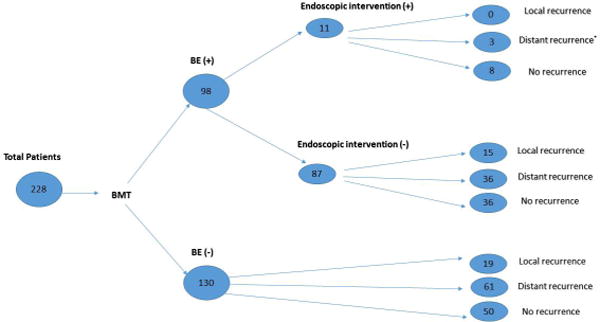
Total Patients with and without BE after bimodality therapy
Characteristics of patients based on the presence of BE after BMT
Patient characteristics are outlined in two groups of BE and without BE in Tables 1 and 2. There was no difference between two groups with regard to age, gender, primary tumor length, and TNM staging. Higher percentage of patients with BE was in AEG1 (59.2%) compared to those without BE who had AEG 2 (55.4%, P<.0001) (Table 1).
Table 1.
Summary of variables before BMT based on Barrett’s status (N=228)
| Covariate | Levels | Barrett’s status after BMT
|
P-value | |
|---|---|---|---|---|
| Yes (n=98) | No (n=130) | |||
|
| ||||
| Age at diagnosis (year) median (range) | – | 67 (31 - 103) | 67 (28 - 85) | .52 |
|
| ||||
| Tumor length at diagnosis (cm) median (range) | – | 5 (1 - 13) | 5 (1 - 13) | .81 |
|
| ||||
| Gender | Male | 93(94.9%) | 115(88.5%) | .09 |
| Female | 5(5.1%) | 15(11.5%) | ||
|
| ||||
| Siewert class | Esophagus | 13(13.3%) | 3(2.3%) | <.0001 |
| AEG1 | 58(59.2%) | 55(42.3%) | ||
| AEG2 | 26(26.5%) | 72(55.4%) | ||
| NA | 1(1%) | 0 | ||
|
| ||||
| Tumor Histology Subtype | SRC | 16(16.3%) | 19(14.6%) | .72 |
| M | 1(1%) | 2(1.5%) | ||
| M&SRC | 3(3.1%) | 5(3.8%) | ||
| NE | 3(3.1%) | 1(0.8%) | ||
| NOS | 75(76.5%) | 103(79.2%) | ||
|
| ||||
| Chronic GERD history | Yes | 60(61.2%) | 78(60%) | . 85 |
| No | 38(38.8%) | 52(40%) | ||
|
| ||||
| Barrett’s before BMT (biopsy) | Yes | 50(51%) | 18(13.9%) | |
| No | 48(49%) | 112(86.2%) | <.0001 | |
|
| ||||
| Barrett’s length before BMT(endoscopy appearance) | LS≥3cm | 38(76%) | 14(63.6%) | |
| SS<3cm | 12(24%) | 8(36.4%) | <0.0001 | |
|
| ||||
| Highest dysplasia before BMT (biopsy) | HG | 34(34.7%) | 12(9.2%) | <.0001 |
| LG | 10(10.2%) | 2(1.5%) | ||
| None | 54(55.1%) | 116(89.2%) | ||
|
| ||||
| Baseline T-staging | T1 | 3(3%) | 3(2.3%) | .27 |
| T2 | 15(15.3%) | 10(7.7%) | ||
| T3 | 74(75.5%) | 109(83.8%) | ||
| T4 | 3(3%) | 3(2.3%) | ||
| TX | 3(3.1%) | 5(3.8%) | ||
|
| ||||
| Baseline N-staging | N0 | 36(36.7%) | 51 (39.2%) | .37 |
| N1 | 54(55.1%) | 62(47.7%) | ||
| N2 | 3(3.1%) | 10(7.7%) | ||
| N3 | 1(1%) | 2(1.5%) | ||
| NX | 4(1.1%) | 5(3.8%) | ||
|
| ||||
| Baseline M-staging | M0 | 88(89.8%) | 110(84.6%% | .12 |
| M1 | 6(6.1%) | 17(13.1%) | ||
| MX | 4(4.1%) | 3(2.3%) | ||
|
| ||||
| Baseline staging | I | 9(9.1%) | 5(3.8%) | .07 |
| II-III | 80(81.6%) | 105(80.8%) | ||
| IV | 6(6.1%) | 17(13.1%) | ||
| NA | 3 (3.1%) | 3(2.3%) | ||
Abbreviations: BMT: bimodality therapy, GERD: gastroesophageal reflux disease, M: mucinous, NE: neuroendocrine; NOS: not otherwise specified; NA: not available, SRC: signet ring cell
Table 2.
a. Summary of categorical variables after BMT, based on Barrett’s status (N=228)
b. Summary of continuous variables after BMT, based on Barrett’s status (N=228)
| Covariate | Levels | Barrett’s status after BMT
|
P-value | |
|---|---|---|---|---|
| Yes (N=98) | No (N=130) | |||
|
| ||||
| Induction chemotherapy | Yes | 31 (31.6%) | 50(38.5%) | |
| No | 67(68.4%) | 80(61.5%) | .29 | |
|
| ||||
| Highest dysplasia after BMT | HG | 29(29.6%) | 0 | <.0001 |
| LG | 30(30.6%) | 1*(0.8%) | ||
| None | 39(39.8%) | 129(99.2%) | ||
|
| ||||
| Recurrence type | Local | 15(15.3%) | 19(14.6%) | |
| Distant | 39(39.8%) | 61(46.9%) | .54 | |
| None | 44(44.9%) | 50(38.5%) | ||
|
| ||||
| Salvage esopaghectomy | Yes | 11 (39.3%) | 13(31%) | |
| No | 17(60.7%) | 29(69%) | .47 | |
|
| ||||
| Survival status | Alive | 47(48%) | 49(37.7%) | .12 |
| Dead | 51 (52%) | 81(62.3%) | ||
| Covariate | Barrett’s status after BMT | N | Median (range) | p-value |
|---|---|---|---|---|
| Frequency of endoscopic biopsy after BMT | Yes | 98 | 4 (2 - 14) | |
| No | 130 | 3 (2 - 11) | 0.01 | |
| Interval between Barrett’s detection after BMT and BMT start-date (months) | Yes | 98 | 5 (2 - 51) | – |
| No | 0 | – | ||
| Interval between BMT start-date and local recurrence (months) | Yes | 15 | 14.23 (6.14 - 95.41) | |
| No | 19 | 14.29 (5.65 - 38.77) | 0.58 | |
| Interval between Barrett’s detection and local recurrence (months) | Yes | 15 | 5.52 (0 - 84.3) | – |
| No | 0 | – | ||
| Duration of follow-up after BMT start-date (months) | Yes | 98 | 43 (9 - 157) | |
| No | 130 | 32 (6 - 146) | 0.007 |
The biopsy reports at least low-grade columnar epithelial dysplasia without reporting any evidence of intestinal metaplasia;
Abbreviations: BMT: bimodality therapy, HG: high grade, LG: low grade
Abbreviation: BMT: bimodality therapy
In comparison of patients with and without BE after BMT, higher rate of BE before BMT was present in the first group than the second one (51% vs.13.9%, P <0.0001). Higher rate of dysplasia (HG and LG) before BMT was present in patients with BE compared to patients without BE (44.9% vs. 10.7 %, P< 0.0001). Also, higher rate of dysplasia after BMT was found in patients with BE (HG in 29.6% and LG in 30.6%, respectively), compared to the group without BE (HG in 0% and LG in 0.8% respectively, P<0.0001) (Table 2).
Recurrence and survival based on the presence of BE after BMT
Patients were followed up for a median duration of 37 months (range: 6-157) after BMT. In patients with BE (N=98), local recurrence was detected in 15 (15.3%) and in patients without BE (N=130), it was detected in 19 (14.6%). There was no significant difference in the overall recurrence type between patients with and without BE (Table 2).
Patients with BE were evaluated based on recurrence type. Variables including age, tumor location, histology subtype, history of chronic GERD and presence of BE/dysplasia before BMT were not associated with the recurrence type (Table 3). In the local recurrence group (N=15) and no-recurrence group (N=44), BE was detected at a median of 6 months after BMT while in distant recurrence group (N=39), BE was detected within a median of 3 months (P=0.007).
Table 3.
Summary of variables before BMT in patients with BE, based on the recurrence (N=98)
| Covariate | Levels | Recurrence
|
P-value | ||
|---|---|---|---|---|---|
| Local (n=15) | Distant (n=39) | None (n=44) | |||
|
| |||||
| Age at diagnosis, (year) median (range) | – | 67 (53 - 83) | 65 (31 - 103) | 68 (50 - 79) | 0.26 |
|
| |||||
| Tumor length at diagnosis (cm), median (range) | – | 5 (1 - 11) | 6 (1 - 13) | 4(2-10) | 0.01 |
|
| |||||
| Gender | Male | 14(93.3%) | 38(97.4%) | 41 (93.1%) | .65 |
| Female | 1(6.7%) | 1(2.6%) | 3(6.9%) | ||
|
| |||||
| Siewert class | Esophagus | 3(20%) | 5(12.8%) | 5(11.4%) | .38 |
| AEG1 | 7(46.7%) | 27(69.2%) | 24(54.5%) | ||
| AEG2 | 5(33.3%) | 6(15.4%) | 15(34.1%) | ||
| NA | 0 | 1(2.6%) | 0 | ||
|
| |||||
| Tumor histology subtype | SRC | 4(26.7%) | 7(18%) | 5(11.4%) | .59 |
| M | 0 | 0 | 1(2.3%) | ||
| M & SRC | 0 | 1(2.6%) | 2(4.5%) | ||
| NE | 1(6.7%) | 2(5.1%) | 0 | ||
| NOS | 10(66.7%) | 29(74.3%) | 36(81.8%) | ||
|
| |||||
| Chronic GERD history | Yes | 8(53.3%) | 24(61.5%) | 28(63.6%) | .78 |
| No | 7(46.7%) | 15(38.5%) | 16(36.4%) | ||
|
| |||||
| Barrett’s before BMT (biopsy) | Yes | 5(33.3%) | 20(51.3%) | 25(56.8%) | |
| None | 10(66.7%) | 19(48.7%) | 19(43.2%) | .29 | |
|
| |||||
| Barrett’s length before BMT (Endoscopy appearance) | LS≥3cm | 5(100%) | 16(72.7%) | 17 (73.9%) | |
| SS<3cm | 0 | 6(27.3%) | 6(26.1%) | 0.41 | |
|
| |||||
| Highest dysplasia before BMT (biopsy) | HG | 3(20%) | 13(33.3%) | 18(40.9%) | .55 |
| LG | 1(6.7%) | 4(10.3%) | 5(11.4%) | ||
| No | 11(73.3%) | 22(56.4%) | 21 (47.7%) | ||
|
| |||||
| Baseline T-staging | T1 | 0 | 1(2.6%) | 2(4.5%) | .03 |
| T2 | 0 | 3 (7.7%) | 12(27.3%) | ||
| T3 | 15 (100%) | 30(76.9%) | 29(65.9%) | ||
| T4 | 0 | 3(7.7%) | 0 | ||
| TX | 0 | 2 (5.1%) | 1(2.3%) | ||
|
| |||||
| Baseline N-staging | N0 | 5(33.3%) | 8(20.5%) | 23(52.3%) | .05 |
| N1 | 10(66.7%) | 27(69.2%) | 17(38.7%) | ||
| N2 | 0 | 1(2.6%) | 2 (4.5%) | ||
| N3 | 0 | 1(2.6%) | 0 | ||
| NX | 0 | 2(5.1%) | 2 (4.5%) | ||
|
| |||||
| Baseline M-staging | M0 | 15(100%) | 31 (79.5%) | 42(95.5%) | .03 |
| M1 | 0 | 6(15.4%) | 0 | ||
| MX | 0 | 2(5.1%) | 2(4.5%) | ||
|
| |||||
| Baseline staging | I | 0 | 1(2.6%) | 8(18.2%) | .005 |
| II-III | 15(100%) | 30(76.9%) | 35(79.5%) | ||
| IV | 0 | 6(15.4%) | 0 | ||
| NA | 0 | 2(5.1%) | 1(2.3%) | ||
Abbreviations: BMT: bimodality therapy, GERD: gastroesophageal reflux disease, M: mucinous, NE: neuroendocrine; NOS: not otherwise specified; NA: not available, SRC: signet ring cell
Endoscopic intervention was performed only in 11 patients (Table 4). 3 cases developed recurrences including 1 case presented with distant metastases 2 years after BMT and two cases presented with both local and distant recurrences (one case within less than one year and the other one 6.5 years after BMT) (Table 4).
Table 4.
Summary of variables after BMT in patients with BE, based on the recurrence (N=98)
| Covariate | Levels | Recurrence
|
P-value | ||
|---|---|---|---|---|---|
| Local (N=15) | Distant (N=39) | None (N=44) | |||
|
| |||||
| Induction chemotherapy | Yes | 5(33.3%) | 17(43.6%) | 9(20.5%) | .08 |
| No | 10(66.7%) | 22 (56.4%) | 35(79.5%) | ||
|
| |||||
| Frequency of endoscopic biopsy after BMT | – | 4 (2 - 14) | 2 (2 - 6) | 5 (2 - 13) | <0.0001 |
|
| |||||
| Frequency of Barrett’s after BMT (Total) | – | 2 (1 - 6) | 2 (1 - 6) | 3 (1 - 9) | 0.02 |
|
| |||||
| Interval between Barrett’s detection after BMT and BMT start-date (months) | – | 6 (2 - 27) | 3 (2 - 29) | 6(2 - 51) | 0.006 |
|
| |||||
| Highest dysplasia after BMT | HG | 7(46.7%) | 13(33.3%) | 9(20.4%) | .37 |
| LG | 3(20%) | 12(30.8%) | 15(34.1%) | ||
| None | 5(33.3%) | 14(35.9%) | 20(45.5%) | ||
|
| |||||
| Frequency of HG dysplasia after BMT (biopsy) | 0 | 8(53.3%) | 26(66.7%) | 35(79.5%) | .12 |
| 1 | 5(33.3%) | 9(23.1%) | 8(18.2%) | ||
| 2 | 1(6.7%) | 4(10.2%) | 1(2.3%) | ||
| 3 | 1(6.7%) | 0 | 0 | ||
|
| |||||
| Frequency of LG dysplasia after BMT (biopsy) | 0 | 5(33.3%) | 15(38.5%) | 20(45.5%) | .30 |
| 1 | 3(20%) | 10(25.7%) | 9(20.5%) | ||
| 2 | 4(26.7%) | 7(17.9%) | 2(4.5%) | ||
| 3 | 3(20%) | 7(17.9%) | 13(29.5%) | ||
|
| |||||
| Local recurrence at same site of previous Barrett’s | Yes | 8(53.3%) | 9(69.2%) | ||
| No | 4(26.7%) | 4(30.8%) | NA | 0.23 | |
| Equivocal | 3(20%) | 0 | |||
|
| |||||
| Local recurrence at same site previous dysplasia | Yes | 6(40%) | 4(30.8%) | ||
| No | 6 (40%) | 9(69.2%) | NA | .14 | |
| Equivocal | 3(20%) | 0 | |||
|
| |||||
| Local recurrence with concurrent Barrett’s | Yes | 10(66.7%) | 6(42.9%) | 0 | .20 |
| No | 5(33.3%) | 8(57.1%) | 0 | ||
|
| |||||
| Endoscopic intervention | Yes | 0 | 3(7.7%) | 8(18.2%) | .10 |
| No | 15(100%) | 36(92.3%) | 36(81.8%) | ||
|
| |||||
| Endoscopic intervention frequency | 0 | 15(100%) | 36(92.3%) | 36(81.9%) | .58 |
| 1 | 0 | 2(5.1%) | 2(4.5%) | ||
| 2 | 0 | 1(2.6%) | 3(6.8%) | ||
| 3 | 0 | 0 | 1(2.3%) | ||
| 8 | 0 | 0 | 2(4.5%) | ||
|
| |||||
| Follow-up duration after BMT (months) | – | 42 (13 - 157) | 26 (9 - 84) | 55 (19 - 151) | <.0001 |
|
| |||||
| Survival status | Alive | 8(53.3%) | 6(15.4%) | 33(75%) | <.0001 |
| Dead | 7(46.7%) | 33(84.6%) | 11(25%) | ||
Abbreviations: BMT: bimodality therapy, LG: low grade, HG: high grad
At the time of data collection, 42.2% of total patients were alive. Median OS was 49 months, with 3-year and 5-year survival rate of 61.3% and 44.4%, respectively. There was no difference in OS between patients with and without BE. Among patients with BE, 53.3% of patients in the local recurrence group were alive compared to 15.4% in distant and 75% in no-recurrence groups. (P<0.0001). Median LRFS in patients with BE was 43.4 months (95% confidence interval [CI], 28.2-60.7 months), with 3-year and 5-year LRFS rate of 55% (95%CI, 46-66%) and 41% (95%CI, 32-53%), respectively.
In the fitted Cox proportional hazards model for OS and LRFS among all 228 patients, BE was not significantly associated with the risk for death (p=0.43) or the risk for local recurrence or death (p=0.84). In the fitted Cox proportional hazards model for OS and LRFS among patients with BE, the endoscopic intervention was not significantly associated with the risk for death (p=0.34) or the risk for local recurrence or death (p=0.63).
Discussion
In this study on patients with EAC who had BMT, the main findings include 1) endoscopic intervention for BE was not associated with decreased risk for local recurrence or death, although, we acknowledge that the number of cases that received this intervention is too small, 2) with a median follow-up of 37 months since BMT, there was no significant difference in recurrence and survival rates, and 3) patients with BE had mostly AEG1 tumor compared to patients without BE had mostly AEG2.
Several studies evaluated the role of endoscopic intervention in decreasing progression rate of BE/LGD to EAC (20–23). However, the data on the beneficial effect of procedure for treatment of BE after BMT is very limited. In our patients with BE, only 11.2% underwent endoscopic procedures for BE/dysplasia and the reason for no procedure in the remainders is unknown. Barthel et al analyzed retrospectively the efficacy and safety of cryoablation for persistent dysplastic BE in 14 patients who achieved complete clinical response after BMT. They reported a significant reduction in median circumferential and maximal Prague criteria accompanied by downgrading of dysplasia in all 14 patients with median number of 1 sessions and median follow-up time of 7 month from initial cryoablation (24).
In our study, BE after BMT was not significantly associated with OS and LRFS. While poorer prognosis was found for patients after trimodality if they showed BE prior to trimodality (25, 26), no study has evaluated the prognostic role of BE after BMT.
In our study, most patients with BE had history of BE before BMT and they also had mostly AEG 1 compared to AEG2 in patients without BE. Tumor location is not assessed in reported studies in patients with BE after BMT. However, Cen et al showed higher rate of AEG 1 in patients with history of BE before trimodality (26).
Among studies that investigated persistent BE after chemoradiation in patients with EAC (24, 27), Barthel et al. identified persistent BE on surveillance endoscopy in 14 out of 16 patients after BMT(24). They reported that surveillance endoscopy every 6 months and beyond detected all cases with reappearance of BE visible both endoscopically and on histology. They also argued that concurrent detection of BE re-appearance with the resolution of therapy-related acute mucosal inflammation supports the persistence of pre-treatment tumor-associated BE, rather than reflux-related recurrence of BE (24). They also suggested that surveillance for persistent BE should begin 6 months after BMT (27). In our study, the median time for the detection of BE was 5 months from the BMT and 48% of patients were detected to have BE at the first biopsy which was taken within 2.7 months after BMT (data no shown).
With regard to risk factors for BE progression, prior studies mostly evaluated clinical risk factors for progression of BE before BMT or TMT rather than risk factors for progression of BE after BMT. Although, male patients are reported to have a higher chance of progression to EAC than female patients (5, 28–31), in patients with BE after BMT, gender was not a significant risk factor neither for the development of BE nor for the recurrence (tables 1&3). With regard to the length of BE, while there are controversial reports on the incidence rate of progression of short segment (SS) BE (0.07-0.61%) and long segment (LS) BE (0.22-0.67%) to EAC (28, 30–35), its prognostic role in recurrence after BMT is not evaluated. In our study, we found significantly more patients with LS BE≥3cm in patients with BE but we did not find any significant difference in the length of BE among local, distant, and no recurrence groups (Table 3).
Dysplasia is one of the most established risk factor for the progression of BE to EAC (36). In our study, patients with BE had significantly higher rate of dysplasia before (HG: 34.7%, LG: 10.2%) and after BMT (HG: 29.6%, LG: 30.6%). However, recurrence type was not significantly associated with dysplasia before and after BMT. (Tables 3&4). During the recent years, biomarkers studies are developing to give a better prediction of progression of BE/dysplasia to EAC (37–39); however, no study is available regarding biomarkers of BE progression to EAC recurrence after BMT.
There are certain limitations in this study. On one hand, we did not find the beneficial role of BE diagnosis and an intervention for it in decreasing recurrence rate during follow-up, but one should notice that progression of BE to EAC might occur even 10 years or more (5) and EAC would be detected in a very long-term follow-up. One more challenging issue is whether the local recurrence is a new EAC or not; although, a new EAC is expected to occur years later compared to relapse. It should be also noted that endoscopic biopsy after chemoradiation might not show an existing residual tumor due to sampling limitations. In a large study, 322 patients with esophageal cancer who underwent preoperative chemoradiation showed negative post-chemoradiation biopsy in 79% while only 21.7% had a pathCR on their surgical specimens (40). The negative predictive value of pathology report after chemoradiation is around 30% (41–43). In addition, the number of our patients with BE before BMT is likely underestimated as BE lesions might not be biopsied during the endoscopy for the initial tumor staging.
All in all, the aim of this study was to look into prognostic role of BE after BMT and if endoscopic intervention makes any difference. Due to the retrospective nature of this study and the mentioned challenging issues, we cannot make a recommendation on the appropriateness of endoscopic intervention for BE/dysplasia after BMT. Panel of biomarkers might be helpful in forecasting recurrence. Further bench and prospective clinical studies might reveal more information in this area.
Acknowledgments
Funding Sources: This research was supported by generous grants from the Caporella, Dallas, Sultan, Park, Smith, Frazier, Oaks, Vanstekelenberg, Planjery, and Cantu families, as well as from the Schecter Private Foundation, Rivercreek Foundation, Kevin Fund, Myer Fund, Dio Fund, Milrod Fund, and The University of Texas MD Anderson Cancer Center (Houston, Texas, USA) multidisciplinary grant program. This research was also supported in part by the National Cancer Institute and Department of Defense awards CA129906. CA 127672, CA138671, and CA172741 and the DOD grants: CA150334 and CA162445 (J.A.A.)
Footnotes
Conflict of Interest: None
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program Research data (1973-2013) National Cancer Institute, Division of Cancer Control and Population Services, Surveillance Research Program, Surveillance Systems Branch; www.seer.cancer.gov. released September 2015 [accessed accessed Aug 1, 2017]. [Internet] [Google Scholar]
- 3.Cossentino MJ, Wong RK. Barrett’s esophagus and risk of esophageal adenocarcinoma. Semin Gastrointest Dis. 2003;14(3):128–35. [PubMed] [Google Scholar]
- 4.Wild CP, Hardie LJ. Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003;3(9):676–84. doi: 10.1038/nrc1166. [DOI] [PubMed] [Google Scholar]
- 5.Sikkema M, Looman CW, Steyerberg EW, Kerkhof M, Kastelein F, van Dekken H, et al. Predictors for neoplastic progression in patients with Barrett’s Esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106(7):1231–8. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 6.Hage M, Siersema PD, van Dekken H, Steyerberg EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39(12):1175–9. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 7.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(3):235–44. doi: 10.1016/j.cgh.2009.10.010. quiz e32. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287(15):1972–81. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 9.Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26(11–12):1465–77. doi: 10.1111/j.1365-2036.2007.03528.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Falk GW, Iyer PG, Gerson LB, Gastroenterology ACo ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network NCC. National Comprehensive Cancer Network. Esophageal and Esophagogastric junction cancers (Version 1) 2017 doi: 10.6004/jnccn.2015.0028. http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed August 1, 2017 version 1. [DOI] [PubMed]
- 12.Sudo K, Taketa T, Correa AM, Campagna MC, Wadhwa R, Blum MA, et al. Locoregional failure rate after preoperative chemoradiation of esophageal adenocarcinoma and the outcomes of salvage strategies. J Clin Oncol. 2013;31(34):4306–10. doi: 10.1200/JCO.2013.51.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorth JA, Pura JA, Palta M, Willett CG, Uronis HE, D’Amico TA, et al. Patterns of recurrence after trimodality therapy for esophageal cancer. Cancer. 2014;120(14):2099–105. doi: 10.1002/cncr.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32(5):385–91. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 15.Taketa T, Xiao L, Sudo K, Suzuki A, Wadhwa R, Blum MA, et al. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology. 2013;85(2):95–9. doi: 10.1159/000351999. [DOI] [PubMed] [Google Scholar]
- 16.Sudo K, Xiao L, Wadhwa R, Shiozaki H, Elimova E, Taketa T, et al. Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol. 2014;32(30):3400–5. doi: 10.1200/JCO.2014.56.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353–61. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL. Paul Meier Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–81. [Google Scholar]
- 19.Cox DR. Regression Models and Life-Tables (with discussion) Journal of the Royal Statistical Society Series B. 1972;34(2):187–220. [Google Scholar]
- 20.Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011;141(2):460–8. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360(22):2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 22.Kroep S, Heberle CR, Curtius K, Kong CY, Lansdorp-Vogelaar I, Ali A, et al. Radiofrequency Ablation of Barrett’s Esophagus Reduces Esophageal Adenocarcinoma Incidence and Mortality in a Comparative Modeling Analysis. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 24.Barthel JS, Kucera S, Harris C, Canchi D, Hoffe S, Meredith K. Cryoablation of persistent Barrett’s epithelium after definitive chemoradiation therapy for esophageal adenocarcinoma. Gastrointest Endosc. 2011;74(1):51–7. doi: 10.1016/j.gie.2011.03.1121. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal B, Swisher SG, Ajani J, Kelly K, Komaki RR, Abu-Hamda E, et al. Differential response to preoperative chemoradiation and surgery in esophageal adenocarcinomas based on presence of Barrett’s esophagus and symptomatic gastroesophageal reflux. Ann Thorac Surg. 2005;79(5):1716–23. doi: 10.1016/j.athoracsur.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Cen P, Correa AM, Lee JH, Le JH, Maru D, Anandasabapathy S, et al. Adenocarcinoma of the lower esophagus with Barrett’s esophagus or without Barrett’s esophagus: differences in patients’ survival after preoperative chemoradiation. Dis Esophagus. 2009;22(1):32–41. doi: 10.1111/j.1442-2050.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 27.Barthel JS, Kucera ST, Lin JL, Hoffe SE, Strosberg JR, Ahmed I, et al. Does Barrett’s esophagus respond to chemoradiation therapy for adenocarcinoma of the esophagus? Gastrointest Endosc. 2010;71(2):235–40. doi: 10.1016/j.gie.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–57. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 30.Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(3):237–49. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 31.Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61(7):970–6. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 32.Wani S, Falk G, Hall M, Gaddam S, Wang A, Gupta N, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9(3):220–7. doi: 10.1016/j.cgh.2010.11.008. quiz e26. [DOI] [PubMed] [Google Scholar]
- 33.Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141(4):1179–86.e1. doi: 10.1053/j.gastro.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 34.Weston AP, Sharma P, Mathur S, Banerjee S, Jafri AK, Cherian R, et al. Risk stratification of Barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol. 2004;99(9):1657–66. doi: 10.1111/j.1572-0241.2004.30426.x. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 36.Bird-Lieberman EL, Dunn JM, Coleman HG, Lao-Sirieix P, Oukrif D, Moore CE, et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology. 2012;143(4):927–35.e3. doi: 10.1053/j.gastro.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 37.Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24(25):4138–48. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 38.Bird-Lieberman EL, Neves AA, Lao-Sirieix P, O’Donovan M, Novelli M, Lovat LB, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat Med. 2012;18(2):315–21. doi: 10.1038/nm.2616. [DOI] [PubMed] [Google Scholar]
- 39.Ross-Innes CS, Chettouh H, Achilleos A, Galeano-Dalmau N, Debiram-Beecham I, MacRae S, et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol. 2017;2(1):23–31. doi: 10.1016/S2468-1253(16)30118-2. [DOI] [PubMed] [Google Scholar]
- 40.Ajani JA, Correa AM, Hofstetter WL, Rice DC, Blum MA, Suzuki A, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23(10):2638–42. doi: 10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu AJ, Goodman KA. Clinical tools to predict outcomes in patients with esophageal cancer treated with definitive chemoradiation: are we there yet? J Gastrointest Oncol. 2015;6(1):53–9. doi: 10.3978/j.issn.2078-6891.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkaria IS, Rizk NP, Bains MS, Tang LH, Ilson DH, Minsky BI, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg. 2009;249(5):764–7. doi: 10.1097/SLA.0b013e3181a38e9e. [DOI] [PubMed] [Google Scholar]
- 43.Miyata H, Yamasaki M, Takiguchi S, Nakajima K, Fujiwara Y, Konishi K, et al. Prognostic value of endoscopic biopsy findings after induction chemoradiotherapy with and without surgery for esophageal cancer. Ann Surg. 2011;253(2):279–84. doi: 10.1097/SLA.0b013e318206824f. [DOI] [PubMed] [Google Scholar]


