Abstract
Chronic stress contributes to the neuropathology of mental health disorders, including those associated with anxiety. The basolateral amygdala (BLA) coordinates emotional behavioral responses through glutamatergic outputs to downstream regions such as the prefrontal cortex (PFC), nucleus accumbens core (NAcc) and bed nucleus of the stria terminalis (BNST). We explored the effects of chronic stress on BLA outputs to the PFC, NAcc and BNST using slice electrophysiology combined with optogenetics in two inbred mouse strains with distinct stress-induced anxiety responses. We found that ten consecutive days of chronic restraint stress enhanced pre-synaptic glutamate release at BLA-to-PFC synapses in C57BL/6J mice, but reduced pre-synaptic glutamate release at these synapses in DBA/2J mice. To assess the behavioral relevance of enhanced glutamate output at BLA-to-PFC synapses, we approximated the effects of chronic stress on the BLA-PFC circuit using optogenetics. We found that photostimulation of the BLA-PFC circuit in unstressed C57BL/6J mice produced persistent (i.e., post-stimulation) increased anxiety-like behavior and hyperactivity in the elevated plus-maze - a profile consistent with prototypical behavioral responses of stressed C57BL/6J mice. These data demonstrate that chronic stress dysregulates the BLA-PFC circuit by altering pre-synaptic glutamate release from BLA outputs, and provide a mechanism by which chronic stress can lead to increased anxiety.
Keywords: Stress, BLA, Amygdala, PFC, Glutamate, Anxiety
1. Introduction
Anxiety disorders consistently rank among the most common mental health concerns in the United States and globally (Kessler et al., 2005, 2009). These disorders are characterized by intense and persistent feelings of worry or panic that are disproportionate to the associated threat and significantly interfere with daily function (American Psychiatric Association, 2013). Though the clinical manifestation of anxiety disorders can vary greatly between patients, chronic stress is considered a major factor in their development and progression (Craske et al., 2017).
Preclinical models have been developed to better understand the contribution of chronic stress to anxiety. Results from studies in rodents reveal that even relatively minor stressors, experienced frequently and over long periods of time, can lead to increased anxiety-like behavior (Caruso et al., 2017; Netto et al., 2002; Strekalova et al., 2004, 2005; Vyas and Chattarji, 2004; Zurita et al., 2000). However, the effect of chronic stress on subsequent behavior can vary considerably depending on numerous environmental and individual factors, including the type of stressor, the length of stress exposure, age of stress exposure, sex, and genetic background.
Comparing inbred mouse strains of distinct genetic backgrounds is a common and informative approach to understanding the influence of genetic variability on a range of behaviors, including those related to anxiety and stress (Crabbe et al., 1998; Crawley et al., 1997; Lowery et al., 2008; Millstein and Holmes, 2007; Paylor and Crawley, 1997; Singh et al., 2007; Szklarczyk et al., 2012). Previous work from our group and others utilizing this approach has revealed that anxiety- and depression-related behaviors vary considerably between several inbred strains, indicating a strong influence of genetic background on these phenotypes (Crawley et al., 1997; Kopp et al., 1999; Mozhui et al., 2010). Notably, strain-dependent variations in these behaviors were observed at baseline, and in some cases, became more pronounced following chronic exposure to stress in a strain-dependent manner (Mozhui et al., 2010). For instance, two of the inbred strains tested in a larger strain-panel, DBA/2J and C57BL/6J, displayed differing levels of anxiety-like behavior under unstressed conditions.
Specifically, DBA/2J mice displayed behaviors consistent with greater anxiety-like behavior, as compared to C57BL/6J mice, consistent with other reports of overall greater negative emotional states in DBA/2J mice relative to C57BL6/J mice in various test assays (Crawley et al., 1997; Miller et al., 2010; Mineur et al., 2006; Thoeringer et al., 2007). Despite these baseline differences, both the DBA/2J and C57BL/6J strains were found to be vulnerable to the anxiety-provoking effects of ten consecutive days of restraint stress (Ihne et al., 2012; Masneuf et al., 2014; Mozhui et al., 2010), albeit manifesting in distinct behavioral responses. Thus, relative to their unstressed counterparts, stressed DBA/2J mice displayed the classical anxiety-related response of avoiding the illuminated compartment in the light/dark exploration test, while stressed C57BL/6J mice spent more time in this aversive area (Mozhui et al., 2010). Importantly, this ostensibly paradoxical response to stress is reversible with prototypical anxiolytic drugs (Ihne et al., 2012), suggesting the distinct anxiety responses in the two mouse strains reflect avoidant or ‘passive’ (DBA/2J) versus ‘active’ or even panic-like (C57BL/6J) response to stress, respectively.
Subsequent analyses found that these stress-induced anxiety responses were associated with divergent patterns of gene expression and measures of BLA glutamate-mediated synaptic plasticity in the basolateral amygdala (BLA) - a key regulator of behavioral responses to stress (Mozhui et al., 2010). Moreover, follow-up studies in C57BL/6J mice provided further evidence that BLA dysregulation is a key component of the neuropathology underlying the stress-induced anxiety-like responses in these mice (Masneuf et al., 2014). However, while these and other data indicate that adaptations in BLA activity follow chronic stress exposure, the consequential effects of these changes on BLA contacts with downstream anatomical targets, and the behavioral implications thereof, are not fully understood.
In the current study, we sought to characterize the pre- and post-synaptic effects of restraint stress in DBA/2J and C57BL/6J mice on three of the BLA’s major projection targets. Using slice ex vivo whole-cell electrophysiology combined with optogenetics, we show that stress has an opposite effect on BLA output to the dorsomedial prefrontal cortex (dmPFC) in DBA/2J and C57BL/6J mice. Establishing the specificity of these effects, stress did not alter BLA output to either the nucleus accumbens core (NAcc) or the bed nucleus of the stria terminalis (BNST) in either strain. Next, using in vivo optogenetics to approximate the stress-induced increase in BLA output to the PFC, we were able to induce a persistent increase in anxiety-like behavior in unstressed C57BL/6J mice. Collectively, these findings demonstrate that strain can modulate-stress induced plasticity within distinct pathways in the brain. In addition, the optogenetic studies in naïve mice provide support for the important role for BLA outputs to the PFC in modulating behavioral responses related to anxiety-like behavior. Notably, the differences seen in optogenetic stimulation of BLA outputs to the PFC in naïve animals, and that following chronic stress, highlights the need for an increased understanding of how dysregulation of this circuit may differentially modulate anxiety-like behavior versus stress-induced anxiety-like behavior.
2. Material and methods
2.1. Subjects
Male C57BL/6J and DBA/2J mice aged 2–3 months obtained from The Jackson Laboratory (Bar Harbor, ME, USA) were used for all experiments. Mice were acclimated for at least one week before the start of experiments to a temperature- (22 ± 3 °C) and humidity- (45 ± 15%) controlled vivarium with a 12-h light/dark cycle (lights on at 07:00). Food and water were available ad libitum except during periods of restraint stress and behavioral testing (as detailed below). All experimental procedures were approved by the NIAAA and UNC Animal and Care and Use Committees, and followed the National Institutes of Health guidelines outlined in ‘Using Animals in Intramural Research.’
2.2. Viral infusion and ferrule implantation
Mice were anesthetized under 1–2% isoflurane and placed in a stereotaxic alignment system (Kopf Instruments, Tujunga, CA, USA) to infuse virus and implant ferrules. AAVs were bilaterally infused into the BLA (coordinates relative to bregma: AP -1.4, ML ±3.22, DV -4.85) using a Neuro Hamilton syringe with a 33 gauge needle (Hamilton, Reno, NV, USA) at the rate of 0.02 μL/min, and were then left in place for an additional 5 min. To express the excitatory channelrhodopsin-2 (Zhang et al., 2007), we used rAAV5/CaMKII-hChR2(H134R)-eYFP (0.35 μL/hemisphere; titer 1 × 10e13). rAAV5/CaMKII-eYFP (0.35 μL/hemisphere; titer 6 × 10e12) served as control virus in behavioral studies. Viruses were obtained from the University of North Carolina vector core (http://www.med.unc.edu/genetherapy/vectorcore). During the same surgery, 200-μm diameter (NA 0.37) ferrules were bilaterally directed at the dmPFC (coordinates relative to bregma: AP -1.9, ML ±1.0, DV -2.0, 20° angle) and chronically implanted by affixing to the skull with dental cement. Ferrule-fiber assembly was constructed according to previously published methods (Bergstrom et al., 2018; Sparta et al., 2011). Mice were single-housed and left undisturbed for 6 weeks prior to testing to allow for recovery and virus expression.
To verify virus expression and ferrule placements at the completion of testing, mice were terminally overdosed with ketamine/xylazine and transcardially perfused with phosphate buffered saline, then 4% paraformaldehyde (PFA). After suspending brains in 4% PFA overnight and 4 °C 0.1 M phosphate buffer for 1–2 days, 50 μm coronal sections were cut with a vibratome (Classic 1000 model, Vibratome, Bannockburn, IL, USA). Cases of missed placement were removed from all datasets.
2.3. Restraint stress
Each day for 10 consecutive days, beginning at 10:00, mice were placed in ventilated 50 mL Falcon tubes for 2 h, in a quiet room. Control mice remained in their home cages. We have previously shown that this procedure results in significantly reduced body weights and alterations in anxiety-like behavior in the DBA/2J and C57BL/6J strains (Ihne et al., 2012; Masneuf et al., 2014; Mozhui et al., 2010).
2.4. Slice electrophysiology
One day following the final exposure to stress, mice were sacrificed via deep isoflurane anesthesia and decapitated. Brain slices containing the BLA, BNST, PFC, or NAcc were prepared as previously described (Crowley et al., 2016; Lowery-Gionta et al., 2015; Masneuf et al., 2014). Briefly, brains were rapidly removed and 300 μM coronal slices were cut on a vibratome (Leica Biosystems, Buffalo Grove, IL, USA) in cold (1–4 °C) sucrose extracellular solution (in mmol/L: 194 sucrose; 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, 26 NaHCO3). Slices were immediately placed in normal artificial cerebrospinal fluid (ACSF, in mmol/L: 124 NaCl, 4.4 KCl, 2 CaCl, 1.2 MgSO4, 1 NaPO4, 10 glucose, 26 NaHCO3) maintained at 30 °C and allowed to recover for at least 1 h. Slices were then placed in a holding chamber and continuously perfused at a rate of 2 mL per min with normal ACSF maintained at 30 °C.
Neurons were visualized using infrared video microscopy (Olympus, Center Valley, PA, USA). Recording electrodes (3–5 MΩ) were pulled with a Flaming-Brown Micropipette Puller (Sutter Instruments, Novato, CA, USA) using thin-walled borosilicate glass capillaries. Signals were acquired by a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized at 10 kHz and analyzed using Clampfit 10.2 software (Molecular Devices, Sunnyvale, CA, USA). Input resistance and access resistance were continuously monitored throughout all experiments, and those in which access resistance fluctuated in excess of 20% were excluded from all data analyses. A maximum of 2 cells were recorded from each animal from each region with one exception: 3 cells were recorded from a single C57BL/6J mouse in the control group for the BLA excitability experiments.
Whole-cell voltage clamp recordings were performed in slices from mice expressing ChR2 in the BLA to assess the effects stress on glutamate transmission at pre- and post-synaptic contacts with target regions in the PFC, NAcc and BNST. Recordings were specifically performed in the dmPFC, nucleus accumbens core and dorsolateral BNST - only in those mice in which ChR2 viral expression was evident both in the BLA and the appropriate output region. The recordings presented herein were made in cells that exhibited excitatory monosynaptic post-synaptic currents that were time-locked to optical stimulation. The maximum LED output capacity for stimulation was 25 mW, however for each cell, the intensity of LED optical stimulation was tailored to isolate a monosynaptic response (up to 5 ms (Crowley et al., 2016)); and was kept consistent for the duration of each experiment.
To assess presynaptic glutamate release, using a cesium methanesulfonate-based intracellular solution (in mM: 135 cesium methanesulfonate, 10 KCl, 1 MgCl2, 0.2 EGTA, 2 QX-314, 4 MgATP, 0.3 GTP, 20 phosphocreatine, pH 7.3, 285–290 mOsmol) with cells held at −55 mV, excitatory post-synaptic currents were evoked by 2 x 5-ms pulses of 490 nM blue light delivered 50 ms apart (Cool LED, Traverse City, MI, USA). The amplitudes of each pair of evoked excitatory post-synaptic currents (eEPSCs) were then used to create a paired-pulse ratio (PPR; amplitude of pulse 2/amplitude of pulse 1) to measure between-group differences in glutamate release probability from presynaptic BLA contacts. To assess postsynaptic glutamate transmission, using a cesium gluconate-based intracellular solution (in mM: 117 D-gluconic acid, 20 HEPES, 0.4 EGTA, 5 TEA, 2 MgCl2, 2 QX-314, 4 Na2ATP, 0.4 Na2GTP, pH 7.3, 285–290 mOsmol) with the GABA-A channel blocker picrotoxin (25 μM) in the bath, excitatory post-synaptic currents were evoked by a 1-ms pulse of 490 nM blue light. Cells were held stably at −70 mV to record AMPA receptor-mediated currents, then were held stably at +40 mv to record NMDA receptor-mediated currents. The amplitudes of AMPA receptor-mediated currents relative to NMDA receptor-mediated currents were used to create an AMPA/NMDA ratio, a measure of postsynaptic glutamate transmission.
Whole-cell current clamp recordings were performed at resting membrane potential (RMP) in slices from surgery-naïve mice to assess the effects of stress on excitability in the BLA. All experiments were conducted in drug-free ACSF using a potassium gluconoate-based intracellular solution (in mM: 135 K + gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na2ATP, 0.4 Na2GTP, pH 7.3, 285–290 mOsmol). After each cell had settled, the experiments were performed to assess the amount of current required to fire an action potential (termed a rheobase), the membrane potential at which an action potential first fired, followed by an experiment to assess the number of action potentials fired at given amount of current (0–200 pA, increased 10 pA per current step).
2.5. Elevated plus-maze test for anxiety-like behavior
The elevated plus-maze test consisted of 2 open arms (30 × 5 cm; 90 lux) and 2 closed arms (30 × 5 × 15 cm; 20 lux) extending from a 5 × 5 cm central area and elevated 47 cm from the ground (San Diego Instruments, San Diego, CA, USA), as previously described (Mozhui et al., 2010; Holmes and Rodgers, 2003). The walls were made from black ABS plastic and the floor from white ABS plastic. A 0.5 cm raised lip around the perimeter of the open arms prevented mice from falling off the maze.
Optical fibers were connected, via the ferrules, to a 473 nm laser diode (OEM Laser Systems, East Lansing, MI, USA) through an FC/PC adapter. The mouse was placed in the center square of the maze, facing an open arm, and allowed to explore the apparatus for a 9-min session. Laser output was controlled using a Master-8 pulse stimulator (A.M.P.I., Jerusalem, Israel) to deliver light trains at 20 Hz, 5 ms pulse-width for 473 nm. Laser power was set to 3–5 mW based on pilot data showing that higher power induced seizure activity in some mice. Light was shone during the first and third (and not the second) 3-min test epochs (as in previous studies – Tye et al., 2011)). Time spent in the open arms, entries into the open and closed arms, and total distance moved in the open and closed arms was recorded using EthoVisionXT software (Noldus Information Technology Inc., Leesburg, VA, USA).
2.6. Statistical analysis
For each strain, differences in PPR, AMPA/NMDA ratios, and excitability measures between Control and Stress groups were analyzed using unpaired t-tests with Welch’s correction applied where appropriate. Repeated measures ANOVAs (group x current step) were used to assess between-group differences in the number of action potentials fired across a range of current for each strain. Repeated measures ANOVAs (group x epoch) were used to assess between- and within-group effects of BLA-PFC activation on behavioral measures obtained in the elevated plus-maze. Sidak’s multiple comparisons post hoc tests were used to analyze significant ANOVA terms. The threshold for significance for all statistical analyses was set at p < 0.05.
3. Results
3.1. Stress effects on presynaptic glutamate release from BLA projections to PFC, NAcc and BNST
Significant alterations in the probability of presynaptic glutamate release from BLA projections were observed following stress, in a manner dependent on both target region and mouse strain. The probability of presynaptic glutamate release was measured by comparing the amplitudes of two events evoked by two light pulses delivered 50 ms apart. The resulting paired pulse ratio (PPR; amplitude of pulse 2/amplitude of pulse 1) is inversely related to presynaptic neurotransmitter release. Therefore, decreases in PPR are reflective of increased neurotransmitter release while increases in PPR are reflective of decreased neurotransmitter release.
In DBA/2J mice, stress significantly increased PPR relative to control conditions in the PFC (Fig. 1a; t(22) = 2.855, p = 0.0092). Stress did not alter PPR relative to control conditions in either the NAcc (Fig. 1b; t(19) = 0.7041, p = 0.4899) or the BNST (Fig. 1c; t(16) = 1.366, p = 0.1908) of DBA/2J mice. In marked contrast, in C57BL/6J mice, stress significantly decreased PPR relative to control conditions in the PFC (Fig. 1d; t(21) = 2.573, p = 0.0177). Again, stress did not alter PPR relative to control conditions in either the NAcc (Fig. 1e; t(15.41) = 1.95, p = 0.0697) or the BNST (Fig. 1f; t(11.66) = 0.2501, p = 0.8069) of the C57BL/6J strain.
Fig. 1. Strain-dependent effects of stress on presynaptic glutamate release from BLA projections.

Traces are normalized to control pulse 1 for each graph. (a) Stressed DBA/2J mice had significantly increased paired pulse ratios (PPR; amplitude of pulse 2/amplitude of pulse 1) relative to controls in the prefrontal cortex (PFC; n = 15 cells from 8 mice for control; n = 9 cells from 7 mice for stress). Effects of stress on PPR was not observed in the nucleus accumbens core (NAcc; b; n = 10 cells from 6 mice for control; n = 11 cells from 5 mice for stress) or bed nucleus of the stria terminalis (BNST; c; n = 6 cells from 6 mice for control; n = 12 cells from 6 mice for stress) in DBA/2J mice. (d) Stressed C57BL/6J mice had significantly decreased PPR relative to control mice in the PFC (n = 12 cells from 7 mice for control; n = 11 cells from 9 mice for stress). Stress effects in PPR were not observed in the NAcc (e; n = 13 cells from 8 mice for control; n = 10 cells from 5 mice for stress) or BNST (f; n = 11 cells from 6 mice for control; n = 10 cells from 5 mice for stress) in C57BL/6J mice. Data are means ± SEM. * denotes significant between-group differences (p < 0.05).
Together, these data demonstrate that glutamate release in the dmPFC is reduced following chronic stress in DBA/2J mice but increased following chronic stress in C57BL/6J mice.
3.2. Stress effects on postsynaptic glutamate transmission at BLA synapses onto PFC, NAcc and BNST neurons
Postsynaptic glutamate transmission in 3 BLA target areas was assessed by comparing the amplitudes of AMPA receptor-mediated events and NMDA receptor-mediated events evoked by a single light pulse. In DBA/2J mice, stress did not significantly alter AMPA/NMDA ratios, relative to controls, in the PFC (Fig. 2a; t(12.2) = 1.167, p = 0.2655), NAcc (Fig. 2b; t(22) = 0.002, p = 0.9984) or BNST (Fig. 2c; t(19) = 0.873, p = 0.3936). Similarly, stress did not significantly alter AMPA/NMDA ratios in the PFC (Fig. 2d; t(18) = 0.5533, p = 0.5869), NAcc (Fig. 2e; t(16) = 1.423, p = 0.1739) or BNST (Fig. 2f; t(16) = 0.4783, p = 0.6389) in C57BL/6J mice.
Fig. 2. Absence of stress effects on postsynaptic glutamate transmission in BLA projections to target regions.
Traces are normalized to control NMDA for each graph. Stressed DBA/2J mice did not have significantly different AMPA/NMDA ratios (amplitude of AMPA current/amplitude of NMDA current) relative to controls in the PFC (a; n = 10 cells from 8 mice for control, n = 10 cells from 7 mice for stress), NAcc (b; n = 12 cells from 8 mice for control; n = 12 cells from 7 mice for stress) or BNST (c; n = 11 cells from 7 mice for control; n = 10 cells from 7 mice for stress). Stressed C57BL/6J mice did not have significantly different AMPA/NMDA ratios, as compared to controls, in the PFC (d; n = 9 cells from 7 mice for control; n = 11 cells from 6 mice for stress), NAcc (e; n = 8 cells from 7 mice for control; n = 10 cells from 6 mice for stress) or BNST (f; n = 9 cells from 6 mice for control; n = 9 cells from 5 mice for stress). Data are means ± SEM.
These data show that significant alterations in postsynaptic AMPA-mediated or NMDA-mediated glutamate transmission at BLA synapses in various target regions did not occur in response to stress, in either DBA/2J or C57BL/6J mice.
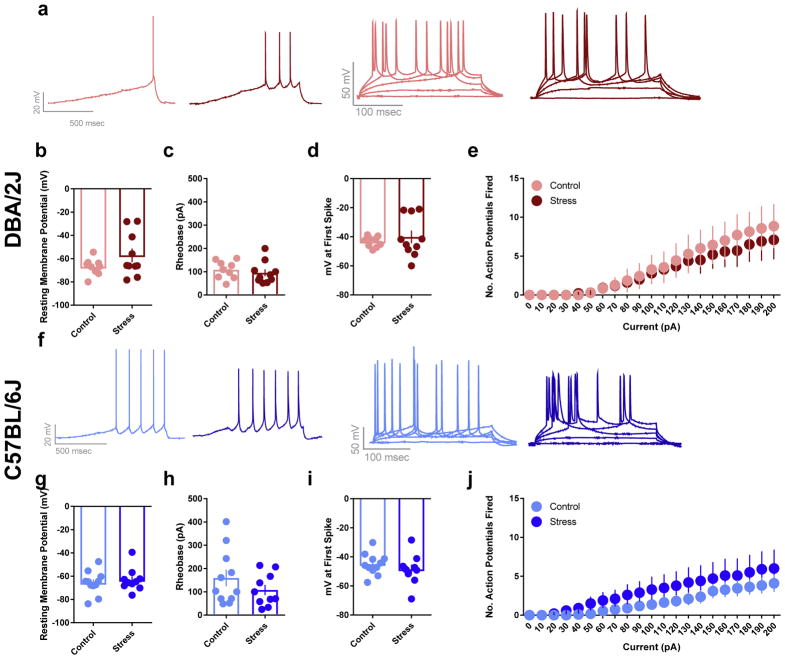
3.3. Stress effects on neuronal excitability in the BLA
The excitability of BLA neurons was assessed following chronic restraint stress by recording the potentials of BLA neuronal membranes. Because stress did not alter the resting membrane potential (RMP) of neurons in either strain, all measures were taken while neurons were at rest. Measures recorded with neurons held at −70 mV did not differ from those recorded at rest (data not shown). Additional measures of excitability obtained included the amount of current needed to fire an action potential (i.e., rheobase), the threshold membrane potential at which an action potential was fired and the number of action potentials fired across a range of currents (0–200 pA, increased in 10 pA increments).
Stress did not alter excitability in BLA neurons in either DBA/2J or C57BL/6J mice. Specifically, stressed DBA/2J mice were similar to controls with regards to RMP (Fig. 3a; t(11.84) = 1.556, p = 0.1460), rheobase (Fig. 3b; t(17) = 0.6587, p = 0.5189), the threshold membrane potential for action potential firing (Fig. 3c; t(10.39) = 0.7272, p = 0.4832), and the number of action potentials fired across a range of currents (Fig. 3d; F(20, 300) = 0.2499, p = 0.9997 for group × current interaction; F(1, 15) = 0.1457, p = 0.7080 for main effect of group; F(20, 300) = 15.54, p < 0.0001 for main effect of current). The same absence of group differences was evident for C57BL/6J mice: RMP (Fig. 3e; t(19) = 0.5806, p = 0.5683), rheobase (Fig. 3f; t(19) = 1.203, p = 0.2439), threshold membrane potential for action potential firing (Fig. 3g; t(19) = 0.912, p = 0.3732), and number of action potentials fired across currents (Fig. 3h; F(20, 380) = 0.5883, p = 0.9208 for group × current interaction; F(1, 19) = 0.9515, p = 0.3416 for main effect of group; F(20, 380) = 14.49, p < 0.0001 for main effect of current).
Fig. 3. Stress effects on BLA neuronal excitability.
Stressed DBA/2J mice did not have significantly different resting membrane potentials (RMP; a), rheobase (b) or threshold for action potential firing (c), as compared to controls (n = 9 cells from 5 mice for control; n = 10 cells from 5 mice for stress). Stressed and control DBA/2J mice also did not differ in the number of action potentials fired across a range of current steps (d; n = 7 cells from 4 mice for control; n = 10 cells from 5 mice for stress). Stressed C57BL/6J mice did not have significantly altered resting membrane potentials (RMP; e), rheobase (f) or threshold for action potential firing (g) relative to controls (n = 11 cells from 6 mice for control; n = 10 cells from 5 mice for stress). Stressed and control C57BL/6J mice also not differ in the number of action potentials fired across a range of current steps (h; n = 11 cells from 5 mice for control; n = 10 cells from 5 mice for stress). Data are means ± SEM.
These data indicate that the excitability of BLA neurons was not altered by stress in either strain of mice.
3.4. Behavioral effects of photostimulating BLA neuronal fibers in PFC in stress-naive C57BL/6J mice
BLA projections to the PFC were manipulated in stress-naive C57BL/6J mice using an optogenetic approach. ChR2-expressing BLA fibers in the mPFC were photostimulated by blue light during specific periods during elevated plus-maze testing (see Fig. 4a and b).
Fig. 4. Effects of BLA-to-PFC photostimulation in stress-naïve C57BL/6J mice.

Channelrhodopsin2-EYFP (ChR2; n = 19) or EYFP control virus (control; n = 15) was injected in to the BLA and optical fibers were implanted in the mPFC, denoted by red x’s (a). Shining blue light in the PFC of mice expressing ChR2 in neurons projecting from the BLA during the initial 3-min (‘ON’ epoch) in the elevated plus-maze (EPM) led to increased locomotor activity on subsequent 3-min light OFF and ON epochs, as compared to controls (b). ChR2-expressing mice also traveled significantly less distance (c), spent significantly less time (d, e) and were less likely to enter (f) the open arms of the EPM, as compared to controls. Data are means ± SEM. * denotes significant within-group post-hoc analyses after two-way repeated measures ANOVA (p < 0.05). + denotes significant within-group post-hoc analyses after one-way repeated measures ANOVA (p < 0.05).
In ChR2, but not eYFP, expressing mice, shining light during the first 3-min epoch significantly increased total distance traveled during the subsequent 3-min light-off and, thereafter, 3-min light-on epochs (Fig. 4c; post hoc tests following group × epoch interaction: F(2, 64) = 5.901, p = 0.0044). ChR2-expressing mice, but not eYFP controls, showed a significant decrease in distance traveled in the open arms during the second (light on) and third (light off) epochs (Fig. 4d; post hoc tests after 2-way ANOVA: main effect of epoch; F(2, 64) = 4.205, p = 0.0192, group; F(1, 32) = 5.902, p = 0.0209, but no group × epoch interaction; F(2, 64) = 1.163, p = 0.3190; and 1-way ANOVA effect of epoch in ChR2-expressing mice; F(2, 36) = 7.677, p = 0.0017). A similar epoch-related pattern of decreases was evident for ChR2-expressing mice, not controls, for percent open arm time (Fig. 4e; post hoc tests after 2-way ANOVA: main effect of group; F(1, 32) = 4.96, p = 0.0331, main effect of epoch; F(2, 64) = 4.307, p = 0.0176, but no group × epoch interaction; F(2, 64) = 0.822, p = 0.4441; and 1-way ANOVA effect of epoch in ChR2-expressing mice; F(2, 36) = 7.085, p = 0.0025), as well as for the ratio of arm entries into the open arms (Fig. 4f; post hoc tests after 2-way ANOVA: main effect of group; F(2, 64) = 1.906, p = 0.1570, main effect of epoch; F(1, 32) = 3.446, p = 0.0727, but no group × epoch interaction; F(2, 64) = 1.67, p = 0.1963; and 1-way ANOVA in ChR2-expressing mice; F(2, 36) = 4.308, p = 0.0210).
These results indicate that BLA-PFC activation in unstressed C57BL6/J mice is sufficient t0 produce increases in EPM anxiety-like behavior and overall locomotion, which persist beyond photostimulation.
4. Discussion
Environmental challenges, including chronic stress, have long been known to engage BLA activity, resulting in altered emotional behavioral states including enhanced anxiety (Burgos-Robles et al., 2017; Likhtik et al., 2014; Masneuf et al., 2014; Mozhui et al., 2010; Tye et al., 2011). However, the consequences of this engagement on brain structures downstream of the BLA remain less clear.
Here, we show that chronic stress alters pre-synaptic glutamate release from BLA projections to the dmPFC in mice. These effects appear to reflect lasting neuroadaptations in the BLA-dmPFC pathway, as they were observed the day after the final stress exposure. Moreover, the effects of stress were highly strain-dependent: stress led to increased glutamate release in C57BL6/J mice and decreased glutamate release in DBA2/J mice. Another important finding was that stress did not change pre-synaptic glutamate release from BLA projections to two other primary output regions, the BNST and the NAcc. Also of note was the observation that stress effects were restricted to pre-synaptic sites, as evidenced by the absence of stress-induced alterations in post-synaptic glutamate transmission from BLA projections to any of the output regions assessed, in either strain. Finally, in agreement with a recent report (Felix-Ortiz et al., 2016), approximating the increased BLA-dmPFC glutamate release after chronic stress in C57BL/6J mice by optogenetically activating this pathway increased anxiety-like behavior in unstressed (C57BL/6J) mice. Indeed, the behavioral effects of photoexcitation were, strikingly, sufficiently potent that they persisted (for at least 3 min) after the offset of light-excitation of BLA fibers in the dmPFC. Even though these effects of stimulation were robust, it would nonetheless be valuable to extend them to additional behavioral assays for anxiety-like behavior and, more generally, other behavioral indices known to be sensitive to the effects of stress and modulated by the PFC, such as reward-seeking and social behavior.
Collectively, these results indicate that dysregulation of pre-synaptic glutamatergic inputs to the dmPFC arising from BLA projections represents at least one plausible mechanism for the enhanced anxiety-like behavior stemming from chronic stress exposure. Nonetheless, it is important to note that the current findings do not discount the involvement of other pathways, for example those arising from the BLA to target regions not studied here, or inputs other than the BLA that innervate the PFC. One candidate is the ventral hippocampus, as it projects to both the BLA and PFC and may be involved in glutamatergic-based adaptations following chronic stress. In this context, there is growing support for the importance of ventral hippocampal inputs to the PFC and BLA in regulating various stress-related behaviors (Felix-Ortiz et al., 2016; Marek et al., 2018).
BLA outputs arise from glutamatergic pyramidal neurons and are under tight control of inhibitory interneurons. During environmental challenges, the dynamic range of BLA neuronal activity afforded by its functional organization shapes behavioral responses. For example, the activity of populations of BLA neurons is increased during the presentation of fear-associated cues to generate the adaptive behavioral response of freezing (Burgos-Robles et al., 2017; Courtin et al., 2014; Gunduz-Cinar et al., 2018; Likhtik et al., 2014). In this way, BLA activity, and consequent alterations in BLA output, may influence the selection of behavioral responses under threat. In turn, dysregulation of BLA activity and its outputs may drive a range of maladaptive behavioral responses to threat including those associated with heightened anxiety (Felix-Ortiz et al., 2016; Likhtik et al., 2014; Tye et al., 2011). This is consistent with our observation that stress differentially alters pre-synaptic glutamate release from BLA-PFC projections in an opposing manner in two inbred mouse strains with distinct baseline and stress-induced anxiety-like responses (Mozhui et al., 2010). In DBA2/J mice, chronic stress results in avoidance of potentially challenging situations and reduced glutamate release from BLA inputs to the PFC, whereas in C57BL6/J mice, stress results in hyperactive, possibly ‘panic-like’ patterns of behavior in anxiety-related tests and, in tandem, leads to exaggerated glutamate release from BLA-PFC projections. It must be noted that in the current study, C57BL6/J mice show patterns of hyperactive exploration but traditional patterns of anxiety-like behavior with respect to open arm aversion, which contrasts with previous reports in which these mice show hyperactivity that is accompanied by reduced open arm aversion (Mozhui et al., 2010). Therefore, it is possible that anxiety induced by optogenetic stimulation of BLA-PFC projections is qualitatively distinct from that which arises spontaneously or is induced by chronic stress exposure, perhaps due to the involvement of other circuits or the strength of optogenetic stimulation versus endogenous stimulation.
The strain-dependent effects of chronic stress on BLA-PFC glutamate release likely arises from distinct patterns of neuro-adaptations within the BLA of DBA2/J and C57BL6/J mice. We previously found that the same chronic stress regimen employed in the current study alters distinct sets of glutamate-signaling genes in each strain and facilitates metaplasticity at glutamatergic synapses in the BLA of DBA2/J, but not C57BL6/J mice (Mineur et al., 2006). Stress-induced dysregulation of BLA function that ultimately promotes increased output to the PFC could manifest in many ways, including via changes in the intrinsic excitability of BLA projection neurons, disrupted excitatory or inhibitory modulation of BLA projection neurons and alterations in mechanisms that regulate glutamate release locally at BLA terminals in the PFC. However, based on the current results, it is unlikely that stress alters BLA-PFC output through changes in the intrinsic excitability of BLA projection neurons, though this mechanism cannot be completely ruled out and has certainly been reported in the context of other stressors (Rau et al., 2015). Rather, previous work from our groups provides support for changes in modulation of BLA projection neurons. In C57BL6/J mice, chronic stress disrupts the balance between excitatory and inhibitory transmission in the BLA, and enhancing inhibitory transmission in this structure by activating GluK1 kainate receptors attenuates the effects of chronic stress on anxiety-like behavior (Masneuf et al., 2014). To extend these observations, further studies are now needed to determine if disruptions in the balance of excitatory and inhibitory transmission in the BLA also occur in the DBA2/J strain and if they are indeed the cause of altered (in the case, decreased) glutamate output to the PFC.
The BLA and PFC share reciprocal connections that intricately govern many behaviors related to emotion. A body of prior work untangling the anatomy of these connections suggest that glutamatergic inputs to the BLA synapse primarily onto PFC inhibitory interneurons, which in turn modulate the activity of PFC projection neurons (Dilgen et al., 2013; Marek et al., 2018; McGarry and Carter, 2016). PFC projection neurons provide ‘top-down’ modulation of many downstream structures throughout the brain, including the BLA, sculpting appropriate fear and anxiety-like responses based on current information about a given threat (Bukalo et al., 2015; Fitzgerald et al., 2015; Likhtik et al., 2014). However, in chronically stressed states, these carefully controlled circuit dynamics can be disrupted (Holmes and Wellman, 2009; Shepard and Coutellier, 2018).
Recent evidence suggests chronic stress enhances BLA activity, resulting in increased inhibitory modulation of the PFC (Shepard and Coutellier, 2018) and, consequently, decreased control of the PFC over the BLA. This could conceivably lead to ‘active’ maladaptive behavioral responses to threat, including the ‘panic-like’ anxiety seen in stressed C57BL/6J mice or the emergence of darting in females (Gruene et al., 2015). An interesting corollary possibility is that reduced glutamate release at BLA-PFC synapses in stressed DBA2/J mice has opposite effects on this circuit: with the net effect of enhancing PFC control over the BLA favoring ‘passive’ behavioral responses to threat, such as behavioral inhibition and avoidance. In line with this possibility, shifting the balance of excitation to inhibition in the PFC towards excitation reduces social interaction (Yizhar et al., 2011) and disinhibition of PFC neurons projecting to the BLA promotes freezing behavior (Courtin et al., 2014; Senn et al., 2014). However, as our experiments were not performed on a specific PFC cell type, it is difficult to determine whether our results reflect stress-induced dysregulation of glutamatergic modulation of PFC interneurons or projection neurons. This will be explored in future experiments.
In sum, we found that chronic stress exposure sufficient to enhance anxiety promotes adaptations in BLA activity that dysregulate glutamate release at BLA to PFC synapses. These effects of stress appear to manifest pre-synaptically and without apparent effects on post-synaptic glutamate transmission mediated by AMPA or NMDA receptors. Using inbred mouse strains with distinct stress-induced anxiety-like phenotypes, we found that chronic stress selectively enhances or reduces glutamate release specifically within the BLA to dmPFC circuit without altering glutamatergic modulation of other two other BLA projection targets, the BNST and NAcc. Finally, optogenetic excitation of the BLA-PFC circuit was sufficient to cause a persistent increase in anxiety-like behavior and hyperactivity in unstressed C57BL6/J mice. These findings add to converging evidence demonstrating that intricate interactions between the BLA and PFC govern behavioral responses to challenging situations, and suggest that significant dysregulation of glutamate release within this circuit may be a key mechanism by which chronic stress precipitates the development of anxiety disorders.
Acknowledgments
The authors have no conflicts of interest to disclose. This work was supported by the U. S. Department of Defense Award W81XWH-10-1-0999 (to TLK and AH; administered by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.), the National Institute on Alcohol Abuse and Alcoholism’s Intramural Research Program (AH), the Bowles Center for Alcohol Studies (TLK), and the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (NIH-NIAAA F32-022549 to EGLG and NIH-NIAAA F31-022280 to NAC).
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D. C: 2013. [Google Scholar]
- Bergstrom HC, Lipkin AM, Lieberman AG, Pinard CR, Gunduz-Cinar O, Brockway ET, Taylor WW, Nonaka M, Bukalo O, Wills TA, Rubio FJ, Li X, Pickens CL, Winder DG, Holmes A. Dorsolateral striatum engagement interferes with early discrimination learning. Cell Rep. 2018;23:1–9. doi: 10.1016/j.celrep.2018.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle N, Colacicco G, Busch E, Patel S, Singewald N, Holmes A. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv. 2015:1. doi: 10.1126/sciadv.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A, Tye KM. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci. 2017;20:824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso MJ, Kamens HM, Cavigelli SA. Exposure to chronic variable social stress during adolescence alters affect-related behaviors and adrenocortical activity in adult male and female inbred mice. Dev Psychobiol. 2017;59:679–687. doi: 10.1002/dev.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher EJ, Cross SJ, Belknap JK. Genetic determinants of sensitivity to diazepam in inbred mice. Behav Neurosci. 1998;112:668–677. doi: 10.1037//0735-7044.112.3.668. [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU. Anxiety disorders. Nat Rev Dis Primers. 2017;3:17024. doi: 10.1038/nrdp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berlin) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, Lowell BB, Whistler JL, Bruchas MR, Kash TL. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilgen J, Tejeda HA, O’Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol. 2013;110:221–229. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Pinard CR, Camp MC, Feyder M, Sah A, Bergstrom HC, Graybeal C, Liu Y, Schluter OM, Grant SG, Singewald N, Xu W, Holmes A. Durable fear memories require PSD-95. Mol Psychiatr. 2015;20:913. doi: 10.1038/mp.2015.44. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015:4. doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Brockway E, Lederle L, Wilcox T, Halladay LR, Ding Y, Oh H, Bushch EF, Kaugars K, Flynn S, Limoges A, Bukalo O, MacPherson KP, Masneuf S, Pinard C, Sibille E, Chesler EJ, Holmes A. Identification of a novel gene regulating amygdala-mediated fear exctinction. Mol Psychiatr. 2018 doi: 10.1038/s41380-017-0003-3. [DOI] [PMC free article] [PubMed]
- Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459:221–230. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology. 2012;62:464–473. doi: 10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Misslin R. Comparative study of emotional behaviour in three inbred strains of mice. Behav Process. 1999;47:161–174. doi: 10.1016/s0376-6357(99)00057-1. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, Holehonnur R, Ploski JE, Fitzgerald PJ, Lynagh T, Lynch JW, Maren S, Sah P. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci. 2018;21:384–392. doi: 10.1038/s41593-018-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, Crowley N, Flynn S, Holmes A, Kash T. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry LM, Carter AG. Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J Neurosci. 2016;36:9391–9406. doi: 10.1523/JNEUROSCI.0874-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One. 2010;5:e14458. doi: 10.1371/journal.pone.0014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety-and depression-related phenotypes in different mouse strains. Neurosci Bio-behav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto SM, Silveira R, Coimbra NC, Joca SR, Guimaraes FS. Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog Neuro-Psychopharmacol Biol Psychiatr. 2002;26:1135–1141. doi: 10.1016/s0278-5846(02)00248-8. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berlin) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J Neurosci. 2015;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Shepard R, Coutellier L. Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol Neurobiol. 2018;55:2591–2602. doi: 10.1007/s12035-017-0528-0. [DOI] [PubMed] [Google Scholar]
- Singh SM, Treadwell J, Kleiber ML, Harrison M, Uddin RK. Analysis of behavior using genetical genomics in mice as a model: from alcohol preferences to gene expression differences. Genome. 2007;50:877–897. doi: 10.1139/g06-118. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyper-locomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16:171–180. doi: 10.1097/00008877-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Szklarczyk K, Korostynski M, Golda S, Solecki W, Przewlocki R. Genotype-dependent consequences of traumatic stress in four inbred mouse strains. Gene Brain Behav. 2012;11:977–985. doi: 10.1111/j.1601-183X.2012.00850.x. [DOI] [PubMed] [Google Scholar]
- Thoeringer CK, Sillaber I, Roedel A, Erhardt A, Mueller MB, Ohl F, Holsboer F, Keck ME. The temporal dynamics of intrahippocampal corticosterone in response to stress-related stimuli with different emotional and physical load: an in vivo microdialysis study in C57BL/6 and DBA/2 inbred mice. Psychoneuroendocrinology. 2007;32:746–757. doi: 10.1016/j.psyneuen.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandao ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]