Abstract
People in the early stages of Alzheimer’s disease and related dementias (ADRD) are encouraged to engage in advance care planning (ACP) while they are still competent to appoint a surrogate decision maker and meaningfully participate in ACP discussions with the surrogate. In this NIH Stage I behavioral intervention development trial, we will adapt and test an efficacious ACP intervention, SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), with people with mild dementia and their surrogates to promote open, honest discussions while such discussions about end-of-life care are possible. We will first adapt SPIRIT (in person) to target people with mild dementia and their surrogates through a process of modification-pretesting-refinement using stakeholders (persons with mild dementia, family caregivers, and clinicians) and experts, including adapting the delivery mode to interactive web-based videoconference format (SPIRIT-remote). Then in a 3-group RCT with 120 patient-surrogate dyads, we will evaluate the feasibility and acceptability of SPIRIT in-person and SPIRIT remote, and preliminary efficacy of SPIRIT compared to usual care on preparedness outcomes for end-of-life decision making (dyad congruence on goals of care, patient decisional conflict, and surrogate decision-making confidence) shortly after the intervention. This Stage I research of SPIRIT will generate valuable insights regarding how to improve ACP for people with mild dementia who will progress to an advanced stage of the disease in the foreseeable future.
Keywords: Alzheimer’s disease, dementia, advance care planning, end-of-life care, randomized trial
INTRODUCTION
Alzheimer's disease and related dementias (ADRD), affects over 5 million individuals in the US [1]. ADRD cannot be prevented and is incurable. Progressive memory loss and impaired reasoning and judgment are its main symptoms [2]. For this reason, people in the early stages of ADRD are encouraged to engage in advance care planning (ACP) while they are still competent to appoint a surrogate decision maker and meaningfully participate in ACP discussions with that surrogate [3, 4]. Yet only a minority (39%) of older adults with early cognitive impairment complete any form of ACP following their diagnosis [5].
The failure to engage in ACP before the window of opportunity closes (i.e., before loss of decision making capacity) has serious adverse consequences and exerts the greatest impact on the surrogate. As a matter of course in ADRD, family members are left to make decisions regarding care transition, tube feeding, and other life-sustaining treatments without input from the person and in the absence of a full understanding of the wishes, values and preferences of the person [6–8]. Unfortunately, the culture of a technological imperative to deliver aggressive or futile medical care to very frail older adults drives end-of-life decision making especially when there has been no ACP [9, 10].
To promote open, honest end-of-life care discussions when they are still possible, we will work with persons with mild dementia and their surrogates to adapt and evaluate an efficacious ACP intervention, SPIRIT (Sharing Patient’s Illness Representations to Increase Trust; NIH Stage I behavioral intervention development [11]). SPIRIT is a brief, scalable patient- and family-centered ACP counseling intervention based on the Representational Approach to Patient Education [12, 13]. Its goal is to promote cognitive and emotional preparation for end-of-life decision making for patients with a serious or life-threatening illness and their surrogates. SPIRIT focuses on having both the person with a serious illness and the surrogate fully understand end-of-life decision making in anticipation of a loss of decision-making capacity.
To produce an effective and maximally implementable ACP intervention for people with early stage dementia and their surrogates, we will first adapt SPIRIT in-person to target people with mild dementia and their surrogates through a process of modification, pretesting, and refinement using stakeholders (people with mild dementia, family caregivers, and clinicians) and experts, including adapting the delivery mode to interactive web-based videoconferencing, SPIRIT remote (Aim 1). SPIRIT is a counseling intervention conducted with the patient and surrogate together to promote authentic dialogue between them (not just to complete an advance directive document). To date, SPIRIT has been tested only in the face-to-face in-person modality. We will develop and test SPIRIT remote using interactive web-based videoconferencing with the goal of providing an efficacious ACP intervention to hard-to-reach populations without compromising the intervention integrity.
Then, in a 3-group randomized controlled trial (RCT) with 120 patient-surrogate dyads, we will evaluate the feasibility and acceptability of SPIRIT in-person and SPIRIT remote, and test their effects compared to usual care (wait-list control) on preparedness outcomes for end-of-life decision making (Aim 2). Our secondary aims are to compare the completion of advance directives among the three treatment conditions at 1-year post-intervention; and using qualitative interviews, in a sample of surrogates from each group, explore the perceived impact of the treatment condition at 1-year post-intervention on preparation for end-of-life decision making.
METHODS
Study design overview
This study is NIH Stage I intervention development research [11] that includes two stages: Stage 1A is to adapt, pretest, and refine SPIRIT, and Stage 1B is to rigorously evaluate the refined SPIRIT’s feasibility/acceptability, and efficacy.
SPIRIT Intervention
SPIRIT is based on the Representational Approach to Patient Education [12, 13]. This approach melds two theories: Leventhal’s common sense model [14] and the conceptual change model [15]. The common sense model proposes that individuals have representations of their illness or health problems. Representations are based on an individual’s experiences, cultural traditions, or media and may not be medically accurate. It is critical to understand a patient’s representations because they filter new learning: representations serve as the cognitive framework that affects whether or not individuals accept or reject new information [12], and whether knowledge leads to behavior change [16, 17], The conceptual change model proposes that the likelihood of learning increases when the individual has an opportunity to reflect and comment on current ideas when the individual is dissatisfied with current ideas or recognizes their limitations, and when alternative information is seen as beneficial [12, 13, 15, 18]. Learning and change can occur through integrating new information into existing representations to fill gaps in understanding, by clarifying existing representations to reduce confusion, or through replacing existing ideas with new information [18, 19]. The scientific premise for this study is that the Representational Approach to Patient Education requires an interventionist to elicit the patient’s existing illness representations before providing new information [12, 13]. Then, the interventionist, the patient, and his/her surrogate have an opportunity to recognize gaps or confusions, and the interventionist can give new information that is specific and relevant, increasing the likelihood that it will be acted upon.
SPIRIT is a two-session, 60-minute, structured psychoeducational intervention, targeting both patient and surrogate. SPIRIT was developed by our team and extensively evaluated in patients with end-stage renal disease, advanced heart failure, and cardiac surgical patients and their surrogates [20–25]. Using a structured intervention guide, the interventionist follows six steps: 1) assess illness representations, 2) identify gaps and concerns, 3) create conditions for conceptual change, 4) introduce replacement information, 5) summarize, and 6) set goals and plan [26].
In its current form, all sessions of SPIRIT are conducted in a private room in a clinic and follow the structured SPIRIT Interview Guide. The goals of SPIRIT are to assist patients to clarify their end-of-life preferences and to help surrogates understand the patient’s wishes and prepare for the surrogate role. Currently SPIRIT has two face-to-face sessions with patient and surrogate together. During the first session (~45 min.), an interventionist assesses the patient’s and surrogate’s cognitive, emotional, and spiritual/religious representations of the patient’s illness, progression, and end-of-life care. This allows the interventionist to provide individualized information about topics, such as the effectiveness of life-sustaining treatment for people with progressive chronic illness (to be adapted for dementia) and assist the person with dementia to examine his/her values about life-sustaining treatment at the end of life. The interventionist also helps the surrogate prepare for end-of-life decision-making and for the emotional burden of decision-making by actively involving the surrogate in the discussion. If the surrogate is someone out of the order of the hierarchical compensatory model [27] (e.g., a sibling is chosen instead of a spouse or child), the interventionist explores potential family conflicts and encourages the dyad to talk with other family members and complete a medical power of attorney. A Goals-of-Care tool is completed at the end of the session to indicate the patient’s preferences.
A brief second session (~15 min.) is conducted about 2 weeks later to address remaining or new concerns and questions raised after the first session. The interventionist documents the patient’s end-of-life preferences and the surrogate’s name and relationship to the patient in the medical record.
Stage 1A: SPIRIT adaptation, pretesting, and refinement
Guided by Stirman’s framework for adaptations of evidence-based interventions [28], we have identified modifications that are needed to target persons with mild dementia (See Table 1). Two investigators (MS and SW) will draft the initial content adaptations of the SPIRIT Interview Guide and work iteratively with the rest of the team members to complete the initial modifications.
Table 1.
Planned Adaptations of SPIRIT
| Modification type | Nature of modification |
|---|---|
| Format/modality | Virtual face-to-face via web-based videoconferencing for SPIRIT remote |
| Setting | Home for SPIRIT remote |
| Content | Tailoring illness trajectory discussion to dementia |
| Tailoring the likely situations requiring end-of-life decision making and types of end-of-life treatment relevant to dementia | |
| Delivery process | Integrating “enhanced consent techniques” [56], such as reducing information load by proceeding in manageable segments/chunks, offering repetition of material, opportunity for rehearsal, and use of targeted questions to verify comprehension prior to eliciting preferences for goals of care [57–59]. |
| Interventionist training and evaluation | Integrating “enhanced consent techniques” Adding use of videoconferencing for SPIRIT remote |
Formative review by a panel of clinicians and content experts
After the initial modification, we will invite a panel of experts (N=5–7) who have not been involved in the development of SPIRIT and who have expertise in the theoretical underpinnings of SPIRIT, advance care planning, dementia, and ACP to provide written feedback on the modified SPIRIT Interview Guide. The panel will review the extent to which the SPIRIT Interview Guide (prescribed activities and interview questions) is relevant to the theoretical core elements and likely to be effective in achieving the intervention purpose (ACP goals) in this new population (persons with mild dementia and their surrogates) [29]. Panel members will rate: (a) the extent to which each activity is pertinent to the intended theoretical element (relevance), (b) the likelihood that each activity will successfully address the intended intervention goal (likely effectiveness), and (c) the extent to which language, nature of activities, interview questions, and enhanced consent techniques are appropriate for the population (appropriateness), on 4-point scales (e.g., 1=irrelevant, 4=essential). The panel will also be asked to comment on the activities and interview questions and to suggest content areas that have been omitted but should be included.
Analyzing review results and refining the intervention
This process will involve both quantitative indicators and the investigative team’s careful judgment. We will compute the Content Validity Index (CVI; 0–1.0) as a quantitative indicator of acceptable content validity at the individual intervention activity level. Intervention activities and interview questions with a CVI < .78 (“excellent” CVI for intervention activities [29]) will be reviewed for the nature of the problem; e.g., if an activity is rated as not relevant to its intended target, the investigative team may consider undertaking a major revision or removing it. If an activity is rated as relevant but not likely to be effective, the team will consider if it can be altered to increase its likely effectiveness. Narrative comments from the panel and follow-up interviews with panel members will be utilized to hone the intervention.
SPIRIT remote
We will adapt SPIRIT to a videoconference format so that patients and surrogates can receive the intervention in their home. We anticipate SPIRIT delivery via videoconferencing will require minimal training of the interventionist and instructions for participants. The equipment needed for videoconferencing includes a computer, a webcam, a headset, a microphone (if not already present in the computer), and the Internet. We will use Zoom, a videoconferencing platform supported in Window, Mac, Linux, and other virtual desktop environments. Zoom also includes a recording module that is consistent with HIPAA security requirements. We chose Zoom because its technology enables high definition, low latency, and is error resilient.
We will send the dyads randomized to SPIRIT remote the necessary equipment (e.g., webcam, two-way earphone) if they do not already have it. The patient and surrogate will need to be in the same room to participate in SPIRIT remote session. Detailed instructions about how to set up the equipment, room setting, a link to Zoom, and call number will be sent by email 1–2 weeks before the scheduled SPIRIT session. A research assistant will call the surrogate to walk through the instructions and problem solve. An observer will sit with the SPIRIT interventionist in every SPIRIT remote session to document and problem solve any technical issues.
Pretesting of SPIRIT in-person, SPIRIT remote, and refinement
Pretesting will involve 20 dyads of patients with mild dementia and their surrogates recruited from a Memory Care Clinic at Emory. Inclusion criteria for patients are: (a) a diagnosis of dementia; (b) a CDR score ≤1 (mild dementia); (c) a recent MoCA score ≥ 13 or MMSE score ≥ 18 (a threshold for ACP [30–33]); (d) have a computer and internet connectivity at home; and (e) have decision-making capacity to consent to a low-risk study (risks only of inconvenience, emotional discomfort or fatigue) determined by a score >14.5 on the University of California San Diego Brief Assessment of Capacity to Consent (UBACC) [34]. The 10-item UBACC screens for decision-making capacity (each item score ranges 0–2), requires less than 5 minutes to administer, and has been validated in people with ADRD [35].
A study invitation letter signed by the clinic director and principal investigator will be mailed to patients meeting the criteria a) through c). This letter will include an opt-out postcard. After 2 weeks, a research staff member will call potential participants who have not returned the opt-out postcard to explain the study and schedule a brief meeting at the clinic during their upcoming return clinic visit. Because nearly all patients with dementia visit the center with a family member, potential surrogates should be easily identifiable. However, to make sure those family members are the persons who are likely to be involved in treatment decision-making as the patient’s condition progresses, we will use a short investigator-developed set of questions [42], a 2-minute Surrogate Selection Guide used in our previous studies. Surrogate eligibility criteria include: (a) 18 years or older (to serve as a surrogate decision-maker, the individual must be an adult); (b) chosen by the patient; (c) have access to a computer and internet connectivity in a private setting, e.g., either the patient’s or the surrogate’s home and being able to use email (to receive URL links to Zoom); and (d) able to understand and speak English. Patient-surrogate dyads without videoconference capability via a home computer or tablet will not be eligible for this study, but we will obtain descriptive statistics from them for comparison purposes and in anticipation of determining the real-world applicability of SPIRIT.
After the willing patients and their family caregiver have checked in at the clinic on the day of their visit, a recruiter will approach them to explain the study purposes and procedures in detail. If they are interested in participation, the recruiter will administer the UBACC to screen the patient for decision- making capacity. With a patient whose UBACC score is higher than 14.5, the recruiter will determine whether the person accompanying the patient is an appropriate surrogate decision-maker using the Surrogate Selection Guide. Written consent will be obtained from each member of the dyad. Upon their consent, baseline assessment, the SPIRIT session, and post-intervention assessment will be scheduled. Each member of the dyad will receive a $30 gift card at completion of the study.
The first 10 dyads will be randomly assigned to SPIRIT in-person or to SPIRIT remote. Dyads will be assigned using a randomization sequence created in SAS 9.4 with a 1:1 allocation using random block sizes of 2 to ensure balance between the two arms. One interventionist trained for both modalities will conduct all sessions. In-person sessions will be conducted in a private room in the clinic. For remote sessions, dyads will participate from their home, and the interventionist will use a videoconferencing workstation in a private room in the School of Nursing. All sessions will be audio-recorded and reviewed by the principal investigator for fidelity assessment. After completion of each session the interventionist will make field notes to document what did or did not go well, reasons for interruptions or difficulties, and contextual factors that cannot be captured in the audio-recording.
Post-intervention assessment (2–3 days post intervention): A research assistant (who is not the interventionist) will call the dyad to complete questionnaires assessing the dyad’s preparedness for end-of-life decision making. Each person will provide his/her answers in private. As done successfully in our previous studies with seriously ill patients, the research assistant will ask the survey items in segments, offer repetition, offer the item response options in segments and verify adequate comprehension (enhanced consent techniques). After the outcome assessment, the research assistant will conduct a brief semi-structured interview (10–15 minutes) with each member of the dyad regarding: the overall experience with SPIRIT; any facets that the participant found helpful/not helpful and the reasons; pacing, length, and modality; and suggestions for improvement. Interviews will be audio-recorded and transcribed.
Analyzing feasibility data and patient/surrogate input: The interview transcripts will be transferred to ATLAS.ti for analysis. Traditional content analysis techniques [36] will be used without preconceived categories [37]. Based on the results from the content analysis of the patient and surrogate interviews and field notes, the investigative team will identify areas of the intervention that are consistently identified as problematic and thus require immediate modifications. The team will refine the intervention before the next set of pretesting.
The second 10 dyads will be randomly assigned to either SPIRIT in-person or SPIRIT remote to evaluate the revised or refined areas of the intervention. The same procedures used for the first 10 dyads will be performed (unless any procedural adjustments have been made based on the initial pretesting). Using the same analytic approach, the team will make final adjustments to the intervention as needed.
Stage 1B: Evaluation of the feasibility/acceptability, and efficacy
Study design and rationale
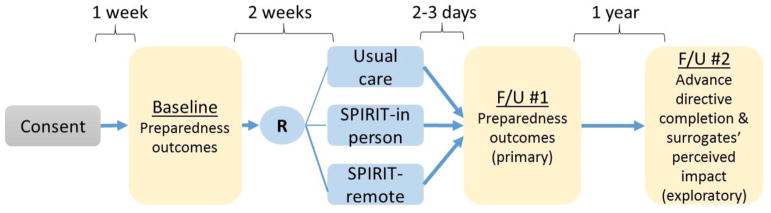
We will conduct an RCT with three groups: SPIRIT in-person, SPIRIT remote, and usual care (wait-list control). We will recruit 120 dyads of patients with early stage dementia and their surrogates. The groups will be stratified by race (white vs non-white). Both SPIRIT in-person and SPIRIT remote will be delivered by trained interventionists. The primary outcomes are patient and surrogate self-reported preparedness for end-of-life decision making. These will be measured at baseline and shortly after the intervention (by phone in the next 2–3 days). Additionally, we will compare 1) the completion rates of advance directives among the three groups, and 2) surrogate perception of the impact of the treatment conditions at 1 year post intervention. The perceived impact will be assessed using semi-structured telephone interviews with surrogates of patients who have progressed to moderate dementia (severely impaired cognitive and daily functioning; a CDR score=2; anticipate 22% based on previous research [38–41], which will be ~26 surrogates) and 20% randomly selected surrogates of patients who have remained in the same stage at 1 year (~18–20 surrogates).
Setting and participants
A total of 120 patient-surrogate dyads will be recruited from the Alzheimer’s Disease Research Center at Emory and Emory Geriatrics Clinics. Patient eligibility criteria include: (a) diagnosed with dementia; (b) a CDR score ≤1 (mild dementia); (c) a MoCA score ≥13 or a MMSE score ≥18; (d) able to understand and speak English; and (e) a UBACC score > 14.5. There will be no age limitation, but nearly all patient participants will likely be 60 or older. Exclusion criteria are (a) lack of an available surrogate, (b) uncompensated hearing deficits, and (c) already has an advance directive (determined by the patient’s medical record and confirmed by the surrogate).
Recruitment and consent procedures
The recruitment process will be similar to that of Stage 1A study. After the willing patients and their family caregiver have checked in at the clinic on the day of their visit, a recruiter will approach dyads to explain the study purposes and procedures in detail. If they are interested in participation, the recruiter will administer the UBACC to screen the patient for decision-making capacity. With a patient whose UBACC score is higher than 14.5, the recruiter will determine whether the person accompanying the patient is an appropriate surrogate decision-maker using the Surrogate Selection Guide. Written consent will be obtained from each member of the dyad.
Randomization
The statistician will generate a randomization scheme using stratified (by race), permuted block randomization with block size 6, using a random-number generator. Dyads will be randomized with equal allocation (1:1:1) to SPIRIT in-person, SPIRIT remote, or usual care. Due to the nature of the intervention, blinding dyads to their group allocation is impossible, but the research staff assessing outcomes will be blind. Immediately after the completion of the baseline assessment by phone, the data collector will open a sealed envelope to identify group assignment and schedule an intervention session to take place ~2 weeks hence for SPIRIT in-person or SPIRIT remote, as well as a follow-up data collection session in the next 2–3 days. Any equipment needed for Skype set up will be shipped if a dyad is assigned to SPIRIT remote.
Comparison condition: Usual care
At the ADRC clinic, an advanced practice nurse provides written information on advance directives to a patient and his/her family caregiver at the diagnosis of a dementing illness, reviews this information, and encourages them to complete one. This typically takes 10 minutes. Patients and their family members may be referred to attorneys who can assist them in completing an advance directive. If completed, the presence of an advanced directive is documented in the electronic medical record and a copy of the document is scanned to the electronic chart. Patients and families may be referred to a support group organized by a social worker in which legal and financial issues are discussed with a lawyer. At the Emory geriatric clinics, physicians or advanced practice providers conduct advance care planning discussion with the patient and family member during an annual wellness visit to explain about advance directives, including completion of those forms. These discussions take about 30 minutes. We will review the patient’s medical records at baseline and biannually to track usual care.
Interventionist training for SPIRIT delivery
Interventionist minimal qualification is having at least 2 years of clinical experience in caring for people with dementia as a nurse (RN or APRN) or social worker. Interventionist training will consist of a 3½–day competency-based program used in our previous trials using training manuals. Module 1 (1 day) focuses on understanding ADRD and end-of-life care issues, communication as key to improving end-of-life care, and the Representational Approach (theoretical underpinnings of SPIRIT); Module 2 (1 day) is a skill-based session on delivery of the SPIRIT intervention (e.g., communication behaviors and enhanced consent techniques), including role plays; Module 3 (1/2 day) focuses on videoconferencing procedures and etiquette, features of Zoom, and handling technical problems. A 2-week practice period is then scheduled for integration of skills. Module 4 (1 day) involves skill-demonstration and certification.
Intervention fidelity and monitoring
To promote consistency and quality of intervention delivery, the SPIRIT Interview Guide will be used during each session. To monitor fidelity, we will use two data sources. The SPIRIT Interview Guide will direct the interventionist to document performance data after each patient-surrogate dyad encounter; the Guide has a checklist of SPIRIT components, including start and finish times, and brief self-evaluation. As in our previous studies, all intervention sessions will be audio-recorded. Every 2 months, 50% of sessions randomly selected from SPIRIT in-person and SPIRIT remote will be reviewed by the PI (approximately 2 sessions from each group). Using the modified Treatment Fidelity Assessment Tool, the interventionist’s adherence to intervention content, process, and duration will be evaluated on a 3-point scale (1=appropriate, 3=skipped). Problems detected including drift from protocol will be discussed with the interventionist and re-training will be provided if adherence is < 80% based on the Fidelity Assessment Tool.
Participant retention
The following strategies will be used to maximize participant retention: (a) obtain backup contact information, (b) make confirmation phone calls 2 days prior to each appointment, (c) make scripted monthly check-in calls, (d) send holiday and special occasion cards, (e) assign the same data collector whenever possible, (f) compensate each member of the dyad as a token of appreciation ($20 at baseline, $25 at post-intervention assessment) and surrogates who complete the 1-year follow- up interview ($30 at 12 months); and (g) use a cell phone matched to the participant’s wireless network provider whenever possible (so that their minutes do not run out).
Data collection and measures
Feasibility
Feasibility of recruiting and retaining patients with mild dementia and surrogates for SPIRIT in-person and SPIRIT remote will be assessed by tracking the numbers of dyads who are eligible and who agree to participate, and reasons for refusal and withdrawals. Feasibility of conducting SPIRIT will be assessed by tracking the number of dyads who complete their session, the number of incomplete or interrupted sessions, and the minutes required to complete all sessions. Of particular relevance to generalizability, we will also explore whether these variables are associated with patients’ MoCA or MMSE scores.
Acceptability
Patient and surrogate acceptability will be assessed using the 10-item ACP Acceptability Questionnaire developed from our previous trial.24 Participants are asked how strongly they agree or disagree (4 to 1) with statements about their experience with SPIRIT sessions, including duration, interactions with the interventionist, level of comfort and satisfaction. Higher scores indicate greater acceptability. Each patient and surrogate will complete this survey following the preparedness outcome measures after the intervention.
Preparedness for end-of-life decision making (measured at baseline and 2–3 days post intervention)
Dyad congruence will be assessed using the Goals-of-Care Tool [21, 23], which has been modified to include two scenarios relevant to the context of ADRD. In the first, the patient has progressed to advanced dementia and develops a severe infection and is admitted to a hospital; the medical team believes recovery unlikely and continuing life-sustaining treatment would no longer be beneficial. There are three response options: “The goals of care should focus on delaying my death no matter what, and thus I want to continue life-sustaining treatment”, “The goals of care should focus on my comfort and peace, and thus I do not want life-sustaining treatment”, and “I am not sure.” In the second scenario, the patient has progressed to advanced dementia and develops a severe infection. The nursing home staff is asking whether the patient should be taken to an ED, which will lead to hospitalization with life-sustaining treatments. Patients and surrogates complete this tool independently and their responses are then compared to determine dyad congruence -- either congruent in both scenarios or incongruent. If both members of the dyad endorse “I am not sure,” they are considered incongruent.
Patient decisional conflict will be measured using the 13-item Decisional Conflict Scale (DCS), a validated measure in the context of end-of-life decision making [20]; higher scores indicate greater difficulty in weighing benefits and burdens of life-sustaining treatments and decision making (range 1–5; Cronbach’s α = 0.8–.93 [20, 21, 23, 43]).
Surrogate decision-making confidence will be measured using the 5-item Decision Making Confidence (DMC) scale (Cronbach’s α = 0.81–0.90 [21, 44]); higher scores reflect greater comfort in performing as a surrogate (0=“not confident at all” to 4=“very confident”). DMC assesses a surrogate’s confidence in: knowing the patient’s wishes, ability to make treatment decisions even in a highly stressful situation, ability to seek information about risks and benefits of medical choices, ability to handle unwanted pressure from others, and ability to communicate with providers about the patient’s wishes.
Completion of advance directives (at 12 months)
A research assistant will review the patient’s medical record to determine if the patient has completed an advance directive (a medical power of attorney or living will) by 12 months. If there is no documentation, the research assistant will call the surrogate to confirm.
Surrogates’ perceived impact of SPIRIT (at 12 months)
Approximately 44 surrogates will participate in a semi-structured interview by phone: approximately 26 surrogates of patients who have progressed to an advanced stage (CDR score > 1; 22% progression rate [38–41]) and 20% randomly selected surrogates (stratified by SPIRIT modality) of patients who have not progressed (~18–20 surrogates). A research assistant will review medical records to identify if a patient’s dementia has progressed. Staging is well documented for dementia patients (they are followed at least every six months) and thus is easily identifiable.
A trained interviewer/research assistant will conduct the interview using the Perceived Impact Interview Guide, which includes questions about surrogates’ experiences with the treatment condition, the perceived impact of the treatment condition on their loved ones and themselves, and what they found most and least helpful and why. This 15–30 minute interview will be audio-recorded and transcribed.
Descriptors and potential covariates (collected at baseline)
Patients and surrogates will each complete a Sociodemographic Profile which includes demographic information and previous end-of-life decision-making experience. Patients’ clinical characteristics, (date of dementia diagnosis, CDR score, MoCA or MMSE scores, and comorbid conditions) will be abstracted from the patient’s medical records.
Data analysis plan
Analyses will be intention to treat with all available data from all participants. Preliminary analyses will include summarizing variables with descriptive statistics. Distributional assumptions will be assessed, and the data transformed as necessary. Baseline characteristics will be examined to explore possible between-group differences using analysis of variance and chi-square tests as appropriate. We will investigate missing data with pattern analysis for data missing at random or missing not at random and use maximum likelihood or multiple imputation appropriate for each type to impute missing values. We will conduct sensitivity analyses to encompass different scenarios of assumptions and evaluate consistency or discrepancy among them.
Feasibility and acceptability
Data on feasibility and acceptability will be summarized using descriptive statistics (%, means [SDs], and 95% CIs). For feasibility, data on recruitment, retention, and completion of SPIRIT sessions will be compared to those from previous SPIRIT trials; e.g., dropouts < 4% (seen in our efficacy trial [45]) will be considered successful retention. SPIRIT will be deemed acceptable to patients and surrogates if >75% of responses exceed an average score ≥3 (of 4) on the acceptability measures.
Preliminary efficacy
SPIRIT effects on the preparedness outcomes
For analysis of dyad congruence, a binary outcome (congruent or not congruent), a generalized mixed effects logistic regression model will be used. A random intercept model will be used to account for dyad level variation over time while treatment group, time (baseline and post-intervention), and treatment x time interaction will be treated as fixed effects. If there is a significant treatment x time interaction (α<0.05) (an overall treatment effect), then two contrasts will be tested for individual treatment effectiveness (usual care vs SPIRIT in-person and usual care vs SPIRIT remote). Adjustments for multiple pairwise comparisons will be made using Tukey’s test. For patient DCS and surrogate DMC scores, we will use linear mixed effects regression models, adjusting for potential covariates as appropriate.
SPIRIT effect on advance directive completion (secondary aim)
For this binary outcome (completed or not completed) measured post intervention, the probability of completion will be estimated for the three groups using logistic regression. Baseline characteristics that differ by group will be adjusted. Adjusted mean completion proportions will be compared between groups; Tukey’s test will adjust for multiple comparisons.
Surrogates’ perceived impact (secondary aim)
As in our previous work [46], qualitative analysis will use content and thematic techniques [47]. Initial coding involves line by line examination, labeling, and organizing of data into segments, preserving detail in participants’ words [48]. To optimize validity, codes and definitions will be reviewed and refined by the research team and applied to subsequent interviews [49]. Related codes will be grouped into categories representing aspects of the surrogates’ experiences. Similarities, differences, and trends across cases will be examined [50]. Then, data will be organized into themes. Discrepancies will be resolved by consensus. To explore differences in themes by dementia progression and by treatment condition, we will count the occurrence of themes (“quantitizing”) [51, 52]; the occurrence of each theme will be counted only once for a participant even if it is mentioned more than once. The data will be graphed to facilitate pattern interpretation [53].
Power calculation and sample size justification
Our sample size of 40 dyads per group can detect preliminary efficacy of SPIRIT in-person or SPIRIT remote (compared to usual care) on two preparedness outcomes based on effect sizes of the SPIRIT intervention in our previous studies [20–23, 25]. For dyad congruence, we observed large effect sizes (OR=4.4–8.7) at 1 or 2 weeks post intervention [21, 25], which would require only 10–20 dyads/group to achieve over 80% power. For patient DCS, a moderate treatment effect (Cohen’s d=.53) was observed [25], which would require 21 patients/group to achieve 80% power. Thus, with a sample size of 40 dyads per group, power to detect similar effect sizes of dyad congruence and patient DCS is excellent (>90%) after accounting for attrition and any unbalances in baseline characteristics. For surrogate DMC, a significant treatment effect was observed at 2 months post intervention [23], but at short-term (2 weeks), the effect size was negligible (Cohen’s d=.02) [25]. Therefore, we plan to carefully investigate trends and obtain an estimate of the effect size for the ADRD population instead of solely focusing on hypothesis testing.
DISCUSSION
In this paper, we have described the study design and methods of an NIH Stage I behavioral intervention development trial of SPIRIT. Stage I behavioral intervention development research can include the generation of a new behavioral intervention as well as the modification, adaptation, or refinement of an existing intervention (Stage IA), and it culminates in feasibility and pilot testing (Stage 1B) [11]. SPIRIT has been developed and tested in three other patient populations [20–25], advanced kidney disease, heart failure, and cardiac surgery. While SPIRIT is likely to be applicable to persons with mild dementia, such an application has never been studied. Therefore, this Stage I behavioral developmental research study will a) modify SPIRIT for individuals with mild dementia and their surrogates, and b) rigorously test its feasibility and demonstrate the hypothesized effect.
These are necessary and highly important steps because the population of persons with mild dementia is distinctly different from the populations in which SPIRIT has been tested and validated. Specifically, mild dementia itself is not life-threatening but persons with this diagnosis will soon lose the ability to advocate for themselves with respect to end-of-life care. In fact, it is customary to exclude people who have been diagnosed with dementia from clinical trials precisely because they may have impaired decision-making capacity. It is possible that SPIRIT may be more emotionally taxing for this population than it is for others with advanced chronic illnesses, or they may not even want to participate in the SPIRIT trial. It is also possible that the SPIRIT interventionist may find it more challenging to conduct SPIRIT sessions with people with early stage of dementia and their surrogates than has been the case when they conduct sessions with persons who are more physically ill and likely closer to end of life. These possibilities necessitate a Stage I study of SPIRIT with this new population.
As in our previous studies of SPIRIT, we chose individual randomization because intervention spillover to the control condition is very unlikely. The intervention will be delivered by a trained interventionist, not a care provider, in a private room at the clinic or via videoconferencing at home, and thus it is nearly impossible for care providers to obtain the knowledge and skill related to SPIRIT to change their ACP practice. We chose race (white vs non-white) as a stratification factor to ensure equal allocation of race to each treatment condition to control for race as a confounding variable [54].
The most challenging aspects of designing this intervention trial were: a) determining an optimal follow-up time point and data collection mode to minimize the potential influence of patients’ cognitive impairment on the outcome assessment, and b) maintaining blinding of data collectors. The first follow-up time point (2–3 days post intervention) is to evaluate the impact of SPIRIT on preparedness outcomes while minimizing the potential influence of the patient’s impaired ability to recall what was discussed during the SPIRIT session (i.e., the patient needs to recall what he/she clarified as goals-of-care preferences). We considered measuring the outcomes immediately following the intervention, but this would preclude blinding the data collector. The second follow-up time (12 months post intervention) was chosen to maximize the number of patients whose conditions might progress to an advanced stage within the study period so that we can explore how surrogates experience having or not having an in-depth ACP discussion before the window of opportunity has closed. Although not ideal, telephone-based data collection was chosen to assure blinding of data collectors and to reduce participants’ travel burden.
As in our previous studies, we chose usual care as a comparison condition rather than an attention placebo control group for two reasons. First, currently there are no methodological standards for constructing attention placebo controls in trials of psychoeducational interventions [55]. Second, in the context of preparing for future medical care and end-of- life decision making, an attention placebo group (information and discussion irrelevant to the context) would not meet the participants’ expectations or motivation to participate in the study, and could cause a high refusal rate, dissatisfaction, and disproportional dropouts [55]. We will offer either SPIRIT in-person or SPIRIT remote per preference to dyads in the control group at the completion of the 1-year follow-up if the patient has not progressed to moderate dementia. The same approach was used in our previous efficacy trial with 12-month follow-ups, and a wait-list control did not result in disproportional dropouts (SPIRIT [5.5%] vs usual care [2.0%]) [23].
Although it is not ideal, in the context of mild dementia, we will focus on short-term preparedness outcomes, rather than on actual end-of-life outcomes such as post-bereavement distress for surrogates, because such efforts are not feasible in this 5-year study due to the protracted nature of dementia trajectories (i.e., death is not imminent). Instead, we will evaluate whether SPIRIT in-person and SPIRIT remote result in an embodiment of the patient’s wishes (i.e., advance directives) by 1-year post intervention as an exploratory aim. In another exploratory aim, we will interview a sample of surrogates at 1 year to assess the perceived impact of the intervention conditions.
The SPIRIT intervention has the potential to serve as a foundation to help family members navigate the decision- making journey as a patient progresses to an advanced stage. It can facilitate open discussions about the trajectory of dementia, offer a deeper understanding about the patient’s values, goals of care, and possible future treatment choices. Testing SPIRIT with people with mild dementia and their surrogates can be used as a model for conducting ACP discussions with all types of dementia regardless of etiology or course. SPIRIT tested in this population will be generalizable to other neurodegenerative disorders in which there is initial mild cognitive impairment followed by progressive dementia. We believe this Stage I behavioral intervention development trial of SPIRIT will generate new insights to fill the critical void of knowledge regarding how to improve ACP for people with mild dementia. As these individuals will inexorably progress to advanced dementia they will likely require complex medical care for some time into the near future.
Figure 1.
Stage 1B Study Design
Acknowledgments
Funding source: This work is supported by NIH/NIA 1R01AG057714-01 (PI, Song), P30 AG101061 (PI, Bennett)
Footnotes
Trial registration: ClinicalTrials.gov NCT03311711, Registered 10/12/2017
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61(6):1–51. [PubMed] [Google Scholar]
- 2.McKhann GM, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay SY, et al. Education and Executive Function Mediate Engagement in Advance Care Planning in Early Cognitive Impairment. J Am Med Dir Assoc. 2015;16(11):957–62. doi: 10.1016/j.jamda.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Cheong K, et al. Advance care planning in people with early cognitive impairment. BMJ Support Palliat Care. 2015;5(1):63–9. doi: 10.1136/bmjspcare-2014-000648. [DOI] [PubMed] [Google Scholar]
- 5.Garand L, et al. Incidence and predictors of advance care planning among persons with cognitive impairment. Am J Geriatr Psychiatry. 2011;19(8):712–20. doi: 10.1097/JGP.0b013e3181faebef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel SE, Kiely DK, Mitchell SL. Satisfaction with end-of-life care for nursing home residents with advanced dementia. J Am Geriatr Soc. 2006;54(10):567–72. doi: 10.1111/j.1532-5415.2006.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biola H, et al. Physician communication with family caregivers of long-term care residents at the end of life. J Am Geriatr Soc. 2007;55(6):846–56. doi: 10.1111/j.1532-5415.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 8.Givens JL, et al. Healthcare proxies of nursing home residents with advanced dementia: decisions they confront and their satisfaction with decision-making. J Am Geriatr Soc. 2009;57(7):1149–55. doi: 10.1111/j.1532-5415.2009.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman SR. Intensive care, old age, and the problem of death in America. Gerontologist. 1998;38(6):715–25. doi: 10.1093/geront/38.6.715. [DOI] [PubMed] [Google Scholar]
- 10.Svanholm JR, et al. Normativity under change: Older persons with implantable cardioverter defibrillator. Nurs Ethics. 2016;23(3):328–38. doi: 10.1177/0969733014564906. [DOI] [PubMed] [Google Scholar]
- 11.Onken LS, et al. Reenvisioning Clinical Science: Unifying the Discipline to Improve the Public Health. Clin Psychol Sci. 2014;2(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–6. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Donovan HS, et al. An update on the representational approach to patient education. J Nurs Scholarsh. 2007;39(3):259–65. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leventhal H, Nerenz D, Steele DS. Illness representations and coping with health threats. In: Baum A, Singer JE, editors. Handbook of psychology and health. Erlbaum; New York: 1984. pp. 221–252. [Google Scholar]
- 15.Posner G, et al. Accommodation of a scientific conception: Toward a theory of conceptual change. Science Education. 1982;66:211–227. [Google Scholar]
- 16.Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88(2):251–8. doi: 10.7326/0003-4819-88-2-251. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman A, Mendelsohn E. Systems of medical knowledge: a comparative approach. J Med Philos. 1978;3(4):314–30. doi: 10.1093/jmp/3.4.314. [DOI] [PubMed] [Google Scholar]
- 18.Hewson M. Patient education through teaching for conceptual change. J Gen Intern Med. 1993;8(7):393–8. doi: 10.1007/BF02600081. [DOI] [PubMed] [Google Scholar]
- 19.Hewson P, Hewson M. The role of conceptual conflict in conceptual change and the design of instruction. Instructional Science. 1984;13(1):1–13. [Google Scholar]
- 20.Song MK, et al. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Medical Care. 2005;43(10):1049–1053. doi: 10.1097/01.mlr.0000178192.10283.b4. [DOI] [PubMed] [Google Scholar]
- 21.Song MK, et al. Randomized controlled trial of SPIRIT: An effective approach to preparing African American dialysis patients and families for end-of-life. Research in Nursing & Health. 2009;32:260–273. doi: 10.1002/nur.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song MK, et al. Effects of an intervention to improve communication about end-of-life care among African Americans with chronic kidney disease. Applied Nursing Research. 2010;23:65–72. doi: 10.1016/j.apnr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Song MK, et al. Advance care planning and end-of-life decision making in dialysis: A randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis. 2015;66(5):813–22. doi: 10.1053/j.ajkd.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzger M, Song MK, Devane-Johnson S. LVAD patients' and surrogates' perspectives on SPIRIT-HF: An advance care planning discussion. Heart Lung. 2016;45(4):305–10. doi: 10.1016/j.hrtlng.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Metzger M, et al. A randomized controlled pilot trial to improve advance care planning for LVAD patients and their surrogates. Heart Lung. 2016;45(3):186–92. doi: 10.1016/j.hrtlng.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song MK, Ward SE. Making visible a theory-guided advance care planning intervention. J Nurs Scholarsh. 2015;47(5):389–96. doi: 10.1111/jnu.12156. [DOI] [PubMed] [Google Scholar]
- 27.Carr D, Khodyakov D. Health care proxies: whom do young old adults choose and why? J Health Soc Behav. 2007;48(2):180–94. doi: 10.1177/002214650704800206. [DOI] [PubMed] [Google Scholar]
- 28.Stirman SW, et al. Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implement Sci. 2013;8:65. doi: 10.1186/1748-5908-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassam-Adams N, et al. A new method for assessing content validity in model-based creation and iteration of eHealth interventions. J Med Internet Res. 2015;17(4):e95. doi: 10.2196/jmir.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazel S, Hope T, Jacoby R. Effect of cognitive impairment and premorbid intelligence on treatment preferences for life-sustaining medical therapy. Am J Psychiatry. 2000;157(6):1009–11. doi: 10.1176/appi.ajp.157.6.1009. [DOI] [PubMed] [Google Scholar]
- 31.Gregory R, et al. Is the degree of cognitive impairment in patients with Alzheimer's disease related to their capacity to appoint an enduring power of attorney? Age Ageing. 2007;36(5):527–31. doi: 10.1093/ageing/afm104. [DOI] [PubMed] [Google Scholar]
- 32.Fazel S, Hope T, Jacoby R. Assessment of competence to complete advance directives: validation of a patient centred approach. BMJ. 1999;318(7182):493–7. doi: 10.1136/bmj.318.7182.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazel S, Hope T, Jacoby R. Dementia, intelligence, and the competence to complete advance directives. Lancet. 1999;354(9172):48. doi: 10.1016/S0140-6736(99)01911-X. [DOI] [PubMed] [Google Scholar]
- 34.Jeste DV, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64(8):966–74. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- 35.Seaman JB, et al. Psychometric Properties of a Decisional Capacity Screening Tool for Individuals Contemplating Participation in Alzheimer's Disease Research. J Alzheimers Dis. 2015;46(1):1–9. doi: 10.3233/JAD-142559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 37.Kondracki NL, Wellman NS, Amundson DR. Content analysis: review of methods and their applications in nutrition education. J Nutr Educ Behav. 2002;34(4):224–30. doi: 10.1016/s1499-4046(06)60097-3. [DOI] [PubMed] [Google Scholar]
- 38.Spackman DE, et al. Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res. 2012;9(9):1050–8. doi: 10.2174/156720512803569046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschanz JT, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19(6):532–42. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behl P, Stefurak TL, Black SE. Progress in clinical neurosciences: cognitive markers of progression in Alzheimer's disease. Can J Neurol Sci. 2005;32(2):140–51. doi: 10.1017/s0317167100003917. [DOI] [PubMed] [Google Scholar]
- 41.Green C, Zhang S. Predicting the progression of Alzheimer's disease dementia: A multidomain health policy model. Alzheimers Dement. 2016;12(7):776–85. doi: 10.1016/j.jalz.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song MK, Ward SE. Disconnect between emergency contacts and surrogate decision-makers in the absence of advance directives. Palliat Med. 2013;27(8):789–92. doi: 10.1177/0269216312474486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song MK, Sereika SM. An evaluation of the Decisional Conflict Scale for measuring the quality of end-of-life decision making. Patient Educ Couns. 2006;61(3):397–404. doi: 10.1016/j.pec.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Song MK, Ward SE, Lin FC. End-of-life decision-making confidence in surrogates of African-American dialysis patients is overly optimistic. J Palliat Med. 2012;15(4):412–7. doi: 10.1089/jpm.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song MK, et al. Advance care planning and end-of-life decision-making in dialysis: A randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis. doi: 10.1053/j.ajkd.2015.05.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song MK, Metzger M, Ward SE. Process and impact of an advance care planning intervention evaluated by bereaved surrogate decision-makers of dialysis patients. Palliat Med. 2017;31(3):267–274. doi: 10.1177/0269216316652012. [DOI] [PubMed] [Google Scholar]
- 47.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. [Google Scholar]
- 48.Patton MQ. Qualitative evaluation and research methods. 3. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 49.Maxwell JA. Qualitative research design: An inteactive approach. Thousand Oak, CA: SAGE; 2013. [Google Scholar]
- 50.Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855–66. doi: 10.1177/104973230201200611. [DOI] [PubMed] [Google Scholar]
- 51.Sandelowski M. Real qualitative researchers do not count: the use of numbers in qualitative research. Res Nurs Health. 2001;24(3):230–40. doi: 10.1002/nur.1025. [DOI] [PubMed] [Google Scholar]
- 52.Tashakkori A, Teddlie C. Handbook of Mixed Methods in Social and Behavioral Sciences. Thousand Oaks, CA: Sage Publications Inc; 2003. [Google Scholar]
- 53.Dickinson WB. Visual displays for mixed methods findings. In: Tashakkori A, Teddlie C, editors. SAGE handbook of mixed methods in social & behavioral research. SAGE; Thousand Oaks, CA: 2010. pp. 469–504. [Google Scholar]
- 54.Song MK, et al. Racial Differences in Outcomes of an Advance Care Planning Intervention for Dialysis Patients and Their Surrogates. J Palliat Med. 2016;19(2):134–42. doi: 10.1089/jpm.2015.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials. 2015;16:150. doi: 10.1186/s13063-015-0679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mittal D, et al. Comparison of two enhanced consent procedures for patients with mild Alzheimer disease or mild cognitive impairment. Am J Geriatr Psychiatry. 2007;15(2):163–7. doi: 10.1097/JGP.0b013e31802dd379. [DOI] [PubMed] [Google Scholar]
- 57.Taub HA, Kline GE, Baker MT. The elderly and informed consent: effects of vocabulary level and corrected feedback. Exp Aging Res. 1981;7(2):137–46. doi: 10.1080/03610738108259796. [DOI] [PubMed] [Google Scholar]
- 58.Grisso T, Appelbaum PS. Mentally ill and non-mentally- ill patients' abilities to understand informed consent disclosures for medication: preliminary data. Law Hum Behav. 1991;15(4):377–88. doi: 10.1007/BF02074077. [DOI] [PubMed] [Google Scholar]
- 59.Okonkwo O, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–35. doi: 10.1212/01.wnl.0000277639.90611.d9. [DOI] [PubMed] [Google Scholar]