Abstract
Tyro3, Axl, and Mertk, referred to as the TAM family of receptor tyrosine kinases, are instrumental in maintaining cell survival and homeostasis in mammals. TAM receptors interact with multiple signaling molecules to regulate cell migration, survival, phagocytosis and clearance of metabolic products and cell debris called efferocytosis. The TAMs also function as rheostats to reduce the expression of proinflammatory molecules and prevent autoimmunity. All three TAM receptors are activated in a concentration-dependent manner by the vitamin K– dependent growth arrest-specific protein 6 (Gas6). Gas6 and the TAM are abundantly expressed in the nervous system. Gas6, secreted by neurons and endothelial cells, is the sole ligand for Axl. ProteinS1 (ProS1), another vitamin K–dependent protein functions mainly as an anti-coagulant, and independent of this function can activate Tyro3 and Mertk, but not Axl. This review will focus on the role of the TAM receptors and their ligands in the nervous system. We highlight studies that explore the function of TAM signaling in myelination, the visual cortex, neural cancers, and multiple sclerosis (MS) using Gas6−/− and TAM mutant mice models.
Keywords: Tyro3, Axl and Mertk receptor tyrosine kinase family; Gas6; ProS1; homeostasis in the nervous system; myelination; inflammation; phagocytosis
1. Introduction
Several excellent reviews already exist detailing the many roles of Tyro3, Axl and Mertk (TAM) receptor tyrosine kinases and their ligands growth arrest-specific protein 6 (Gas6) and ProteinS1 (ProS1) in the maintenance of homeostasis within the innate immune system (Lemke, 2013; Lu & Lemke, 2001; Rothlin, Carrera-Silva, Bosurgi, & Ghosh, 2015; Rothlin & Lemke, 2010; Seitz & Matsushima, 2010). Identification of the TAM family in the vertebrate nervous system was first characterized in the 1990s by Lai and Lemke making the TAM family a relatively recent kinase family ripe for exploration (Lai & Lemke, 1991). Defects in TAM signaling disrupt cellular processes that regulate homeostasis, phagocytosis, and inflammation and can result in autoimmune diseases (Gohlke, et al., 2009; Rothlin, et al., 2015; Scott, et al., 2001). Defects in TAM signaling have been reported in cancer, colitis, lupus, and multiple sclerosis (MS) as well as mouse models of these diseases (Gohlke, et al., 2009; Rothlin, et al., 2015; Scott, et al., 2001). In this review, we will focus our attention on the role of the TAMs in the peripheral and central nervous systems detailing the role of TAM signaling in stem cells, the retina, Schwann cells and oligodendrocytes, and their roles in myelination, inflammation, phagocytosis and cancers of neural cells. We will review the expression of TAMs in gliomas and review possible therapeutics being explored. As the focus of our laboratory is multiple sclerosis (MS) we will present an overview of MS, defining the lesions types, mouse models that share aspects of pathology with MS, and how the TAM signaling has been implicated in MS pathophysiology.
2. Tyro3, Axl and Mertk signaling pathways and their ligands
2.1. Tyro3, Axl and Mertk (TAM)
Tyro3, Axl and Mertk comprise a family of cell adhesion molecule-related receptor tyrosine kinases, where one or more family members are often co-expressed on the same cell type (Graham, Dawson, Mullaney, Snodgrass, & Earp, 1994; Lai, Gore, & Lemke, 1994; Lai & Lemke, 1991; Lemke & Lu, 2003; O’Bryan, et al., 1991). The extracellular domain of the TAM family members contain two tandem immunoglobulin (Ig)-like repeats and two fibronectin type-III repeats previously observed in cell adhesion molecules or receptor tyrosine phosphatases, but not in receptor tyrosine kinases (Figure 1), (Graham, et al., 1994; Lai, et al., 1994; Lu & Lemke, 2001; O’Bryan, et al., 1991; Prasad, et al., 2006).
Figure 1. Structural characterization of the TAM receptors and their ligands.
TAM receptors have immunoglobulin (Ig)-like domains (green) and fibronectin repeats (orange) in the extracellular domain. A single pass transmembrane domain (red), an intracellular cytoplasmic domain containing a consensus kinase domain (yellow) and sites of tyrosine autophosphorylation (purple). The consensus sites for recruitment of signaling molecules including p85 subunit of PI3 kinase (pYXXM), Grb2 pYXN, and reported phosphatases are also shown. The structure of Gas6 and ProS1 is designated in blue. The ligands contain an amino terminal Gla domain that is γ-carboxylated in the presence of Vitamin K, four EGF-like repeats, and a carboxy-terminal sex hormone-binding globulin-like (SHBG-like) module that can bind and activate the TAM receptors.
The extracellular portion of the transmembrane domains of Axl and Mertk contain cleavage sites for ADAM10 (Axl only) and ADAM17 (Axl and Mertk) that can result in soluble forms of both receptors (O’Bryan, Fridell, Koski, Varnum, & Liu, 1995; Thorp, et al., 2011). The TAMs have a single hydrophobic transmembrane domain, and the intracellular region contains a highly conserved consensus sequence KW(I/L)A(I/L)ES within the tyrosine kinase catalytic domain. These structurally conserved regions and the conserved sequence places these three homologous type I receptor tyrosine kinases in its own family. The TAM kinase domain shares closest homology with the kinase domain of PDGFR (Robinson, Wu, & Lin, 2000). Upon activation by their ligand or self-activation, autophosphorylation of tyrosine residues adjacent to consensus sequences in the cytosolic domain recruit several signaling molecules including Grb2 (pYXN), the regulatory subunit of phosphoinositide 3 kinase (pYXXM; PI3 kinase) (J. G. Weinger, et al., 2008a) and Ras (Fridell, et al., 1996). The downstream signaling can result in enhanced cell migration/proliferation, cell survival, dampened inflammatory response, cellular debris clearance due to enhanced phagocytosis. The signaling pathway responsible for cell survival is largely through the PI3K–Akt pathway. Upon ligand activation and phosphorylation, the p85 subunit of PI3K binds to receptor phosphotyrosine residues that then lead to activation of the Akt survival pathway (Binder & Kilpatrick, 2009a; J. G. Weinger, et al., 2008a). The same pathway can be alternatively engaged by Grb2 binding and recruitment of PI3K and promote downstream Akt1 activation to promote cell survival (J.G. Weinger, et al., 2008). In vitro studies showed that Gas6/Axl mediated survival signaling requires NFκB (Demarchi, Verardo, Varnum, Brancolini, & Schneider, 2001; Hasanbasic, Cuerquis, Varnum, & Blostein, 2004). The Y867 residue within the cytoplasmic domain of Mertk was shown to mediate the binding of Grb2, which recruited the p85 of PI 3-kinase. When the Y867 site of Mertk was mutated and transfected into cells activation of PI3 kinase and NFκB were significantly reduced (Georgescu, Kirsch, Shishido, Zong, & Hanafusa, 1999; Tibrewal et al., 2008).
Figure 2 depicts several of the signaling pathways attributed to TAM signaling. Axl activation by Gas6/ProS1 recruits Grb2 and SOS resulting in activation of ERK, cell migration and proliferation; left (Allen, et al., 1999). Activation of Axl and Mertk results in the recruitment of PI3K via its regulatory subunit activates the AKT cell survival pathway (J. G. Weinger, et al., 2008b). In dendritic cells physical interaction with the R1 subunit of the type I interferon (IFN) receptor and Axl results in the expression of suppressor of cytokine signaling regulators SOCS1 and SOCS3 that reduce expression of proinflammatory molecules (Rothlin, Ghosh, Zuniga, Oldstone, & Lemke, 2007). Further, we found that SOC1/SOCS3 and the cytokines they regulate are differentially expressed in the CNS (Ray, et al., 2017a). TAM receptors have been shown to heterodimerize both in cis and trans, and Axl interacts with Tyro3 and Mertk (Seitz, Camenisch, Lemke, Earp, & Matsushima, 2007). In addition to interacting with the IFN receptor ((Rothlin, et al., 2007), Axl interacts with the epidermal growth factor receptor (EGFR; (Meyer, Miller, Gertler, & Lauffenburger, 2013; Vouri, et al., 2016).
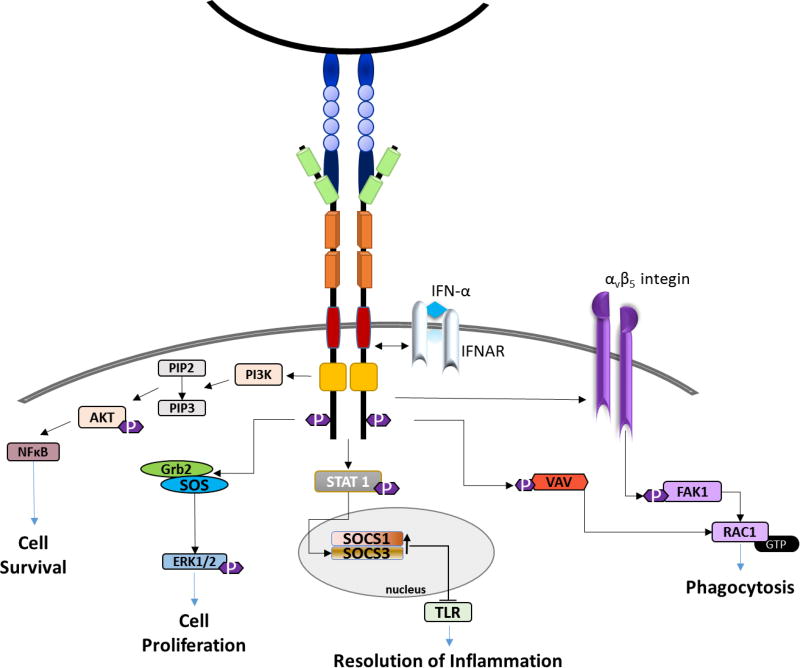
Figure 2. TAM receptor signaling cascade.
TAM receptors regulate cell survival/proliferation, inflammation and phagocytosis. The Axl receptor interacts with multiple SH2-containing signaling molecules, including Grb2 and PI3K, as well as STAT1 and αv β5-integin. Axl interaction with the interferon (IFN)-α/β receptor results in the phosphorylation and activation of STAT1, and increased SOCS1 and SOCS3 transcription. This reduces Toll-like receptor (TLR) activation and decreases the expression of proinflammatory molecules. Axl and Mertk are both important for phagocytosis of cell and myelin debris.
2.2. TAM and ligand tissue distribution and expression in the nervous system
TAM receptors are expressed in a variety of cell types and tissues. For a detailed review of TAM expression see (A. M. Pierce & Keating, 2014; Rothlin, et al., 2015); (Lemke, 2013). Several studies have demonstrated the presence of the TAMs and their ligands in the vertebrate nervous system, and while we will note their expression in other cell types our focus is on the nervous system (Lai & Lemke, 1991; Prieto, O’Dell, Varnum, & Lai, 2007; Prieto, Weber, & Lai, 2000; J. Wang, et al., 2011; Y. Zhang, et al., 2014; Y. Zhang, et al., 2016).
Axl, Tyro3, Mertk and Pros1 mRNA expression were detected in developing mouse cortex at E15.5 (J. Wang, et al., 2011). In situ hybridization studies of rat CNS tissue demonstrated the presence of TAM kinases in the nervous system, and noted that Axl and Tyro3 are expressed in the white matter during myelination. Tyro3 expression is very low in rat embryos, but its expression increases during early postnatal stages and parallels synaptogenesis. Tyro3 expression has been detected in granule neurons and Bergman glia (Prieto, et al., 2000). Tyro3 is highly expressed in the adult nervous system (Prieto, et al., 2000).
Axl is expressed in cells of the brain, heart, skeletal muscle, kidney, testis, liver, platelets, endothelial cells, monocytes/macrophages, and microglia (A. M. Pierce & Keating, 2014). Axl is expressed early in nervous system development, and although its expression decreases in the adult animal, it is expressed in the adult CNS with high expression in the cerebellum and hippocampus (Prieto, et al., 2000). Axl expression is high in migrating gonadotrophin-releasing hormone (GnRH) neurons (Allen, et al., 1999; Fang, Xiong, James, Gordon, & Wierman, 1998). Axl and Tyro3 are mainly in low-expressing GnRH expressing migratory neurons, and Mertk is upregulated in high GnRH expressing neurons shown to be post-migratory (A. Pierce, et al., 2008). Axl and Mertk are expressed by microglia and play a role in eliminating apoptotic newborn neurons in the subventricular zone (Fourgeaud, et al., 2016).
Mertk is expressed in astrocytes, microglia, testis, lung, retina, skeletal muscle, kidney, prostate, heart, brain, dendritic cells, monocytes/macrophages, NK and natural killer T cells, and (A. M. Pierce & Keating, 2014). Similar to Axl, Mertk is expressed at low but constant levels throughout development. Depending upon the cell type, the receptors can form homophilic interactions or be activated by the ligand (Bellosta, Zhang, Goff, & Basilico, 1997; Chan, Mather, McCray, & Lee, 2000; Crosier & Crosier, 1997; Goruppi, Ruaro, Varnum, & Schneider, 1999).
A comparison of human and mouse transcriptomes identified the expression levels of Tyro3, Axl Mertk and their ligands. The laboratory of the late Ben Barres performed transcriptome studies with human progenitor and mature human astrocytes and compared them with mouse, as well as RNA-seq data from murine cerebral cortex (Y. Zhang, et al., 2014; Y. Zhang, et al., 2016). Both murine and human cortex express high levels of Tyro3 on newly formed and myelinating oligodendrocytes, astrocytes, neurons and endothelial cells. In mouse cortex, Axl was expressed at highest levels in mature astrocytes, OPCs, microglia, endothelial cells and neurons. In human cortex, Axl mRNA was highest in mature astrocytes, microglia, oligodendrocytes, endothelial cells and neurons. Mertk expression was highest in microglia and mature astrocytes in both human and murine cortex, but was also detected in OPCs and oligodendrocytes. In murine cortex, Gas6 expression was detected at highest levels in microglia followed by neurons, OPCs, endothelial cells, newly formed oligodendrocytes, astrocytes and myelinating oligodendrocytes. In humans, Gas6 was highly expressed in microglia, endothelial cells, fetal and mature astrocytes, neurons and oligodendrocytes. In murine cortex, ProS1 was expressed in endothelial cells and microglia, OPCs and astrocytes; whereas ProS1 was also expressed at high levels in human neurons.
3. TAM ligands and a requirement for Vitamin K
3.1. Vitamin K is essential for TAM ligand activation and for additional functions in the nervous system
Vitamin K is an essential fat-soluble vitamin for nervous system function. Vitamin K exists naturally in two biologically active forms, vitamin K1 (phylloquinone) and vitamin K2 (menaquinone). Phylloquinones and menaquinones are not toxic in animal studies but it is not recommended to use menadione (vitamin K3) therapeutically (Marcus R, 2001). Vitamin K is expressed at high concentrations in brain cell membranes where it participates in the synthesis of sphingolipids, an important class of lipids essential for myelination (Ferland, 2012). Vitamin K is essential for the activation of the TAM kinase ligands ProS1 and Gas6, which are the major vitamin-K dependent proteins in the nervous system (Tsou, et al., 2014).
3.2. Growth arrest specific protein 6 (Gas6)
As noted, Gas6 is a vitamin K-dependent growth factor that is abundantly expressed in the nervous system. Gas6 contains an N-terminal Gla domain that is γ-carboxylated in a vitamin-K-dependent manner. The gamma-carboxylation allows binding to phosphatidylserine (PtdSer) (M. Huang, et al., 2003). This posttranslational modification is essential for its activity (Hasanbasic, Rajotte, & Blostein, 2005; Nagata, et al., 1996). Gas6 also contains four epidermal growth factor (EGF)-like domains and two laminin-G domains (Figure 1). Binding of Gas6 to the two immunoglobulin domains of Axl receptor is through the laminin-G domain binding and occurs as a heterodimeric ligand:receptor complex.
Gas6 was cloned following its up-regulation in confluent cell lines where it protected cells from death after complete serum withdrawal, in the absence of significant DNA synthesis (Bellosta, et al., 1997; Goruppi, et al., 1999; Schneider, King, & Philipson, 1988; Stitt, et al., 1995; Varnum, et al., 1995). Gas6 is widely expressed in the CNS where it has important physiologically relevant functions (Allen, et al., 1999; Avanzi, et al., 1998; Crosier & Crosier, 1997; Fourgeaud, et al., 2016; Healy, et al., 2016; Prieto, et al., 2000).
Gas6 is expressed in and secreted by neurons including Purkinje cells in the cerebellum. (Allen, et al., 1999; Prieto, et al., 2000). In the spinal cord, Gas6 is expressed by motor neurons and large neurons of the dorsal root ganglia; neural injury upregulates TAMs in Schwann cell (R. Li, et al., 1996; O’Guin, et al., 2014). Gas6 is concentrated along the plasma membrane of resting endothelial cells where its proposed physiologic function is to serve an anti-inflammatory role (Avanzi, et al., 1998).
3.3. ProteinS1 (ProS1)
Evolutionarily, Gas6 and ProS1 are paralogs. The duplication event that generated them may have occurred early in metazoan evolution, perhaps 600 Myr ago (Studer, Opperdoes, Nicolaes, Mulder, & Mulder, 2014). Analogous to Gas6, ProS1 is also a secreted soluble, vitamin K-dependent protein that is γ-carboxylated within the N-terminal Gla domain. The Gla domain confers the ability of ProS1 to bind phosphatidylserine on the surface of apoptotic cells (Hasanbasic, et al., 2005; Nakano, et al., 1997), and the C-terminal sex hormone-binding globulin-like module can bind and activate Tyro3 and Mertk (Nagata, et al., 1996; Nyberg, He, Hardig, Dahlback, & Garcia de Frutos, 1997). ProS1 phosphorylates Tyro3 and Mertk, but not Axl (Prasad, et al., 2006; Stitt, et al., 1995). ProS1 also contains the four EGF-like domains and the two laminin-G domains similar to Gas6 (Stitt, et al., 1995; Suleiman, Negrier, & Boukerche, 2013). ProS1 is a key plasma protein which plays a critical role in anti-coagulation and phagocytosis of apoptotic cells (Anderson, et al., 2003; Benzakour & Kanthou, 2000; Marlar & Neumann, 1990). In serum ProS1 is a co-factor that can block factor Va, VIIIa, and Xa activity regulating blood clotting, a function independent of TAM signaling. Gas6 does not play a direct role in clotting (Foley & Conway, 2013), and the TAMs are not involved in the anti-coagulate function of ProS1. ProS1 null mice are embryonic lethal as a result of coagulopathy and vascular dysgenesis (Burstyn-Cohen, Heeb, & Lemke, 2009). Mutations in the ProS1 gene result in thrombolytic events and an increased risk of death (Cho, et al., 2012; Heeb, Gandrille, Fernandez, Griffin, & Fedullo, 2008). ProS1 is also expressed by activated T cells (Lemke & Rothlin, 2008; A. M. Pierce & Keating, 2014). In a colitis model, T cells secrete ProS1 which can reduce inflammation by binding to TAM receptors on macrophages in the periphery (Carrera Silva, et al., 2013). In rats, ProS1 shows protective effects in neurons against stroke (D. Liu, Guo, Griffin, Fernandez, & Zlokovic).
4. The affinity of Gas6 for the TAM receptors
Gas6 as a ligand for the TAM receptors was identified in 1995 (Varnum, et al., 1995). The relative affinity of Gas6 for Tyro3, Axl and Mertk receptors is Axl>Tyro3>Mertk. Gas6 is the sole ligand for Axl. The receptor binding site of Gas6 maps to the first two receptor Ig-domains of Axl. The major Gas6 binding site is found only on the Axl receptor while the minor binding site is conserved by Tyro3 and Mertk (T. Sasaki, et al., 2006). Receptor monomers on the surface of opposing cells can dimerize through homophilic interactions with a large cluster of molecules generating a cooperative effect sufficient for stable adhesion (Heiring, Dahlback, & Muller, 2004).
While Gas6 and ProS1 can activate TAMs as soluble factors, it can also opsonize phosphatidylserine (PS) on apoptotic cells and serve as bridging molecules between apoptotic cells and TAMs (Tsou, et al., 2014). As part of a study to characterize the TAM ligands, reporter cell lines expressing chimeric TAM receptors were generated. Based on the screening system it was determined that each TAM has a unique pattern of interaction with and activation by GAS6 and PROS1. Each interaction was differentially affected by the presence of apoptotic cells, phosphatidylserine containing lipid vesicles, and enveloped virus, and that γ-carboxylation of both Gas6 and ProS1 are required for the full activation of TAM. Further, soluble extracellular Ig-like TAM domains sequesters the ligand and act as ligand antagonists (Tsou, et al., 2014).
4.1. A role for Gas6 in human oligodendrocyte survival, maturation and myelination
As a result of the expression of secretion of Gas6 by neurons, and reports of Gas6 as a neurotrophic growth factor (Funakoshi, Yonemasu, Nakano, Matumoto, & Nakamura, 2002), our laboratory examined whether Gas6/Axl interactions may enhance adhesion between axons and oligodendrocytes during myelination where high levels of both ligand and receptor are observed (Shankar, et al., 2003; Shankar, et al., 2006). RT-PCR studies show that Tyro3, Axl and Mertk are expressed in human oligodendrocytes, and all three receptors are expressed in human fetal brain and spinal cord (Shankar, et al., 2003).
Investigators who study primate oligodendrocyte maturation and myelination know that unlike rodent, human oligodendrocytes require axonal contacts for initiation of myelin synthesis. While rat oligodendrocytes in culture are stable and will express myelin basic protein (MBP), a major myelin protein necessary for myelination in the CNS, human and primate oligodendrocytes in culture undergo spontaneous apoptosis ~5–7 days after plating indicating that the signaling pathways and molecular events required for human oligodendrocyte survival and maturation have not been elucidated fully. Using O4, the sulfatide antibody specific for oligodendrocyte progenitor cells (OPCs), we immunopanned O4+ oligodendrocytes from human fetal spinal cord, and screened cDNA microarray libraries to identify novel receptors that may be involved in OPC preservation and enhance myelination. This led to the identification of high levels of Axl and Mertk expression on human O4+ OPCs (Shankar, et al., 2003).
An earlier study identified Gas6 as a growth factor for human Schwann cells in the peripheral nervous system (R. Li, et al., 1996). As a result of that study, human OPCs were plated in the presence of recombinant human (rh) rhGas6. The study found that Gas6 serves as a survival factor, not a mitogenic factor, for human fetal oligodendrocytes, and that enriched oligodendrocyte primary cell cultures generated from human fetal spinal cord were viable with fewer apoptotic OPCs 6 days after the administration of rhGas6. The study found that in the presence of rhGas6, there was a twofold increase in mature oligodendrocyte proteins relative to cells grown in the absence of Gas6. The effect was abolished in the presence of an Axl-Fc decoy, but not in the presence of the receptor fusion molecule TrkA-Fc. Additional studies determined that PI3-kinase inhibitors were able to block the anti-apoptotic effect of rhGas6, while MEK/ERK inhibitors had no effect (Shankar, et al., 2003).
Based on the observation that rhGas6 sustained human fetal oligodendrocyte viability by Gas6 activation of TAM receptors and downstream signaling via the PI3-kinase/Akt pathway (J. G. Weinger, et al., 2008a), additional studies determined that this signaling protected against TNFα–mediated cytotoxicity in human oligodendrocytes, and in oligodendrocytes prepared from WT and Tyro3−/− mice, but not Axl−/− mice (Shankar, et al., 2006). These studies led us to examine whether Gas6 would enhance myelination in human co-cultures established from human fetal dorsal root ganglia (DRGs) and brain-derived OPCs. DRG neurons course both in the peripheral and central nervous system, and both rat Schwann cell and oligodendrocyte/DRG co-cultures myelinate axons (Kleitman N, 1991). The addition of rhGas6 enhanced survival, maturation and myelination in human dorsal root ganglia (DRG) and OPC co-cultures (O’Guin, et al., 2014). The addition of rhGas6 to co-cultures significantly increased the number of myelin basic protein-positive (MBP+) oligodendrocytes with membranous processes parallel to and ensheathing axons after 14 days compared to co-cultures maintained in defined medium. The addition of rhGas6 did not increase the total number of MBP+ oligodendrocytes/culture, suggesting that rhGas6 is not mitogenic in this context.
RhGas6 also significantly increased the length of MBP+ oligodendrocyte processes in contact with and wrapping axons. Similar to its role in the CNS, multiple oligodendrocytes were in contact with a single axon and several processes from one oligodendrocyte ensheathed multiple axons. We determined by 3D- analysis that oligodendrocyte membranes completely encircled the axons. Forskolin activates adenylate cyclase and increases the concentration of intracellular cAMP. As a second messenger cAMP activates cAMP-dependent protein kinase A which can control transcription, protein phosphorylation, and ion transport (Montminy, 1997). Forskolin increased Gas6 expression in Sertoli cells (Chan, et al., 2000). We examined whether forskolin alone and in combination with rhGas6 would be beneficial when added to the human co-cultures. When added to the defined medium forskolin (5 µM) enhanced the number of MBP+ oligodendrocytes with membranous processes parallel with the axon. The addition of rhGas6 plus forskolin further enhanced oligodendrocyte process ensheathment and wrapping of axons; Mann-Whitney p=0.015 (Figure 3). Thus, forskolin enhances process outgrowth in human DRG/oligodendrocyte co-culture, and in combination with Gas6 enhances MBP+ OL ensheathment of axons (O’Guin, 2014).
Figure 3. Forskolin and rhGas6 enhance MBP+ oligodendrocyte process ensheathment of axons.
MBP+ OLs (green) ensheathing multiple neurofilament positive (NF+) axons (red) in co-cultures containing forskolin (5µM) (A–D), and forskolin and rhGas6 (2.6 nM) (E-H). Images showing MBP+ OLs wrapping NF+ axons were captured by confocal microscopy and Volocity software. Mann-Whitney p=0.015
Electron microscopy supported confocal Z-series microscopy demonstrating axonal ensheathment by MBP+ oligodendrocyte membranous processes in Gas6-treated co-cultures; however, compact myelin in the treated cultures was not observed (O’Guin, et al., 2014). This suggests that additional factors are required for compact myelin formation, but that rhGas6 is important for events in myelination in humans and aspects of non-compact myelin formation.
Purified rat OPC cultures, and rat DRG/OPC co-cultures supported the role of Gas6 in myelination, and showed that Gas6−/− mice have a delay in remyelination following cuprizone-induced demyelination (Binder, et al., 2011). Discussion of studies in mouse models is detailed in below.
With regard to the ligands, ProS1 is expressed by adult neurostem cells, and was shown to be a negative regulator of neural stem and progenitor cell self-renewal by negatively regulating Bim-1 signaling in both adult and embryonic neural stem cells. Deletion of ProS1 in neural stem cells increases cell proliferation (Burstyn-Cohen, 2017; Zelentsova-Levytskyi, et al., 2017; Zelentsova, et al., 2017).
5. Multiple Sclerosis: A role for the TAMs and their ligands
5.1. Multiple Sclerosis (MS): definition, symptoms and clinical course
MS is a debilitating immune-mediated neurological disease affecting young adults between 20 and 40 years of age (Lassmann, 2011; Lassmann, Bruck, & Lucchinetti, 2007; Noseworthy, Lucchinetti, Rodriguez, & Weinshenker, 2000; Sospedra & Martin, 2005). The most recent estimates of MS prevalence show more than 400,000 individuals in the U.S. and over 2.3 million people worldwide are affected (Browne, et al., 2014; Dilokthornsakul, et al., 2016). The ratio of females to males is ~3.4:1. The precise target of attack of peripheral immune cells is unclear. However, a major focus of research is on the influx of inflammatory T cells and monocytes that enter the CNS and attack the myelin sheath, exposing the resident cells of the CNS to insult. The damage resulting from the ongoing inflammation include activated resident glia, including astrocytes and microglia, that produce both proinflammatory and anti-inflammatory molecules, destruction of the myelin sheath, loss of oligodendrocytes, and damage to the axonal nerve fibers. Current diagnostics include magnetic resonance imaging (MRI) screening, the presence of oligoclonal bands in cerebrospinal fluid (CSF), the use of evoked potential tests to measure nerve response, and biomarkers, however, there is no definitive diagnostic test for MS (Lim, et al., 2017; Paty, 1988; Teunissen, Malekzadeh, Leurs, Bridel, & Killestein, 2015).
5.1.1. Symptoms of MS
Individuals with MS have different symptoms and presentations, and in fact, no two patients present in an identical manner. Among the most common symptoms, and frequently the earliest symptoms include optic neuritis, sensory abnormalities such as numbness and paresthesia in a limb, motor weakness in an extremity, and fatigue. Other symptoms include bladder, bowel and sexual dysfunction, spasticity usually in flexor spasms, sleep disorders, depression and cognitive impairment, reviewed in (El-Moslimany & Lublin, 2008).
5.1.2. Clinical Course of MS
The clinical course of MS is heterogeneous and includes inflammation, the expression of proinflammatory cytokines and chemokines, gliosis, demyelination, axonal damage and neuronal loss in brain and spinal cord (Compston & Coles, 2008; El-Moslimany & Lublin, 2008; Lassmann, et al., 2007). The different presentations of clinical symptoms fall into broad classifications and are referred to as clinical isolated syndrome (CIS), relapsing-remitting MS (RRMS), primary progressive PPMS, and secondary progressive SPMS. Individuals classified as CIS experience their first relapse and have lesion(s) on an MRI with recovery. Until individuals experience another episode they remain classified as CIS. The majority of in dividuals with MS (85%) initially develop a relapsing-remitting disease course. The National Multiple Sclerosis Society (https://www.nationalmssociety.org) states that RRMS is the most common disease course. This classification refers to clearly defined disease relapses with increasing neurologic symptoms, brain/spinal cord lesions on MRIs, followed by full or partial recovery. During remission there is no disease progression. CNS deficits often accumulate following relapses and as the disease progresses there are cumulative deficits. For example, following an attack and demyelination, intact axons can be remyelinated by mature oligodendrocytes. However, after subsequence attacks of demyelination and remyelination, the myelin sheath surrounding axons become progressively thinner and never appear as robust as prior to the demyelinating event. Oftentimes individuals with RRMS progress to a secondary progressive clinical course. During SPMS individuals progress with or without relapses or remissions. 10%–15% of individuals with MS develop a primary progressive clinical course in which there is a gradual and continuous worsening of symptoms with no clear remissions. Until recently, there were no effective treatments for PPMS; however, a 2017 study determined that the humanized monoclonal antibody orcrelizumab that depleted CD20-expressing B cells was associated with lower rates of clinical and MRI progression than placebo (El-Moslimany & Lublin, 2008; Montalban, et al., 2017). Approximately 15 percent of people with MS are diagnosed with PPMS. Individuals with PPMS have a worsening of neurologic function and an accumulation of disability from the onset of symptoms, without early relapses or remissions (https://www.nationalmssociety.org).
5.2. Morphology of the MS lesion
5.2.1. The chronic MS plaque
The most common type of MS lesion is the chronic plaque which is observed at autopsy in individuals who die after a prolonged course of disease (Lucchinetti, Bruck, Rodriguez, & Lassmann, 1996; Ludwin & Raine, 2008; Prineas, 1985). This well circumscribed lesion is found in both the brain and spinal cord, often periventricular in location. The chronic plaque varies in size and while mainly in white matter, it often extends into gray matter structures. Histologically, chronic plaques have a sharp demarcation between the normal myelinated tissue and the demyelinated plaque. Demyelination is observed in tissue sections by staining with luxol fast blue or by immunochemical stains against the major myelin proteins seen in the CNS, namely MBP and proteolipid protein (PLP). Oligodendrocyte loss is widespread although OPCs can be observed within the lesion (Wolswijk, 1997, 1998, 2000, 2002). A major characteristic of the chronic plaque is the extensive gliosis and meshwork of glial fibrillary acid protein (GFAP)+ astrocytic processes. Due to the extensive demyelination, naked or unmyelinated axons are surrounded by GFAP+ astrocytes processes, hence the term gliotic or sclerotic plaque.
5.2.2. The active or acute MS plaque
The acute plaque is characterized by myelin loss, and the lesion edge is less defined than the chronic plaque. Axons tend to be devoid of myelin, and naked axons are visible by electron microscopy. Additionally, oligodendrocytes expressing early developmental markers as well as thinly remyelinated axonal fibers are observed. The lesion contains lymphocytic infiltrates including debris-filled macrophages, CD4+ and CD8+ T cells, MHC-II+ cells, as well as activated resident microglia and reactive astrocytes. Axonal damage is often observed in acute lesions identified by the expression of non-phosphorylated neurofilament protein and amyloid precursor protein, indicative of defects in axonal transport.
5.3. Expression of TAMs and their ligands in MS tissue
The first study to examine the expression of TAMs and their ligands in MS lesions was performed by analyzing protein homogenates isolated from chronic active and chronic silent lesions (J.G. Weinger, Omari, Marsden, Raine, & Shafit-Zagardo, 2009). TAM and Gas6 protein expression was compared to normal appearing tissue from the contralateral region of the brain from the same individual, as well as homogenates from other neurologic diseases by immunoblot analysis. Cell type expression was determined by immunohistochemistry. The study determined that levels of full-length, membrane-bound Mertk (205 kDa), soluble Mertk (~150 kDa), and soluble Axl (80 kDa) were all significantly elevated in homogenates from established multiple sclerosis lesions comprised of both chronic active and chronic silent lesions. Soluble Axl was significantly elevated in chronic silent lesion samples whereas in normal tissue homogenates there was minimal to undetectable expression. Full-length Mertk was significantly increased in protein homogenates isolated from chronic silent lesions and soluble Mertk was significantly elevated in protein homogenates from chronic active tissue.
In addition, increased levels of soluble Axl and Mertk were associated with increased levels of mature ADAM17, mature ADAM10, and Furin proteins that are associated with Axl and Mertk cleavage and solubilization. Despite the increase in soluble Axl and Mertk, full-length Axl and Mertk were not decreased in MS lesions. Full-length Mertk was increased in chronic silent lesions and elevated in chronic active lesions, suggesting either more full-length Axl and Mertk were being synthesized or more full-length Axl and Mertk were being introduced into the lesion via infiltrating or proliferating cells. If as it appears that full-length membrane-bound Axl and Mertk receptors are not depleted but there was an increase in soluble forms of these receptors, it is plausible that any beneficial effects afforded by Gas6 ligation and activation of full-length Axl and Mertk was impeded by the soluble Axl and Mertk acting as decoy receptors and sequestering Gas6. Protein homogenates from chronic active samples having the most soluble Axl and soluble Mertk had minimal to no Gas6 expression by immunoblot analysis. The combined data suggest that there is dysregulation of protective Gas6 receptor signaling in MS lesions.
Brain sections from primary progressive and secondary progressive MS patients and non-neurological controls were incubated with Axl, Mertk, and Tyro3 antibodies. While there was low level of both Axl and Mertk expression in normal brain tissue, Axl expression was elevated on astrocytes and oligodendrocytes in chronic active lesions by double- label immunohistochemistry with Axl and PDGFRα, or GFAP and Axl antibodies. Mertk expression was elevated on astrocytes in chronic active lesions where astrocyte proliferation is known to occur and full-length Mertk is elevated. In chronic silent lesions, Axl was elevated on microglia verified by double-staining for Axl and Iba1. Mertk expression was elevated on astrocytes and oligodendrocytes in chronic silent lesions. In all samples from other neurologic diseases (OND), there were no differences in Axl, Mertk, or Tyro3 immunoreactivity relative to normal control tissue. In addition, no differences in Tyro3 immunoreactivity was detected in brain sections containing chronic active or chronic silent lesions (J.G. Weinger, et al., 2009). These data suggest that Axl and Mertk are playing important signaling roles in multiple cells types within the CNS during episodes of inflammation and repair.
While the failure to effectively activate membrane-bound TAMs likely results in an inability to dampen the immune response, as well as initiate efficient clearance of cell debris necessary for protecting cells of the CNS from damage and for efficient remyelination, the abovementioned study explored the TAMs and Gas6 in static tissue. Yet, there are additional studies that report cleavage of TAM family members resulting in increased inflammation and delayed recovery (Cai, et al., 2017; Cai, et al., 2016).
An International Multiple Sclerosis Genetics Consortium has shown a SNP polymorphism in Mertk associated with MS susceptibility (Ma, Stankovich, Kilpatrick, Binder, & Field, 2011; Sawcer, et al., 2011). A refinement of the Mertk SNP study, demonstrated that one of the variants showed increased expression of MERTK in monocytes. Increased expression of MERTK was associated with either increased or decreased risk of developing MS, which was dependent upon the HLA-DRB1*15:01 status (Binder, et al., 2016). As noted previously, phagocytosis and clearance of apoptotic cells is mediated by Mertk and a reduction in the expression Mertk in myeloid cells resulted in inefficient clearance of apoptotic cells in myeloid organs (Scott, et al., 2001). Mertk in myeloid cells was shown to function in regulating the clearance of myelin debris by phagocytosis (Healy, et al., 2016). Further, monocyte derived macrophages from controls and patients with RRMS and SPMS were assessed for the phagocytosis of myelin debris and both mRNA and protein expression of TAM family members. Individuals with MS were able to efficiently phagocytose red blood cells but demonstrated reduced capacity to phagocytose human myelin. Macrophage showed lower levels of both Mertk and Gas6 suggesting a defect in phagocytosis by macrophages of individuals with MS. Treatment with TGFβ could restore phagocytosis and expression of the receptor and the ligand (Healy, et al., 2017). Thus, several independent reports point to Mertk as a defective signaling pathway in individuals with MS.
Total and free ProS1 in plasma from individuals with MS and controls from two independent cohorts correlated with the MS Severity Score. Only free ProS1 can activate Tyro3 and Mertk. When compared with controls, total ProS1, but not free ProS1, was decreased in individuals with MS. Interestingly, there was a decrease in both total and free ProS1 levels relative to control samples in female MS individuals. Individuals with very low levels of free ProS1 in plasma had higher MS severity scores and increased disease severity.
The decrease in plasma concentration of soluble Mertk observed in females with MS did not correlate with ProS1 levels (Ma, et al., 2015). The function of free ProS1 is as an anti-coagulant and a co-factor for Protein C and tissue factor pathway inhibitor (TFPI) that inhibits FactorXa. ProS1 binds to C4b–binding protein (C4BP) regulates complement and the activity of ProS1. The C4BP beta chain short consensus repeat-2 contributes to the interaction of C4BP with ProS (van de Poel, Meijers, Dahlback, & Bouma, 1999); surfaces (Trouw, Nilsson, Goncalves, Landberg, & Blom, 2005). When bound the ProS1-C4BP complex is limited as an anti-coagulant and in addition, cannot associate with Tyro3 or Mertk (Nyberg, et al., 1997). C4BP isoforms without the beta chain do not bind ProS1 (Garcia de Frutos, Alim, Hardig, Zoller, & Dahlback, 1994). Humans have a ProS1-C4BP complex in plasma; however, mice, rabbits and cattle do not. Alpha-only C4BP molecules do not bind ProS1 and appear to function in dendritic cell maturation (Olivar, et al., 2013). With the regard to the study by Ma et al (Ma, et al., 2015), the concentration of C4BP isoforms in the MS patients is not known. Perhaps, the group of patients with low free ProS1 and higher MSSS are a sub-group with inflammation-induced elevation in C4BP expression and decreased free ProS1.
The concentration of Gas6 in plasma and CSF in 65 individuals undergoing a spinal tap during a relapse of RRMS was compared to the levels with 40 individuals having non-inflammatory, non-autoimmune neurological diseases. The study found that the concentration of Gas6 in CSF was inversely correlated with the severity of relapse in RR-MS patients; however, it did not predict the subsequent course of the disease (Sainaghi, et al., 2013).
A recent study examined the expression of microglia gene expression from material isolated from normal appearing white matter (NAWM), and compared the gene expression profile with microglia isolated from the center of active MS lesions containing high infiltrates of foamy macrophages. Gas6 was increased >5-fold in the lesion relative to the NAWM (Zrzavy, et al., 2017). Thus, when considering the combined studies, expression of AXL and MERTK, and ligands GAS6 and PROS1 are elevated in MS tissue, and strongly support a functional and physiologic role for signaling through this family of receptor tyrosine kinases, likely in reducing inflammation, debris clearance and restoring cellular homeostasis.
5.4. Therapeutic administration of rhGas6 as a potential treatment strategy for MS and other nervous system disease applications
It is clear from several human studies that both the ligands and the TAM receptors are altered in MS lesions and plasma, and that changes in free ProS1, Gas6, soluble forms of Axl and Mertk correlate with neurologic deficits and more severe disease pathology. In vitro human co-cultures demonstrate a beneficial effect of Gas6 in myelination (O’Guin, et al., 2014), yet a lack of total understanding of this important signaling pathway limits it use as a therapy. Gas6 is synthesized in the CNS where it secreted by neurons and endothelial cells, yet it has a very short half-life. Administration of Gas6 to the CNS via an Omari pump would be impractical in this age when pills have superseded injections as a means of MS treatment. Murine erythroblasts can released Gas6 in response to the hormone erythropoietin. In transgenic mice with insufficient Epo production and chronic anemia, Gas6 can synergized with Epo to restore hematocrit levels. Yet, there is concern that Epo could result in bleeds in the CNS over time (Angelillo-Scherrer, et al., 2008). At this time, an understanding of how best to locally stimulate Gas6 expression to activate the appropriate TAM receptor is an important strategy for future treatments.
6. Mouse Models Sharing Pathologic Aspects of MS are used to study TAM Kinases and their ligands in the CNS
6.1. Myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (MOG-induced EAE)
is an in vivo chronic mouse model analyzing the interactions between cells of the CNS and cells of the immune system. MOG-induced EAE examines aspects of disease observed in MS; permitting researchers to analyze mice in which there is an influx of T cells and monocytes/macrophages into the CNS. With some phenotypes similar to MS, EAE is an inflammatory demyelinating disease that affects all cellular elements of the CNS, and in which neuronal/axonal damage and demyelination are consequences of immune-mediated inflammation. To generate the model, mice are sensitized with an emulsion of MOG35–55 peptide and complete Freund’s adjuvant, followed by an intraperitoneal injection of pertussis toxin (Ptx). Two days later the mice receive a second Ptx injection and mice develop signs of disease by day 10 post-MOG injection. The mice are scored on a 0–5 scale: on disease, 1= flaccid tail; 2= hindlimb weakness; 3= hindlimb paralysis; 4= hindlimb and front limb paralysis; 5= moribund (Gruber, et al., 2014; Pluchino, et al., 2009).
6.2. PLP Model of RR-EAE
Similar to MOG-induced EAE, the PLP model of RR-EAE is characterized clinically by the development of ascending flaccid hind limb paralysis. Each mouse is graded daily, and mice are scored on a 0 to 5 scale (Miller, Karpus, & Davidson, 2010). The acute phase of disease is when there is the first clinical sign of disease, in which mice show ascending paralysis following active disease induction of SJL mice via PLP139–151-induced disease. The remission phase is clinical improvement following a clinical episode defined as a reduction in clinical score for at least 2 days after the peak score of the acute phase, or of a disease relapse. Relapse is defined as the phase of increasing clinical disease seen after remission or an increase of at least one grade in clinical score maintained for at least 2 days after remission has occurred. The majority of mice retain some neurological deficits and disease progression will occur over repeated relapse/remission phases.
6.3. Cuprizone Model of Demyelination/Remyelination
The cuprizone model is used to study changes in myelination, inflammation and axonal integrity within the corpus callosum, focusing on changes that occur within the CNS, and not as a result of disruption of the blood-brain-barrier (Hiremath, et al., 1998; Matsushima & Morell, 2001). There are no T cells infiltrates in the brain during cuprizone treatment although there are macrophages and neutrophil infiltrates. Inflammation is predominantly the result of microglial and astrocyte activation. Mice are fed a diet of cuprizone in powdered chow (0.2% w/w) for six weeks and then given standard chow and monitored over time through 3 weeks post-cuprizone withdrawal. Mice treated with cuprizone have no ill effects from the treatment, having focal demyelination in the corpus callosum. Remyelination is restored 3 weeks post cuprizone withdrawal (Blakemore, 1973a, 1973b; Jurevics, et al., 2002; Matsushima & Morell, 2001; Scurlock & Dawson, 1999). Cuprizone treatment induces demyelination of the corpus callosum by 4 weeks resulting in reactive gliosis, accumulation of microglia and macrophages (Hiremath, et al., 1998), TNFα expression (Arnett, et al., 2001; Mason, Xuan, Dragatsis, Efstratiadis, & Goldman, 2003), and mRNAs coding for many cytokine/chemokine genes, including MIP-1 gamma, monocyte chemoattractant protein-1 (MCP-1), MIP-1, CCR5, CIP3, SOCS3 are observed (Jurevics, et al., 2002). Apoptotic death of mature oligodendrocytes during cuprizone intoxication is significantly delayed in TNF-α knock-out mice indicating a role for TNFα in this process (Arnett, et al., 2001).
6.4. Lysolecithin model of demyelination
For the lysolecithin model, mice are focally injected with the detergent lysolecithin which results in focal demyelination and recovery over time. The mice have no overt clinical signs and are very active and normal in appearance. Mice are studied both during demyelination and remyelination (Monteiro de Castro, Deja, Ma, Zhao, & Franklin, 2015; Rusielewicz, et al., 2014).
7. TAM deficient mice
Tyro3, Axl and Mertk single, double and triple knockout (KO) mice have been generated as some cells express more than one TAM receptor (Lu, et al., 1999). All single KO, double KO and triple KO mice are viable indicating that the TAM receptors are not required for embryogenesis. There are reports of varying degrees of developmental maturation, and impairment in cell viability and homeostasis in some of the KOs, depending on the tissue type (Akkermann, et al., 2017; D'Cruz, et al., 2000; Lu, et al., 1999; Lu & Lemke, 2001). Many of the phenotypes are summarized in the review by Graham et al (Graham, DeRyckere, Davies, & Earp, 2014) and only those pertinent to the nervous system will be discussed in this review.
7.1. Tyro3−/− mice
Tyro3, also called Sky or Rse, was identified by cDNA cloning and shown to have homology to Axl. Tyro3 localizes to human chromosome 15, adjacent to its pseudogene (Fujimoto & Yamamoto, 1994; Lai, et al., 1994; Mark, et al., 1994; Ohashi, Mizuno, Kuma, Miyata, & Nakamura, 1994). The upstream region of the coding exon is highly GC-rich and contains potential recognition sites for the Sp1 trans-acting factor, but lacks TATA and CAAT boxes (M. Sasaki & Enami, 1996). Tyro3 expression is greatest in the post-natal and adult murine brain, highly expressed in neurons in the neocortex, cerebellum, and hippocampus. In situ hybridization studies in adult rat brain identified Tyro3/Sky transcripts within neuronal of the inner granular layer of the olfactory bulb, CA-1 area of the hippocampus, granule cell layer of the cerebellum, tenia tectum and cingulate gyrus neurons, and wide regions of cortex layers II-VI (Ohashi, Honda, Ichinomiya, Nakamura, & Mizuno, 1995; Prieto, et al., 2007; Prieto, et al., 2000). In mature neurons Tyro3 was found in dendrites, and in the soma; and in axons and growth cones of immature neurons (Prieto, et al., 2007). Tyro3 expression correlates with synaptogenesis and myelination. Similar to Axl, Tyro3 was proposed to play a role in adhesion and signaling. Immunoprecipitation studies demonstrated that phosphorylated Tyro3 was in a complex with Src family members (Toshima, Ohashi, Iwashita, & Mizuno, 1995). While early studies showed that Tyro3 is expressed solely in neurons, additional studies indicate that Tyro3 is expressed in white matter regions and on oligodendrocytes where it is required for myelination (Akkermann, et al., 2017; Prieto, et al., 2000).
In the peripheral nervous system, Tyro3 bound to the non-receptor tyrosine kinase Fyn was shown to regulate myelination by Schwann cells (Miyamoto, et al., 2015). Using DRGs from Tyro3−/− mice there was reduced myelin thickness produced by Schwann cell co-cultures. Interestingly, Fyn was shown to be down-regulated in Tyro3-knockout mice suggesting that Tyro3, acting through Fyn, regulates myelination. Fyn was found to be upregulated early during oligodendrocyte progenitor cell (OPC) differentiation and to require the expression of tyrosine phosphorylated proteins on mature oligodendrocytes for myelination including p190RhoGAP (Osterhout, Wolven, Wolf, Resh, & Chao, 1999; Wolf, Wilkes, Chao, & Resh, 2001).
Using the lysolecithin model, and cell cultures, Gas6 induced STAT3 activation in OPCs or oligodendrocytes, due to Tyro3 signaling; however, the direct involvement of Tyro3 was not shown by inhibition studies or in KO mice (Goudarzi, Rivera, Butt, & Hafizi, 2016). Using Tyro3−/− mice, another study showed that Tyro3 is important for myelin growth and that there were fewer myelinated axons and less myelin thickness in the optic nerve of Tyro3−/− mice during initiation of myelination. Further, oligodendrocytes isolated from Tyro3−/− mice produced significantly fewer myelin segments relative to oligodendrocytes from WT mice; the mechanism of action was believed to be by ERK1 indicating that Tyro3 can affect several signaling pathways including ERK1 and Fyn (Akkermann, et al., 2017; Wolf, et al., 2001).
While cuprizone-induced demyelination and remyelination in Tyro3−/− mice showed no differences in demyelination or remyelination relative to the WT mice (Hoehn, et al., 2008), it is possible that the thickness of the myelin sheaths was reduced in the null mice. However, there was no difference in the g-ratio of WT and Tyro3−/− mice as determined by electron microscopy. Additionally, in the absence of Tyro3 expression, there was no significant difference in clinical scores between WT and Tyro3−/− mice during acute and chronic phases of EAE, although there was a significant increase in Axl expression in Tyro3−/− mice during EAE but not in naïve mice (J. G. Weinger, et al., 2011). As EAE is predominantly a model of inflammation, it is possibly that the model is not optimal to examine subtly changes in demyelination.
7.2. Axl−/− mice
Studies with a sciatic nerve injury model showed an increase in Axl following lesioning, suggesting a role for Axl in survival and protection against apoptosis (Funakoshi, et al., 2002). In fibroblasts, Axl signaling protects against apoptosis induced by serum deprivation, myc overexpression, or TNFα, but did not protect from ultraviolet irradiation or staurosporine (Bellosta, et al., 1997). Our laboratory has determined that Gas6, Axl and Mertk, but not Tyro3 or ProS1 are up-regulated over the course of MOG-induced EAE (Gruber, et al., 2014). RNA isolated from spinal cords of WT mice sensitized with MOG35–55 peptide was examined by qRT-PCR after 1, 4, 8, and 20 days of consecutive clinical scores. Relative to naive mice, Gas6 mRNA expression was significantly increased in mice with clinical scores for 8 consecutive days. Mertk RNA expression was significantly increased in mice with clinical scores for 4 and 8 days, time points that correlate with the influx in monocyte/macrophages and glial activation.
When Axl−/− mice were compared to WT mice during EAE, Axl−/− mice had a significantly more severe clinical course during the acute phase of EAE, with significantly more spinal cord lesions, larger inflammatory cuffs, more demyelination, and axonal damage. The lesions in the spinal cord of the Axl−/− mice had inefficient clearance of myelin debris as assessed by more intense Oil-Red-O staining. There were fewer activated microglia/macrophages surrounding lesions in Axl−/− mice relative to WT mice suggesting the loss of Axl affected migration of the microglia to the site to circumscribe the damage and to clear cell debris. During acute EAE, Axl−/− spinal cords trended toward increased proinflammatory cytokine and chemokine expression of TNFα, MCP1, and RANTES relative to WT spinal cord but only TNFα was consistently significantly higher. As part of this study Tyro3−/− were similar in their disease course to the WT mice (J. G Weinger, et al., 2011).
During cuprizone treatments of WT mice, TAM receptors and their respective ligands were examined by qRT-PCR over the course of cuprizone diet and 1-week recovery (Ray, et al., 2017b). Gas6 mRNA expression was highest at 4-weeks cuprizone treatment, decreased at 5-weeks ingestion, and continued to decrease through 1-week recovery. Similarly, Axl mRNA peaked at 4-weeks cuprizone and steadily decreased over time returning to baseline 1-week after removal of cuprizone from the diet. Tyro3 expression was significantly increased after 4-weeks on a cuprizone diet, declined by 5-weeks and remained at naïve levels until at 1-week recovery, when Tyro3 mRNA increased again. At 4-weeks cuprizone, Mertk mRNA was increased ~15-fold relative to naïve WT mRNA, and continued to decrease over the course of cuprizone treatment, returning to near-baseline levels after 1-week recovery.
There is a reduction in remyelination in the corpus callosum of Axl −/− mice 3 weeks after cuprizone withdrawal relative to WT littermate controls. The lag in remyelination is contributed to inefficient clearance of debris as determined by OilRedO staining. In the corpora callosa of WT mice there were Oil Red O+ staining at 4-week cuprizone treatment indicative of ongoing phagocytosis. At the same time point Axl−/− mice had minimal Oil Red O+ staining, and significantly more TUNEL+/ASPA+ mature oligodendrocytes than the WT. By 6-week cuprizone treatment, the Axl−/− corpora callosa showed significantly more Oil Red O+ staining than in the WT demonstrative of a lag in the clearance of neutral lipids and myelin debris. Likely as a result of delayed clearance of debris, there were significantly more damaged axons in the Axl−/− mice at 3-week post-cuprizone withdrawal relative to WT mice. At this time point, the number of dystrophic axons and axons containing autophagosome-like vacuoles/mouse was increased in the Axl−/− corpus callosum relative to the WT mice. At 5-week post-cuprizone withdrawal, SMI32+ non-phosphorylated neurofilament immunoreactivity and swollen axons were still present in the Axl−/− corpora callosa, indicating that similar to what is observed in MS lesions, delayed clearance of apoptotic oligodendrocytes and myelin debris prolongs axonal damage and remyelination.
7.3. Mertk−/− mice
Mertk−/− mice have impaired clearance of infused apoptotic cells and develop progressive lupuslike autoimmunity, with antibodies to chromatin, DNA, and IgG (Cohen, et al., 2002; Scott, et al., 2001). However, the CNS has not been examined for signs of neuropsychiatric lupus. Mertk−/− mice have not been reported to be examined in the cuprizone or EAE models. Mertk mice have increased hematopoietic cells including macrophages, T cells, dendritic cells, plasmacytoid dendritic cells and B cells in the peritoneal cavity, a site of autoreactive B cells prior to their transit to other immune sites. Mice lacking Tyro3 or Axl do not show the same dysregulation in peritoneal cell number or distribution of cells or the autoimmune phenotype (Seitz, Camenisch, Lemke, Earp, & Matsushima, 2007).
Mertk regulates the activation of Rac1 and Cdc42 and the downstream cytoskeletal changes necessary for the phagocytosis of debris by macrophages (Mahajan & Earp, 2003). Using a yeast two-hybrid assay, Mertk was found to interact with the carboxyl-terminal region of the guanine nucleotide-exchange factor (GEF) VAV1. The interaction was independent of a requirement for tyrosine phosphorylation, although the binding site was the SH2 domain of VAV1. Upon ligand activation of Mertk, tyrosine phosphorylation of VAV1 resulted in the release of VAV1 from Mertk; kinase-dead Mertk failed to release VAV1. In human monocytes, activation of Mertk released VAV1 from Mertk and stimulated GDP replacement by GTP on RhoA family members, that regulated local activation of Rac1, Cdc42, and RhoA resulting in cytoskeletal changes that potentially impact clearance of debris that results in clearance of pigmented retinal epithelial cells (Mahajan & Earp, 2003) The retina and the visual cortex of Mertk−/− mice have been extensively examined, see the Retina section below.
7.4. Gas6−/− mice
The beneficial effects of Gas6 during myelination and in the context of the cuprizone model (Binder & Kilpatrick, 2009b; Binder, et al., 2011) were detailed in the myelination section and will not be discussed here except to state that Gas6 plays an essential role in activating the TAM receptors to maintain homeostasis, efferocytosis, and remyelination. Gas6−/− mice appear normal but had increased expression of cytokines known to inhibit erythropoiesis including IL-10, IL-13, IL-1α, IL-1β, IL-6, and TNFα in plasma and supernatant of bone-marrow-derived macrophages (Angelillo-Scherrer, et al., 2008).
In the context of MOG-induced EAE, Gas6−/− spinal cords had higher clinical scores during late peak to early chronic disease, with significantly more SMI32+ axonal swellings, and greater numbers of Iba1+ microglia/macrophages, when compared to WT spinal cords. Flow cytometry showed that during acute disease, Gas6−/− spinal cords had more macrophages infiltrates that WT spinal cords. In addition, qRT-PCR determined that when compared with WT spinal cords, spinal cords from Gas6−/− mice had increased expression of several proinflammatory molecules after having clinical scores for 8 consecutive days. Consistent with the cuprizone studies, GAS6 is protective during EAE, and deletion of Gas6 increases inflammation, and compromises axonal integrity and myelination (Gruber, et al., 2014). The combined data strongly suggest that Gas6 activation could be therapeutic for demyelinating disease. Gas6 is an approximately 80 kDa protein with post-translational modifications that likely increases its mass, making it inefficient to administer Gas6 in the periphery and to efficiently cross the BBB. Further, similar to cytokines such as INFβ, the half-life of Gas6 is short. Therefore, a small molecule which can stimulate Gas6 in the CNS would be a better approach to transiently activate Gas6.
In the periphery, Gas6 increases erythropoietin expression. Erythropoietin (EPO) was shown to be anti-inflammatory and to reduce clinical scores in rat and mouse models of EAE (Agnello, et al., 2002; Cervellini, Ghezzi, & Mengozzi, 2013; W. Li, et al., 2004; J. Zhang, et al., 2005). Gas6 enhanced erythropoietin receptor signaling by activating Akt. In a transgenic mouse model of chronic anemia resulting from insufficient EPO production, Gas6 synergized with EPO and restored normal hematocrit levels (Angelillo-Scherrer, et al., 2005). EPO, produced in the kidney, bone marrow and spleen, is a glycoprotein cytokine/hormone that stimulates erythroid progenitor production and maturation. EPO was shown to induce murine erythroblasts to release Gas6. However, due to erythropoietin’s role in increasing hematocrit and red cell mass, the entire molecule is not considered therapeutic. A library of cyclic and linear EPO-derived fragments were generated, and screened for beneficial effects during MOG-induced EAE, and PLP-induced EAE. In C57Bl6 mice, high doses of full-length EPO treatment did not result in altered hematocrit during MOG-induced EAE. However, SJL/J mice treated during PLP-induced EAE showed increased hematocrit, and greater than 50% of treated mice died after 5–7 days of high-dose EPO (Chen, et al., 2010; W. Li, et al., 2004; Yuan, Wang, Lu, Maeda, & Dowling, 2015). Desialylated EPO and carbamylated EPO, modified forms of EPO, are neuroprotective during MOG-induced EAE (Adembri, et al., 2008; Mun & Golper, 2000). One 19-mer JM-4 peptide fragment from the EPO-AB loop containing 2 cysteine residues showed beneficial effects during EAE, and did alter hematocrit. JM-4 peptide fragment blocked expansion of monocyte/dendritic antigen-presenting cell and T helper 17 cell populations, decreased proinflammatory cytokine production, and increased expansion of Tregs (Yuan, et al., 2015). Although Gas6 levels were not examined in any of these studies, it is feasible that modified forms of EPO might be beneficial at inducing Gas6 expression in the CNS during EAE.
7.5. Gas6−/− Axl−/− Double KO (DKO)
Due to the cross-talk between the TAM receptors and ProS1’s ability to activate Tyro3 and Mertk, as well as Tyro3 and Mertk’s ability to each activate Axl by heterodimerization, we have generated Gas6−/− Axl−/− DKO mice were generated to directly assess the Gas6/Axl signaling pathway permitting us to address whether retention of the ProS1 signaling axis through Tyro3 and Mertk can compensate for the loss of Gas6/Axl signaling. In addition, Axl is unavailable for activation and interaction with INFR and EGFR both shown to interact and signal with Axl (Meyer, Miller, Gertler, & Lauffenburger, 2013; Rothlin, et al., 2007; Seitz & Matsushima, 2010; Vouri, et al., 2016).
DKO pups are normal relative to age-matched WT C57Bl6J, Axl+/+Gas6−/− and Axl+/−Gas6−/−littermates, and upon weaning, all mice had no overt phenotype. In the CNS, naïve 8–10 week old DKO and WT mice have comparable myelination and equal numbers of axons and oligodendrocytes in the corpus callosum. Myelination in the spinal cord is similar in both groups of mice.
DKO and WT mice were examined at multiple times during acute cuprizone-induced demyelination and during recovery. Electron micrographs of WT and DKO corpus callosum showed that DKO mice have extensive axonal swellings containing autophagosomes, autophagolysosomes, and multivesicular bodies, fewer myelinated axons, and membranous inclusions in glia at 3-weeks recovery, following a 6-week cuprizone diet (Figure 4).
Figure 4. Relative to WT mice, Gas6−/−Axl−/− DKO mice have swollen axons, fewer myelinated axons, and glial aggregates in the corpus callosum at 6-weeks cuprizone treatment+3-weeks recovery.
Electron micrographs of WT mice (A) show abundant remyelinated axons relative to the DKO mice (B,C). The arrow (B) points to a swollen axon containing autophagosomes, autophagolysosomes, and multivesicular bodies. Abnormal myelin figure (arrowhead) and aggregated membranous structure (asterisk) consistent with a zebra body is shown in (C).
Although the number of astrocytes and microglia/macrophages were the same in the DKO and WT groups of mice, the DKO mice had significantly more proinflammatory cytokine expression and altered suppressor of cytokine signaling (SOCS) mRNA expression relative to WT corpora callosa, supporting a role for Gas6/Axl signaling in proinflammatory cytokine suppression. At 5-and 6-weeks cuprizone treatment, DKO mice had significant motor deficits when compared to WT mice on balance beams and in a negative geotaxis assay. These data suggest that Gas6/Axl signaling plays an important role in maintaining axonal integrity and regulating and reducing CNS inflammation that impairs remyelination and is not compensated for by ProS1/Tyro3/Mertk signaling (Ray, et al., 2017a).
7.6. Tyro3−/−, Axl−/−, Mertk−/− triple KO mice
Tyro3, Axl, Mertk triple knock-out mice have neurologic abnormalities, physiological deficits, and autoimmune defects. Increased apoptosis and cellular degeneration are apparent in the CNS and male mice produce no sperm (Lu, et al., 1999). The mice appear to have a lupus-like phenotype, likely as result of loss of Mertk, since Mertk−/− mice produce antibodies to chromatin, DNA, and IgG (Cohen, et al., 2002). The BBB of TAM TKO mice are compromised and show increased permeability to Evans blue and fluorescent-dextran. As a result of the compromised BBB, the TKO mice show increased T cell infiltrates into the brain, ubiquitin-reactive aggregates in the hippocampus, damage of the hippocampal mossy fibers, lipofuscin within neurons throughout the brain, and neuronal apoptotic death as a result of the increased inflammation that included elevated TNF-α and specific autoantibodies deposited onto brain blood vessels (Q. Li, Lu, Lu, Tian, & Lu, 2013).
8. Gas6 administration to the CNS of mice during EAE and cuprizone
The above studies have led us to explore whether the in vivo administration of recombinant human Gas6 administered to WT mice would protect against oligodendrocyte death in the cuprizone model. The rationale for starting in this model versus EAE is the proximity of the corpus callosum to the ventricular space making it a perfect system to administer signaling molecules and monitor the effect at multiple stages of cuprizone-induced toxicity and recovery. Using a 14-day minipump connected to a cannula implanted into the corpus callosum, rhGas6 or PBS was administered onto the corpus callosum. Gas6 treatment resulted in more efficient repair following cuprizone-induced injury. Relative to PBS-treated mice, Gas6 treatment of WT mice showed minimal OilRedO+ droplets, fewer SMI32+ and APP-positive axonal swellings, and enhanced remyelination. Increasing the concentration of rhGas6 enhanced its beneficial effects (Tsiperson, Li, Schwartz, Raine, & Shafit-Zagardo, 2010).
As demonstrated in Figure 5, administration of rhGas6 to WT mice via AAV2/9 viral vectors or cannula (Gruber, et al., 2014) also proved to be beneficial following MOG-induced EAE. Using a 30 day minipump, artificial cerebral spinal fluid (ACSF) - or rhGas6-treated WT mice were compared during MOG-induced EAE. Relative to ACSF-treated mice, GAS6-treated mice had significantly reduced clinical scores during peak and chronic EAE; mice were sacrificed and examined 28 days after continual delivery. Spinal cords of mice receiving icv GAS6 for 28 days had preserved SMI31(+) phosphorylated neurofilament immunoreactivity, and significantly fewer SMI32(+) axonal swellings and spheroids, as well as less demyelination relative to ACSF-treated mice. The study also examined whether administration of interferon beta (IFNβ), a regimen used by individuals with MS, and shown to work in concert with Axl to reduced inflammation, would be beneficial when given in combination with Gas6. Alternate-day subcutaneous IFNβ injection in combination with rhGas6 did not enhance GAS6 treatment effectiveness (Gruber, et al., 2014).
Figure 5. Gas6-AAV9 reduces clinical scores during acute but not chronic EAE.
A. Immunofluorescent staining and microscopy show GFP staining in the cervical spinal cord of male mice injected IV with GFP-AAV2/9. Bar is 50 µm. B. Mice were sensitized with MOG35–55 peptide and Ptx on day 0, and Ptx on day 2. Six days after the second Ptx injection, equivalent AAV9 viral particles were injected IV into the tail vein in a 0.2ml volume. Mice were scored on a 0–5 scale. There was no significant difference in the onset of disease of the groups of mice. Therefore, data from two experiments was normalized to the onset of clinical signs of disease once a clinical score of 1 was obtained. *significance p<0.05, Mann-Whitney. C. qRT-PCR demonstrates increased Gas6 expression in liver, but not in brain or spinal cord at day 32 post-MOG injection. D. SMI32 staining of ventral spinal cord of GFP-AAV9 and Gas6-AAV9 mice shows no significant difference in SMI32+ axonal swellings; p>0.05. Visualization is by DAB. Bar is 200 µm. E. Quantification of the mean number of SMI32+ axonal swellings in ventral lumbar spinal cords of multiple GFP-AAV9 (n=4) and Gas6-AAV9 mice (n=5) mice; p>0.05. Gas6 and GFP were cloned into AAV2/9, grown and purified by the Penn Vector Core University of Pennsylvania. The vector was obtained by Dr. Brian Kaspar are driven by the chicken beta actin CMV hybrid promoter. AAV2/9 contains AAV2’s inverted terminal repeats with the AAV9 capsid.
An unpublished study showed that addition of rhGas6 via icv cannula to Axl−/− mice was not beneficial during EAE (Figure 6). The data demonstrate the importance of Gas6/Axl signaling within the CNS and its beneficial effects during EAE.
Figure 6. Administration of rhGas6 to Axl−/− mice is not beneficial.
Axl−/−mice (n=8/group)) were sensitized with MOG35–55 peptide. Six days after the second pertussis toxin injection, minipumps were connected to the cannula, inserted under the scapula,
8.1. Loss/defects in TAM receptors in hypothalamic neuronal populations contribute to alterations in reproduction and appetite
The hypothalamus is responsible for multiple metabolic processes including sleep, hunger, regulating body temperature, circadian rhythms, thirst and fatigue. The hypothalamus links the autonomic nervous system with the endocrine system by secreting releasing hormones, also called neurohormones, which regulate pituitary hormone function. Gonadotropin releasing hormone (GnRH) neurons in the hypothalamus are responsible for normal female reproductive function. Axl and Tyro3 mediate the survival and appropriate targeting of GnRH neurons to the ventral forebrain, and contribute to normal reproduction in female mice. Axl/Tyro3 null mice have loss of GnRH neurons and sex hormone-induced gonadotropin surge resulting in estrous cycle abnormalities, with delayed sexual maturation, including delayed vaginal opening (A. Pierce, et al., 2008; A. Pierce, et al., 2011; Salian-Mehta, et al., 2014).
Recently, an arginine to tryptophan (R7W) mutation in the TYRO3 gene (C to T) was identified and it was determined that Tyro3 act as a modifier which contributes to severity of the weight loss phenotype in anorexic (anx/anx) mice. Abnormal Tyro3 mRNA localization is observed in anx/anx brains by postnatal day 19. Insertion of wild-type Tyro3 transgene, but not mutant Tyro3 transgene, in anx/anx mice increased the weight, the number of appetite-stimulating neuropeptide Y-expressing neurons and the lifespan of the Tyro3-transgenic anx/anx mice at P19, but not the mutant Tyro3 trangene, suggesting that Tyro3 has a modifying role in the hypothalamic appetite regulatory circuitry (Kim, et al., 2017).
9. The role of the TAM kinases and their ligands at the Blood Brain Barrier (BBB)
The blood–brain barrier (BBB) which lines capillaries is a dynamic, semipermeable barrier that efficiently separates the circulating blood from the brain. The BBB is comprised of non-fenestrated brain capillary endothelial cells (BCECs) which allow for the passive diffusion of water, some gases, amino acids, glucose, hormones and lipid-soluble molecules, and restricts larger molecules and pathogens (Abbott, Ronnback, & Hansson, 2006). The BCECs are sealed by tight junctions that restrict entry of compound and limit entry of peptides and certain drugs. The BCECs are sustained by pericytes which are embedded in the vascular basement membrane and by astrocyte endfeet (Abbott, Patabendige, Dolman, Yusof, & Begley, 2010; Armulik, et al., 2010). BCECs, pericytes, and astrocytes synthesize and deposit proteins extracellularly which form the vascular basement membrane (Gautam, Zhang, & Yao, 2016; Sixt, et al., 2001; Thomsen, Birkelund, Burkhart, Stensballe, & Moos, 2017; Thomsen, Routhe, & Moos, 2017; Yao, Chen, Norris, & Strickland, 2014).
ProS1 is expressed by vascular endothelial cells (ECs), which function in coagulation, and vascular development (Benzakour & Kanthou, 2000; Carmeliet, 2001; Fair, Marlar, & Levin, 1986; Stern, Brett, Harris, & Nawroth, 1986). Lack of ProS1 in mice causes lethal coagulopathy, ischemic/thrombotic injuries, vascular dysgenesis, and BBB disruption with intracerebral hemorrhage. A beneficial role for ProS1 at the BBB was shown using human brain endothelial cells. In this system, ProS1 inhibited BBB breakdown. RNAi treatment, and blocking antibodies to Tyro3, Axl, and Mertk, as well as brain endothelial cells from Tyro3−/−, Axl−/− and Mertk−/−mice, showed that Tyro3 mediates ProS1 vascular protection. 2-photon in vivo imaging showed that ProS1 blocked post-ischemic BBB disruption in Tyro3+/+, Axl−/−, and Mer−/− mice, but not in Tyro3−/− mice, or Tyro3+/+ mice receiving low-dose of the S1P1-specific antagonist W146, indicating that ProS1 maintains BBB integrity by signaling through Tyro3 and SIP1 receptor (Zhu, et al., 2010). This study supports a protective role for ProS1/Tyro3 at the BBB. An earlier study determined that Axl is significantly elevated in response to vascular injury (Melaragno, et al., 1998). Although ProS1 cannot directly activate Axl it is feasible that Tyro3 heterodimerizes with Mertk and/or Axl to activate Axl in response to vascular injury.
The entry of viruses into the brain was examined in TAM-deficient mice. Many enveloped viruses display phosphatidylserine on the outer leaflet of their membranes, possibly enabling TAM receptor activation allowing for viral entry and replication via downregulation of antiviral responses. Mice lacking Mertk−/−Axl−/−, but not Tyro3, were most vulnerable to infection with neuroinvasive West Nile and La Crosse encephalitis viruses, where increased blood-brain barrier permeability and enhanced virus entry and infection in the brain were observed. A study using the Mertk−/−Axl−/− knockout mouse model showed that their expression was required for interferon-beta (IFNβ) activity and for maintaining BBB integrity (Miner, et al., 2015).
10. Mertk expression in the retina
As a result of light and stress, the outer segment of photoreceptor cells are continually shed, renewed and cleared by retinal pigment cells (Young & Bok, 1969). Multiple studies have explored the TAM receptors and ligands expressed in the retina, as a result of studies from the Royal College of Surgeons (RCS) rats that demonstrated a role for Mertk and an association with inherited retinal degeneration. As RCS rats mature they became blind as a result of a failure of retinal pigment epithelium to efficiently phagocytize shed photoreceptor outer segments, resulting in the progressive loss of rod and cone photoreceptors (Bok & Hall, 1971). RCS rats were found to have mutations in Mertk resulting in retinitis pigmentosa-like disease similar to humans (Abu-Safieh, et al., 2013; D’Cruz, et al., 2000; Duncan, et al., 2003; X. Liu, Zhang, He, Zhao, & Su, 2015; Nandrot, et al., 2000). Somatic gene transfer of adenovirus-containing Mertk via sub-retinal injection reversed the phenotype in the RCS rats, and provided genetic evidence for Mertk having a crucial role in retinal pigment epithelial cell clearance of the outer segment of photoreceptor cells (Vollrath, et al., 2001). While Gas6 and recombinant ProS1 can facilitate phagocytosis of outer segments of cultured rat retinal pigment epithelial cells via a Mertk dependent pathway, Gas6−/− mice do not become blind suggesting that ProS1, or additional ligands may signal to Mertk to clear shed photoreceptor cells (Hall, Obin, Heeb, Burgess, & Abrams, 2005; Parinot & Nandrot, 2016; Prasad, et al., 2006). Retinal deletion of either Gas6 or ProS1 also results in normal photoreceptor number in retinae; however, deletion of both ProS1 and Gas6 reproduces photoreceptor death that is observed in Mertk mutant mice (Burstyn-Cohen, et al., 2012).
As a result of the RCS rat and in vitro retinal pigment epithelial cell culture studies, several ligands have been reported to activate Mertk and induce phagocytosis. Tubby and tubby-like protein 1 (Tulp1) are bridging molecules that bind to Mertk and facilitate phagocytosis of retinal pigment epithelium. Tulp1 was found to interact with Tyro3, Axl and Mertk, and tubby binds only to Mertk. Excess soluble Mertk extracellular domain can block tubby- or Tulp1-mediated phagocytosis (Caberoy, Zhou, & Li, 2010). Galectin-3 (Gal-3) was also shown to be a ligand for Mertk using a phagocytosis-based functional cloning assay strategy. Mertk co-immunoprecipitated with Gal3, and Gal3 stimulated the phagocytosis of debris and apoptotic cells by both retinal pigment epithelial cells and macrophages with concomitant activation and phosphorylation of Mertk. Similar to the tubby/Tulp1 study, Gal-3-mediated phagocytosis was abrogated by excessive soluble Mertk extracellular domain and lactose (Caberoy, Alvarado, Bigcas, & Li, 2012). Thus, in the retina, several ligands can activate Mertk to induce the clearance of cell debris. Homeostatic phagocytosis, similar to the necessity for clearance of the outer segment of the photoreceptors cells of the retina, is a function contributed to Mertk in several cells of the nervous and immune systems (Scott, et al., 2001; Zagorska, Traves, Lew, Dransfield, & Lemke, 2014). The rapid clearance of apoptotic cells reduces the risk of tissue destruction as a result of prolonged inflammation that can lead to autoimmunity.
11. A role for Mertk and MEGF10 in synapse pruning in developing and adult brain
Astrocyte arrays of transcripts enriched in select pathways identified Mertk and multiple EGF-like-domains 10 protein (MEGF10) as highly expressed in the phagocytic pathway. Both proteins localize to astrocytes in developing and adult astrocytes where they function to phagocytize and prune synapses, predominantly dendritic spines, in the somatosensory cortex. In the adult CNS, both excitatory and inhibitory synapses are pruned by astrocytes in retinogeniculate connections of the visual cortex. As part of this study, ProS1 was shown to activate Mertk in astrocytes in vitro (Chung, et al., 2013), and the studies highlighted the involvement of astrocytes and functional Mertk and MEGF10 in remodeling synapses and importantly, their ability to prune neural circuits in the developing and adult brain. The combined studies from the RCS rat, the mutant Mertk mice, and studies in the immune system (Zagorska, et al., 2014) all support a role for Mertk in homeostatic clearance of debris and remodeling. In the context of the central nervous system, efficient clearance of debris and synaptic pruning strategically place astrocytes in the vicinity for efficient sanitation and to minimalize damage as a result of a lag in clearance of debris. Microglia participate in homeostatic synapse pruning in the healthy, intact CNS where they scan the local environment and interact with astrocytes and neurons (Schafer, Lehrman, & Stevens, 2013). It is likely that Mertk functions in pruning within microglia similar to its role in astrocytes. This has significant implications for diseases such as MS, where timely clearance of damaged and dying cells and debris increase the likelihood of efficient repair. Synaptic sculpting is essential for learning and memory in the healthy brain where microglia and astrocytes function in developing and maturing synapses and in neurological diseases where cognitive deficits correlate with prolonged CNS damage. Studies in mouse frontal cortex demonstrated that changes in microglia activation and enhanced phagocytosis of synaptic elements but no neuroinflammation are observe in mice with chronic sleep restriction for approximately 5 days (Bellesi, et al., 2017). This potentially could result in further microglial activation and brain dysfunction. Serial scanning by electron microscopy combined with 3D measurements showed that presynaptic components of large synapses associated with astrocytic process were altered in mice with sleep deprivation, and chronic sleep restriction relative to wakefulness suggesting that phagocytosis and synaptic activity accounts for changes in clearance of what the authors refer to as “heavily-used synapses”. In addition, correlative studies showed Mertk expression was increased after a few hours of wakefulness and some astrocytic processes were Mertk-positive suggesting that increased Mertk expression is associated with acute and chronic sleep loss. Isolation of cortical synaptoneurosomes from mice with wakefulness, acute, and chronic sleep loss showed increased Mertk protein expression but was not linked to duration of sleep loss (Bellesi, et al., 2017).
12. TAM receptors/ligands in Stem Cells
TAM receptors and their ligands have been shown to regulate the identity, survival, proliferation, and differentiation of neuronal stem cells. Using a dual reporter strategy to track doublecortin (DCX)-red fluorescent protein (RFP) and Nestin-green fluorescent protein (GFP), TAM family members were selectively Nestin+DTX neural progenitor cells relative to DCX+ cells (J. Wang, et al., 2011) which was confirmed (Gely-Pernot, et al., 2012). Further, TAM receptors were found to be important for the maintenance of cortical neural progenitor cells, as Axl−/− Mertk−/− double knockout or Axl inhibition caused more progenitor cells to leave the cell cycle and led to the accumulation of more migrating neurons beyond the ventricular zone (VZ)/subventricular zone (SVZ) (J. Wang, et al., 2011). Using multiple strategies, a dichotomous role for TAM ligands Gas6 and Pros1 was identified where Gas6 promotes SVZ stem cells proliferation and/or pool maintenance and Pros1 inhibits SVZ cell proliferation in vivo (Gely-Pernot, et al., 2012). Further, Pros1 was identified as a pleiotropic modulator of embryonic and adult NSC quiescence and differentiation wherein Pros1 acts as a negative regulator or NSPC self-renewal and controls NSPC differentiation via Bmi-1 signaling (Zelentsova-Levytskyi, et al., 2017; Zelentsova, et al., 2017). Neurospheres generated from Tyro3−/−Axl−/−Mertk−/− triple knockout mice demonstrate slower growth, decreased proliferation and increased apoptosis possibly due to decreased trophic support and this data is consistent with a report that Gas6 can replace nerve growth factor in PC12 cell growth and differentiation (Ji, et al., 2014; Zheng, et al., 2009). Finally, TAM receptors likely affect adult brain neurogenesis indirectly by suppressing microglial inflammatory signaling and efferocytosis (Fourgeaud, et al., 2016; Ji, et al., 2013).
13. Nervous System Tumors: Contribution of Tyro3, Axl, Mertk and their ligands to tumor migration and invasion
Tumors of the peripheral nervous system (PNS) and the CNS are aggressively studied to identify altered genes and proteins that can be targeted to limit tumor growth, migration, invasion as well as their role in the resistance to cancer therapies. Below, we highlight the types of tumors which express one or more TAM family member and/or ligand and address potential roles in the tumor progression and treatments. One or more of the TAM receptors are overexpressed in several human cancers, including neuroblastomas, breast, and head and neck cancers, and is associated with progression, invasion, and resistance to therapy (Debruyne, et al., 2016; Giles, et al., 2013; Meyer, et al., 2013). This overview is by no means meant to be a comprehensive review of the field but rather, to note the potential role(s) of TAM family members, and to highlight that several of the proposed cancer therapies are targeting family members; reviewed in (Akalu, Rothlin, & Ghosh, 2017; Linger, Keating, Earp, & Graham, 2010). Mutations in TAM family members have not been found in any cancer type. The upregulation likely reflects it role in cell migration and phagocytosis. While the primary focus of tumor treatment is the shrinkage or total elimination of the tumor, contemplation of the many important roles of the TAMs in the CNS, including cell survival and myelination, should be considered as post-cancer therapies often result in significant neuropathies including axonal degeneration of distal nerve endings resulting in dying back of axons (Fukuda, Li, & Segal, 2017).
13.1. Gliomas
are a diverse group of tumors which encompass astrocytomas, oligodendrogliomas, mixed glioma often referred to as oligoastrocytomas, brain stem gliomas, ependymomas, and glioblastoma multiforme (GBMs). GBMs are the most aggressive group of brain tumors with an average survival time of 12 months, as a result of poor responsiveness to available treatments. Epithelial-to-mesenchymal transition (EMT) characterized as the transdifferentiation of epithelial cells into invasive migratory mesenchymal cells underlies cancer progression and poor patient survival. Several glioma studies have targeted Axl, since high expression of Axl in glioblastoma cells support a role in EMT and migration; reviewed in (Nakada, et al., 2013). Phospho-Axl was shown to be expressed in 74% of newly diagnosed individuals with GBM (Onken, et al., 2017). Expression of phospo-Axl in the tumor vasculature and areas of hypercellularity were associated with a significant decrease in survival. As such, numerous studies using multiple approaches have shown that Axl, Mertk and their ligands appear in microarrays and proteomic studies and strongly correlate with glioma progression, migration and chemoresistance (Argaw, et al., 2012; Cheng, et al., 2015; Hill, Moreno, Lam, Haqqani, & Kelly, 2009; Hutterer, et al., 2008; Keating, et al., 2010; Knubel, et al., 2014; Krause, et al., 2015; Martinho, Zucca, & Reis, 2015; Onken, et al., 2016; Ott, et al., 2012; Rogers, et al., 2012; Staflin, Zuchner, Honeth, Darabi, & Lundberg, 2009; Sufit, et al., 2016; Vajkoczy, et al., 2006; Vouri, An, Birt, Pilkington, & Hafizi, 2015; Y. Wang, et al., 2013; Yen, et al., 2016). Mesenchymal gliomas tended to express highest levels of MERTK (Popova, et al., 2014). In addition, one study showed a correlation between high expression of Mertk in individuals with t(1;19)-positive acute lymphoblastic leukemia and a subsequent tumor infiltration into the CNS (Krause, et al., 2015).
cDNA expression arrays identified TYRO3 among genes down-regulated to less than 50% of normal levels by gene expression profiling of diffuse astrocytomas (H. Huang, et al., 2000). Tyro3 is expressed at very high levels in the adult CNS, and its downregulation is consistent with the dedifferentiated phenotype taken on by gliomas. While not extensively studied in nervous system tumors, Tyro3 was reported to induce the expression of a master regulator of EMT transition SNAI1in colon cancer (Chien, et al., 2016).
13.2. Peripheral nerve tumors
13.2.1. Schwannomas
are tumors of the PNS, and are classified as benign nerve sheath tumors composed of Schwann cells, the cell type that produces myelin proteins that generate the myelin sheath that wrap peripheral axons. Gas6 and Axl activation of NFκB was found to correlate with pathological proliferation, adhesion and survival in Schwannoma (Ammoun, et al., 2014). Following nerve crush Schwann cells up-regulate Axl and Mertk, and Schwann cells lacking Axl and Mertk have significantly impaired myelin phagocytosis. The study showed that Axl along with Mertk, MEGF10 and LRP1 are involved in the clearance of myelin debris from Schwann cells (Brosius Lutz, et al., 2017). As noted by Brosius Lutz, unlike Schwann cells, oligodendrocytes cannot contribute to myelin debris clearance since they lack phagocytic machinery (Brosius Lutz & Barres, 2014; Brosius Lutz, et al., 2017; Cahoy, et al., 2008; Gomez-Sanchez, et al., 2015).
13.2.2. Neurofibromas
are benign, small localized tumors along the sheath of peripheral nerve; they tend to grow near the budding of the nerve, and are prevalent in individuals with Neurofibromatosis type 1. Examination of Axl expression in malignant peripheral nerve sheath tumor (MPNST) cell lines and primary MPNST tissues found that there was an increase in Axl expression when compared to normal human Schwann cells. Also, soluble Axl protein was detected in the conditioned media from MPNST cell lines. When treatments targeted tumor growth, there was a reduction in soluble Axl relative to the controls (Johansson, et al., 2014). Relative to less aggressive dermal neurofibromas, soluble Axl is significantly increased in human plexiform neurofibroma tumors, supporting a role of sAXL as a marker for neurofibromatosis type 1-related tumor burden.
13.3. ProS1
Targeting of PROS1 as a therapeutic strategy in GBM treatment was proposed following siRNA treatment in LN18 glioma cells. Relative to untreated cells, PROS1 knockdown by siRNA showed decreased cell proliferation, increased apoptosis, a reduction in cell migration and invasion, and a downregulation of GAS6 and TAM receptors (Che Mat, et al., 2016). While promising, a potential concern with targeting ProS1 is its protective role at the BBB. While GBMs and gliomas are brain-derived, it is possible that other confounding prodromal insults might develop as a result of a compromised innate immune response.
13.4. Tumor Treatment Strategies
Resistance to chemotherapy or altered cellular functions that enhance metastasis including EMT transition, migration, invasion, and angiogenesis serve as significant challenges for cancer treatment. Due to the roles of the TAMs in the nervous system, major focus on the potential therapeutic treatments resulting in Axl and Mertk inhibition in solid tumors are ongoing, including small molecule tyrosine kinase inhibitors, siRNAs, monoclonal antibodies, and fusion proteins (Linger, et al., 2010). The use of immunotherapies for treatment of solid tumors that are unresponsive to standard radiation and chemotherapy, include the use of patient’s genetically engineered T cells that express chimeric antigenic receptors, monoclonal antibodies to block adaptive immune checkpoints, and vaccines. Current studies are considering the TAMs and their role in the regulation of the innate immune response as candidates for checkpoint blockade as targets of cancer immunotherapy. An advantage of using direct Axl and Mertk inhibitors is the potential for action on both tumor cells as well as cells in the tumor microenvironment where resident macrophages express TAM receptors. Also, tumor-infiltrating dendritic cells and macrophages express significantly higher levels of Gas6 than normal tissue macrophages, indicating that Gas6 and TAM family members expressed by stromal immune cells contribute to tumor growth and metastasis (Loges, et al., 2010). Studies show that inhibition of Axl and Mertk lead to increased chemosensitivity to temozolomide, carboplatin, and vincristine in astrocytoma cells, and to cisplatin and vincristine in neuroblastoma cells (Keating, et al., 2010; Y. Li, Wang, Bi, Zhao, & Yu, 2015). There are inhibitors for TAM members, including R428 or BGB324 for Axl (Ghosh, et al., 2011; Hector, et al., 2010; Holland, et al., 2010; Y. Li, et al., 2009; Rettew, et al., 2012), and UNC2025 and UNC1666 for Mertk; although, there is some cross-reactivity with other kinases such as FLT3 (Cummings, et al., 2015; Lee-Sherick, et al., 2015; von Massenhausen, et al., 2016; W. Zhang, et al., 2014). A 2017 review highlights the role of TAM family members as “tumor drivers and mediators of chemoresistance”, and reviews how targeted therapeutic strategies might be used to improve cancer therapy (Vouri & Hafizi, 2017). It should be noted that inhibitors targeting TAMs for CNS tumors must cross the BBB to be effective, although there may be some barrier compromise as a result of tumor progression.
Long-term use of TAM inhibitors would not be recommended. Rats with mutations in Mertk become blind as a result of an inability to clear shed photoreceptor cells and mice with mutations in Mertk have retinitis pigmentosa-like disease (Abu-Safieh, et al., 2013; X. Liu, et al., 2015; Nandrot, et al., 2000). As noted, neuropathies are reported following several cancer therapy regiments, and thus, the many significant roles in which the TAMs participate within the nervous system should be considered. It is feasible that autoimmunity might develop as a result of inhibiting TAM kinase functions and their roles in regulating the innate immune response and tissue homeostasis. Finally, because TAM receptors restrict pathogenesis of neuroinvasive viruses, care should be taken when considering TAM receptor antagonists, which are in various stages of development as cancer therapies (Miner, et al., 2015; Suarez, et al., 2013; Verma, Warner, Vankayalapati, Bearss, & Sharma, 2011).
14. TAM receptors in CNS viral infection
The TAM receptor kinases play a variety of complex, non-synonymous roles that contribute both positively and negatively in viral immunity. These functions include phosphatidylserine (PtdSer) mediated viral entry, suppression of the innate immune system, and maintenance of the BBB. As previously described, the TAM receptors mediate efferocytosis of apoptotic cells by bridging the exposed PtdSer via Gas6 and or Pros1. Similarly, enveloped viruses displaying PtdSer, including but not limited to, Dengue, West Nile, Ebola, Marburg and Zika, utilize this mechanism to mediate entry into TAM expressing cells (Hamel, et al., 2015; Morizono, et al., 2011; Shimojima, et al., 2006). Additionally, TAM receptor activation can dampen inflammatory signaling by innate immune cells including macrophages, microglia and dendritic cells (Grommes, et al., 2008; Lemke & Rothlin, 2008; Rothlin, et al., 2007; Zagorska, et al., 2014). Therein, enveloped viruses, flaviviruses and pseudotyped retroviruses, can take advantage of this function by disabling the innate immune responses in dendritic cells by direct activation of TAM receptors by dampening of type I interferon signaling (Bhattacharyya, et al., 2013).
TAM receptors may also play protective roles in CNS viral immunity. For instance the role of TAMs in the BBB was described in a previous section; however, TAM signaling in neuroinvasive West Nile and La Crosse viral infection was demonstrated by Miner et al 2015. The loss of Mertk and/or Axl enhanced viral infection, and this was driven by increased BBB permeability (Miner, et al., 2015). Also, infected cells exposing PtdSer on their outer leaflet can also trigger phagocytosis of surveilling microglia and subsequent activation of the inflammatory response by innate immune cells via a TAM-dependent mechanism (Tufail, et al., 2017). This was shown to be the case when replication-incompetent adenovirus 5 was injected into the CNS triggering phospholipid scramblase 1 activity in infected cells, resulting in PtdSer externalization and targeting infected cells for TAM-dependent microglial phagocytosis (Tufail, et al., 2017).
With the emerging enveloped flavivirus Zika dominating the news headlines the role for TAM receptors and specifically Axl has become a very active area of study. Zika virus displays neurotropism and neurological manifestations particularly when infection occurs during fetal development. The combination of high TAM receptor expression in neural and glial cells during development, and TAM receptors described roles in enveloped virus infectivity made Axl a plausible viral entry receptor in neural stem cells (Broutet, et al., 2016; Dick, 1952; Nowakowski, et al., 2016). While it is widely shown that Axl can play a role in Zika virus infection, more in- depth studies have led to conflicting reports on the degree to which Axl plays in Zika virus infectivity. First, it has been clearly demonstrated that by using a knockout strategy Axl and the TAM receptors are not required for Zika virus infection in mice (Hastings, et al., 2017) (Z. Y. Wang, et al., 2017). However, in human cells, inhibition of Axl blocks Zika virus infection (Hamel, et al., 2015; S. Liu, DeLalio, Isakson, & Wang, 2016; Meertens, et al., 2017; Retallack, et al., 2016; Savidis, et al., 2016). Further, it was demonstrated in human glial cells that Axl plays a role in both promoting viral entry and antagonizing protective type I interferon signaling (Meertens, et al., 2017), and CNS cells are more predisposed to infection (Cumberworth, et al., 2017). However, a genetic ablation strategy does not protect human neural progenitor cells and cerebral organiods from Zika virus infection (Wells, et al., 2016). A well-reasoned hypothesis explaining the difference between mouse and human cells was described (Hastings, et al., 2017), wherein mouse TAM receptors may not in fact serve as Zika virus receptors, there may be other entry receptors in mice that are not expressed by human cells, and/or human cells in vivo may also express redundant entry receptors but in vitro cells fail to recapitulate the expression of the viral entry receptors present in vivo. Additionally, different cell types may express different entry receptors such that in vitro systems may not adequately reflect in vivo infectivity and may explain some differences observed in the literature. Finally, while to our knowledge heretofore unexplored, TAM receptor signaling provides a strong cell-survival signal; therefore, cells which are infected by enveloped viruses may create an autocrine feedforward cycle of TAM receptor activation permitting persistent infection. If this occurs, it would provide evidence that treatment TAM inhibitors could diminish certain chronic viral infections by allowing for normal cell death and removing the negative regulation of interferon signaling. However, due to the diverse roles that TAM receptors play in CNS viral immunity it is critical to carefully consider pharmacological modulation of these signaling pathways.
15. Conclusions, Perspectives and Future Studies
In conclusion, the TAM receptors and their ligands are critical for diverse roles in the nervous system as well as in the immune system. There are still many avenues remaining to be explored including the regulation of their downstream signaling, and diverse groups of binding partners.
There are likely still many functions and surprises left in store for this important receptor tyrosine kinase family, including consideration of additional potential binding partners for Axl and the other receptors in the CNS. Using a yeast two-hybrid screen for protein-protein interactions, human Axl cytoplasmic domain was found to interact with C1-TEN, an SH2-containing C1 domain-containing phosphatase and TENsin homologue focal adhesion protein, expressed in liver, muscle and human brain (Hafizi, Alindri, Karlsson, & Dahlback, 2002; Kikuno, et al., 1999). Axl and C1-TEN interaction was confirmed by co-immunoprecipitation (Hafizi, Alindri, Karlsson, & Dahlback, 2002). Stable expression of C1-TEN in HEK293 cells showed that C1-TEN negatively regulated Akt signaling, similar to phosphatase and tensin homologue deleted from chromosome 10 (PTEN; (Hafizi, Ibraimi, & Dahlback, 2005; Leslie & Downes, 2002), and that p62/SQSTM1 negatively regulates C1-TEN expression and degradation, but no other tensins; thus linking C1-TEN to autophagy (Koh, et al., 2014). Gas6 Axl signaling can induce autophagy (Han, et al., 2016) making it feasible for oligodendrocytes to eliminate some of their cell content by autophagy. Perhaps, microglia and astrocytes can aid in the phagocytosis of myelin debris following a demyelinating event. Therefore, Gas6 TAM receptor signaling and recruitment of its binding partners should be further explored in the context of remyelination.
Additionally, an intriguing report by Healy et al 2017, noted a defect in the clearance of myelin debris by macrophages generated by individuals with RRMS and SPMS. These studies strongly support a neuroimmune function for TAM receptors and their ligands in the etiology of MS and possibly other autoimmune diseases (Healy, et al., 2017; Healy, et al., 2016).
Finally, at the time of writing this review, several exciting reports identifying microglial phenotypes clearly demonstrate the importance of TAM receptors in homeostatic and possible pathological functions of microglia in neurological disease. These reports indicate the clear relevance of current and future study of these pathways’ function in health and disease, as this work could lead to future therapeutic strategies for neuroinflammatory and/or neurodegenerative diseases (Butovsky, et al., 2014; Keren-Shaul, et al., 2017; Krasemann, et al., 2017; Mathys, et al., 2017).
Acknowledgments
We thank Ms. Hillary Guzik for help with images, Ms. Xheni Nishu for help with electron microscopy, Dr. Bryan Kaspar for AAV2/9 vectors, and Dr. Cedric Raine for his expert advice on electron microscopy. We are grateful to Dr. Pablo Garcia deFrutos for educating us on the functional differences between total and free ProS1, and guiding us toward appropriate references. We thank Ms. Kathleen O’Guin for her help in establishing the human co-cultures.
Abbreviations
- ACSF
artificial cerebral spinal fluid
- APP
amyloid precursor protein
- BBB
blood-brain-barrier
- BCECs
brain capillary endothelial cells
- C57Bl6/J
strain of mice for MOG-induce EAE and cuprizone studies
- CNS
central nervous system
- DKO
Gas6−/− Axl−/− double knockout mice
- EAE
experimental autoimmune encephalopathy
- ECs
endothelial cells
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EM
electron microscopy
- EPO
erythropoietin
- Gal3
Galectin-3
- Gas6
growth arrest-specific protein 6
- GBM
glioblastoma multiforme
- GFAP
glial fibrillary acidic protein, astrocyte-specific protein
- H+E
hematoxylin and eosin stain
- Iba1
ionized calcium adaptor binding protein-1
- ICV
intracerebroventricular
- IF
immunofluorescence
- IFNR
interferon-alpha/beta receptor (IFNR)
- IHC
immunohistochemistry
- IP
intraperitoneal
- IV
intravenous
- KO
knockout
- MA
Macroautophagy
- MBP
myelin basic protein
- MEGF10
multiple EGF-like-domains 10 protein
- Mertk
Mer receptor tyrosine kinase
- MMPs
matrix metalloproteases
- MOG
myelin oligodendrocyte glycoprotein
- MOG-induced EAE
myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis
- MPNST
malignant peripheral nerve sheath tumor
- MS
Multiple Sclerosis
- NAWM
normal appearing white matter
- NF
neurofilament
- OLIG1 and OLIG2
oligodendrocyte lineage transcription factor 1 and 2
- OLs
oligodendrocytes
- OPCs
oligodendrocyte progenitor cells
- PDGFRα
platelet-derived growth factor receptor α
- PI3K
Phosphoinositide 3-kinase
- PLP
proteolipid protein
- pNF
phosphorylated neurofilament protein
- PNS
peripheral nervous system
- ProS1
ProteinS1
- PtdSer
phosphatidylserine
- Ptx
pertussis toxin
- RCS mice
Royal College of Surgeons mice
- rhGas6
recombinant human Gas6
- RRMS
relapsing remitting Multiple Sclerosis
- Secs
seconds
- SIP1
sphingosine-1-phosphate
- siRNA
small (or short) interfering RNA
- SOCS
Suppressor of cytokine signaling
- SVZ
subventricular zone
- STAT
Signal transducer and activator of transcription
- TAM receptors
Tyro3, Axl, Mertk tyrosine kinase receptors
- Tulp1
tubby like protein 1
- WT
wildtype mice, C57Bl6/J mice, same background as KO mice;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no actual or potential conflict of interest including any financial, personal or other relationships with individuals or organizations within three years of initiating the work that could inappropriately influence, or be perceived to influence, the study design or data interpretation.
Literature Cited
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, Al-Zahrani J, Al-Abdi L, Hashem M, Al-Tarimi S, Sebai MA, Shamia A, Ray-Zack MD, Nassan M, Al-Hassnan ZN, Rahbeeni Z, Waheeb S, Alkharashi A, Abboud E, Al-Hazzaa SA, Alkuraya FS. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23:236–247. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, Pellegrini-Giampietro DE. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36:975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276:165–177. doi: 10.1111/imr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkermann R, Aprico A, Perera AA, Bujalka H, Cole AE, Xiao J, Field J, Kilpatrick TJ, Binder MD. The TAM receptor Tyro3 regulates myelination in the central nervous system. Glia. 2017;65:581–591. doi: 10.1002/glia.23113. [DOI] [PubMed] [Google Scholar]
- Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201. doi: 10.1210/mend.13.2.0230. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton DA, Hafizi S, Hanemann CO. Axl/Gas6/NFkappaB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2014;33:336–346. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- Angelillo-Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, Herbert JM, Lemke G, Goff SP, Matsushima GK, Earp HS, Vesin C, Hoylaerts MF, Plaisance S, Collen D, Conway EM, Wehrle-Haller B, Carmeliet P. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest. 2005;115:237–246. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelillo-Scherrer A, Burnier L, Lambrechts D, Fish RJ, Tjwa M, Plaisance S, Sugamele R, DeMol M, Martinez-Soria E, Maxwell PH, Lemke G, Goff SP, Matsushima GK, Earp HS, Chanson M, Collen D, Izui S, Schapira M, Conway EM, Carmeliet P. Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest. 2008;118:583–596. doi: 10.1172/JCI30375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Avanzi GC, Gallicchio M, Bottarel F, Gammaitoni L, Cavalloni G, Buonfiglio D, Bragardo M, Bellomo G, Albano E, Fantozzi R, Garbarino G, Varnum B, Aglietta M, Saglio G, Dianzani U, Dianzani C. GAS6 inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J Neurosci. 2017;37:5263–5273. doi: 10.1523/JNEUROSCI.3981-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellosta P, Zhang Q, Goff SP, Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–2397. doi: 10.1038/sj.onc.1201419. [DOI] [PubMed] [Google Scholar]
- Benzakour O, Kanthou C. The anticoagulant factor, protein S, is produced by cultured human vascular smooth muscle cells and its expression is up-regulated by thrombin. Blood. 2000;95:2008–2014. [PubMed] [Google Scholar]
- Bhattacharyya S, Zagorska A, Lew ED, Shrestha B, Rothlin CV, Naughton J, Diamond MS, Lemke G, Young JA. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Fox AD, Merlo D, Johnson LJ, Giuffrida L, Calvert SE, Akkermann R, Ma GZ, Perera AA, Gresle MM, Laverick L, Foo G, Fabis-Pedrini MJ, Spelman T, Jordan MA, Baxter AG, Foote S, Butzkueven H, Kilpatrick TJ, Field J. Common and Low Frequency Variants in MERTK Are Independently Associated with Multiple Sclerosis Susceptibility with Discordant Association Dependent upon HLA-DRB1*15:01 Status. PLoS Genet. 2016;12:e1005853. doi: 10.1371/journal.pgen.1005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Kilpatrick TJ. TAM Receptor Signalling and Demyelination. Neurosignals. 2009;17:277–287. doi: 10.1159/000231894. [DOI] [PubMed] [Google Scholar]
- Binder MD, Xiao J, Kemper D, Ma GZ, Murray SS, Kilpatrick TJ. Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PLoS One. 2011;6:e17727. doi: 10.1371/journal.pone.0017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF. Demyelination of the superior cerebellar peduncle in the mouse induced by cuprizone. J Neurol Sci. 1973a;20:63–72. doi: 10.1016/0022-510x(73)90118-4. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973b;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell. 2014;28:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Chung WS, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, Barres BA. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1710566114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, Espinal M, Low N, Dye C. Zika Virus as a Cause of Neurologic Disorders. N Engl J Med. 2016;374:1506–1509. doi: 10.1056/NEJMp1602708. [DOI] [PubMed] [Google Scholar]
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T. TAM receptor signaling in development. Int J Dev Biol. 2017;61:215–224. doi: 10.1387/ijdb.160285tb. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest. 2009;119:2942–2953. doi: 10.1172/JCI39325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76:1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Alvarado G, Bigcas JL, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227:401–407. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. Embo j. 2010;29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127:564–568. doi: 10.1172/JCI90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016;113:6526–6531. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Biomedicine. Clotting factors build blood vessels. Science. 2001;293:1602–1604. doi: 10.1126/science.1064981. [DOI] [PubMed] [Google Scholar]
- Carrera Silva EA, Chan PY, Joannas L, Errasti AE, Gagliani N, Bosurgi L, Jabbour M, Perry A, Smith-Chakmakova F, Mucida D, Cheroutre H, Burstyn-Cohen T, Leighton JA, Lemke G, Ghosh S, Rothlin CV. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39:160–170. doi: 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellini I, Ghezzi P, Mengozzi M. Therapeutic efficacy of erythropoietin in experimental autoimmune encephalomyelitis in mice, a model of multiple sclerosis. Methods Mol Biol. 2013;982:163–173. doi: 10.1007/978-1-62703-308-4_10. [DOI] [PubMed] [Google Scholar]
- Chan MC, Mather JP, McCray G, Lee WM. Identification and regulation of receptor tyrosine kinases Rse and Mer and their ligand Gas6 in testicular somatic cells. J Androl. 2000;21:291–302. [PubMed] [Google Scholar]
- Che Mat MF, Abdul Murad NA, Ibrahim K, Mohd Mokhtar N, Wan Ngah WZ, Harun R, Jamal R. Silencing of PROS1 induces apoptosis and inhibits migration and invasion of glioblastoma multiforme cells. Int J Oncol. 2016;49:2359–2366. doi: 10.3892/ijo.2016.3755. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Wang YL, Lo WT, Wu CC, Hsieh CW, Huang CF, Lan YH, Wang CC, Chang DM, Sytwu HK. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:210–223. doi: 10.1111/j.1365-2249.2010.04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Phillips E, Kim SH, Taylor D, Hielscher T, Puccio L, Hjelmeland AB, Lichter P, Nakano I, Goidts V. Kinome-wide shRNA screen identifies the receptor tyrosine kinase AXL as a key regulator for mesenchymal glioblastoma stem-like cells. Stem Cell Reports. 2015;4:899–913. doi: 10.1016/j.stemcr.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CW, Hou PC, Wu HC, Chang YL, Lin SC, Lin SC, Lin BW, Lee JC, Chang YJ, Sun HS, Tsai SJ. Targeting TYRO3 inhibits epithelial-mesenchymal transition and increases drug sensitivity in colon cancer. Oncogene. 2016;35:5872–5881. doi: 10.1038/onc.2016.120. [DOI] [PubMed] [Google Scholar]
- Cho EJ, Kim YC, Hwang JH, Lee H, Park SS, Kim SY, Kim S, Chin HJ. Inherited protein S deficiency due to a novel nonsense mutation in the PROS1 gene in the patient with recurrent vascular access thrombosis: A case report. Kidney Res Clin Pract. 2012;31:72–75. doi: 10.1016/j.krcp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Crosier KE, Crosier PS. New insights into the control of cell growth; the role of the AxI family. Pathology. 1997;29:131–135. doi: 10.1080/00313029700169744. [DOI] [PubMed] [Google Scholar]
- Cumberworth SL, Barrie JA, Cunningham ME, de Figueiredo DPG, Schultz V, Wilder-Smith AJ, Brennan B, Pena LJ, Freitas de Oliveira Franca R, Linington C, Barnett SC, Willison HJ, Kohl A, Edgar JM. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected. Acta Neuropathol Commun. 2017;5:50. doi: 10.1186/s40478-017-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CT, Zhang W, Davies KD, Kirkpatrick GD, Zhang D, DeRyckere D, Wang X, Frye SV, Earp HS, Graham DK. Small Molecule Inhibition of MERTK Is Efficacious in Non-Small Cell Lung Cancer Models Independent of Driver Oncogene Status. Mol Cancer Ther. 2015;14:2014–2022. doi: 10.1158/1535-7163.MCT-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Debruyne DN, Bhatnagar N, Sharma B, Luther W, Moore NF, Cheung NK, Gray NS, George RE. ALK inhibitor resistance in ALK(F1174L)-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene. 2016;35:3681–3691. doi: 10.1038/onc.2015.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J Biol Chem. 2001;276:31738–31744. doi: 10.1074/jbc.M104457200. [DOI] [PubMed] [Google Scholar]
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86:1014–1021. doi: 10.1212/WNL.0000000000002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, Vollrath D. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44:826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- El-Moslimany H, Lublin F. Clinical features in multiple sclerosis. In: Raine MHCS, Hohlfeld R, editors. Multiple Sclerosis: A Comprehensive Text. Edinburgh: Saunders Elsevier; 2008. pp. 1–23. [Google Scholar]
- Fair DS, Marlar RA, Levin EG. Human endothelial cells synthesize protein S. Blood. 1986;67:1168–1171. [PubMed] [Google Scholar]
- Fang Z, Xiong X, James A, Gordon DF, Wierman ME. Identification of novel factors that regulate GnRH gene expression and neuronal migration. Endocrinology. 1998;139:3654–3657. doi: 10.1210/endo.139.8.6221. [DOI] [PubMed] [Google Scholar]
- Ferland G. Vitamin K, an emerging nutrient in brain function. Biofactors. 2012;38:151–157. doi: 10.1002/biof.1004. [DOI] [PubMed] [Google Scholar]
- Foley JH, Conway EM. Gas6 gains entry into the coagulation cascade. Blood. 2013;121:570–571. doi: 10.1182/blood-2012-11-468678. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu ET. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996;16:135–145. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Yamamoto T. brt, a mouse gene encoding a novel receptor-type protein-tyrosine kinase, is preferentially expressed in the brain. Oncogene. 1994;9:693–698. [PubMed] [Google Scholar]
- Fukuda Y, Li Y, Segal RA. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front Neurosci. 2017;11:481. doi: 10.3389/fnins.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68:150–160. doi: 10.1002/jnr.10211. [DOI] [PubMed] [Google Scholar]
- Garcia de Frutos P, Alim RI, Hardig Y, Zoller B, Dahlback B. Differential regulation of alpha and beta chains of C4b–binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood. 1994;84:815–822. [PubMed] [Google Scholar]
- Gautam J, Zhang X, Yao Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep. 2016;6:36450. doi: 10.1038/srep36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gely-Pernot A, Coronas V, Harnois T, Prestoz L, Mandairon N, Didier A, Berjeaud JM, Monvoisin A, Bourmeyster N, De Frutos PG, Philippe M, Benzakour O. An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche. Stem Cells. 2012;30:719–731. doi: 10.1002/stem.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Shishido T, Zong C, Hanafusa H. Biological effects of c-Mer receptor tyrosine kinase in hematopoietic cells depend on the Grb2 binding site in the receptor and activation of NF-kappaB. Mol Cell Biol. 1999;19:1171–1181. doi: 10.1128/mcb.19.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011;117:1928–1937. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD, Stuart LM, Goodall GJ, Leedman PJ. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther. 2013;12:2541–2558. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- Gohlke PR, Williams JC, Vilen BJ, Dillon SR, Tisch R, Matsushima GK. The receptor tyrosine kinase MerTK regulates dendritic cell production of BAFF. Autoimmunity. 2009;42:183–197. doi: 10.1080/08916930802668586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene. 1999;18:4224–4236. doi: 10.1038/sj.onc.1202788. [DOI] [PubMed] [Google Scholar]
- Goudarzi S, Rivera A, Butt AM, Hafizi S. Gas6 Promotes Oligodendrogenesis and Myelination in the Adult Central Nervous System and After Lysolecithin-Induced Demyelination. ASN Neuro. 2016:8. doi: 10.1177/1759091416668430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–140. doi: 10.1007/s11481-007-9090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber RC, Ray AK, Johndrow CT, Guzik H, Burek D, de Frutos PG, Shafit-Zagardo B. Targeted GAS6 delivery to the CNS protects axons from damage during experimental autoimmune encephalomyelitis. J Neurosci. 2014;34:16320–16335. doi: 10.1523/JNEUROSCI.2449-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Alindri F, Karlsson R, Dahlback B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun. 2002;299:793–800. doi: 10.1016/s0006-291x(02)02718-3. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Ibraimi F, Dahlback B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. Faseb j. 2005;19:971–973. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Bae J, Choi CY, Choi SP, Kang HS, Jo EK, Park J, Lee YS, Moon HS, Park CG, Lee MS, Chun T. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy. 2016;12:2326–2343. doi: 10.1080/15548627.2016.1235124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–1213. doi: 10.1152/ajpheart.00020.2004. [DOI] [PubMed] [Google Scholar]
- Hasanbasic I, Rajotte I, Blostein M. The role of gamma-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3:2790–2797. doi: 10.1111/j.1538-7836.2005.01662.x. [DOI] [PubMed] [Google Scholar]
- Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, Parnell LA, Cao B, Mysorekar IU, Rothlin CV, Fikrig E, Diamond MS, Iwasaki A. TAM Receptors Are Not Required for Zika Virus Infection in Mice. Cell Rep. 2017;19:558–568. doi: 10.1016/j.celrep.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy LM, Jang JH, Won SY, Lin YH, Touil H, Aljarallah S, Bar-Or A, Antel JP. MerTK-mediated regulation of myelin phagocytosis by macrophages generated from patients with MS. Neurol Neuroimmunol Neuroinflamm. 2017;4:e402. doi: 10.1212/NXI.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy LM, Perron G, Won SY, Michell-Robinson MA, Rezk A, Ludwin SK, Moore CS, Hall JA, Bar-Or A, Antel JP. MerTK Is a Functional Regulator of Myelin Phagocytosis by Human Myeloid Cells. J Immunol. 2016;196:3375–3384. doi: 10.4049/jimmunol.1502562. [DOI] [PubMed] [Google Scholar]
- Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, Feldmann G, Hong SM, Haffner MC, Meeker AK, Holland SJ, Yu J, Heckrodt TJ, Zhang J, Ding P, Goff D, Singh R, Roa JC, Marimuthu A, Riggins GJ, Eshleman JR, Nelkin BD, Pandey A, Maitra A. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10:1009–1018. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb MJ, Gandrille S, Fernandez JA, Griffin JH, Fedullo PF. Late onset thrombosis in a case of severe protein S deficiency due to compound heterozygosity for PROS1 mutations. J Thromb Haemost. 2008;6:1235–1237. doi: 10.1111/j.1538-7836.2008.02994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiring C, Dahlback B, Muller YA. Ligand recognition and homophilic interactions in Tyro3: structural insights into the Axl/Tyro3 receptor tyrosine kinase family. J Biol Chem. 2004;279:6952–6958. doi: 10.1074/jbc.M311750200. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Moreno MJ, Lam JC, Haqqani AS, Kelly JF. Identification of secreted proteins regulated by cAMP in glioblastoma cells using glycopeptide capture and label-free quantification. Proteomics. 2009;9:535–549. doi: 10.1002/pmic.200800257. [DOI] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Hoehn HJ, Kress Y, Sohn A, Brosnan CF, Bourdon S, Shafit-Zagardo B. Axl−/− mice have delayed recovery and prolonged axonal damage following cuprizone toxicity. Brain Res. 2008;1240:1–11. doi: 10.1016/j.brainres.2008.08.076. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, Zhang J, Ding P, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- Huang H, Colella S, Kurrer M, Yonekawa Y, Kleihues P, Ohgaki H. Gene expression profiling of low-grade diffuse astrocytomas by cDNA arrays. Cancer Res. 2000;60:6868–6874. [PubMed] [Google Scholar]
- Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, Seaton B, Furie BC. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, Kostron H, Stockhammer G, Ullrich A. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- Ji R, Meng L, Jiang X, Cvm NK, Ding J, Li Q, Lu Q. TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS One. 2014;9:e115140. doi: 10.1371/journal.pone.0115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Tian S, Lu HJ, Lu Q, Zheng Y, Wang X, Ding J, Li Q, Lu Q. TAM receptors affect adult brain neurogenesis by negative regulation of microglial cell activation. J Immunol. 2013;191:6165–6177. doi: 10.4049/jimmunol.1302229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G, Peng PC, Huang PY, Chien HF, Hua KT, Kuo ML, Chen CT, Lee MJ. Soluble AXL: a possible circulating biomarker for neurofibromatosis type 1 related tumor burden. PLoS One. 2014;9:e115916. doi: 10.1371/journal.pone.0115916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevics H, Largent C, Hostettler J, Sammond DW, Matsushima GK, Kleindienst A, Toews AD, Morell P. Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J Neurochem. 2002;82:126–136. doi: 10.1046/j.1471-4159.2002.00954.x. [DOI] [PubMed] [Google Scholar]
- Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, Salzberg DB, Foreman NK, Liang X, Thorburn A, Graham DK. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9:1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. e1217. [DOI] [PubMed] [Google Scholar]
- Kikuno R, Nagase T, Ishikawa K, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:197–205. doi: 10.1093/dnares/6.3.197. [DOI] [PubMed] [Google Scholar]
- Kim DY, Yu J, Mui RK, Niibori R, Taufique HB, Aslam R, Semple JW, Cordes SP. The tyrosine kinase receptor Tyro3 enhances lifespan and neuropeptide Y (Npy) neuron survival in the mouse anorexia (anx) mutation. Dis Model Mech. 2017;10:581–595. doi: 10.1242/dmm.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N WP, Bunge RP. Tissue Culture methods for the study of myelination. In: Goslin GBaK., editor. Culturing Nerve Cells. Cambridge MA: MIT Press; 1991. pp. 337–377. [Google Scholar]
- Knubel KH, Pernu BM, Sufit A, Nelson S, Pierce AM, Keating AK. MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget. 2014;5:1338–1351. doi: 10.18632/oncotarget.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A, Park D, Jeong H, Lee J, Lee MN, Suh PG, Ryu SH. Regulation of C1-Ten protein tyrosine phosphatase by p62/SQSTM1-mediated sequestration and degradation. Cell Signal. 2014;26:2470–2480. doi: 10.1016/j.cellsig.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C, Butovsky O. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity. 2017;47:566–581. doi: 10.1016/j.immuni.2017.08.008. e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S, Pfeiffer C, Strube S, Alsadeq A, Fedders H, Vokuhl C, Loges S, Waizenegger J, Ben-Batalla I, Cario G, Moricke A, Stanulla M, Schrappe M, Schewe DM. Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central nervous system (CNS) Blood. 2015;125:820–830. doi: 10.1182/blood-2014-06-583062. [DOI] [PubMed] [Google Scholar]
- Lai C, Gore M, Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9:2567–2578. [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Review: the architecture of inflammatory demyelinating lesions: implications for studies on pathogenesis. Neuropathol Appl Neurobiol. 2011;37:698–710. doi: 10.1111/j.1365-2990.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sherick AB, Zhang W, Menachof KK, Hill AA, Rinella S, Kirkpatrick G, Page LS, Stashko MA, Jordan CT, Wei Q, Liu J, Zhang D, DeRyckere D, Wang X, Frye S, Earp HS, Graham DK. Efficacy of a Mer and Flt3 tyrosine kinase small molecule inhibitor, UNC1666, in acute myeloid leukemia. Oncotarget. 2015;6:6722–6736. doi: 10.18632/oncotarget.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003;15:31–36. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295. doi: 10.1016/s0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Lu H, Tian S, Lu Q. Systemic autoimmunity in TAM triple knockout mice causes inflammatory brain damage and cell death. PLoS One. 2013;8:e64812. doi: 10.1371/journal.pone.0064812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Chen J, Hammonds G, Phillips H, Armanini M, Wood P, Bunge R, Godowski PJ, Sliwkowski MX, Mather JP. Identification of Gas6 as a growth factor for human Schwann cells. J Neurosci. 1996;16:2012–2019. doi: 10.1523/JNEUROSCI.16-06-02012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Maeda Y, Yuan RR, Elkabes S, Cook S, Dowling P. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann Neurol. 2004;56:767–777. doi: 10.1002/ana.20274. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Bi S, Zhao K, Yu C. Inhibition of Mer and Axl receptor tyrosine kinases leads to increased apoptosis and improved chemosensitivity in human neuroblastoma. Biochem Biophys Res Commun. 2015;457:461–466. doi: 10.1016/j.bbrc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, Kallop D, Ludlam MJ, Pei L. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, Bessede A, Brew BJ, Guillemin GJ. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger RM, Keating AK, Earp HS, Graham DK. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets. 2010;14:1073–1090. doi: 10.1517/14728222.2010.515980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Guo H, Griffin JH, Fernandez JA, Zlokovic BV. Protein S confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 107:1791–1796. doi: 10.1161/01.CIR.0000058460.34453.5A. [DOI] [PubMed] [Google Scholar]
- Liu S, DeLalio LJ, Isakson BE, Wang TT. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ Res. 2016;119:1183–1189. doi: 10.1161/CIRCRESAHA.116.309866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Y, He Y, Zhao J, Su G. Progress in histopathologic and pathogenetic research in a retinitis pigmentosa model. Histol Histopathol. 2015;30:771–779. doi: 10.14670/HH-11-596. [DOI] [PubMed] [Google Scholar]
- Loges S, Schmidt T, Tjwa M, van Geyte K, Lievens D, Lutgens E, Vanhoutte D, Borgel D, Plaisance S, Hoylaerts M, Luttun A, Dewerchin M, Jonckx B, Carmeliet P. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–274. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin KS, Raine CS. The neuropathology of multiple sclerosis. In: Raine CS, editor. Multple sclerosis: A comprehensive text. Philadelphia: Saunders Elsevier; 2008. pp. 151–177. [Google Scholar]
- Ma GZ, Giuffrida LL, Gresle MM, Haartsen J, Laverick L, Butzkueven H, Field J, Binder MD, Kilpatrick TJ. Association of plasma levels of Protein S with disease severity in multiple sclerosis. Mult Scler J Exp Transl Clin. 2015;1:2055217315596532. doi: 10.1177/2055217315596532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma GZ, Stankovich J, Kilpatrick TJ, Binder MD, Field J. Polymorphisms in the receptor tyrosine kinase MERTK gene are associated with multiple sclerosis susceptibility. PLoS One. 2011;6:e16964. doi: 10.1371/journal.pone.0016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan NP, Earp HS. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem. 2003;278:42596–42603. doi: 10.1074/jbc.M305817200. [DOI] [PubMed] [Google Scholar]
- Marcus R CA. Fat soluble vitamins. In: Hardman LLJG, editor. The Pharmacological Basis of Therapeutics. 10. McGraw-Hill; 2001. pp. 1773–1791. [Google Scholar]
- Mark MR, Scadden DT, Wang Z, Gu Q, Goddard A, Godowski PJ. rse, a novel receptor-type tyrosine kinase with homology to Axl/Ufo, is expressed at high levels in the brain. J Biol Chem. 1994;269:10720–10728. [PubMed] [Google Scholar]
- Marlar RA, Neumann A. Neonatal purpura fulminans due to homozygous protein C or protein S deficiencies. Semin Thromb Hemost. 1990;16:299–309. doi: 10.1055/s-2007-1002683. [DOI] [PubMed] [Google Scholar]
- Martinho O, Zucca LE, Reis RM. AXL as a modulator of sunitinib response in glioblastoma cell lines. Exp Cell Res. 2015;332:1–10. doi: 10.1016/j.yexcr.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai LH. Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep. 2017;21:366–380. doi: 10.1016/j.celrep.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Le Charpentier T, Hafirassou ML, Zamborlini A, Cao-Lormeau VM, Coulpier M, Misse D, Jouvenet N, Tabibiazar R, Gressens P, Schwartz O, Amara A. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017;18:324–333. doi: 10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- Melaragno MG, Wuthrich DA, Poppa V, Gill D, Lindner V, Berk BC, Corson MA. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res. 1998;83:697–704. doi: 10.1161/01.res.83.7.697. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol, Chapter. 2010;15 doi: 10.1002/0471142735.im1501s88. Unit 15.11. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464–1472. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Torii T, Takada S, Ohno N, Saitoh Y, Nakamura K, Ito A, Ogata T, Terada N, Tanoue A, Yamauchi J. Involvement of the Tyro3 receptor and its intracellular partner Fyn signaling in Schwann cell myelination. Mol Biol Cell. 2015;26:3489–3503. doi: 10.1091/mbc.E14-05-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Monteiro de Castro G, Deja NA, Ma D, Zhao C, Franklin RJ. Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination. Am J Pathol. 2015;185:2431–2440. doi: 10.1016/j.ajpath.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen IS. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun KC, Golper TA. Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif. 2000;18:13–17. doi: 10.1159/000014403. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Nakada M, Kita D, Teng L, Pyko IV, Watanabe T, Hayashi Y, Hamada J. Receptor tyrosine kinases: principles and functions in glioma invasion. Adv Exp Med Biol. 2013;986:143–170. doi: 10.1007/978-94-007-4719-7_8. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem. 1997;272:29411–29414. doi: 10.1074/jbc.272.47.29411. [DOI] [PubMed] [Google Scholar]
- Nandrot E, Dufour EM, Provost AC, Pequignot MO, Bonnel S, Gogat K, Marchant D, Rouillac C, Sepulchre de Conde B, Bihoreau MT, Shaver C, Dufier JL, Marsac C, Lathrop M, Menasche M, Abitbol MM. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol Dis. 2000;7:586–599. doi: 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, He X, Hardig Y, Dahlback B, Garcia de Frutos P. Stimulation of Sky tyrosine phosphorylation by bovine protein S--domains involved in the receptor-ligand interaction. Eur J Biochem. 1997;246:147–154. doi: 10.1111/j.1432-1033.1997.t01-2-00147.x. [DOI] [PubMed] [Google Scholar]
- O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, 3rd, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Guin KN, Gruber RC, Raine CS, Guzik HM, Poulos BK, Shafit-Zagardo B. Gas6 enhances axonal ensheathment by MBP+ membranous processes in human DRG/OL promyelinating co-cultures. ASN Neuro. 2014;6:e00135. doi: 10.1042/AN20130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan JP, Fridell YW, Koski R, Varnum B, Liu ET. The transforming receptor tyrosine kinase, Axl, is post-translationally regulated by proteolytic cleavage. J Biol Chem. 1995:270. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Honda S, Ichinomiya N, Nakamura T, Mizuno K. Molecular cloning and in situ localization in the brain of rat sky receptor tyrosine kinase. J Biochem. 1995;117:1267–1275. doi: 10.1093/oxfordjournals.jbchem.a124854. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Mizuno K, Kuma K, Miyata T, Nakamura T. Cloning of the cDNA for a novel receptor tyrosine kinase, Sky, predominantly expressed in brain. Oncogene. 1994;9:699–705. [PubMed] [Google Scholar]
- Olivar R, Luque A, Naranjo-Gomez M, Quer J, Garcia de Frutos P, Borras FE, Rodriguez de Cordoba S, Blom AM, Aran JM. The alpha7beta0 isoform of the complement regulator C4b–binding protein induces a semimature, anti-inflammatory state in dendritic cells. J Immunol. 2013;190:2857–2872. doi: 10.4049/jimmunol.1200503. [DOI] [PubMed] [Google Scholar]
- Onken J, Torka R, Korsing S, Radke J, Krementeskaia I, Nieminen M, Bai X, Ullrich A, Heppner F, Vajkoczy P. Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget. 2016;7:9876–9889. doi: 10.18632/oncotarget.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken J, Vajkoczy P, Torka R, Hempt C, Patsouris V, Heppner FL, Radke J. Phospho-AXL is widely expressed in glioblastoma and associated with significant shorter overall survival. Oncotarget. 2017;8:50403–50414. doi: 10.18632/oncotarget.18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Litzenburger UM, Sahm F, Rauschenbach KJ, Tudoran R, Hartmann C, Marquez VE, von Deimling A, Wick W, Platten M. Promotion of glioblastoma cell motility by enhancer of zeste homolog 2 (EZH2) is mediated by AXL receptor kinase. PLoS One. 2012;7:e47663. doi: 10.1371/journal.pone.0047663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinot C, Nandrot EF. A Comprehensive Review of Mutations in the MERTK Proto-Oncogene. Adv Exp Med Biol. 2016;854:259–265. doi: 10.1007/978-3-319-17121-0_35. [DOI] [PubMed] [Google Scholar]
- Paty D, Noseworthy J, Eber GC. Diagnosis of multiple sclerosis. In: Paty EGD, editor. Multiple Sclerosis. Philadelphia, PA: FA Davis; 1988. pp. 48–134. [Google Scholar]
- Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, Wierman ME. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol. 2008;22:2481–2495. doi: 10.1210/me.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Xu M, Bliesner B, Liu Z, Richards J, Tobet S, Wierman ME. Hypothalamic but not pituitary or ovarian defects underlie the reproductive abnormalities in Axl/Tyro3 null mice. Mol Cell Endocrinol. 2011;339:151–158. doi: 10.1016/j.mce.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AM, Keating AK. TAM receptor tyrosine kinases: Expression, disease and oncogenesis in the central nervous system. Brain Res. 2014;1542:206–220. doi: 10.1016/j.brainres.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, t Hart B, Vescovi A, Martino G. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. 2009;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Popova SN, Bergqvist M, Dimberg A, Edqvist PH, Ekman S, Hesselager G, Ponten F, Smits A, Sooman L, Alafuzoff I. Subtyping of gliomas of various WHO grades by the application of immunohistochemistry. Histopathology. 2014;64:365–379. doi: 10.1111/his.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Prieto AL, O’Dell S, Varnum B, Lai C. Localization and signaling of the receptor protein tyrosine kinase Tyro3 in cortical and hippocampal neurons. Neuroscience. 2007;150:319–334. doi: 10.1016/j.neuroscience.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol. 2000;425:295–314. [PubMed] [Google Scholar]
- Prineas J. The neuropathology of multiple sclerosis: Demyelinating diseases. In: Koetsier VPJC, Bruyn GW, Klawans HL, editors. Handbook of clinical neurology. Vol. 3. Amsterdam: Elsevier; 1985. pp. 213–257. [Google Scholar]
- Ray AK, DuBois JC, Gruber RC, Guzik HM, Gulinello ME, Perumal G, Raine C, Kozakiewicz L, Williamson J, Shafit-Zagardo B. Loss of Gas6 and Axl signaling results in extensive axonal damage, motor deficits, prolonged neuroinflammation, and less remyelination following cuprizone exposure. Glia. 2017;65:2051–2069. doi: 10.1002/glia.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, Pollen AA, Mandel-Brehm C, Nowakowski TJ, Kriegstein AR, DeRisi JL. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113:14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew AN, Young ED, Lev DC, Kleinerman ES, Abdul-Karim FW, Getty PJ, Greenfield EM. Multiple receptor tyrosine kinases promote the in vitro phenotype of metastatic human osteosarcoma cell lines. Oncogenesis. 2012;1:e34. doi: 10.1038/oncsis.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- Rogers AE, Le JP, Sather S, Pernu BM, Graham DK, Pierce AM, Keating AK. Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene. 2012;31:4171–4181. doi: 10.1038/onc.2011.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusielewicz T, Nam J, Damanakis E, John GR, Raine CS, Melendez-Vasquez CV. Accelerated repair of demyelinated CNS lesions in the absence of non-muscle myosin IIB. Glia. 2014;62:580–591. doi: 10.1002/glia.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainaghi PP, Collimedaglia L, Alciato F, Molinari R, Sola D, Ranza E, Naldi P, Monaco F, Leone M, Pirisi M, Avanzi GC. Growth arrest specific gene 6 protein concentration in cerebrospinal fluid correlates with relapse severity in multiple sclerosis. Mediators Inflamm. 2013;2013:406483. doi: 10.1155/2013/406483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian-Mehta S, Xu M, Pierce A, Bliesner B, Tobet S, Wierman ME. Loss of Growth arrest specific gene 6 (Gas6) results in altered GnRH neuron migration, delayed vaginal opening and sexual maturation in mice. Mol Cell Endocrinol. 2014;393:164–170. doi: 10.1016/j.mce.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Enami J. Structure and expression of a murine homologue of sky receptor tyrosine kinase gene. J Biochem. 1996;120:264–270. doi: 10.1093/oxfordjournals.jbchem.a021408. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Gohring W, Ullrich A, Timpl R, Hohenester E. Structural basis for Gas6-Axl signalling. Embo j. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, John SP, Aker AM, Renzette N, Robbins DR, Guo Z, Green S, Kowalik TF, Brass AL. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Rep. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’Alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’Hooghe M B, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppa V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Ruckert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sorensen PS, Sondergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvanen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Scurlock B, Dawson G. Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: role of ceramide in apoptosis. J Neurosci Res. 1999;55:514–522. doi: 10.1002/(SICI)1097-4547(19990215)55:4<514::AID-JNR11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- Seitz HM, Matsushima GK. Dendritic cells in systemic lupus erythematosus. Int Rev Immunol. 2010;29:184–209. doi: 10.3109/08830181003602507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SL, O’Guin K, Cammer M, McMorris FA, Stitt TN, Basch RS, Varnum B, Shafit-Zagardo B. The growth arrest-specific gene product Gas6 promotes the survival of human oligodendrocytes via a phosphatidylinositol 3-kinase-dependent pathway. J Neurosci. 2003;23:4208–4218. doi: 10.1523/JNEUROSCI.23-10-04208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SL, O’Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci. 2006;26:5638–5648. doi: 10.1523/JNEUROSCI.5063-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Staflin K, Zuchner T, Honeth G, Darabi A, Lundberg C. Identification of proteins involved in neural progenitor cell targeting of gliomas. BMC Cancer. 2009;9:206. doi: 10.1186/1471-2407-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D, Brett J, Harris K, Nawroth P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: the synthesis and release of protein S. J Cell Biol. 1986;102:1971–1978. doi: 10.1083/jcb.102.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Studer RA, Opperdoes FR, Nicolaes GA, Mulder AB, Mulder R. Understanding the functional difference between growth arrest-specific protein 6 and protein S: an evolutionary approach. Open Biol. 2014:4. doi: 10.1098/rsob.140121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez RM, Chevot F, Cavagnino A, Saettel N, Radvanyi F, Piguel S, Bernard-Pierrot I, Stoven V, Legraverend M. Inhibitors of the TAM subfamily of tyrosine kinases: synthesis and biological evaluation. Eur J Med Chem. 2013;61:2–25. doi: 10.1016/j.ejmech.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Sufit A, Lee-Sherick AB, DeRyckere D, Rupji M, Dwivedi B, Varella-Garcia M, Pierce AM, Kowalski J, Wang X, Frye SV, Earp HS, Keating AK, Graham DK. MERTK Inhibition Induces Polyploidy and Promotes Cell Death and Cellular Senescence in Glioblastoma Multiforme. PLoS One. 2016;11:e0165107. doi: 10.1371/journal.pone.0165107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman L, Negrier C, Boukerche H. Protein S: A multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol. 2013;88:637–654. doi: 10.1016/j.critrevonc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, Malekzadeh A, Leurs C, Bridel C, Killestein J. Body fluid biomarkers for multiple sclerosis--the long road to clinical application. Nat Rev Neurol. 2015;11:585–596. doi: 10.1038/nrneurol.2015.173. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Birkelund S, Burkhart A, Stensballe A, Moos T. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J Neurochem. 2017;140:741–754. doi: 10.1111/jnc.13747. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17722436. 271678×17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of theMer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK) J Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, Birge RB. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283:3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- Toshima J, Ohashi K, Iwashita S, Mizuno K. Autophosphorylation activity and association with Src family kinase of Sky receptor tyrosine kinase. Biochem Biophys Res Commun. 1995;209:656–663. doi: 10.1006/bbrc.1995.1549. [DOI] [PubMed] [Google Scholar]
- Trouw LA, Nilsson SC, Goncalves I, Landberg G, Blom AM. C4b–binding protein binds to necrotic cells and DNA, limiting DNA release and inhibiting complement activation. J Exp Med. 2005;201:1937–1948. doi: 10.1084/jem.20050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiperson V, Li X, Schwartz GJ, Raine CS, Shafit-Zagardo B. GAS6 enhances repair following cuprizone-induced demyelination. PLoS One. 2010;5:e15748. doi: 10.1371/journal.pone.0015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou WI, Nguyen KQ, Calarese DA, Garforth SJ, Antes AL, Smirnov SV, Almo SC, Birge RB, Kotenko SV. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem. 2014;289:25750–25763. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Cook D, Fourgeaud L, Powers CJ, Merten K, Clark CL, Hoffman E, Ngo A, Sekiguchi KJ, O’Shea CC, Lemke G, Nimmerjahn A. Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia. Neuron. 2017;93:574–586. doi: 10.1016/j.neuron.2016.12.021. e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, Kiessling F, Eichelsbacher U, Essig M, Read TA, Erber R, Ullrich A. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103:5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poel RH, Meijers JC, Dahlback B, Bouma BN. C4b–binding protein (C4BP) beta-chain Short Consensus Repeat-2 specifically contributes to the interaction of C4BP with protein S. Blood Cells Mol Dis. 1999;25:279–286. doi: 10.1006/bcmd.1999.0255. [DOI] [PubMed] [Google Scholar]
- Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001;98:12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Massenhausen A, Sanders C, Thewes B, Deng M, Queisser A, Vogel W, Kristiansen G, Duensing S, Schrock A, Bootz F, Brossart P, Kirfel J, Heasley L, Bragelmann J, Perner S. MERTK as a novel therapeutic target in head and neck cancer. Oncotarget. 2016;7:32678–32694. doi: 10.18632/oncotarget.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouri M, An Q, Birt M, Pilkington GJ, Hafizi S. Small molecule inhibition of Axl receptor tyrosine kinase potently suppresses multiple malignant properties of glioma cells. Oncotarget. 2015;6:16183–16197. doi: 10.18632/oncotarget.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouri M, Croucher DR, Kennedy SP, An Q, Pilkington GJ, Hafizi S. Axl-EGFR receptor tyrosine kinase hetero-interaction provides EGFR with access to pro-invasive signalling in cancer cells. Oncogenesis. 2016;5:e266. doi: 10.1038/oncsis.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouri M, Hafizi S. TAM Receptor Tyrosine Kinases in Cancer Drug Resistance. Cancer Res. 2017;77:2775–2778. doi: 10.1158/0008-5472.CAN-16-2675. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang H, Young AG, Qiu R, Argalian S, Li X, Wu X, Lemke G, Lu Q. Transcriptome analysis of neural progenitor cells by a genetic dual reporter strategy. Stem Cells. 2011;29:1589–1600. doi: 10.1002/stem.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Moncayo G, Morin P, Jr, Xue G, Grzmil M, Lino MM, Clement-Schatlo V, Frank S, Merlo A, Hemmings BA. Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene. 2013;32:872–882. doi: 10.1038/onc.2012.104. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Wang Z, Zhen ZD, Feng KH, Guo J, Gao N, Fan DY, Han DS, Wang PG, An J. Axl is not an indispensable factor for Zika virus infection in mice. J Gen Virol. 2017;98:2061–2068. doi: 10.1099/jgv.0.000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Brosnan CF, Loudig O, Goldberg MF, Macian F, Arnett HA, Prieto AL, Tsiperson V, Shafit-Zagardo B. Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J Neuroinflammation. 2011;8:49. doi: 10.1186/1742-2094-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Gohari P, Yan Y, Backer JM, Varnum B, Shafit-Zagardo B. In brain, Axl recruits Grb2 and the p85 regulatory subunit of PI3 kinase; in vitro mutagenesis defines the requisite binding sites for downstream Akt activation. J Neurochem. 2008;106:134–146. doi: 10.1111/j.1471-4159.2008.05343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Omari KM, Marsden K, Raine CS, Shafit-Zagardo B. Upregulation of soluble Axl and Mer receptor tyrosine kinases negatively correlates with Gas6 in established multiple sclerosis lesions. Submitted. 2009 doi: 10.2353/ajpath.2009.080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, Kane LT, Ye C, Kaykas A, Eggan K. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell. 2016;19:703–708. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte precursor cells in chronic multiple sclerosis lesions. Mult Scler. 1997;3:168–169. doi: 10.1177/135245859700300221. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123(Pt 1):105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SY, Chen SR, Hsieh J, Li YS, Chuang SE, Chuang HM, Huang MH, Lin SZ, Harn HJ, Chiou TW. Biodegradable interstitial release polymer loading a novel small molecule targeting Axl receptor tyrosine kinase and reducing brain tumour migration and invasion. Oncogene. 2016;35:2156–2165. doi: 10.1038/onc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Wang B, Lu W, Maeda Y, Dowling P. A Distinct Region in Erythropoietin that Induces Immuno/Inflammatory Modulation and Tissue Protection. Neurotherapeutics. 2015;12:850–861. doi: 10.1007/s13311-015-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelentsova-Levytskyi K, Talmi Z, Abboud-Jarrous G, Capucha T, Sapir T, Burstyn-Cohen T. Protein S Negatively Regulates Neural Stem Cell Self-Renewal through Bmi-1 Signaling. Front Mol Neurosci. 2017;10:124. doi: 10.3389/fnmol.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelentsova K, Talmi Z, Abboud-Jarrous G, Sapir T, Capucha T, Nassar M, Burstyn-Cohen T. Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis. Stem Cells. 2017;35:679–693. doi: 10.1002/stem.2522. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Cui Y, Chen J, Lu M, Elias SB, Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang W, DeRyckere D, Hunter D, Liu J, Stashko MA, Minson KA, Cummings CT, Lee M, Glaros TG, Newton DL, Sather S, Zhang D, Kireev D, Janzen WP, Earp HS, Graham DK, Frye SV, Wang X. UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. J Med Chem. 2014;57:7031–7041. doi: 10.1021/jm500749d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA, 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zhang L, Lu Q, Wang X, Yu F, Wang X, Lu Q. NGF-induced Tyro3 and Axl function as survival factors for differentiating PC12 cells. Biochem Biophys Res Commun. 2009;378:371–375. doi: 10.1016/j.bbrc.2008.11.049. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140:1900–1913. doi: 10.1093/brain/awx113. [DOI] [PMC free article] [PubMed] [Google Scholar]