Abstract
Objectives
Phenotypes differ between late and early-onset systemic lupus erythematosus (SLE). Prior studies suggested that there may be more pulmonary disease among late-onset patients. Our objective was to perform a systematic review and meta-analysis to evaluate the differences in pulmonary manifestations in late- versus early-onset SLE.
Methods
We searched the literature using PubMed, CINAHL, Web of Science, Cochrane Library, and EMBASE. We excluded studies that did not include American College of Rheumatology SLE classification criteria, an early-onset SLE comparison group, or those that defined late-onset SLE as <50 years of age. We rated study quality using the Newcastle Ottawa Quality Scale. We used Forest plots to compare odds ratios (95% confidence intervals) of pulmonary manifestations by age. Study heterogeneity was assessed using I2.
Results
Thirty-nine studies, representing 10,963 early-onset and 1,656 late-onset patients with SLE, met eligibility criteria. The odds of developing several pulmonary manifestations were higher in the late-onset group. Interstitial lung disease (ILD) was nearly three times more common (OR 2.56 (1.27, 5.16)). Pleuritis (OR 1.53 (1.19, 1.96)) and serositis (OR 1.31 (1.05, 1.65)) were also more common in the late-onset group. The mean Newcastle Ottawa Quality Scale score for study quality was moderate (6.3 ± 0.7, scale 0–9).
Conclusions
Pulmonary manifestations of SLE were more common in late-onset SLE patients compared to their younger peers, in particular ILD and serositis. Age-related changes of the immune system, tobacco exposure, race, and possible overlap with Sjögren’s syndrome should be examined in future studies.
Keywords: Systemic lupus erythematosus (SLE), pulmonary manifestations, interstitial lung disease (ILD), pleuritis, serositis
INTRODUCTION
Systemic lupus erythematosus (SLE) is a pleomorphic autoimmune disease that often begins in early life. Presentation ranges from rashes and arthralgia to life-threatening lung and kidney involvement. Late-onset SLE is a distinct classification that begins in patients ≥50 years old. Prior meta-analyses report significant differences in the clinical manifestations between late and early-onset SLE patients, including fewer cutaneous manifestations and more sicca symptoms [1, 2]. A recent meta-analysis demonstrated increased pulmonary manifestations in adult-onset lupus patients compared to childhood-onset patients, suggesting a higher risk with increasing age [3]. Late-onset lupus patients were not included in this study, however. Other studies have suggested increased pulmonary involvement in late-onset patients as well [4], but conclusions have been limited by sample size. In the multiethnic prospective LUMINA cohort (n=626), age was an independent risk factor for development of pulmonary damage in patients with SLE [5].
Moreover, in non-lupus populations, lung fibrosis increases with advanced age, raising our interest in examining these relationships in lupus [6]. Pulmonary involvement is common in SLE, and pulmonary features are the presenting symptom in 5% of patients [7]. The most common pulmonary manifestation, pleuritis, occurs in up to 50% of all lupus patients. Chronic interstitial lung disease (ILD) occurs in up to 13% of lupus patients, typically later in the disease course [8]. Other pulmonary manifestations of SLE including acute pneumonitis, diffuse alveolar hemorrhage, pulmonary hypertension, shrinking lung syndrome, and pulmonary embolism are less common and often difficult to classify independently from antiphospholipid antibody syndrome or medication complications [8]. Although some studies have suggested more pulmonary disease in the late-onset group [4, 9], we found no large dedicated meta-analysis that quantified the relative odds of lung involvement in late- versus early-onset SLE. Such information could have important implications for the diagnosis, screening, and prognosis in older adults with SLE.
We aimed to conduct a systematic review and meta-analysis to compare the odds of pulmonary involvement, including serositis, pleuritis, ILD, pulmonary embolism (PE), and pulmonary hypertension in late versus early-onset lupus patients.
MATERIALS AND METHODS
Literature Search Inclusion Criteria
We performed a systematic review of the literature to identify articles comparing clinical manifestations of patients with late- versus early-onset lupus as described in our previous work [2]. We included the studies used in our prior meta-analysis that had data on pulmonary manifestations. Additionally we performed an electronic search of the literature in PubMed, CINAHL, and EMBASE using keyword subject headings “late-onset systemic lupus erythematosus” then “systemic lupus erythematosus,” “pulmonary,” and “late-onset” together and then “systemic lupus erythematosus,” “lung,” and “late-onset” together to determine if any relevant studies had been published through December 2016. Inclusion criteria were: (A) confirmed SLE using American College of Rheumatology (ACR) criteria and (B) data on pulmonary findings of (C) late-onset SLE, defined as ≥ 50 years of age versus early-onset SLE. Eligible study designs included cohort and case-control studies that presented results in percentages. Exclusion criteria included (A) no requirement for SLE patients to meet ACR classification criteria, (B) no inclusion of early-onset controls, and (C) definition of late-onset SLE as <50 years.
Data
Data was extracted by two authors (JM and CB) and included date of publication, study location (country and population vs hospital or clinic based), study type (cohort vs case-control study), follow-up period, and late-onset age definition. Additional data, including counts of pulmonary manifestations, were extracted with the agreement of a second author. The number of late-onset patients with ILD, serositis, pleuritis, PE, or pulmonary hypertension were identified. We also included a category of “any pulmonary manifestation” attributable to SLE which included a composite of ILD, serositis, pleurisy, pulmonary hemorrhage, PE, pneumonitis, or shrinking lung, to compare odds ratios in late- versus early-onset SLE.
Quality Assessment
Methodological quality of eligible studies and risk of bias were evaluated using the Newcastle Ottawa Quality Assessment Scale for cohort and case control studies [10]. The scale assesses cohort selection and comparisons between groups (cases and controls), outcomes, and adequacy of follow-up. Two reviewers rated each study, assigning a score out of 9 possible points. Discrepancies in scores were resolved by consensus with a third reviewer. Inter-rater reliability of two reviewers was calculated.
Statistical Analysis
To compare the odds of pulmonary manifestations in late- versus early-onset SLE, we used random effects models. We created Forest plots to summarize composite data, generating odds ratios and corresponding 95% confidence intervals for each pulmonary manifestation. Heterogeneity between studies was evaluated using the I2 statistic with 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. Funnel plots were reviewed to detect publication bias. We performed additional sub-group analyses by excluding case-control studies, to determine the relative risk of each pulmonary manifestation. All analyses were performed using R software version 3.1.2 with the package “meta.”
RESULTS
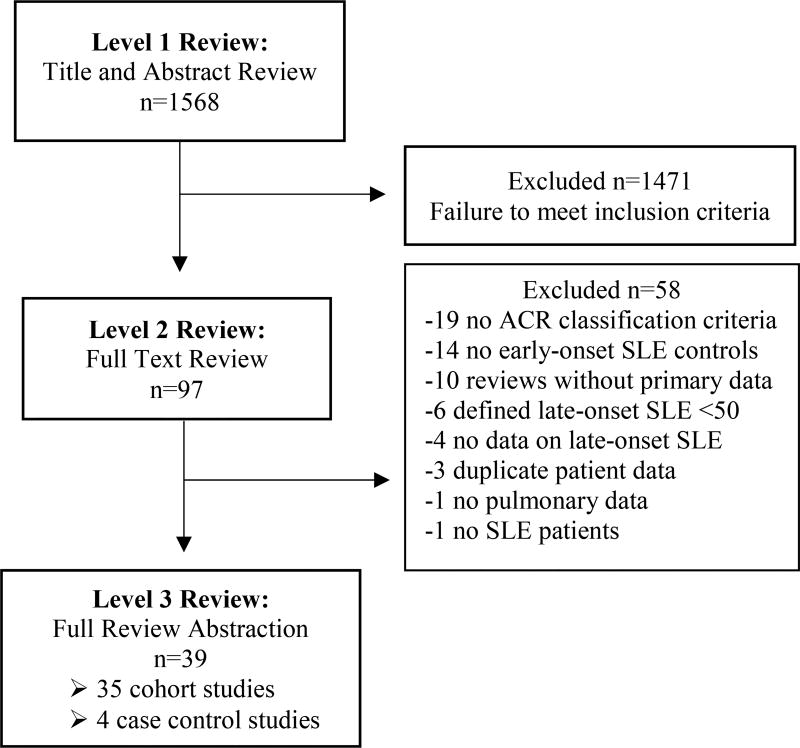
The PubMed, CINAHL, and EMBASE literature search for our meta-analysis yielded 1,568 potential articles of which 97 articles merited full-text review for application of exclusion criteria. Ultimately, we included 39 studies in this meta-analysis, encompassing 35 cohort and four case-control studies (see Figure 1).
Figure 1.
Study selection process with description of study inclusion and exclusions during the three-level review for the systematic review and meta-analysis.
See Table 1 listing the 39 studies included in the systematic review and meta-analysis [4, 9, 11–47].
Table 1.
Descriptions of studies included in meta-analysis
| Study | Location; setting |
Study type |
Years | Early- onset SLE (n) |
Late- onset SLE (n) |
Late- onset age |
Quality † |
|---|---|---|---|---|---|---|---|
| Alonso [11] | Spain; I | CO | ‘87-‘06 | 91 | 59 | ≥50* | 6 |
| Antolin [12] | Spain; I | CO | ‘80-‘92 | 165 | 29 | >50* | 7 |
| Appenzell [13] | Brazil; I | CC | ‘74-‘05 | 60 | 16 | ≥50* | 7 |
| Boddaert [9] | France; O | CO | ‘80-‘00 | 114 | 47 | ≥50* | 6 |
| Cartella [14] | Italy; O | CO | ‘76-‘08 | 495 | 40 | ≥50 | 6 |
| Cervera [15] | Europe; P | CO | ‘80-‘90 | 910 | 90 | >50* | 6 |
| Chen [16] | Taiwan; I/O | CO | ‘98-‘08 | 50 | 19 | ≥60* | 6 |
| Choi [17] | Korea; I | CO | ‘05-‘13 | 176 | 25 | >50* | 5 |
| Cooper [18] | USA; P | CC | ‘97-‘99 | 195 | 61 | ≥50 | 6 |
| Costallat [19] | Brazil; O | CO | ‘73-‘92 | 262 | 10 | ≥50* | 6 |
| das Chagas Medeiros [20] | Brazil; I | CO | ‘74-‘13 | 398 | 16 | >50* | 7 |
| Dimant [21] | USA; I/O | CO | ‘66-‘76 | 218 | 16 | >50* | 6 |
| Domenech [22] | England;I/O | CO | ‘85-‘91 | 232 | 15 | ≥50* | 6 |
| Feng [23] | China; I | CO | ? | 1659 | 131 | ≥50* | 5 |
| Font [24] | Spain; I/O | CO | ‘61-‘91 | 252 | 48 | ≥50 | 6 |
| Gomez [25] | Spain; P | CO | ‘92-? | 272 | 91 | ≥50* | 6 |
| Hashimoto [26] | Japan; O | CO | ‘55-‘85 | 549 | 21 | ≥50* | 6 |
| Ho [27] | China; O | CO | ‘71-‘97 | 100 | 25 | >50* | 7 |
| Iwazu[28] | Japan; I | CO | ’85-‘04 | 25 | 12 | ≥50 | 7 |
| Jacobsen [29] | Denmark; P | CO | ‘75-‘95 | 410 | 103 | ≥50 | 6 |
| Janwityanujit [30] | Thailand; I | CO | ‘90-‘92 | 359 | 27 | ≥50* | 7 |
| Jeleniewicz [31] | Poland; I/O | CO | ‘04-‘14 | 108 | 20 | ≥50 | 5 |
| Karoubi Nordon [32] | France; O | CC | ‘95-‘03 | 11 | 11 | ≥50 | 8 |
| Lalani [33] | Canada; P | CO | ‘05-? | 1367 | 161 | ≥50* | 6 |
| Liu [34] | Taiwan; I/O | CO | ‘77-‘86 | 207 | 11 | ≥50* | 7 |
| Maddison [35] | England; O | CO | ? | 93 | 19 | >60* | 6 |
| Mak [4] | China; I | CO | ‘85-‘95 | 89 | 13 | >50* | 6 |
| Mok [36] | China; I/O | CO | ‘91-‘03 | 263 | 22 | >50* | 8 |
| Pu [37] | Taiwan; I | CO | ‘88-‘98 | 152 | 42 | ≥50 | 6 |
| Sayarlioglu [38] | Turkey; O | CO | ‘78-‘01 | 100 | 20 | ≥50* | 6 |
| Shaikh [39] | Malaysia; O | CO | ‘76-‘92 | 52 | 17 | >50 | 6 |
| Sousa [40] | Portugal; I | CO | ‘12-‘13 | 89 | 89 | ≥50 | 6 |
| Stefanidou [41] | Greece; P | CO | ‘89-‘07 | 430 | 121 | ≥50* | 6 |
| Takayasu [42] | Brazil; O | CO | ‘89-? | 192 | 7 | ≥50 | 6 |
| Tang [43] | China; O | CO | ‘86-‘08 | 100 | 35 | ≥50 | 6 |
| Tomic-Lucic [44] | Serbia; O | CC | ? | 30 | 30 | ≥50 | 7 |
| Voulgari [45] | Greece; O | CO | ‘81-‘00 | 398 | 90 | ≥55* | 7 |
| Wilson [46] | USA; O | CO | ‘70-‘78 | 49 | 17 | ≥50* | 7 |
| Xu [47] | China; I | CO | ‘00-‘08 | 241 | 30 | ≥50 | 6 |
|
| |||||||
| TOTAL | 10,963 | 1,656 | |||||
SLE= Systemic lupus erythematosus; I=Inpatient; O=Outpatient; P=Population; CO=Cohort; CC=Case control study type.
Indicates early-onset group including age <18.
Scored according to Newcastle Ottawa Quality Score criteria [10].
Studies reflected geographically and ethnically diverse populations. Most studies (27 of 39) included individuals <18 years of age in the early onset SLE comparison group. The mean ± standard deviation Newcastle Ottawa Quality Scale score of the 39 included articles was 6.3 ± 0.7 with a maximum possible score of 9 points. Inter-rater reliability for these quality scores was k=0.96 with two independent reviewers. Lower scores were generally due to not including an inception cohort and/or lack of description for those lost to follow-up.
Meta-analysis Results
Our pooled cohorts included 1,656 patients with late-onset SLE and 10,963 patients with early onset SLE. We used random effects models to compare odds of ILD, pleuritis, serositis, PE, pulmonary hypertension, and any pulmonary manifestation (Table 2) between early and late onset SLE groups.
Table 2.
Meta-analysis summary statistics for pulmonary findings in late- versus early-onset systemic lupus erythematosus
| Pulmonary manifestation |
Total cases |
Late- onset* |
Early- onset* |
OR (95% CI) | Heterogeneity I2** (%), p-value |
|---|---|---|---|---|---|
| Interstitial lung disease | 82 | 29/294 | 53/1267 | 2.56 (1.27, 5.16) | 26, 0.234 |
| Pleuritis | 1212 | 223/939 | 989/6415 | 1.53 (1.19, 1.96) | 36, 0.069 |
| Serositis | 3184 | 499/1589 | 2685/10048 | 1.31 (1.05, 1.65) | 61, <0.001 |
| Pulmonary embolism | 10 | 5/64 | 5/166 | 2.73 (0.78, 9.60) | 0, 0.502 |
| Pulmonary hypertension | 5 | 1/36 | 4/160 | 1.72 (0.22, 13.49) | 0, 0.404 |
| Any | 3074 | 533/1645 | 2541/10852 | 1.70 (1.36, 2.14) | 60, <0.001 |
OR=Odds ratio; CI=Confidence interval.
Numerator is cases with manifestation present, denominator is total for each age category in which this manifestation was examined.
I2 interpretation: low heterogeneity ≤25%, moderate 50%, and high >75%
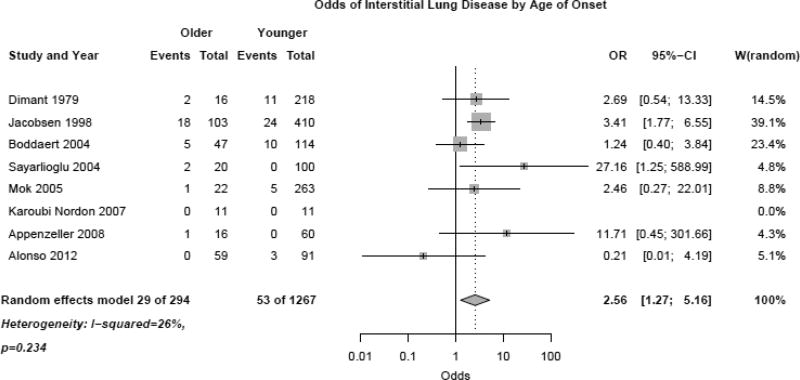
The late-onset group had increased incidence of ILD (Figure 2, OR 2.56 (1.27, 5.16)) with relatively low heterogeneity between studies (I2 26%, p=0.234).
Figure 2.
Odds of interstitial lung disease in late-onset versus early-onset systemic lupus erythematosus patients using a random effects model.
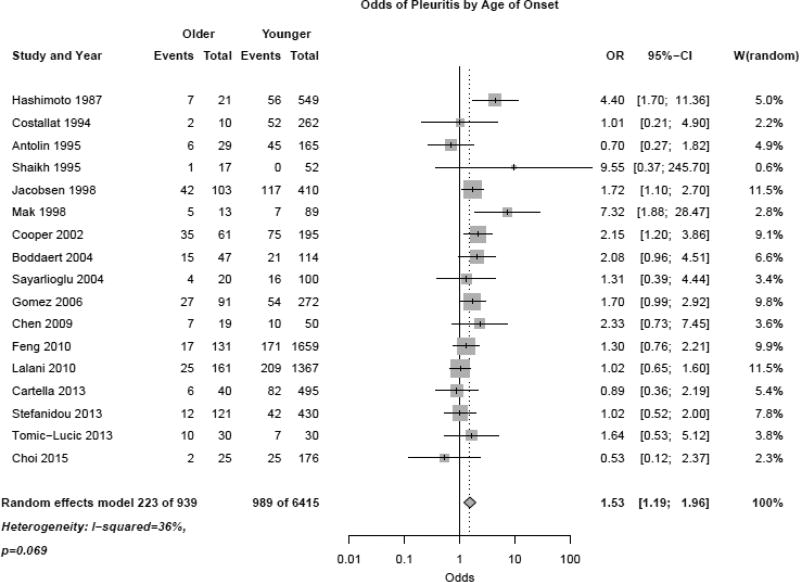
Exclusion of case-control studies still indicated a higher risk of ILD in the late-onset group (RR 2.21, 95% CI 1.14, 4.31). Pleuritis was also more common in the late-onset population (Figure 3, OR 1.53 (1.19, 1.96)).
Figure 3.
Odds of pleuritis in late-onset versus early-onset systemic lupus erythematosus patients using random effects model.
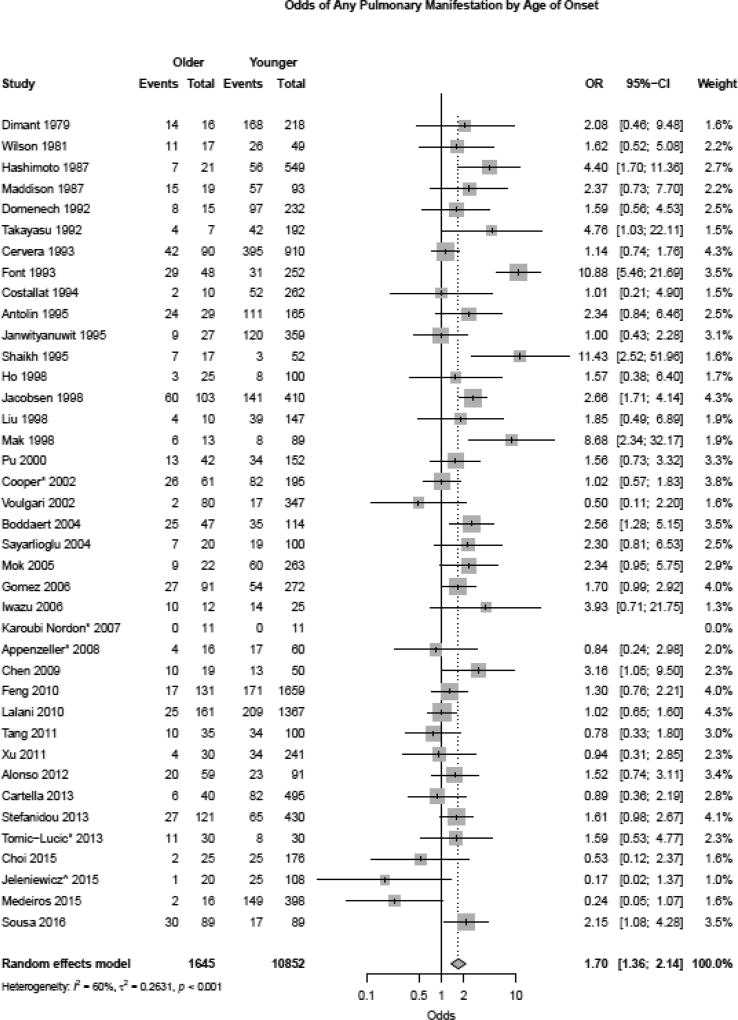
Heterogeneity was slightly higher for pleuritis but did not reach statistical significance (I2 36%, p=0.069). Exclusion of case-control studies from the analysis showed similar higher risk of pleuritis in the late-onset group (RR 1.39, 95% CI 1.12, 1.73). Serositis (which included both pleuritis and pericarditis) was significantly more common in late-onset compared to early-onset SLE patients as well (OR 1.31 (1.05, 1.65)), however, there was moderate heterogeneity (I2 61%, p<0.001). A sensitivity analysis excluding case-control studies did not change the risk of serositis in late-onset lupus (RR 1.26, 95% CI 1.07, 1.49). PE and pulmonary hypertension trended towards being more common in the late-onset group but did not reach statistical significance (OR 2.73 (0.78, 9.60) and OR 1.72 (0.22, 13.49)). Finally, the OR for any pulmonary manifestation was greater in the late-onset group (Figure 4, OR 1.70 (1.36, 2.14)), although there was moderate study heterogeneity (I2 60%, p<0.001).
Figure 4.
Odds of all pulmonary manifestations in late-onset versus early-onset systemic lupus erythematosus patients using random effects model.
Exclusion of case-control studies confirmed these findings, with any pulmonary manifestation being more likely in the older-onset age group (RR 1.47, 95% CI 1.27, 1.70).
DISCUSSION
Results of our meta-analysis indicate that late-onset SLE patients are more likely than early-onset patients to develop pulmonary manifestations. Older onset SLE patients had a nearly three-fold increased odds of ILD, similar to age trends in idiopathic ILD. Overall, findings were consistent with prior SLE cohort studies [4, 9, 21] and a prior 1989 meta-analysis [1] which included 170 late-onset cases compared with our 1,645 late-onset cases. It is known that idiopathic ILD is strongly associated with advanced age [6]. Similar physiology likely plays a significant role in the observed increased incidence of lung disease in the late-onset SLE group.
Although many mechanisms have been proposed to explain increases in pulmonary disease in late-onset patients, the exact pathogenesis remains unclear. Age has multiple effects on both the innate and adaptive immune systems, contributing to immune senescence of the lung. Aging alters innate immunity by several mechanisms including impairing the ability of neutrophils to kill phagocytosed organisms, increasing neutrophil apoptosis and proinflammatory cytokines including TNFα, IL-1, and IL-6, and reducing anti-inflammatory cytokine IL-10 and NK cell function despite an increase in absolute number[48]. The aging adaptive immune system also undergoes changes including decreased thymic production of naïve T cells and resulting compensatory proliferation of post-thymic T-cells (memory cells), which causes a shift to a chronic, proinflammatory state [48]. In addition to this, in aging murine models, T-cells have higher affinity receptors for self-peptide MHC [48, 49]. Age-related telomere shortening has also been associated with pulmonary fibrosis [48].
Although advanced age likely impacts development of ILD in the late-onset group, several studies have suggested that a possible SLE-Sjögren’s Syndrome (SLE-SS) overlap syndrome might also be associated with our findings [35, 50]. Our prior meta-analysis reported that late-onset SLE patients more commonly experience sicca symptoms [2]. Others have reported late-onset SLE patients have similar HLA types to primary SS; specifically both have increased frequency of HLA DRB1*0301, whereas those with SLE but no sicca symptoms had increased DRB1*1501 and DQB1*0602 [35, 50, 51].
Several authors propose a distinct SLE-SS overlap syndrome phenotype in older adults, which could contribute to increased risk of ILD [2, 35, 52]. Another group reported that 26 of 283 (9%) incident SLE patients met criteria for both SLE and SS [50]. In that study, compared to the SLE without SS, the SLE-SS patients were older and had higher frequency of anti-Ro, anti-La, anti-dsDNA antibodies and less renal disease [50]. Given that ILD occurs much more frequently in pSS (9–75% reported prevalence)[53] than in SLE (1–15% prevalence)[52], a SLE-SS overlap syndrome might explain the observed increased incidence of ILD in late-onset SLE patients.
In addition to a possible Sjogren’s overlap phenotype in late-onset SLE, factors such as duration of disease, environmental exposures, and smoking trends over time might also correlate with SLE-ILD [52]. Temporal trends reported by the Centers for Disease Control and Prevention show declines in tobacco use from 37% of the general US population in 1974, to 19% in 2011, predicting lower tobacco exposure in young lupus patients. Likewise, younger patients likely have less cumulative exposure to infectious triggers or other neo-antigens.
Pleuritis and serositis also occurred more frequently in the late-onset group. One hypothesis for this finding is that males are represented more in the late-onset group, though manifestations were not stratified by gender in our analysis. Men have an increased incidence of serositis when compared to age-matched females (OR 1.5–2.0) [15]. Additionally, some studies find that serositis is more common in people of European descent [54], particularly in older women [50].
Strengths of this study are the inclusion of a large-pooled multinational cohort representing 1,645 late onset patients and the use of rigorous meta-analysis methods. The quality of studies was good, with a mean rating of 6.3 ± 0.7 using the Newcastle Ottawa Scale. As with any study, one must also consider limitations, including those related to the methodological qualities of the primary studies. First, inconsistent methods were used to diagnose pulmonary involvement across studies, which may explain varying rates of subclinical pulmonary disease. Specifically, the methods used for diagnosing ILD were not described in most studies, so accuracy of diagnosis is uncertain. Likewise, some studies did not distinguish between pleuritis and pericarditis, potentially leading to misclassification of serositis as a pulmonary manifestation or over reporting based on symptoms above. The majority of studies did not specify the time to development of pulmonary manifestations after disease onset and survival bias may have affected the results. Information bias is also possible; shorter lengths of follow-up in either SLE group might reduce the observed frequency of pulmonary involvement. There was moderate heterogeneity between studies in regards to serositis and “any pulmonary manifestation,” which was not explained with the removal of case-control studies.
CONCLUSIONS
Our pooled analysis demonstrates increased odds of pulmonary manifestations, especially ILD, in late-onset SLE patients compared to their younger peers. Factors that likely contribute to this discovery are increased age, tobacco exposure, immune senescence, and the potential role of an SLE-SS overlap disease phenotype in older patients. Clinicians should recognize that late-onset patients are more likely to have ILD, and screen for the condition when appropriate. Future studies should prospectively investigate the odds of ILD in SLE patients, and perhaps identify and compare rates in SLE-SS overlap patients to further investigate these findings.
Acknowledgments
Authors would like to thank Sara Fitz MD for help with original study selection, and Natalie Wietfeldt, Zaher Karp, Aimée Wattiaux, and Amanda Perez for help with manuscript preparation.
Funding: Bartels received K23 time support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number AR062381 during this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Bartels currently receives unrelated institutional grant funding from Independent Grants for Learning and Change (Pfizer).
Abbreviations
- SLE
Systemic lupus erythematosus
- ILD
Interstitial lung disease
- PE
Pulmonary embolism
- ACR
American College of Rheumatology
- SLE-SS
SLE-Sjogren’s
- pSS
Primary Sjogren’s syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors have no direct financial, consultant, or institutional conflict of interest pertaining to this manuscript.
References
- 1.Ward MM, Polisson RP. A meta-analysis of the clinical manifestations of older-onset systemic lupus erythematosus. Arthritis Rheum. 1989;32(10):1226–32. doi: 10.1002/anr.1780321007. [DOI] [PubMed] [Google Scholar]
- 2.Medlin JL, et al. A systematic review and meta-analysis of cutaneous manifestations in late- versus early-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2016;45(6):691–697. doi: 10.1016/j.semarthrit.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bundhun PK, Kumari A, Huang F. Differences in clinical features observed between childhood-onset versus adult-onset systemic lupus erythematosus: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(37):e8086. doi: 10.1097/MD.0000000000008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7(1):23–28. doi: 10.1191/096120398678919723. [DOI] [PubMed] [Google Scholar]
- 5.Bertoli AM, et al. Systemic lupus erythematosus in a multiethnic US Cohort LUMINA XLVIII: factors predictive of pulmonary damage. Lupus. 2007;16(6):410–7. doi: 10.1177/0961203307079042. [DOI] [PubMed] [Google Scholar]
- 6.Meyer KC, et al. Management of idiopathic pulmonary fibrosis in the elderly patient: addressing key questions. CHEST Journal. 2015;148(1):242–252. doi: 10.1378/chest.14-2475. [DOI] [PubMed] [Google Scholar]
- 7.Torre O, Harari S. Pleural and pulmonary involvement in systemic lupus erythematosus. La Presse Médicale. 2011;40(1):e41–e51. doi: 10.1016/j.lpm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Kamen DL, Strange C. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med. 2010;31(3):479–88. doi: 10.1016/j.ccm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Boddaert J, et al. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine. 2004;83(6):348–59. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 10.Wells GA, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 2018 January 11];2011 Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 11.Alonso MD, et al. Late-onset systemic lupus erythematosus in Northwestern Spain: differences with early-onset systemic lupus erythematosus and literature review. Lupus. 2012;21(10):1135–48. doi: 10.1177/0961203312450087. [DOI] [PubMed] [Google Scholar]
- 12.Antolin J, et al. Systemic lupus erythematosus: clinical manifestations and immunological parameters in 194 patients. Subgroup classification of SLE. Clinical rheumatology. 1995;14(6):678–85. doi: 10.1007/BF02207936. [DOI] [PubMed] [Google Scholar]
- 13.Appenzeller S, Pereira DA, Costallat LT. Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus. 2008;17(11):1023–8. doi: 10.1177/0961203308089695. [DOI] [PubMed] [Google Scholar]
- 14.Cartella S, et al. Evaluation of mortality, disease activity, treatment, clinical and immunological features of adult and late onset systemic Lupus erythematosus. Autoimmunity. 2013;46(6):363–368. doi: 10.3109/08916934.2013.794793. [DOI] [PubMed] [Google Scholar]
- 15.Cervera R, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine. 1993;72(2):113–124. [PubMed] [Google Scholar]
- 16.Chen TL, et al. Systemic Lupus Erythematosus in the Elderly. International journal of gerontology. 2009;3(2):108–113. [Google Scholar]
- 17.Choi J, et al. Comparison of clinical and serological differences among juvenile- adult- and late-onset systemic lupus erythematosus in Korean patients. Lupus. 2015;24(12):1342–9. doi: 10.1177/0961203315591024. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11(3):161–7. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 19.Costallat LT, Coimbra AM. Systemic lupus erythematosus: clinical and laboratory aspects related to age at disease onset. Clinical Experimental Rheumatology. 1994;12(6):603–607. [PubMed] [Google Scholar]
- 20.das Chagas Medeiros M, et al. Clinical and immunological aspects and outcome of a Brazilian cohort of 414 patients with systemic lupus erythematosus (SLE): comparison between childhood-onset, adult-onset, and late-onset SLE. Lupus. 2015;25(4):355–63. doi: 10.1177/0961203315606983. [DOI] [PubMed] [Google Scholar]
- 21.Dimant J, et al. Systemic lupus erythematosus in the older age group: computer analysis. Journal of the American Geriatrics Society. 1979;27(2):58–61. doi: 10.1111/j.1532-5415.1979.tb03342.x. [DOI] [PubMed] [Google Scholar]
- 22.Domenech I, et al. Systemic lupus erythematosus in 50 year olds. Postgraduate medical journal. 1992;68(800):440–4. doi: 10.1136/pgmj.68.800.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng JB, et al. Gender and age influence on clinical and laboratory features in Chinese patients with systemic lupus erythematosus: 1,790 cases. Rheumatology International. 2010;30(8):1017–1023. doi: 10.1007/s00296-009-1087-0. [DOI] [PubMed] [Google Scholar]
- 24.Font J, et al. Systemic lupus erythematosus: a clinical and immunological study of 300 patients. Med Clin (Barc) 1993;100(16):601–5. [PubMed] [Google Scholar]
- 25.Gomez J, et al. Systemic lupus erythematosus in Asturias, Spain: clinical and serologic features. Medicine. 2006;85(3):157–68. doi: 10.1097/01.md.0000224711.54886.b1. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto H, et al. Differences in clinical and immunological findings of systemic lupus erythematosus related to age. Journal of Rheumatology. 1987;14(3):497–501. [PubMed] [Google Scholar]
- 27.Ho CT, et al. Late onset systemic lupus erythematosus in southern Chinese. Annals of the rheumatic diseases. 1998;57(7):437–40. doi: 10.1136/ard.57.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwazu Y, et al. Clinical characteristics of patients with elder-onset lupus nephritis. Nihon Jinzo Gakkai Shi. 2006;48(4):345–53. [PubMed] [Google Scholar]
- 29.Jacobsen S, et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus.I. Disease manifestations and analyses of clinical subsets. Clinical rheumatology. 1998;17(6):468–77. doi: 10.1007/BF01451282. [DOI] [PubMed] [Google Scholar]
- 30.Janwityanujit S, et al. Age-related differences on clinical and immunological manifestations of SLE. Asian Pac J Allergy Immunol. 1995;13(2):145–9. [PubMed] [Google Scholar]
- 31.Jeleniewicz R, Suszek D, Majdan M. Clinical picture of late-onset systemic lupus erythematosus in a group of Polish patients. Polskie Archiwum Medycyny Wewnętrznej. 2015;125(7–8):538–544. doi: 10.20452/pamw.2963. [DOI] [PubMed] [Google Scholar]
- 32.Karoubi Nordon E, et al. Late onset systemic lupus erythematosus: a new approach. Lupus. 2007;16(12):1011–1014. doi: 10.1177/0961203307077148. [DOI] [PubMed] [Google Scholar]
- 33.Lalani S, et al. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. The Journal of rheumatology. 2010;37(1):38–44. doi: 10.3899/jrheum.080957. [DOI] [PubMed] [Google Scholar]
- 34.Liu HW, et al. Subset of systemic lupus erythematosus with late onset. Gaoxiong yi xue ke xue za zhi. 1988;4(10):547–52. [PubMed] [Google Scholar]
- 35.Maddison PJ. Systemic lupus erythematosus in the elderly. The Journal of rheumatology. Supplement. 1987;14(Suppl 13):182–7. [PubMed] [Google Scholar]
- 36.Mok CC, et al. Long-term survival of southern Chinese patients with systemic lupus erythematosus: a prospective study of all age-groups. Medicine. 2005;84(4):218–224. doi: 10.1097/01.md.0000170022.44998.d1. [DOI] [PubMed] [Google Scholar]
- 37.Pu SJ, et al. The clinical features and prognosis of lupus with disease onset at age 65 and older. Lupus. 2000;9(2):96–100. doi: 10.1191/096120300678828109. [DOI] [PubMed] [Google Scholar]
- 38.Sayarlioglu M, et al. Characteristics of patients with late onset systemic lupus erythematosus in Turkey. International journal of clinical practice. 2005;59(2):183–187. doi: 10.1111/j.1742-1241.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 39.Shaikh SK, Wang F. Late-onset systemic lupus erythematosus: clinical and immunological characteristics. The Medical journal of Malaysia. 1995;50(1):25–31. [PubMed] [Google Scholar]
- 40.Sousa S, et al. Clinical features and long-term outcomes of systemic lupus erythematosus: Comparative data of childhood, adult and late-onset disease in a national register. Rheumatol Int. 2016;36(7):955–60. doi: 10.1007/s00296-016-3450-2. [DOI] [PubMed] [Google Scholar]
- 41.Stefanidou S, et al. Clinical expression and course in patients with late onset systemic lupus erythematosus. Hippokratia. 2013;17(2):153–156. [PMC free article] [PubMed] [Google Scholar]
- 42.Takayasu V, et al. Systemic lupus erythematosus in the aged: clinical and laboratory characteristics. Rev Hosp Clin Fac Med Sao Paulo. 1992;47(1):6–9. [PubMed] [Google Scholar]
- 43.Tang Z, et al. Late onset lupus nephritis: analysis of clinical manifestations and renal pathological features in Chinese patients. Rheumatology international. 2011;31(12):1625–9. doi: 10.1007/s00296-010-1536-9. [DOI] [PubMed] [Google Scholar]
- 44.Tomic-Lucic A, et al. Late-onset systemic lupus erythematosus: clinical features, course, and prognosis. Clinical Rheumatology. 2013;32(7):1053–1058. doi: 10.1007/s10067-013-2238-y. [DOI] [PubMed] [Google Scholar]
- 45.Voulgari PV, et al. Gender and age differences in systemic lupus erythematosus. A study of 489 Greek patients with a review of the literature. Lupus. 2002;11(11):722–729. doi: 10.1191/0961203302lu253oa. [DOI] [PubMed] [Google Scholar]
- 46.Wilson HA, et al. Age influences the clinical and serologic expression of systemic lupus erythematosus. Arthritis & Rheumatism. 1981;24(10):1230–1235. doi: 10.1002/art.1780241002. [DOI] [PubMed] [Google Scholar]
- 47.Xu YX, et al. Late onset lupus nephritis in Chinese patients: classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Lupus. 2011;20(8):801–8. doi: 10.1177/0961203310397563. [DOI] [PubMed] [Google Scholar]
- 48.Meyer KC. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Seminars in respiratory and critical care medicine. 2010 doi: 10.1055/s-0030-1265897. [DOI] [PubMed] [Google Scholar]
- 49.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. The Journal of Immunology. 2004;172(1):40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 50.Hochberg MC, et al. Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine (Baltimore) 1985;64(5):285–95. [PubMed] [Google Scholar]
- 51.Manoussakis MN, et al. Sjogren's syndrome associated with systemic lupus erythematosus: clinical and laboratory profiles and comparison with primary Sjogren's syndrome. Arthritis and rheumatism. 2004;50(3):882–91. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 52.Mittoo S, et al. Systemic lupus erythematosus-related interstitial lung disease. Current Rheumatology Reviews. 2010;6(2):99–107. [Google Scholar]
- 53.Parambil JG, et al. Interstitial lung disease in primary Sjogren syndrome. CHEST Journal. 2006;130(5):1489–1495. doi: 10.1378/chest.130.5.1489. [DOI] [PubMed] [Google Scholar]
- 54.Thumboo J, et al. A comparative study of the clinical manifestations of systemic lupus erythematosus in Caucasians in Rochester, Minnesota, and Chinese in Singapore, from 1980 to 1992. Arthritis Care & Research. 2001;45(6):494–500. doi: 10.1002/1529-0131(200112)45:6<494::aid-art374>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]