Abstract
Background
Clostridium difficile (CD) infection (CDI) increases patient morbidity, mortality and healthcare costs. Antibiotic treatment induces gut dysbiosis and is both a major risk factor for CD colonization and treatment of CDI. Probiotics have been trialed to support commensal gut microbiota and reduce CDI. This study investigated commensal microbe Faecalibacterium prausnitzii (FP) and a prebiotic, both known to yield butyrate and be anti-inflammatory and immunomodulatory, on CD colonization and gut integrity in mice.
Methods
Mice were randomly grouped and supplemented daily with FP, prebiotic, FP + prebiotic, FP/prebiotic supernatant, or saline throughout the entire study. Following treatment with clindamycin for 3 days, mice were exposed to CD. Feces were collected at baseline, the day after antibiotic, and 1, 3, and 5 days after CD exposure and cultured for bacterial overgrowth and CD colonization. On days 1 and 5 after CD exposure, mice were randomly euthanized, and proximal colon was dissected for histological analysis and preparation of RNA for analysis of proinflammatory and anti-inflammatory cytokines.
Results
Although all mice exhibited bacterial overgrowth and CD colonization, bacterial burden resolved quicker in the FP + prebiotic group. This was associated with induction and resolution of innate immune responses, anion exchanger, and tight junction protein preservation in proximal colon. CD toxin virulence potential was questionable as expression of CD toxin B receptor was depleted in the FP + prebiotic group.
Conclusion
Supplementation with anti-inflammatory butyrate-supporting commensal bacteria and prebiotic may support innate immune responses and minimize bacterial burden and negative effects during antibiotic and CD exposure.
Keywords: antibiotics, butyrate, Clostridium difficile, innate immunity, intestine, microbiome, prebiotic, probiotics
Clinical Relevancy Statement
Clostridium difficile (CD) is a significant healthcare-associated pathogen with rising infection rates in U.S. hospitals and long-term care facilities. Gut microbiota are known to markedly influence host biology. Depleting gut microbiota and its beneficial metabolic and fermentation by-products, antibiotics are both a risk factor and treatment for CD infection. With a need to alleviate consequences linked with commensal gut microbial perturbations caused by antibiotics and subsequent CD infection, this study supplemented a commensal anti-inflammatory butyrate-supporting bacteria and prebiotic during antibiotic and CD exposure in mice and found effective innate immune responses, enhanced resolution of bacterial overgrowth, and protection of gut integrity in supplemented mice. These results imply that supplementation with commensal anti-inflammatory butyrate-supporting bacteria and prebiotic during antibiotic therapy may offer therapeutic benefit to preserving innate immune responses necessary to combat CD pathogenicity.
Introduction
Clostridium difficile (CD) infection (CDI) is debilitating to patients and extremely costly, with symptoms ranging from diarrhea to fulminant colitis, toxic megacolon, and death.1 In the United States, CDI is increasing, linked to 14,000 deaths annually.2 CDI risk factors include antibiotic therapy, hospitalization, gastrointestinal procedures and surgery, and advanced age.2 Ironically, because antibiotic exposure is a major risk factor for CDI, treatment involves further antibiotic therapy. To date, the best preventative measure for CDI is restricting inappropriate antibiotic usage.2
Bacteroidetes and Firmicutes are the 2 most abundant bacterial phyla in adults.3,4 Firmicutes phyla contain lactic acid and butyrate-producing bacteria (Clostridium clusters XIVa and IV). Types and proportions of bacteria vary within the gut, likely regulated by intestinal microenvironment and motility.5,6 Gut microbiota provide colonization resistance to pathogenic bacteria.5,7 Antibiotics alter colonization resistance likely by disturbing ecological, metabolic, and immunological functions of the gut microbiota- host ecosystem, which creates a niche that favors CD germination and growth.8 Characterization in global changes in microbial community structure associated with CDI and CD-negative nosocomial diarrhea find depletion in Firmicutes phyla abundance and diversity compared with healthy microbiota.9 Notably, butyrate-producing members of the Clostridia class were significantly depleted in CDI and CD-negative mouse microbiota, but Enterococcus and Lactobacillus were unusually abundant. 9 In addition, corresponding with depleted butyrate-producing bacteria, mice highly contagious with CDI also had altered short-chain fatty acid (SCFA) profiles characterized by a proportional reduction in butyrate and acetate, and an increase in formate, lactate, and succinate levels.10 Increased succinate and lactate correlated with an increase in succinate- and lactate-producing bacteria, respectively.10
Prebiotics are indigestible carbohydrates, which upon fermentation beneficially alter gut microbiota composition and produce substrate-specific amounts of SCFA acetate, propionate, and butyrate.11 Of various carbohydrates tested, resistant starch yields a higher molar ratio of butyrate compared with other prebiotics.12 Although the least abundant SCFA in the colonic lumen, butyrate is the most dynamic. Butyrate induces epithelial cell proliferation in normal intestinal tissue, but decreases cell proliferation and increases apoptosis in colon cancer cells,13 stimulates electrolyte and water absorption,14,15 is the primary fuel source for colonocytes and improves gut barrier function,16 and is anti-inflammatory and immunomodulatory through inhibition of transcription factor nuclear factor-κB.17 Absence of butyrate in intestinal tissue is associated with inflammation and mucosal atrophy.14,18
Probiotics are beneficial bacteria that, when consumed in adequate amounts, positively affect the host.19 Although not fully elucidated, probiotics are proposed to compete with pathogenic microbes for available nutrients and epithelial binding sites, decrease luminal pH, making it less favorable for pathogenic bacteria, modulate the immune response, and reestablish intestinal barrier function.19 Probiotic effects are strain specific, making the choice of probiotic crucial for therapeutic success. Various strains and combinations of probiotics have been attempted, with variable effects, to treat and prevent antibiotic-associated and CD-associated diarrhea with Lactobacillus species most commonly tested.
Faecalibacterium prausnitzii (FP) is an anaerobic commensal butyrate-producing bacterium and a dominant member of the Clostridium leptum subgroup known to have anti-inflammatory properties.20 FP is widely distributed in the intestine and accounts for approximately 5% of total fecal microbiota in healthy adults.20 Many diseases are associated with depleted levels of FP, including CDI.20 Because antibiotics provoke gut dysbiosis and CD induces diarrhea, intestinal inflammation, and potential mucosal injury, the aim of this study was to investigate the effects of oral supplementation with a butyrate-producing bacteria (FP) and butyrate-yielding prebiotic on bacterial colonization and colonic health in mice treated with antibiotics and exposed to CD.
Materials and Methods
Reagents
Sodium butyrate, potato starch (PS; Sigma-Aldrich, St. Louis, MO). All primers for quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) were synthesized by Integrated DNA Technologies (Coralville, IA). Antibodies were purchased from the following sources: anti–Na+/H+ exchanger isoform 3 (NHE3), anti–zona occludin-1 (ZO-1), anti–sodium-coupled monocarboxylate transporter 1 (SLC5A8), and anti–frizzled class receptor 7 (FZD7) from Abcam (Cambridge, MA); anti-occludin from Hycult Biotechnologies (Plymouth Meeting, PA); anti-claudin-3, Alexa Fluor 488, and 568 IgG from Invitrogen (Camarillo, CA).
CD Strain
VA17 is an epidemic North American pulsed-field gel electrophoresis type 1 (NAP1) CD strain with high virulence. CD spores were prepared as previously described,21 and animals were treated orally with 4 log10 colony-forming units (CFU) in 10 µL of phosphate-buffered saline (PBS) applied to a mouse treat.
Treatment Design
Treatment included butyrate-producing bacteria (FP; ATCC 27766)22 6 log10 CFU/10 µL and potato starch (PS) (20% w/v). This commensal strain was chosen because it was isolated from human feces and based on its ability to hydrolyze PS, yield butyrate, and utilize lactate.23 Bacteria were grown in conditions and medium as recommended by ATCC, briefly at 37°C in anaerobic gas biological mix of 80% nitrogen, 10% carbon dioxide, 10% hydrogen; ATCC pre-reduced chopped meat broth (ATCC 1703). Overnight culture of FP was aliquoted into 1-mL volumes and lyophilized using a Speed Vac concentrator (Savant, Farmingdale, NY). Lyophilized samples were reconstituted in pre-reduced PBS and plated onto chopped meat agar slant to confirm growth. FP aliquots were kept at −80°C and reconstituted in 100 µL of PBS just before mouse treatment each day. Viability of FP during this treatment period was confirmed via plating sample exposed to room air onto chopped meat agar slant. Brucella broth dilution minimum inhibitory concentration (MIC) of common antibiotics for FP and CD strains were determined using standard methods for susceptibility testing of anaerobic bacteria (Table 1).24
Table 1.
Minimum Inhibitory Concentrations Against Common Antibiotics for Faecalibacterium prausnitzii and Clostridium difficile Strains Used in This Study.
| F. prausnitzii | C. difficile | |
|---|---|---|
| MIC | MIC | |
| Cefoxitin | <2 | 250 |
| Clindamycin | <2 | 500 |
| Piperacillin/tazobactam | 8 | 15.63 |
| Vancomycin | <2 | <2 |
| Metronidazole | <2 | <2 |
| Positive control | Positive | Positive |
| Negative control | Negative | Negative |
MIC, minimum inhibitory concentration.
In Vivo Mouse Model of CD Colonization Resistance and Bacterial Overgrowth
A previously described mouse model evaluating recovery of CD colonization resistance after antibacterial treatment was used.25 Female CF-1 mice (Harlan Sprague-Dawley/ENVIGO, Indianapolis, IN), 8–10 weeks old and weighing 25–30 g, were housed individually in microisolator cages and fed sterilized Teklad Global 18% Protein extruded rodent diet (Harlan Teklad, Madison, WI).
Mice were randomized into the following supplemented treatment groups:
Control: received saline (10 µL) daily
FP: received 6 log10 CFU/10 µL daily
PS: received 20% w/v (20 µL) daily
FP+PS: received 6 log10 CFU/10 µL + 20% w/v (20 µL) daily
Supernatant of FP+PS: received media supernatant of FP+PS grown together (10 µL)
All treatments were provided orally, but to minimize stress to the animals, rather than providing treatments via oral gavage, they were applied to mouse treat. Before the start of the trial, mice were trained for 5 days to consume a mouse treat (Bacon Yummie, Bio-Serve, Prospect, CT) within 20–30 minutes of provision. Mice were provided their assigned supplemented treats daily throughout the entire trial, beginning with the first dose of the antibiotic clindamycin.
After treat training, mice in each treatment group received daily subcutaneous injections (0.1 mL total volume) of clindamycin (1.4 mg/d) for 3 days. The dose of antibiotic was equal to the usual human dose administered over a 24-hour period. Three days after the last dose of clindamycin, all mice were orally exposed to VA17 (4 log10 CFU/10 µL). Fresh stool specimens were collected on days 1, 3, and 5 after CD exposure, and concentration of enterococcus, gram-negative bacteria, and CD was measured by plating serially diluted stool samples on selective agar as previously described.25 Colonization resistance was deemed intact in clindamycin-treated mice if there was no significant increase in concentrations of enterococcus, gram-negative bacteria, and CD in stool in comparison with baseline levels. On day 5 after CD exposure, mice (n = 10) were randomly euthanized by CO2 asphyxiation, and proximal colon was dissected and frozen in optimal cutting temperature medium (OCT; Sakura Finetek USA, Torrance, CA) or stored in RNA later (Ambion, Austin, TX) for further analysis. Proximal colon was analyzed because this is a main location of CD pathogenicity. In addition, on day 1 after CD exposure, a subset of animals were randomly chosen from each treatment group (n = 3) and euthanized with tissue dissection, preservation, and analysis as described earlier. All animal experiments were approved by the Louis Stokes Cleveland Department of Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
Immunohistochemistry
Frozen sections of proximal colon embedded in OCT solution were mounted on glass slides that were coded before analysis. Slides were air-dried and fixed with 4% paraformaldehyde, then washed with 1 × PBS. Sections were blocked with 2% bovine serum albumin (dissolved in 1 × PBS) containing 0.1% Triton X-100, then blocked again with 2% bovine serum albumin only. Slides were incubated with primary antibodies overnight at 4°C, washed with 1 × PBS, incubated with secondary antibodies, then washed with 1 × PBS and mounted with DAPI-containing mounting media (Vectashield H-1200; Vector Laboratories, Burlingame, CA). Fluorescent images were acquired using a Leica confocal microscope with 3 images captured per slide.
Quantitative Real-Time Reverse Transcription PCR
Total RNA was isolated from proximal colon, and 2 µg of total RNA was reverse transcribed using the RETROscript kit (Invitrogen Ambion, Vilnius, Lithuania) with random decamers as primer. In brief, to melt the RNA secondary structure, we incubated the RNA and random decamer at 80°C for 3 minutes, cooled to 42°C, then added the remaining reaction mixture components. During a 60-minute incubation at 42°C, first-strand synthesis was performed, followed by a 10-minute incubation at 92°C to inactivate the Moloney Murine Leukemia virus-reverse transcriptase enzyme. Real-time PCR amplification was performed using PowerSYBR qRT-PCR kits (Applied Biosystems, Foster City, CA) on QuantStudio 5 analyzer (Applied Biosystems) for primers: monochemoattractant protein-1 (MCP1), neutrophil elastase (ELANE), interleukin-8 (IL-8), IL-1β, IL-10, toll-like receptor-2 (TLR2), inducible nitric oxide synthase (iNOS), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and 18S (Table 2). Relative amount of target messenger RNA (mRNA) was determined using the comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S or GAPDH and represented as fold-change relative to saline-treated mice.
Table 2.
Primer Sequences.
| Primer | Forward | Reverse |
|---|---|---|
| ELANE | CAG AGG CGT GGA GGT CAT TT | GAA GAT CCG CTG CAC AGA GA |
| GAPDH | AGG TCG GTG TGA ACG GAT TTG | TGT AGA CCA TGT AGT TGA GGT CA |
| IL-1β | ATG GCA ACT GTT CCT GAA CTC AAC T | CAG GAC AGG TAT AGA TTC TTT CCT TT |
| IL-8 | GCG CCC AGA CAG AAG TCA TAG | AGC CTT GCC TTT GTT CAG TAT C |
| IL-10 | GGC GCT GTC ATC GAT TTC TC | TGC TCC ACT GCC TTG CTC TTA |
| iNOS | GTT CTC AGC CCA ACA ATA CAA GA | GTG GAC GGG TCG ATG TCA C |
| MCP1 | AGG TCC CTG TCA TGC TTC TG | TCT GGA CCC ATT CCT TCT TG |
| TLR2 | TGC TTT CCT GCT GGA GAT TT | TGT AAC GCA ACA GCT TCA GG |
| 18S | ACG GAA GGG CAC CAC CAG GA | CAC CAC CAC CCA CGG AAT CG |
ELANE, neutrophil elastase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; iNOS, inducible nitric oxide synthase; MCP1, monochemoattractant protein-1; TLR2, toll-like receptor-2.
Statistical Analysis
All data are expressed the mean ± standard error of the mean (SEM). Stool culture data include 12–13 mice per treatment group; mRNA data include 3 mice/group for time point CD + 1 day and 10 mice/group for CD + 5 days. Student t test was used for parametric analysis of 2 groups; analysis of variance was used for comparison of multiple groups with a Tukey’s post hoc test for multiple comparisons. Statistical significance was defined as P < .05.
Results
Antibiotic Treatment Promoted Bacterial Overgrowth
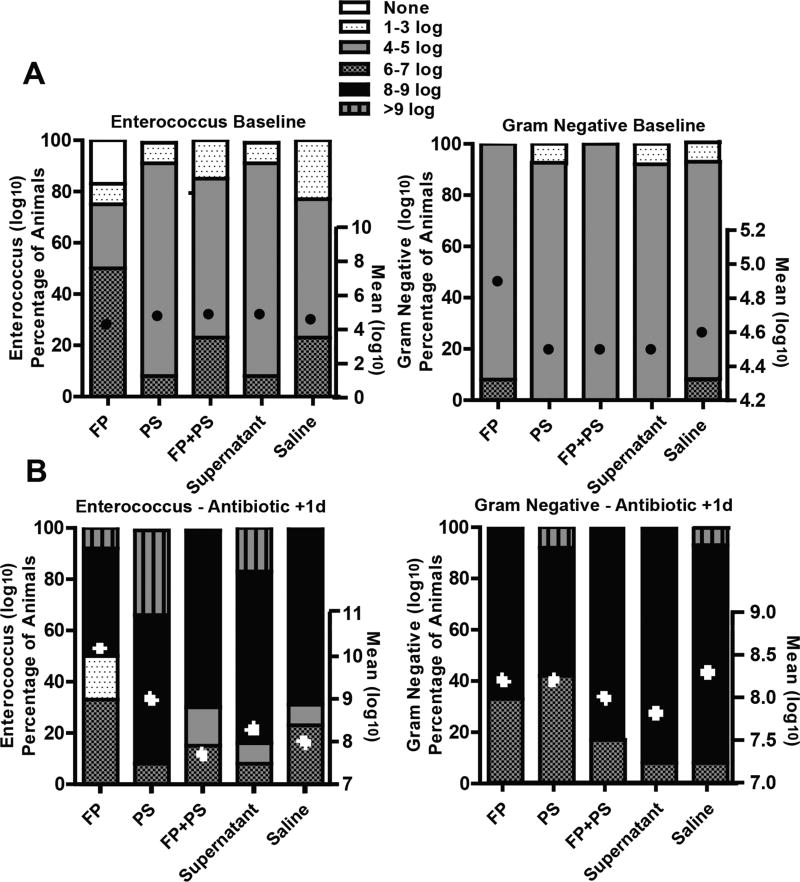
The fecal contents of mice were similar in enterococcus and gram-negative bacteria concentration at baseline (approximately 4 log10 CFU/g) (Figure 1A). Following 3 days of clindamycin, the mean value of both enterococcus and gram-negative in fecal content significantly increased in all treatment groups (approximately 8 log10 CFU/g) (Figure 1B). Of note, not all animals in each group colonized equally.
Figure 1.
Effect of antibiotic treatment on bacterial overgrowth. Mice were randomized into groups and supplemented daily with Faecalibacterium prausnitzii (FP), potato starch (PS), FP+PS, supernatant, or saline. Mice were treated with daily subcutaneous injections of clindamycin (1.4 mg/d) for 3 days. Following transfer to clean cages, fresh feces were collected, and concentration of enterococcus and gram-negative bacteria was measured by plating serially diluted samples on selective agar at (A) baseline before antibiotics and (B) 1 day after last dose of antibiotic. Data are presented as mean log10 CFU/g ± SEM and percentage of animals in each group with range of log10 CFU/g set as none, 1–3, 4–5, 6–7, 8–9, and >9 log10 CFU/g. n = 12–13 animals/group.
Bacterial Overgrowth and CD Colonization
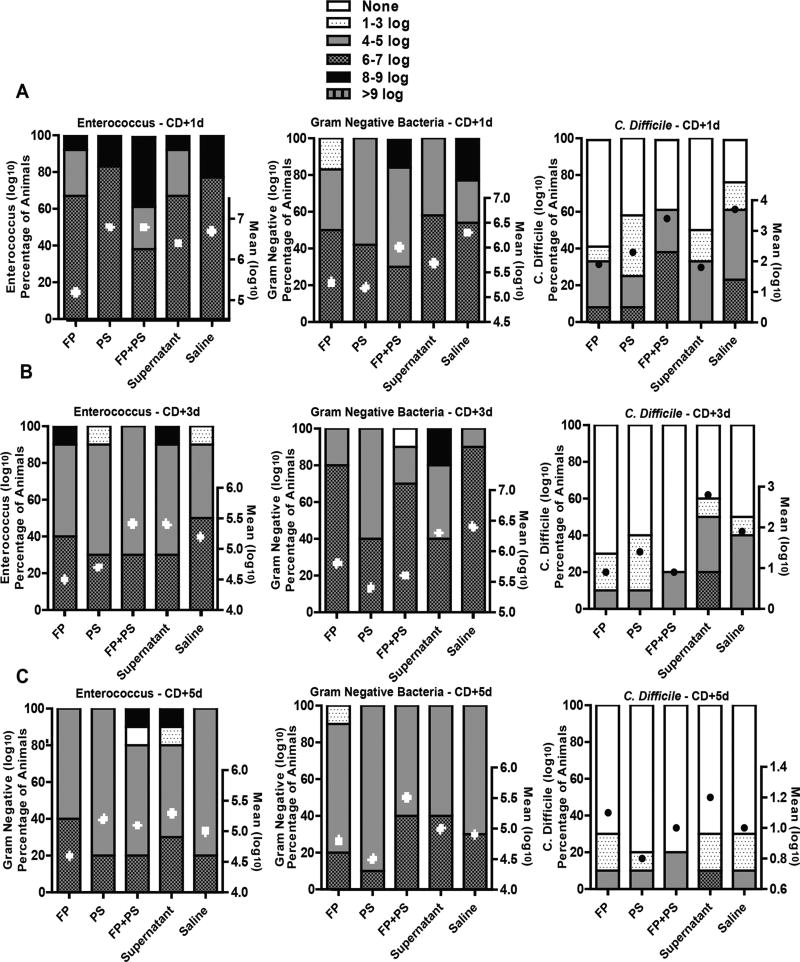
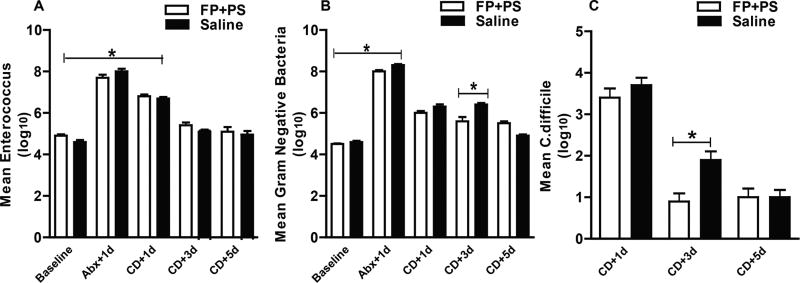
Bacterial overgrowth of enterococcus and gram-negative bacteria persisted throughout the study period (Figure 2). As expected, overgrowth diminished and was to near-baseline levels by 9 days after the last dose of clindamycin (approximately 5 log10 CFU/gm). Exposure to CD (4 log10 CFU) 3 days after the last dose of clindamycin supported fecal growth of CD, where the mean CFU/g for all treatment groups was 2.6 log10 ± 0.7. CD colonization diminished during the study period and was to near-baseline levels 5 days after exposure (1 log10 CFU/g ± 0.6). Although mean CFU per gram colonization was not statistically different between the groups at each time point, interestingly, there was variability among the animals within each treatment group in regard to the incidence and/or level of bacterial overgrowth and colonization (Figure 2). Where 60% of animals in the supernatant group, 50% in saline, 40% in PS, and 30% in FP were colonized with CD 3 days after exposure, the FP+PS treatment group exhibited clearance of CD in all but 20% of animals (2/10 mice) 3 days after exposure to CD. Although clearance of enterococcus was similar between the FP+PS and saline animals (Figure 3A), the FP+PS animals showed significant improvement in colonization with both gram-negative bacteria and CD 7 days after the last dose of clindamycin and 3 days after CD exposure compared with the saline-treated animals (Figure 3B, 3C).
Figure 2.
Bacterial colonization after Clostridium difficile (CD) exposure. Mice were randomized into groups and treated with clindamycin as described in Figure 1. Three days after the last dose of clindamycin, mice were orally exposed to VA17 (4 log10 CFU/mL). Mice received the randomized supplements daily. Concentration of enterococcus, gram-negative bacteria, and CD was measured by plating serially diluted fresh stool samples on selective agar on (A) day 1, (B) day 3, and (C) day 5 after CD challenge. Data are presented as mean log10 CFU/g ± SEM, and percentage of animals in each group with range of log10 CFU/g set as none, 1–3, 4–5, 6–7, 8–9, and >9 log10 CFU/g. n = 10 animals/group. FP, Faecalibacterium prausnitzii; PS, potato starch.
Figure 3.
Effect of Faecalibacterium prausnitzii (FP) and potato starch (PS) on bacterial colonization recovery. Mice received supplementation with FP+PS or saline and received clindamycin and Clostridium difficile (CD) as described in Figures 1 and 2. Fresh stool was cultured on selective agar for concentration of enterococcus, gram-negative bacteria, and CD measured by plating serially diluted samples on (A) day 1, (B) day 3, and (C) day 5 after CD challenge. Data are presented as mean log10 CFU/g ± SEM. *P < .05.
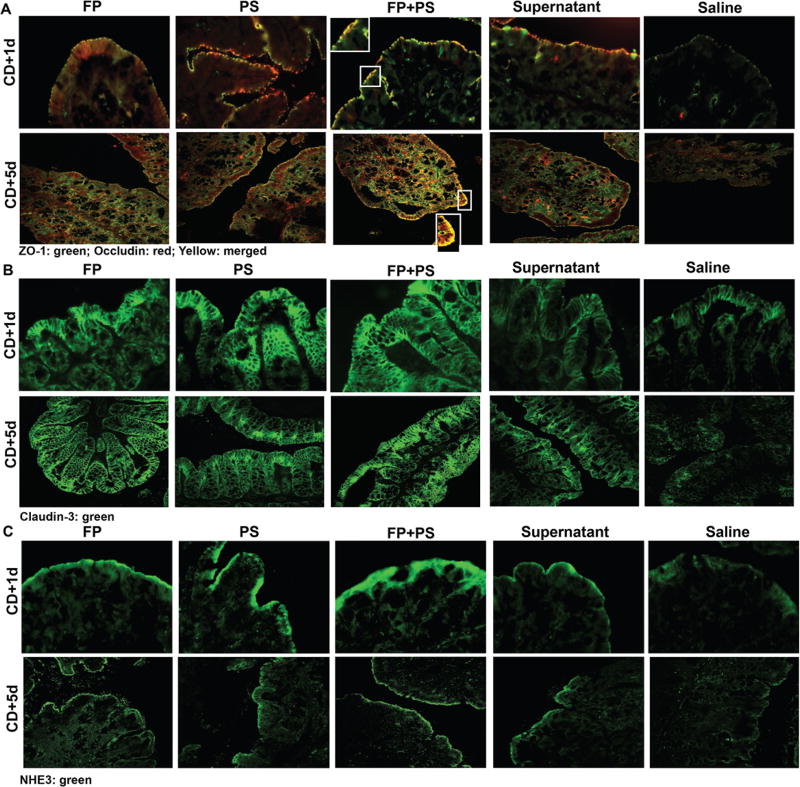
FP and PS Mitigated CD-Induced Disruption of an Anion Exchanger and Tight Junction Proteins
NHE3 is a sodium-hydrogen ion antiporter previously shown to be downregulated in germ-free mice, by antibiotic treatment and in patients with CDI.26–28 Loss of NHE3 is associated with dissociation of intestinal tight junction (TJ) proteins.27 To assess colonic expression of NHE3 and intestinal epithelial integrity, we assessed cell-to-cell junctional markers occludin, ZO-1, claudin-3, and NHE3 by immunofluorescence. Clindamycin treatment followed by CD exposure caused delocalization of TJ proteins (ZO-1, occludin, claudin-3), as well as the anion exchanger NHE3 in proximal colon of control mice (Figure 4). Cosupplementation with PS, FP, PS+FP, and supernatant maintained immunoreactive staining intensity of NHE3 and TJ proteins and colocalization of ZO-1 and occludin, with the PS+FP appearing most robust. Protein expression visually appeared intact at both 1 and 5 days after CD exposure in the supplemented groups; however, the depleted levels observed in the saline group at day 1 seemed to diminish further by day 5.
Figure 4.
Effects of Clostridium difficile (CD) on tight junction protein and an anion exchanger expression in proximal colon. Mice were treated as described in Figures 1 and 2. Proximal colon was collected and embedded in optimal cutting temperature medium (OCT) for histology on days 1 and 5 after CD exposure. (A) Occludin (red), zona occludin-1 (ZO-1; green), (B) claudin-3 (green), and (C) Na+/H+ exchanger isoform 3 (NHE3; green) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. A selected area was cropped and enlarged. All images were acquired using a 40 × objective. Images are representative of at least replicate images captured per mouse in 3–6 mice per treatment group. FP, Faecalibacterium prausnitzii; PS, potato starch.
FP Plus PS Effect on Butyrate Transporter and Pathogen Receptors
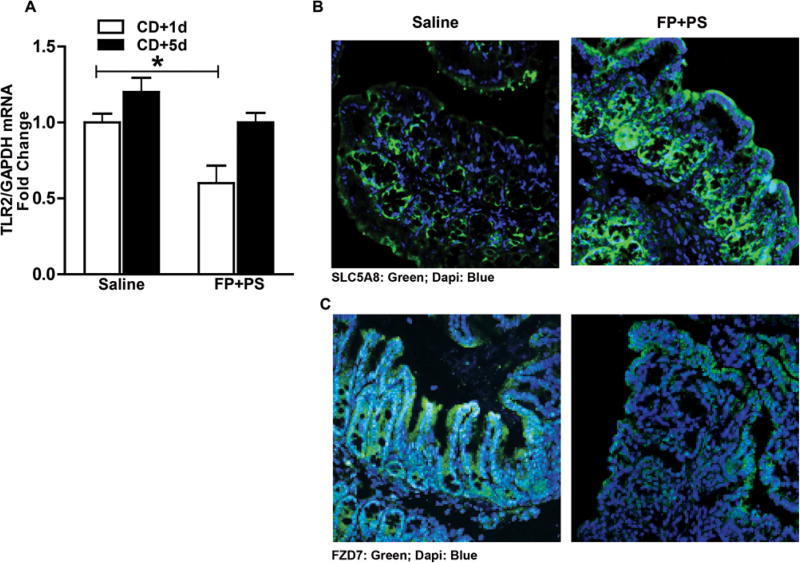
SLC5A8 transports butyrate via a Na+-dependent electrogenic process, and its expression is downregulated in the absence of luminal butyrate.26,27 We examined the expression of SLC5A8 via immunohistochemistry in proximal colon as a surrogate marker for butyrate presence because of FP and PS supplementation and found it maintained immunoreactive staining intensity, but that this intensity was depleted in saline-treated mice (Figure 5B).
Figure 5.
Comparison of Faecalibacterium prausnitzii (FP) plus potato starch (PS) with saline on butyrate transporter and pathogen receptors. Mice were treated as described in Figures 1 and 2. Proximal colon was collected and used to prepare RNA or embedded in optimal cutting temperature medium (OCT) for histology on days 1 and 5 after Clostridium difficile (CD) exposure. (A) Expression of TLR2 messenger RNA was detected in proximal colon of mice using quantitative real-time reverse transcription polymerase chain reaction. Data are the mean fold-change ± SEM. *P < .05. (B) SLC5A8 (green) and (C) FZD7 (green) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. All images were acquired using a 40 × objective. Images are representative of at least replicate images captured per mouse in 6 mice per treatment group at time point 5 days after CD exposure.
Following colonization, CD toxins A and B (TcdA and TcdB) are released. For these toxins to exert their effects, they first bind to receptors in the intestine. TLR2 is a membrane protein that recognizes pathogen-associated molecular patterns, particularly from gram-positive bacteria. FZD7 was recently identified as a physiologically relevant TcdB receptor in the colonic epithelium.29 To determine whether CD colonization had the potential to exert a physiological response and subsequent infection by interacting with these pathogen receptors, we examined expression of TLR2 and FZD7 in proximal colon. Two time points were evaluated: 1 and 5 days after CD exposure. Mice cotreated with FP+PS had reduced TLR2 mRNA expression 1 day after CD exposure compared with saline-treated animals, and mRNA expression levels were similar at 5 days after CD between groups (Figure 5A). Immunohistochemistry analysis showed FZD7 expression in colonic epithelium in saline-treated mice 5 days after CD exposure; however, FZD7 immunoreactive staining intensity was reduced in FP+PS-treated mice (Figure 5B).
FP Plus PS Supported Immune Response After CD Exposure
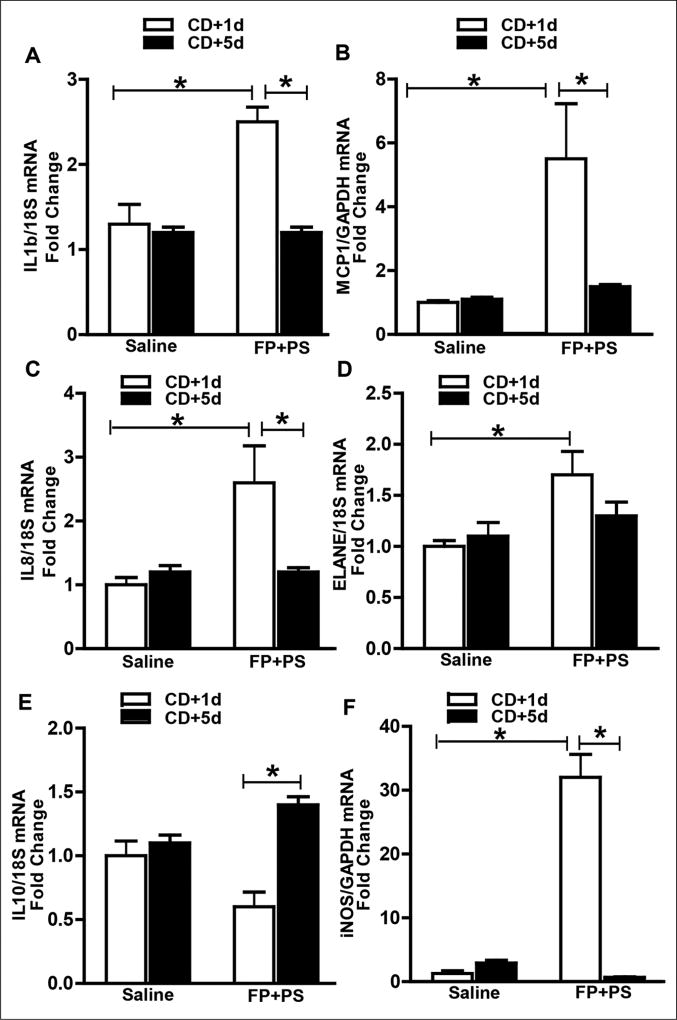
An active immune response is required for bacterial clearance and tissue repair, and chemokine function is necessary for immune cell recruitment. Therefore, we assessed cytokine/chemokine mRNA expression in proximal colon 1 and 5 days after CD exposure in saline- and FP+PS-treated mice. Mice in the saline group did not exhibit an induction in chemokines IL-1β, MCP1, or IL-8 at either time point. In contrast, mice cosupplemented with FP+PS exhibited an induction of these chemokines 1 day after CD exposure, which were then reduced at day 5 (Figure 6A – C). This chemokine induction was associated with an induction in ELANE mRNA and iNOS, markers for neutrophils and monocytes, respectively. Both ELANE and iNOS mRNA were blunted in saline-treated mice at day 1 and did not change by day 5 (Figure 6D, 6F). Although the anti-inflammatory cytokine IL-10 mRNA was low in the saline group at both time points, IL-10 mRNA levels in the FP+PS group increased between days 1 and 5 after CD exposure (Figure 6E).
Figure 6.
Effect of Faecalibacterium prausnitzii (FP) plus potato starch (PS) on chemokine and cytokines following Clostridium difficile (CD) exposure. Mice were treated as described in Figures 1 and 2. Proximal colon was collected and used to prepare RNA. (A–F) Expression of interleukin (IL)-1β, monochemoattractant protein-1 (MCP1), IL-8, neutrophil elastase (ELANE), IL-10, and inducible nitric oxide synthase (iNOS) messenger RNA were detected in proximal colon of mice using quantitative real-time reverse transcription polymerase chain reaction. Data are the mean fold-change ± SEM. *P < .05.
Discussion
We report for the first time the beneficial effects of oral supplementation of FP and PS against negative effects on colonic health induced by antibiotic and CD exposure in mice. Whereas antibiotic treatment and a single CD exposure caused fecal bacterial overgrowth and colonization, clearance of bacterial burden was accelerated in mice supplemented with FP and PS. This was associated with protection of intestinal TJ proteins, preservation of an anion exchanger, and acute induction and resolution of innate immune response.
Current theory suggests antibiotic-induced depletion of the dominant commensal bacterial phyla Bacteroidetes and Firmicutes, and increased Proteobacteria composition renders the intestinal environment susceptible to CD colonization. Butyrate-producing bacteria are found in the Clostridia class of the Firmicutes phyla, and depletion of butyrate-producing bacteria are noted to occur during CDI in both animals and humans.9,28,30,31 Our studies were consistent with other reports in that antibiotic treatment induced overgrowth of enterococci and gram-negative bacteria, and allowed for CD colonization.31 Other studies report clindamycin-induced bacterial overgrowth can last up to 28 days.31 Notably, although bacterial overgrowth and CD colonization occurred in all treatment groups, we found by day 3 after CD exposure that animals supplemented with a butyrate-producing bacteria and a butyrate-yielding prebiotic exhibited accelerated colonization recovery compared with those treated with saline. In addition, CD colonization was not evenly distributed within each treatment group, indicating that animals appeared to respond individually to CD exposure. This is similar to reports found in human studies confirming the individuality of colonization patterns in vivo.30 This may be due to animals having differing responses to antibiotic-induced bacterial overgrowth, which may create variances between animals within treatment groups to colonize with CD. Because CD is shed in stool and mice are coprophagous animals, some animals may have been reexposed to CD more frequently than others, and thus also account for variances.
Butyrate is pleiotropic, known to support water and electrolyte absorption, immune function, and intestinal barrier integrity.15–17 Our prior work demonstrates that supplementation with tributyrin, a triglyceride prodrug of butyrate, during broad-spectrum antibiotic treatment in mice protects against intestinal losses of a sodium-coupled butyrate transporter, butyrate receptor, TJ proteins, and the NHE3.27 NHE3 is essential for intestinal absorption of sodium and water. TcdB was shown to inhibit NHE3 by dephosphorylation and redistribution of ezrin, a protein that anchors NHE3 to the cytoskeleton, resulting in loss of NHE3 from the apical membrane.32 Engevik et al28 showed decreased NHE3 expression in colonic biopsies from patients with CDI, which associated with increased sodium content and alkalinity in CD+ stool. CD thrived in this environment demonstrating increased proliferation, whereas resident commensal bacteria Clostridium butyricum, Blautia producta, and FP did not and were thus depleted.28
After CD colonization, secreted virulence factors, toxins A and B (TcdA and TcdB), bind to host receptors, are internalized into the enterocyte cytoplasm via endocytosis, and become enzymatically active.28,32 Intestinal epithelial barrier damage is attributed to actions of TcdA and TcdB.32 Although we found animals supplemented with FP and PS still colonized with CD, we wanted to determine the potential for toxin virulence. As expected, expression of the newly identified TcdB receptor (FZD7) was highly expressed in the proximal colon of saline-treated animals. However, TcdB expression was diminished in mice supplemented with FP and PS. This decreased TcdB receptor expression was associated with preservation of a sodium-coupled butyrate transporter, SLC5A8, and TJ protein complexes in the proximal colon. Our prior work demonstrates that when commensal gut bacteria are depleted, butyrate transporter expression is also depleted, and that transporter expression returns to physiological levels with the return of commensal bacteria and luminal butyrate, which coincides with intact TJ barrier.26,27 Taken together, we propose in our studies supplementation with butyrate-enhancing FP and PS maintained apical membrane expression of SLC5A8 and NHE3; therefore, physiological colonic anion levels were maintained after CD exposure. By maintaining an environment unfavorable for CD to thrive, colonization clearance was accelerated, and due to lack of virulent toxin release, TcdB receptor was diminished and TJ proteins were preserved in mice supplemented with FP and PS. Butyrate and FP supplementation have both been associated with preservation of TJ proteins.27,33
Although the severity of CDI can be influenced by the adaptive immune response, CDI onset, progression, and overall prognosis are impacted by innate immune responses to CD toxins.32 Clinically it is not the overall toxin burden that predicts poor outcomes, but rather the magnitude and duration of the inflammatory response triggered by CDI.34 Demonstrated in mice with impaired innate immune capacity, following CD exposure animals were more vulnerable to CDI due to their inability to clear the initial infection and appropriately handle bacterial translocation across compromised intestinal epithelial barrier.35–37
Induction of an efficient innate immune response that involves release of inflammatory mediators and neutrophil recruitment is required for swift clearance of CD and protection against commensal bacterial translocation.32,38 Cell recruitment, or chemotaxis, is a highly regulated, receptor-mediated process in which cells migrate to chemokines, bacterial components, and complement factors. Macrophages, mucosal expression, and chemoattractant activity of transforming growth factor-β and IL-8 provide continuous recruitment of monocytes to the lamina propria in intestinal mucosa.39 Following infection, monocytes are recruited to the lamina propria of the intestine, where they differentiate into major producers of iNOS.40 Most known as a microbicidal and inflammatory effector pathway in macrophages, iNOS is also found in other cell types including normal colonic epithelium.41 Inflammatory cytokines, hypoxia, and microbial products can induce iNOS, whereas anti-inflammatory cytokines suppress iNOS gene transcription.42 Our studies found that saline-treated animals did not mount an immune response after CD exposure and colonization, and had associated losses of intestinal integrity at both time points evaluated. Conversely, we show that although animals supplemented with FP and PS did colonize with CD, they also exhibited an early induction and then resolution of chemokines. These chemokine changes associated with clearance of bacterial burden and preservation of markers of intestinal integrity. The initial robust immune response must be adequately controlled to limit persistent and collateral tissue damage. IL-10, a critical immunoregulatory cytokine, limits and downregulates inflammatory responses. During lipopolysaccharide-induced innate immune response, macrophages and neutrophils are the major IL-10-producing cells.43 We found that although saline-treated animals did not mount an immune response to chemokines, they also did not show any altered expression of anti-inflammatory cytokine IL-10. However, animals treated with FP and PS exhibited elevations in anti-inflammatory cytokine (IL-10) at day 5, the same time when proinflammatory mediators were resolved. Taken together, these effects tie into prior knowledge that butyrate and FP have known immunomodulatory and anti-inflammatory properties exhibiting protective effects against acute colitis.11,20,44,45
In summary, maintaining a balanced inflammatory response to counteract infection while limiting collateral tissue injury is likely beneficial during CD infection. We find protective effects of FP and PS against antibiotic-induced bacterial overgrowth and CD colonization after a single exposure in mice. These effects appear to be linked with an intact innate immune response that accelerates clearance of bacterial burden and protects against intestinal injury. Because recurrent CD infection carries high morbidity and mortality, further studies investigating preventative strategies directed toward protecting the gut microbial ecosystem and intestinal integrity against antibiotic effects are warranted.
Acknowledgments
Financial disclosure: This work was supported in part by National Institutes of Health National Institute on Alcohol Abuse and Alcoholism grant 4R00AA023266-03 (to G.A.M.C.); Division of Infectious Diseases & HIV Medicine at Case Western Reserve University and University Hospitals Case Medical Center, with funds from STERIS Corporation (to G.A.M.C.); Cleveland Clinic Children’s Hospital Mark Lauer Pediatric Research Grant (to G.A.M.C.); and the Case Western Reserve University/Cleveland Clinic CTSA (UL1RR024989).
Footnotes
Conflicts of interest: None declared. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Statement of Authorship
G.A.M. Cresci and C. Donskey contributed to the conception and design of the research; J. Cadnum contributed to the design of the research; G. A.M. Cresci, J. Cadnum, B. Glueck, S. Roychowdhury, M. Obrenovich contributed to the acquisition of the data; G.A.M. Cresci, C. Donskey, J. Cadnum contributed to the interpretation of the data; G. A.M. drafted the manuscript. All other authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
References
- 1.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447. doi: 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sehulster L, Chinn RYW. Guidelines for environmental infection control in health-care facilities. MMWR Recomm Rep. 2003;52(RR10):1–42. Available from: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_excerpt.html. [PubMed] [Google Scholar]
- 3.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathogens. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and Bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 5.Backhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Hostbacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 6.Hooper L, Midtvedt T, Gordon J. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 7.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 8.Theriot CM, Koenigsknecht MJ, Carlson PE, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antharam VC, Li EC, Ishmael A, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr. 2002;73:415s–420s. doi: 10.1093/ajcn/73.2.415s. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez OC, Lederer HM, Rombeau JL. Butyrate and the colono-cyte. Dig Dis Sci. 1996;41:727–739. doi: 10.1007/BF02213129. [DOI] [PubMed] [Google Scholar]
- 14.Thangaraju M, Cresci G, Anath S, Digby G, Lambert N, Mellinger J, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder HJ. Role of colonic short-chain fatty acid transport and diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 16.Ploger S, Stumpff F, Penner G, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann NY Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 17.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 18.Hass R, Busche R, Luciano L, Reale E, Engelhardt WV. Lack of butyrate is associated with induction of Bax and subsequent apoptosis in the proximal colon of guinea pig. Gastroenterology. 1997;112:875–881. doi: 10.1053/gast.1997.v112.pm9041249. [DOI] [PubMed] [Google Scholar]
- 19.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 20.Miquel S, Martin R, Rossi O, et al. Faecalibaterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2014;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 21.US Environmental Protection Agency Office of Pesticide Programs. Standard Operating Procedure for Production of Spores of Clostridium difficile for Use in the Efficacy Evaluation of Antimicrobial Agents, SOP Number: MB-28-04. Office of Pesticide Programs, Microbiology Laboratory, Environmental Science Center: Ft. Meade, MD. 2014 Jun 19; Available from: https://www.epa.gov/sites/production/files/2014-12/documents/mb-28-04.pdf.
- 22.Duncan SH, Hold GL, Harmsen H, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb.nov. Int J Syst Evol Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 23.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72:3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Approved standard M7-A5. 5. National Committee for Clinical Laboratory Standards; Wayne, PA: 2000. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Anaerobically. [Google Scholar]
- 25.Pultz NJ, Donskey CJ. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob Agents Chemother. 2005;49:3529–3532. doi: 10.1128/AAC.49.8.3529-3532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cresci G, Thangaraju M, Mellinger J, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- 27.Cresci G, Nagy L, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. J Parent Enteral Nutr. 2013;37:763–774. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engevik MA, Engevik KA, Yacyshyn MB, et al. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol. 2015;308:G497–G509. doi: 10.1152/ajpgi.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao L, Zhang J, Meraner P, et al. Frizzled proteins are colonic epithelial receptors for C. difficile B. Nature. 2016;538:350–355. doi: 10.1038/nature19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ. Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. mBio. 2016;7:e01965–16. doi: 10.1128/mBio.01965-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buffie CG, Jarchum I, Equinda M, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi H, Szaszi K, Coady-Osberg N, et al. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol. 2004;123:491–504. doi: 10.1085/jgp.200308979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Hirota SA. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol Immunol. 2015;63:193–202. doi: 10.1016/j.molimm.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laval L, Martin R, Natividad JN, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyperpermeability in mice. Gut Microbes. 2015;6:1–9. doi: 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Feghaly R, Stauber JL, Deych E, Gonzalez C, Tarr PI, Haslam DB. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. J Clin Dis. 2013;56:1713–1721. doi: 10.1093/cid/cit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa M, Yamazaki T, Kamada N, et al. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol. 2011;186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 37.Ryan A, Lynch M, Smith SM, et al. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7:e1002076. doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun. 2012;80:2989–2996. doi: 10.1128/IAI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buonomo EL, Petri WA. The microbiota and immune response during Clostridium difficile infection. Anaerobe. 2016;14:79–84. doi: 10.1016/j.anaerobe.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smythies LE, Maheshwari A, Clements R, et al. Mucosal IL-8 and TGFβ recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80:492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 41.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perner A, Andresen L, Normark M, Rask-Madsen J. Constitutive expression of inducible nitric oxide synthase in the normal human colonic epithelium. Scand J Gastroenterol. 2002;37:944–948. doi: 10.1080/003655202760230919. [DOI] [PubMed] [Google Scholar]
- 43.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pils MC, Bleich A, Prinz I, et al. Commensla gut flora reduces susceptibiolity to experimentally induced colitis via T-cell-derived interleukin-10. Inflamm Bowel Dis. 2011;10:2038–2046. doi: 10.1002/ibd.21587. [DOI] [PubMed] [Google Scholar]
- 45.Miquel S, Leclerc M, Martin R, et al. Identification of Metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015;6:e00300–15. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]