Abstract
Adult neurogenesis in mammals is a tightly regulated process where neural stem cells (NSCs), especially in the subgranular zone (SGZ) of the hippocampal dentate gyrus, proliferate and differentiate into new neurons that form new circuits or integrate into old circuits involved in episodic memory, pattern discrimination, and emotional responses. Recent evidence suggests that changes in the hyaluronan (HA)-based extracellular matrix of the SGZ may regulate neurogenesis by controlling NSC proliferation and early steps in neuronal differentiation. These studies raise the intriguing possibility that perturbations in this matrix, including HA accumulation with aging, could impact adult neurogenesis and cognitive functions, and that alterations to this matrix could be beneficial following insults to the central nervous system that impact hippocampal functions.
Keywords: neurogenesis, neural stem cell, perineuronal net, hyaluronan
Introduction
Neural stem/progenitor cells (NSCs) in both the developing and adult brain are multipotent self-renewing cell populations that give rise to neurons and glial cells in the mammalian central nervous system (CNS). During early development, NSCs reside in the neural tube and undergo numerous rounds of self-renewal before dividing asymmetrically, giving rise to early populations of neurons and radial glial cells [1]. Later in development, NSCs that reside in the ventricular zone and subventricular zone differentiate predominantly into astrocytes, then oligodendrocytes during perinatal periods. In the adult CNS, NSCs reside in NSC niches including the subventricular zone lining the lateral ventricles and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus [2]. These NSCs are heterogeneous with regards to their localization and their patterns of differentiation [3], and are capable of neurogenesis and gliogenesis throughout life in response to a variety of stimuli ranging from learning to injury.
A variety of extracellular signals in NSC niches, including the ligands of receptor tyrosine kinases, have been implicated in regulating NSC proliferation, differentiation and survival [4, 5]. Components of the extracellular matrix (ECM) have also been implicated in dynamically regulating NSC behaviors during both embryonic development and in adults. For example, NSC niches include a basal lamina that is enriched in collagen and laminin [6–8]. In adult NSC niches, the basal lamina regulates the adhesion of NSCs to the ECM. Extracellular glycoproteins such as tenascin C also play roles in regulating NSC within NSC niches. Disruption of tenascin C results in increased generation of astrocytes due to altered responsiveness to fibroblast growth factor-2 and epidermal growth factor [9]. Finally, proteoglycans have been implicated in regulating a number of NSC behaviors. Proteoglycans consist of a core protein and covalently attached glycosaminoglycan chains. While they have signaling properties of their own, they also can bind other extracellular factors, including signaling molecules, membrane proteins, and components of the ECM to influence NSC migration, differentiation, and survival [10].
Another major constituent of the CNS ECM is hyaluronan (or hyaluronic acid, HA). HA is a large unbranched, non-sulfated glycosaminoglycan composed of repeating disaccharide units of N-glucuronic acid and N-acetylglucosamine. HA acts as a backbone for numerous proteoglycans in the CNS including neurocan, aggrecan, and versican [11, 12]. However, HA is also found at cell surfaces independent of proteoglycans where it binds to various transmembrane and extracellular receptors and HA-binding proteins. HA is synthesized at the inner face of the plasma membrane and secreted into the extracellular space by a family of transmembrane proteins known as HA synthases (HASs). In some instances, HA can remain tethered to synthases or to transmembrane HA receptors. Mammals have three such synthase genes, HAS1, HAS2 and HAS3. HA catabolism, on the other hand, is carried out by hyaluronidases (HYALs) and HA depolymerizing proteins that differ in their cellular localization and pH optima. Mammals possess multiple hyaluronidase and HA depolymerizing genes, including HYAL-1 through HYAL-5, PH20/SPAM1, transmembrane protein 2 (TMEM2), and Cell Migration-Inducing hyaluronan binding Protein (CEMIP) (also called HYBID and KIAA1199) [13–15]. In addition, humans carry a hyaluronidase pseudogene designated PHYAL-1 [13].
Changes in HA synthesis and catabolism play numerous roles in CNS development and homeostasis (e.g. [16–19]). In this review, we discuss recent data supporting distinct roles for HA in adult neurogenesis and, subsequently, in regulating neuronal function within perineuronal nets. We further explore the possibility that changes in the HA matrix with aging or following a variety of insults to the CNS can significantly impact neuronal differentiation as well as learning, memory, and behavior.
Disruption of HA signaling influences hippocampal function
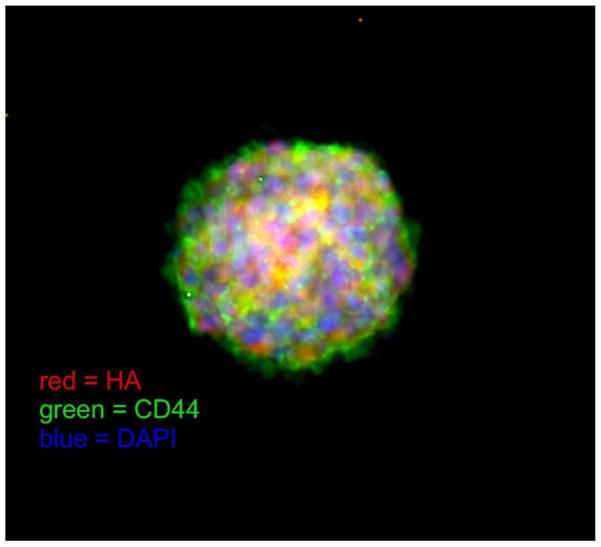
The hippocampal dentate gyrus contributes to the formation of episodic memories (e.g. the times, places, emotions and contexts associated with specific events) as well as pattern separation and emotional control. Each of these functions have been linked to alterations in adult neurogenesis in the SGZ [20]. A role for the HA-based ECM in regulating adult neurogenesis in the dentate gyrus is supported by the finding that HA is enriched in the SGZ [21, 22]. Interestingly, HA is synthesized by NSCs themselves, which express all three mammalian HA synthases [22] (Fig. 1). Furthermore, a major transmembrane HA receptor, the CD44 glycoprotein, is also expressed in the dentate gyrus especially by NSCs [22–24] (Fig. 1).
Fig. 1.
A whole-mount mouse SGZ-derived neurosphere labeled with HA (red, using a biotinylated HA-binding protein), CD44 (green) and DAPI (blue; to stain cell nuclei). As shown here, some of the outermost cells in neurospheres, which are undifferentiated NSCs, express both CD44 and HA (yellow staining). The degree to which this co-localization occurs varies from one neurosphere to another.
A functional role for HA in hippocampal activity was first demonstrated by Kochlamazashvili and co-workers [25] who found that disruption of HA in the hippocampus by intra-hippocampal injection of a hyaluronidase impaired hippocampus-dependent contextual fear conditioning. We subsequently found that mice lacking CD44 show hippocampus-dependent memory impairments [26]. Specifically, CD44 null mice demonstrated impaired hippocampus-dependent spatial memory retention in the probe trial following the first hidden-platform training day in the Morris water maze. There were no genotype differences in swim speeds during the water maze training sessions with the visible or hidden platform. However, some sensorimotor impairments were seen in other behavioral tests. In the inclined screen and balance beam tests, CD44 null animals moved less than wild type mice, while in the wire hang test, CD44 null mice fell off of the wire faster than wild type mice. No genotype differences were observed in tests of emotional learning and memory [26]. These data support an important role for CD44 in hippocampal spatial memory retention and in locomotor and sensorimotor functions.
More recently, Barzilay and co-workers [27] demonstrated that CD44 null mice have increased susceptibility to stress-induced anxiety and demonstrate increased anhedonia and despair compared to wild type mice. These authors also reported that CD44 null nice display reduced cortical serotonergic and striatal dopaminergic turnover, and that the hippocampal expression of another HA receptor, the receptor for HA-mediated motility (RHAMM) is reduced in non-stressed CD44 null mice compared with wild type animals [27]. Although this study did not directly examine changes in adult neurogenesis in CD44 null mice under these conditions, these findings, in conjunction with the previous studies discussed above, collectively support a role for HA and HA receptors in hippocampal neuron function and neurogenesis.
HA regulates NSC proliferation and neuronal differentiation in the SGZ
A study of NSCs on scaffolds made of HA and collagen indicated that HA could play a role in potentiating NSC differentiation [28]. Another study, using HA-laminin hydrogels, demonstrated that the HA-based ECM can regulate NSC migration in response to stromal cell-derived factor-1-alpha (SDF1α) via the CXCR4 receptor [29]. To address the specific roles of HA and CD44 in hippocampal neurogenesis, we recently examined the phenotypes of CD44 null SGZ-derived NSCs and of NSCs treated with a hyaluronidase both in vitro and in vivo. We found that CD44-null NSCs or hyaluronidase-treated wild type NSCs demonstrate increased proliferation rates and delayed neuronal maturation in vitro [22]. Treatment of NSCs with high molecular weight HA (>2 MDa) correspondingly inhibited NSC proliferation in wild type but not CD44-null NSCs [22]. Consistent with these in vitro results, mice lacking CD44 and wild type mice sterotactically injected with hyaluronidase into the SGZ demonstrated increased NSC proliferation, as assessed by Ki-67 and bromodeoxyuridine labeling, and delayed neuronal differentiation. In addition, CD44-null mice display a significant reduction in c-fos expression throughout the dentate gyrus granule cell layer after spatial learning [22], consistent with the notion that these excess immature neurons that accumulate in the granule cell layer may lack functional connectivity. All together, these data indicate that HA in the SGZ signals through CD44 expressed by NSCs to regulate NSC proliferation and early steps in neuronal differentiation.
HA regulates the activities of mature neurons
The mechanisms by which HA-CD44 signaling influences hippocampal neurogenesis are unclear. HA binding to CD44 may be one of many signaling events in the SGZ that cooperate to regulate the timing, onset, and progression of neurogenesis [30]. Alternatively, CD44 activation by HA may directly stimulate intracellular signaling to regulate NSC proliferation and differentiation. Interestingly, CD44 and HA may also play direct roles in regulating the activities of mature neurons. For example, we found that the baseline calcium levels in CD44 null neurons are significantly higher than those in wild type neurons, and that CD44 null neurons are less sensitive to KCl stimulation than wild type cells (our own unpublished findings). This phenotype is reminiscent of the phenotype observed in aged neurons [31–32] and in neurons affected by neurodegenerative disorders [33]. These data suggest that HA-CD44 interactions can influence the activities of calcium channels in mature neurons and further change hippocampal functions [25, 34]. However, we find that although CD44 expression is high in NSCs, it becomes undetectable as hippocampal neurons differentiate [22]. Thus, an alternative possibility is that cells from CD44 null animals aberrantly differentiate, resulting in altered calcium levels within the cells and altered hippocampal activity.
Unlike HA in NSC niches, many of the functions of HA in mature neurons have been linked to the role of HA in perineuronal nets (PNNs), specialized structures that wrap around neurons and proximal dendrites, with openings where synaptic inputs contact their underlying cells [35]. PNNs were first described as reticular structures by Golgi in the late 1800s [36, 37]. They are found in the CNS in a wide range of species, ranging from frogs to birds to mammals, including humans [37–39]. They form during development, and their overarching function is to stabilize synapses during adulthood and generally inhibit plasticity (see below). Despite their original discovery over 100 years ago, only recently has there been a focus on the role of PNNs in physiological brain functions, such as learning and memory, as well as in many pathologies, including schizophrenia, Alzheimer’s disease, stroke, epilepsy, autism, drug addiction, and spinal cord injury [40]. Overall, PNNs play key roles in neural development, synaptogenesis, neuroprotection, and experience-dependent synaptic plasticity [37, 41–44]. The increased excitement about understanding the role of PNNs in plasticity stems from findings that the removal of PNNs restores juvenile-like states of plasticity, as discussed in greater detail below and outlined in several reviews [35, 45–47].
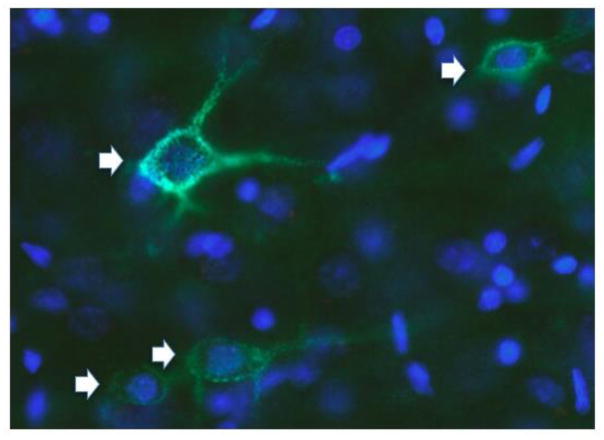
PNNs are formed by the contribution of four families of ECM molecules. (1) HA is a significant component of PNNs, but unlike NSC niches, HA in PNNs appears to mostly form a backbone onto which other PNN molecules bind [11, 48]; (2) Chondroitin sulfate proteoglycans (CSPGs; primarily of the lectican family, including aggrecan, brevican, and versican), which bind via their amino-terminus to HA [49–52]. The composition of CSPGs in PNNs has been distinguished from that present in the loose ECM by using extraction procedures employing relatively harsh treatment (6 M urea), which separates components in PNNs from those in the loose ECM [53]. Further, the composition of these CSPGs varies within different brain regions [45, 54]. (3) Tenascins, proteins that form multimers and cross-link the lecticans to help stabilize PNNs [55]; and (4) HA and proteoglycan link proteins (HAPLNs; HAPLN1, 3 and 4 are found in the CNS). These proteins bind to both the HA backbone and CSPGs to stabilize PNNs [49, 56–58]. PNNs are most commonly identified by staining them with the plant lectin, Wisteria floribunda agglutinin (WFA; Fig. 2), which is thought to bind to terminal N-acetylgalactosamines beta 1 residues of glycoproteins [59], most likely on aggrecan; other methods for staining PNNs have also been used but probably bind to the same residues [59–62].
Fig. 2.
Neurons in a section from mouse prefrontal cortex labeled with Wisteria floribunda agglutinin (WFA; green; arrows) to label PNNs and DAPI (blue; to stain cell nuclei). This image demonstrates the extent to which PNNs surround both neuron cell bodies (green staining surrounding DAPI-labeling) and processes.
The HA synthesizing enzymes, HAS1, HAS2, and HAS3, are found in neurons with PNNs [45]. The different isoforms of HAS synthesize HA chains of different lengths [63, 64], and these may in turn impart PNNs with different functions that vary among brain areas. Which particular HAS enzymes are expressed on PNN-surrounded neurons varies dependent on the brain area and time point during development [45, 47]. For example, the spinal cord expresses HAS1 and HAS3, with only HAS3 in adult spinal cord [65]. In the mouse cortex and hippocampus, although all three enzymes are expressed, there is localization of HAS2 and HAS3 predominantly on cell bodies [66].
The mechanisms by which PNNs are attached to their underlying neurons has been examined in detail in the cerebellum [49]. Three HA receptors located in the CNS, CD44, RHAMM, and LYVE-1 [67], were examined, but none of these receptors was found in the PNNs of the cerebellum. The authors concluded that, of the three isoforms of HAS in mammals [68], neurons surrounded by PNNs have HA attached to their synthesizing enzyme, in this case, HAS2 and HAS3, as HAS1 was not found in PNNs in the cerebellum. The attachment of PNNs to their underlying cells via membrane HAS enzymes is supported by the observation that HEK cells can be transfected to generate HA and aggrecan, and these HEK cells in turn produce PNNs [56].
The removal of PNNs is accomplished most commonly by treatment with the bacterial enzyme called chondroitinase ABC (Ch-ABC; EC 4.2.2.4) from Proteus vulgaris NCTC 4636. This enzyme acts on CSs and CSPGs, and it also removes HA, HA binding proteins, and N-acetylgalactosamine [69, 70] as well as the HA binding region that binds proteins in the PNN [69]. The effects of Ch-ABC on PNNs are known to be long lasting: the enzyme remains active for several days [71]. At 4 weeks post-Ch-ABC treatment, some PNNs re-appear but their normal appearance does not occur for 5 months [69]. It remains unknown whether PNNs reappear on the same neurons or with the same composition. Hyaluronidase treatment also reduces PNN staining by WFA, consistent with its removal of two key components, aggrecan and link proteins [72]. However, the effects are much shorter lasting, with reappearance after several days [73]. While hyaluronidase treatment reduces the intensity of PNN staining by WFA, it does not influence the ECM that wraps around the initial segment of axons [74].
The role of HA in PNNs may depend on the cell type and the contents of the ECM
In many brain regions, PNNs are found mostly around fast-spiking, parvalbumin (PV)-containing GABAergic interneurons [75–78]. However, an increasing number of studies demonstrate that PNNs also surround glycinergic neurons [79] as well as glutamatergic neurons [49, 80–84], which can be both parvalbumin positive or negative [80, 81]. PNNs are located throughout the brain and spinal cord, but are unevenly distributed [85]. They form during development at different rates across the brain and spinal cord [86, 87] and are completed by early adulthood in the cortex of rodents [88], with differences in developmental rates among cortical subregions. Neural activity promotes PNN development, which occurs through changes in potassium and calcium conductance and through activation of glutamate receptors (N-methyl-D-aspartate receptors and calcium-permeable AMPA receptors) [77, 86, 89].
Interestingly, the development of PNNs occurs precisely at the time when critical period plasticity ends and when brain plasticity is greatly reduced. The core components of PNNs (detailed below) increase during this critical window of development [90]. PNNs have been heavily studied for their contributions to critical period plasticity within the visual system, motor system, and somatosensory system [88, 91–93]. PNNs limit plasticity in adulthood, but they can be degraded to reinstate juvenile-like states of plasticity, allowing for axon sprouting and regeneration of function in damaged neurons.
Several knockout (KO) mouse models have been generated to determine the contribution of the various HA-containing lecticans to PNNs. Versican V2 (a splice variant), neurocan, or brevican KO mice have largely normal PNNs [94], whereas aggrecan KO mice do not stain for WFA, indicating that aggrecan is a key contributor to PNN formation [62]. Given that these proteoglycans are absent during development, it is difficult to know how compensatory changes might preserve PNN structure. Overall though, the diversity of lecticans (e.g., [95]) and other components of PNNs, such as link proteins that are critical for PNN formation [58] and the length of the HA chain, are expected to confer different functions of PNNs across different regions of the brain and spinal cord, although exactly what these functions are remain unknown. In addition, other features of PNNs allow for further complexity. For example, the lecticans contain chondroitin sulfate (CS) chains that are sulfated at different positions during development, and this is believed to regulate plasticity in terms of axonal growth and guidance [96–99]. In addition, the sulfation pattern of CS chains continues to change in old age, conferring an even greater inhibitory function to PNNs than is found in younger adults [100].
To date, the best studies examining the function of HA in PNNs have relied on hyaluronidase digestion of the ECM. Each of these studies provide clues about potential roles for HA in PNNs and associated ECM. An early study found that chronic exposure to hyaluronidase caused severe neurological dysfunction, such as rigidity, unresponsiveness and grand mal seizures [101]. The underlying mechanism of these phenomena is unclear due to the complex nature of the neuronal networks involved. However, more recent studies with better controlled delivery of hyaluronidases have revealed more specific roles linked to changes in the PNN ECM. For example, hyaluronidase treatment in the auditory cortex promotes cognitive flexibility in an auditory learning task in gerbils [102]. Hyaluronidase also decreased calcium influx through L-type channels in hippocampal dendrites and abolished long-term potentiation (LTP) in hippocampal (CA1 and CA3) synapses [25]. Addition of HA to hyaluronidase-treated hippocampal neuron cultures restored LTP, indicating that HA acts through L-type calcium channels to modulate synaptic function in the CA1/CA3 regions of the hippocampus. Digestion of HA also increases hippocampal sharp wave ripples observed during slow wave sleep [103], and a combination of Ch-ABC with hyaluronidase impairs fear memory when infused into the medial prefrontal cortex or dorsal hippocampus [104].
One area where HA in PNNs may play a critical role is in limiting the mobility of AMPA receptors (AMPARs) in the postsynaptic membrane. The rapid movement of AMPARs between junctional and extrajunctional sites allows desensitized receptors to be replaced by “fresh” responsive receptors. Treatment of mature PNN with hyaluronidase restored mobility of the AMPAR and synaptic plasticity [105]. This same study reported that PNN degradation did not affect the migration of NMDA receptors. However, there is evidence that the functional properties of NMDA receptors are affected by disruption of PNNs. Schweitzer and co-workers [106] reported that the PNN regulated the composition of NMDAR subunits, designated as GluN2A and GluN2B. During the development of the visual cortex and hippocampus, NMDARs are composed of the more adaptable GluN2B but after the end of the critical period the less plastic GluN2A predominates. In dissociated hippocampal culture the expression of GluN2A and GluN2B follow a similar time course (GluN2B being replaced by GluN2A). The dominant expression of GluN2A in mature cultures can be reversed treating the cells with hyaluronidase.
Each of these studies suggests that HA and HA-associated proteoglycans within PNNs could impact different aspects of neuronal activity. However, with the exception of knockout mice deficient in CSPGs or link protein, all studies to date have used hyaluronidase or Ch-ABC to assess the function of PNNs. A drawback to this approach is that the loose ECM is also digested, bringing into question whether damage to the loose ECM contributes to the neural plasticity effects. In addition, in studies that rely entirely on hyaluronidases, it is unclear if the physiological effects of hyaluronidases are due to loss of high molecular weight HA within PNNs or associated loose ECM, physiological effects of specific sizes of HA digestion products, or more generalized disruption of HA-associated proteoglycans. Future studies will need to develop more advanced methods to manipulate PNNs and their component, including HA, by targeting their removal in a cell- and brain region-specific manner.
How changes in the HA-based ECM can impact neurogenesis and neuronal functions
The manipulation of HA and HA receptors in the SGZ and in PNNs could have a number of therapeutic benefits following different insults to the CNS that lead to altered neurogenesis or neuronal function. For example, there is a dramatic increase in NSC proliferation and an accumulation of immature neurons in the SGZ following seizures [107–109]. These changes are remarkably similar to the phenotypes observed in CD44 null mice and in wild type mice in which HA was disrupted in the SGZ [22]. Animals with seizure-induced increases in NSC proliferation are prone to subsequent seizures and associated cognitive difficulties [19, 27, 107, 110–113]. Furthermore, HA and levels of hyaluronan synthase-3 are altered in the hippocampus following seizures in rats [108] while degradation of HA with a hyaluronidase or treatment with CD44 function-blocking antibodies altered seizure-induced mossy fiber sprouting [109]. Recent studies support the notion that the cells demonstrating aberrant mossy fiber sprouting are aberrant adult-born dentate granule cells [114]. It is possible, therefore, that altering HA digestion or CD44 expression on in the SGZ or on mossy fibers following seizures could help limit seizure-associated cognitive deficits.
Excess accumulation of HA and CD44 overexpression could also influence changes in adult neurogenesis with aging. Neurogenesis in the SGZ decreases with aging, possibly contributing to age-related cognitive decline [115–120]. Decreased neurogenesis with aging is at least partly linked to reduced NSC proliferation. A number of studies have suggested that age-related changes in the NSC microenvironment could limit adult neurogenesis in old age [120–123]. Several studies have demonstrated that HA accumulates in the CNS with aging and in individuals suffering from age-related cognitive dysfunction [22, 124–126]. Importantly, we find that HA accumulates in the SGZ and the rest of the dentate gyrus with aging in mice [22]. This accumulation may, therefore, contribute to reductions in NSC proliferation and affect new neuron maturation, leading to age-related changes in hippocampal function. Developing strategies to prevent HA accumulation or excessive activation of CD44 in the SGZ could therefore be interesting approaches to prevent age-related declines in neurogenesis and associated cognitive disturbances.
Interestingly, the controlled regulation of HA and other components within PNNs may have the potential to influence conditions whose underlying cause is aberrant neuronal function. Consistent with the ability of PNNs to restrict plasticity in adulthood, their removal with either Ch-ABC or hyaluronidase restores several types of plasticity. Ch-ABC treatment restores ocular dominance plasticity in the visual cortex of adult animals [88], enhances object recognition memory [127], enhances reversal learning in the auditory cortex [102], promotes recovery of motor learning after spinal cord injury [128] and cortical ischemia [129], and influences extinction of fear conditioning [130]. However, removal of PNNs has also been shown to prevent certain types of plasticity imposed by strong stimuli, including shock used for fear conditioning and drugs of abuse. Removal of PNNs prevents fear conditioning in the medial prefrontal cortex or dorsal hippocampus [104].
The removal of PNNs also prevents the effects of drugs of abuse, including the consolidation/reconsolidation of drug-associated memories and the erasure of memories [131–133]. We hypothesize that removal of PNNs is effective at both promoting or preventing plasticity depending on the type of stimuli used to induce plasticity. When relatively weak, physiological stimuli such as light or training of motor pathways are used to activate neurons, PNN removal allows for new synapse formation onto the underlying cells that restores function (e.g, ocular dominance plasticity). On the other hand, PNN removal may block the effects of stronger stimuli, including shock used in fear conditioning or drugs of abuse. Drugs of abuse induce metaplasticity [134], and this metaplasticity alters the ability of natural stimuli to further alter plasticity. In this case, PNN removal may prevent the drug-induced changes to restore the excitatory:inhibitory balance, likely by altering cell firing [135] and/or other molecules that bind to PNNs to regulate homeostatic plasticity [74].
PNNs are highly dynamic even during adulthood. For example, environmental enrichment in animals, in which rodents are housed together and given novel toys and exercise wheels, has been shown to either reduce or increase the number and/or the intensity of PNN staining by WFA [136–137]. Several different drugs of abuse have demonstrated increases or decreases in the intensity of PNN staining, depending on treatment and brain area [138–40]. The Sorg lab, (unpublished) and others [141] have even observed diurnal/circadian changes in the intensity of PNNs, suggesting a highly dynamic regulation of these structures. In some cases, some of the PNN components have been measured for what underlies these dynamic changes after exposure to drugs of abuse [139, 142], but multiple mechanisms may regulate the staining intensity of PNNs, including how tightly condensed the PNNs are, the sulfation patterns of the CSPGs, and the ability to capture inhibitory molecules or those that promote plasticity (e.g., BDNF). Regulation of HAS1-3 on neurons bearing PNNs produce HA that serves as the critical backbone structure of PNNs, but these have not yet been explored in the context of these dynamic changes. Future studies will need to dissect the contribution of these enzymes and the mechanisms by which they may be regulated, and in turn, how the properties of PNN-enwrapped neurons are altered.
Conclusions and Future Research Directions
Studies to date have demonstrated that HA, HA-associated ECM (including proteoglycans) and HA receptors each play roles in neurogenesis and in regulating the activities of mature neurons (Fig. 3). These roles are distinct depending on the point in neuronal differentiation, the location of the cells, and on age. For NSCs, HA appears to play a crucial role in regulating NSC expansion and early stages of neuronal differentiation and maturation. In mature neurons, HA is a component of PNNs where it contributes to the regulation of neuronal activity predominantly as a component of proteoglycans within PNNs. The growing evidence that HA is altered or elevated following CNS insults and during normative aging implicate HA and HA receptors as important players in CNS disease pathogenesis and injury responses.
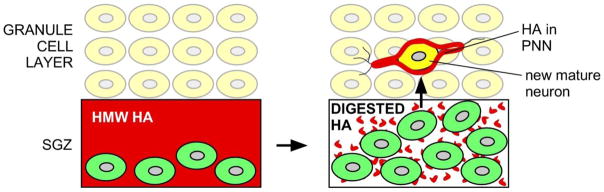
Fig. 3.
Distinct roles for HA in adult hippocampal neurogenesis and in mature neurons. HA (red) in the SGZ is believed to typically exist as a high molecular weight (HMW) molecule that keeps NSCs (green) in a quiescent state through signaling by CD44 expressed by NSCs. When NSCs need to expand in number, HA is digested, possibly by hyaluronidases like CEMIP, either relieving NSCs from the quiescence signals generated by HMW HA or by inducing proliferation in response to HA digestion products (small red dots). These NSCs migrate into the granule cell layer and differentiate into new neurons (yellow cell) that assemble PNNs with HA (red color surrounding the new neuron) around themselves. HA within these PNNs is also likely to be dynamically regulated to influence neuronal activity.
It is intriguing to speculate that HA synthesis and catabolism are dynamically regulated during the course of normal CNS activity. For example, given that HA restricts NSC proliferation in the SGZ and prevents neurogenesis [22], it is possible that hyaluronidases or other mechanisms transiently digest the HA in NSC niches in an activity-dependent manner to promote NSC expansion and subsequent differentiation during learning or in response to other stimuli. What remains unclear is how and which hyaluronidases may be involved in such dynamic regulation. One candidate is CEMIP, which is expressed in the brain and has been linked to hippocampal learning and memory functions [143]. It is also unclear if the effects of HA or HA digestion in either the SGZ or in PNNs is due entirely to high molecular weight forms of HA, the disruption of such HA, or the accumulation of specific sizes of HA digestion products. Indeed, HA breakdown products that can signal through Toll-like receptors have been implicated in regulating the maturation of oligodendrocyte progenitors in CNS white matter [144, 145]. Finally, it will be important to understand which receptors regulate the activities of HA in both NSC niches and in PNNs. While CD44 appears to be crucial for regulating NSC proliferation in the SGZ, roles for other receptors in subsequent stages of neuronal differenation and in mature neurons have not been fully investigated. Future studies that address these issues will provides insights into the precise mechanisms by which the HA-based ECM influences neurogenesis and neuron activity. Understanding these mechanisms will also make HA and HA receptors excellent candidates for a variety of therapeutic interventions aimed at restoring neurogenesis and neuronal functions in the damaged CNS.
Highlights.
The glycosaminoglycan hyaluronan (HA) regulates neural stem cell proliferation and differentiation as well as neuronal activity in mature neurons
The HA-based extracelluar matrix is dynamically regulated and can be influenced by a variety of stimuli and insults to the central nervous system
HA and HA receptors may be effective targets for restoring neurogenesis and neuronal function in the injured brain or spinal cord.
Acknowledgments
This work was supported by P51 OD011092 for the operation of the Oregon National Primate Research Center, MS160144 from the Congressionally Directed Medical Research Programs and RG 4843A5/1 from the National Multiple Sclerosis Society (to LSS)
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- CEMIP
Cell Migration-Inducing hyaluronan binding Protein
- CNS
central nervous system
- ECM
extracellular matrix
- HA
hyaluronan
- HAPLN
HA and proteoglycan link proteins
- HAS
hyaluronan synthase
- TMEM2
transmembrane protein 2
- HYAL
hyaluronidase
- NSCs
neural stem cells
- PNNs
perineuronal nets
- SGZ
subgranular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morest DK, Silver J. Precursors of neurons, neuroglia, and ependymal cells in the CNS: what are they? Where are they from? How do they get where they are going? Glia. 2003;43:6–18. doi: 10.1002/glia.10238. [DOI] [PubMed] [Google Scholar]
- 2.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–42. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Rushing G, Ihrie RA. Neural stem cell heterogeneity through time and space in the ventricular-subventricular zone. Front Biol (Beijing) 2016;11:261–284. doi: 10.1007/s11515-016-1407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20:416–23. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazanis I ffrench-Constant C. Extracellular matrix and the neural stem cell niche. Dev Neurobiol. 2011;71:1006–17. doi: 10.1002/dneu.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird J, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem cells (Dayton, Ohio) 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 8.Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: Fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 9.Karus M, Denecke B, Wiese S, Faissner A. The extracellular matrix molecule tenascin C modulates expression levels and territories of key patterning genes during spinal cord astrocyte specification. Development. 2011;138:5321–31. doi: 10.1242/dev.067413. [DOI] [PubMed] [Google Scholar]
- 10.Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia. 2015;63:1330–49. doi: 10.1002/glia.22839. [DOI] [PubMed] [Google Scholar]
- 11.Oohashi T, Edamatsu M, Bekku Y, Carulli D. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol. 2015;274:134–44. doi: 10.1016/j.expneurol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Smith PD, Coulson-Thomas VJ, Foscarin S, Kwok JC, Fawcett JW. “GAG-ing with the neuron”: The role of glycosaminoglycan patterning in the central nervous system. Exp Neurol. 2015;274:100–14. doi: 10.1016/j.expneurol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem. 2017;292:7304–7313. doi: 10.1074/jbc.M116.770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A. 2013;110:5612–7. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polansky JR, Toole BP, Gross J ffrench-Constant C. Brain hyaluronidase: changes in activity during chick development. Science. 1974;183:862–4. doi: 10.1126/science.183.4127.862. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio LO, Dietrich CP. Changes of sulfated mucopolysaccharides and mucopolysaccharidases during fetal development. J Biol Chem. 1981;256:9205–10. [PubMed] [Google Scholar]
- 18.Shibata S, Cho KH, Kim JH, Abe H, Murakami G, Cho BH. Expression of hyaluronan (hyaluronic acid) in the developing laminar architecture of the human fetal brain. Ann Anat. 2013;195:424–30. doi: 10.1016/j.aanat.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci. 2014;34:6164–76. doi: 10.1523/JNEUROSCI.3458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toda T, Gage FH. Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2017 Nov 29; doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 21.Fuxe K, Tinner B, Chadi G, Härfstrand A, Agnati LF. Evidence for a regional distribution of hyaluronic acid in the rat brain using a highly specific hyaluronic acid recognizing protein. Neurosci Lett. 1994;169:25–30. doi: 10.1016/0304-3940(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 22.Su W, Foster SC, Xing R, Feistel K, Olsen RH, Acevedo SF, Raber J, Sherman LS. CD44 Transmembrane Receptor and Hyaluronan Regulate Adult Hippocampal Neural Stem Cell Quiescence and Differentiation. J Biol Chem. 2017;292:4434–4445. doi: 10.1074/jbc.M116.774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oishi K, Ito-Dufros Y. Angiogenic potential of CD44+ CD90+ multipotent CNS stem cells in vitro. Biochem Biophys Res Commun. 2006;349:1065–72. doi: 10.1016/j.bbrc.2006.08.135. [DOI] [PubMed] [Google Scholar]
- 24.Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS ONE. 2013;8:e53109. doi: 10.1371/journal.pone.0053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM, Westenbroek R, Engel AK, Catterall WA, Rusakov DA, Schachner M, Dityatev A. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67:116–28. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raber J, Olsen RH, Su W, Foster S, Xing R, Acevedo SF, Sherman LS. CD44 is required for spatial memory retention and sensorimotor functions. Behav Brain Res. 2014;275:146–9. doi: 10.1016/j.bbr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barzilay R, Ventorp F, Segal-Gavish H, Aharony I, Bieber A, Dar S, Vescan M, Globus R, Weizman A, Naor D, Lipton J, Janelidze S, Brundin L, Offen D. CD44 Deficiency Is Associated with Increased Susceptibility to Stress-Induced Anxiety-like Behavior in Mice. J Mol Neurosci. 2016;60:548–558. doi: 10.1007/s12031-016-0835-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang TW, Spector M. Development of hyaluronic acid based scaffolds for brain tissue engineering. Acta Biomaterialia. 2009;5:2371–2384. doi: 10.1016/j.actbio.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW, Stabenfeldt SE. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017;60–61:206–216. doi: 10.1016/j.matbio.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosher KI, Schaffer DV. Influence of hippocampal niche signals on neural stem cell functions during aging. Cell Tissue Res. 2018;371:115–124. doi: 10.1007/s00441-017-2709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, DeLorenzo RJ. Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neuroscience Letters. 2007;418:77–81. doi: 10.1016/j.neulet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajieva P, Kuhlmann Ch, Luhmann HJ, Behla C. Impaired calcium homeostasis in aged hippocampal neurons. Neuroscience Letters. 2008;451:119–123. doi: 10.1016/j.neulet.2008.11.068. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP, Engle MG, Rychlik B. Effects of elevated intracellular calcium levels on the cytoskeleton and tau in cultured human cortical neurons. Mol Chem Neuropathol. 1991;15:117–42. doi: 10.1007/BF03159951. [DOI] [PubMed] [Google Scholar]
- 34.Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–49. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fawcett JW. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog Brain Res. 2015;218:213–26. doi: 10.1016/bs.pbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Spreafico R, De Biasi S, Vitellaro-Zuccarello L. The perineuronal net: a weapon for a challenge. J Hist Neurosci. 1999;8:179–85. doi: 10.1076/jhin.8.2.179.1834. [DOI] [PubMed] [Google Scholar]
- 37.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–5. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 38.Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaal B, Rácz É, Juhász T, Holló K, Matesz C. Distribution of extracellular matrix macromolecules in the vestibular nuclei and cerebellum of the frog, Rana esculenta. Neuroscience. 2014;258:162–73. doi: 10.1016/j.neuroscience.2013.10.080. [DOI] [PubMed] [Google Scholar]
- 40.Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, Miquel M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci. 2016;36:11459–11468. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 42.Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253:194–213. doi: 10.1016/j.neuroscience.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 43.Suttkus A, Morawski M, Arendt T. Protective Properties of Neural Extracellular Matrix. Mol Neurobiol. 2016;53:73–82. doi: 10.1007/s12035-014-8990-4. [DOI] [PubMed] [Google Scholar]
- 44.McRae PA, Porter BE. The perineuronal net component of the extracellular matrix in plasticity and epilepsy. Neurochem Int. 2012;61:963–72. doi: 10.1016/j.neuint.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–9. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–89. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 47.Miyata S, Kitagawa H. Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulphate. J Biochem. 2015;157:13–22. doi: 10.1093/jb/mvu067. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–89. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–77. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–53. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 51.Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol. 1999;410:256–64. [PubMed] [Google Scholar]
- 52.Matsui F, Nishizuka M, Yasuda Y, Aono S, Watanabe E, Oohira A. Occurrence of a N-terminal proteolytic fragment of neurocan, not a C-terminal half, in a perineuronal net in the adult rat cerebrum. Brain Res. 1998;790:45–51. doi: 10.1016/s0006-8993(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 53.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 54.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Morawski M, Dityatev A, Hartlage-Rübsamen M, Blosa M, Holzer M, Flach K, Pavlica S, Dityateva G, Grosche J, Brückner G, Schachner M. Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Philos Trans R Soc Lond B Biol Sci. 2014;369:20140046. doi: 10.1098/rstb.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwok JC, Carulli D, Fawcett JW. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem. 2010;114:1447–59. doi: 10.1111/j.1471-4159.2010.06878.x. [DOI] [PubMed] [Google Scholar]
- 57.Koppe G, Brückner G, Härtig W, Delpech B, Bigl V. Characterization of proteoglycan-containing perineuronal nets by enzymatic treatments of rat brain sections. Histochem J. 1997;29:11–20. doi: 10.1023/a:1026408716522. [DOI] [PubMed] [Google Scholar]
- 58.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 59.Härtig W, Brauer K, Bigl V, Brückner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635:307–11. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- 60.Härtig W, Brauer K, Brückner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;10:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Mulligan KA, van Brederode JF, Hendrickson AE. The lectin Vicia villosa labels a distinct subset of GABAergic cells in macaque visual cortex. Vis Neurosci. 1989;2:63–72. doi: 10.1017/s0952523800004338. [DOI] [PubMed] [Google Scholar]
- 62.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–27. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 63.Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997;272:8957–61. doi: 10.1074/jbc.272.14.8957. [DOI] [PubMed] [Google Scholar]
- 64.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–92. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 65.Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci. 2008;27:1373–90. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Li ZX, Jin T, Wang ZY, Zhao P. Tau Pathology Promotes the Reorganization of the Extracellular Matrix and Inhibits the Formation of Perineuronal Nets by Regulating the Expression and the Distribution of Hyaluronic Acid Synthases. J Alzheimers Dis. 2017;57:395–409. doi: 10.3233/JAD-160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 68.Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–9. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 69.Bruckner G, Bringmann A, Härtig W, Köppe G, Delpech B, Brauer K. Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp Brain Res. 1998;121:300–10. doi: 10.1007/s002210050463. [DOI] [PubMed] [Google Scholar]
- 70.Hamai A, Hashimoto N, Mochizuki H, Kato F, Makiguchi Y, Horie K, Suzuki S. Two distinct chondroitin sulfate ABC lyases. An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem. 1997;272:9123–30. doi: 10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- 71.Lin R, Kwok JC, Crespo D, Fawcett JW. Chondroitinase ABC has a long-lasting effect on chondroitin sulphate glycosaminoglycan content in the injured rat brain. J Neurochem. 2008;104:400–8. doi: 10.1111/j.1471-4159.2007.05066.x. [DOI] [PubMed] [Google Scholar]
- 72.Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. doi: 10.1002/glia.440130406. [DOI] [PubMed] [Google Scholar]
- 73.Tona A, Bignami A. Effect of hyaluronidase on brain extracellular matrix in vivo and optic nerve regeneration. J Neurosci Res. 1993;36:191–9. doi: 10.1002/jnr.490360209. [DOI] [PubMed] [Google Scholar]
- 74.Frischknecht R, Chang KJ, Rasband MN, Seidenbecher CI. Neural ECM molecules in axonal and synaptic homeostatic plasticity. Prog Brain Res. 2014;214:81–100. doi: 10.1016/B978-0-444-63486-3.00004-9. [DOI] [PubMed] [Google Scholar]
- 75.Härtig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–72. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Schuppel K, Brauer K, Härtig W, Grosche J, Earley B, Leonard BE, Brückner G. Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res. 2002;958:448–453. doi: 10.1016/s0006-8993(02)03569-2. [DOI] [PubMed] [Google Scholar]
- 77.Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–88. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 78.Yamada J, Ohgomori T, Jinno S. Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur J Neurosci. 2015;41:368–78. doi: 10.1111/ejn.12792. [DOI] [PubMed] [Google Scholar]
- 79.Härtig W, Singer A, Grosche J, Brauer K, Ottersen OP, Brückner G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res. 2001;899:123–33. doi: 10.1016/s0006-8993(01)02211-9. [DOI] [PubMed] [Google Scholar]
- 80.Horii-Hayashi N, Sasagawa T, Hashimoto T, Kaneko T, Takeuchi K, Nishi M. A newly identified mouse hypothalamic area having bidirectional neural connections with the lateral septum: The perifornical area of the anterior hypothalamus enriched in chondroitin sulfate proteoglycans. Eur J Neurosci. 2015;42:2322–34. doi: 10.1111/ejn.13024. [DOI] [PubMed] [Google Scholar]
- 81.Meszar Z, Girard F, Saper CB, Celio MR. The lateral hypothalamic parvalbumin-immunoreactive (PV1) nucleus in rodents. J Comp Neurol. 2012;520:798–815. doi: 10.1002/cne.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wegner F, Härtig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, Brückner G. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–14. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, Rossi F, Miquel M. The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol. 2015;20:941–55. doi: 10.1111/adb.12223. [DOI] [PubMed] [Google Scholar]
- 84.Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J Neurosci. 2016;36:6312–20. doi: 10.1523/JNEUROSCI.0245-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seeger G, Brauer K, Härtig W, Brückner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994;58:371–88. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 86.Bruckner G, Grosche J. Perineuronal nets show intrinsic patterns of extracellular matrix differentiation in organotypic slice cultures. Exp Brain Res. 2001;137:83–93. doi: 10.1007/s002210000617. [DOI] [PubMed] [Google Scholar]
- 87.Bruckner G, Grosche J, Schmidt S, Härtig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–29. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 88.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 89.Kalb RG, Hockfield S. Induction of a neuronal proteoglycan by the NMDA receptor in the developing spinal cord. Science. 1990;250:294–6. doi: 10.1126/science.2145629. [DOI] [PubMed] [Google Scholar]
- 90.Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- 91.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–67. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye Q, Miao QL. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013;32:352–63. doi: 10.1016/j.matbio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fässler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–42. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–47. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok JC. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One. 2011;6:e21499. doi: 10.1371/journal.pone.0021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–22. S1–2. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–91. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kitagawa H, Tsutsumi K, Tone Y, Sugahara K. Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J Biol Chem. 1997;272(50):31377–81. doi: 10.1074/jbc.272.50.31377. [DOI] [PubMed] [Google Scholar]
- 100.Foscarin S, Raha-Chowdhury R, Fawcett JW, Kwok JCF. Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging (Albany NY) 2017;9:1607–1622. doi: 10.18632/aging.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Custod JT, Young IJ. Cat brain mucopolysaccharides and their in vivo hyaluronidase digestion. J Neurochem. 1968;15:809–13. doi: 10.1111/j.1471-4159.1968.tb10326.x. [DOI] [PubMed] [Google Scholar]
- 102.Happel MF, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci U S A. 2014;111:2800–5. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun ZY, Bozzelli PL, Caccavano A, Allen M, Balmuth J, Vicini S, Wu JY, Conant K. Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus. 2017 Sep 16; doi: 10.1002/hipo.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hylin MJ, Orsi SA, Moore AN, Dash PK. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem. 2013;20:267–73. doi: 10.1101/lm.030197.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 106.Schweitzer B, Singh J, Fejtova A, Groc L, Heine M, Frischknecht R. Hyaluronic acid based extracellular matrix regulates surface expression of GluN2B containing NMDAreceptors. Sci Rep. 2017;7:10991. doi: 10.1038/s41598-017-07003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 1997;94:10432–7. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–40. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- 110.Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roszkowska M, Skupien A, Wójtowicz T, Konopka A, Gorlewicz A, Kisiel M, Bekisz M, Ruszczycki B, Dolezyczek H, Rejmak E, Knapska E, Mozrzymas JW, Wlodarczyk J, Wilczynski GM, Dzwonek J. CD44 - a novel synaptic cell adhesion molecule regulating structural and functional plasticity of dendritic spines. Mol Biol Cell. 2016 doi: 10.1091/mbc.E16-06-0423. pii, mbc.E16-06-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Okun E, Griffioen KJ, Son TG, Lee JH, Roberts NJ, Mughal MR, Hutchison E, Cheng A, Arumugam TV, Lathia JD, van Praag H, Mattson MP. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J Neurochem. 2010;114:462–74. doi: 10.1111/j.1471-4159.2010.06778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gray WP, Sundström LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–9. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 115.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 117.Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 118.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jenkins HG, Bachelard HS. Developmental and age-related changes in rat brain glycosaminoglycans. J Neurochem. 1988;51:1634–40. doi: 10.1111/j.1471-4159.1988.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 125.Cargill R, Kohama SG, Struve J, Su W, Banine F, Witkowski E, Back SA, Sherman LS. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol Aging. 2012;33:830, e13–24. doi: 10.1016/j.neurobiolaging.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, Sonnen JA, Larson EB, Montine TJ. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011;70:465–76. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, Bussey TJ, Fawcett JW, Pizzorusso T, Saksida LM. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci. 2013;33:7057–65. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao RR, Fawcett JW. Combination treatment with chondroitinase ABC in spinal cord injury--breaking the barrier. Neurosci Bull. 2013;29:477–83. doi: 10.1007/s12264-013-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gherardini L, Gennaro M, Pizzorusso T. Perilesional Treatment with Chondroitinase ABC and Motor Training Promote Functional Recovery After Stroke in Rats. Cereb Cortex. 2013;25:202–12. doi: 10.1093/cercor/bht217. [DOI] [PubMed] [Google Scholar]
- 130.Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 131.Xue Y-X, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L. Depletion of Perineuronal Nets in the Amygdala to Enhance the Erasure of Drug Memories. J Neurosci. 2014;34:6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35:4190–202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blacktop JM, Todd RP, Sorg BA. Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology. 2017;118:124–136. doi: 10.1016/j.neuropharm.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–9. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–81. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 137.Slaker M, Barnes J, Sorg BA, Grimm JW. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS One. 2016;11:e0168256. doi: 10.1371/journal.pone.0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vazquez-Sanroman D, Carbo-Gas M, Leto K, Cerezo-Garcia M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Rossi F, Miquel M. Cocaine-induced plasticity in the cerebellum of sensitised mice. Psychopharmacology (Berl) 2015;232:4455–67. doi: 10.1007/s00213-015-4072-1. [DOI] [PubMed] [Google Scholar]
- 139.Chen H, He D, Lasek AW. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39:1930–8. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vazquez-Sanroman DB, Monje RD, Bardo MT. Nicotine self-administration remodels perineuronal nets in ventral tegmental area and orbitofrontal cortex in adult male rats. Addict Biol. 2017;22:1743–1755. doi: 10.1111/adb.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pantazopoulos H, Yildiz E, Seltzer P, Turiak L, Zaia J, Berretta S, Ardent M. Microglial associated circadian regulation of perineuronal net composition. Neuropsychopharmacology. 2016;41:S420, T217. [Google Scholar]
- 142.Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, Riga D, Wiskerke J, Binnekade R, Stegeman M, Schoffelmeer AN, Mansvelder HD, Smit AB, De Vries TJ, Spijker S. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010;35:2120–33. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshino Y, Ishisaka M, Tsuruma K, Shimazawa M, Yoshida H, Inoue S, Shimoda M, Okada Y, Hara H. Distribution and function of hyaluronan binding protein involved in hyaluronan depolymerization (HYBID, KIAA1199) in the mouse central nervous system. Neuroscience. 2017;347:1–10. doi: 10.1016/j.neuroscience.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 144.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–60. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–80. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]