Abstract
Arousal plays a central role in a wide variety of phenomena, including wakefulness, autonomic function, affect and emotion. Despite its importance, it remains unclear as to how the neural mechanisms for arousal are organized across them. In this article, we review neuroscience findings for three of the most common origins of arousal: wakeful arousal, autonomic arousal, and affective arousal. Our review makes two overarching points. First, research conducted primarily in non-human animals underscores the importance of several subcortical nuclei that contribute to various sources of arousal, motivating the need for an integrative framework. Thus, we outline an integrative neural reference space as a key first step in developing a more systematic understanding of central nervous system contributions to arousal. Second, there is a translational gap between research on non-human animals, which emphasizes subcortical nuclei, and research on humans using non-invasive neuroimaging techniques, which focuses more on gross anatomical characterizations of cortical (e.g. network architectures including the default mode network) and subcortical structures. We forecast the importance of high-field neuroimaging in bridging this gap to examine how the various networks within the neural reference space for arousal operate across varieties of arousal-related phenomena.
Arousal involves one of the largest and most complex sets of biological changes in the body. It is linked to major daily oscillations in the environment, including light and temperature, and corresponding behavioral activities [e.g. when to be awake, pay attention, search for food, avoid predators, rest and digest, [1]. Despite its centrality to surviving and thriving, research has yet to produce an integrative and generalizable neural model of arousal, for at least two reasons.
First, arousal is a heterogeneous construct [2]. When studying wakeful arousal, researchers focus on the brain regions that are critical for transitioning to wakefulness from sleep (and vice versa), and often use electroencephalography (EEG) de-synchronization as a biomarker of wakefulness [3]. When studying autonomic arousal, the focus is on brain control of (and responses to) peripheral autonomic responses, including changes in heart rate, pupil dilation, and electrodermal responses [4, 5]. And in affective arousal, researchers typically study the state of engagement with salient or evocative stimuli [6], often measured through self-reports of felt arousal [e.g. 2, 7, 8, 9].
A second reason we are currently without a general neural model of arousal can be found in the challenge of identifying homologies across species. Research in non-human animals has developed a rich and detailed model of brainstem thalamic and hypothalamic nuclei involved in wakeful arousal, but it remains unclear how this model relates to autonomic and affective arousal. The location of these nuclei is guided by the use of a stereotaxic framework [10, e.g., 11] in part to facilitate lesion and intra-cortical electrode positioning [e.g. 12]; the accuracy of these procedures can be corroborated with histological staining after sacrificing the animals. In contrast, conventional neuroimaging studies in humans have focused on the cortical and larger subcortical correlates of arousal, with less attention paid to smaller subcortical nuclei that are more difficult to localize in humans, such as the small yet complex brainstem and diencephalic structures that are the focus of studies in non-human animals. Most of these nuclei are not clearly visible with conventional neuroimaging techniques used on (living) humans. The resolution and contrast needed to resolve the location and build preliminary stereotaxic atlases of these key nuclei are just now being developed using high and ultra-high (e.g. 7 Tesla) field MRI scanners [13–22].
In this paper, we briefly review the neuroanatomic substrates underlying wakeful, autonomic, and affective arousal to reveal their overlapping neural architectures. On the basis of research in non-human animals and humans, we propose a distributed neural reference space for arousal whose nodes combine in different ways to create varieties of arousal. We highlight the importance of recently established high-resolution neuroimaging techniques as a methodology for conducting studies to better examine generalization and differentiation across formulations of arousal and across species. Our review emphasizes the brain regions that prior work suggests are important for arousal, but this does not imply that these areas are uniquely involved in arousal [e.g., 23]. In other words, we describe findings showing that activity in areas such as the amygdala, the cingulate cortex, and the insula, along with the many other areas are all associated with arousal, but these areas may also play a role in other mental phenomena that are not the focus of this article.
1 The neuroanatomic substrate for arousal: Findings from studies of non-human animals
A range of findings in non-human animals suggest that there is overlap between neural pathways involved in between wakeful, autonomic, and affect arousal. Below, we briefly review some of the main areas involved with respect to wakeful arousal and further note their involvement in autonomic and affective arousal varieties.
1.1 Wakefulness
In the past 68+ years, several brainstem nuclei have been implicated in the maintenance of wakefulness (typically measured as widespread cortical EEG de-synchronization) based on invasive functional studies involving stimulation and recording from neurons directly, lesion studies, and on the basis of whether a nucleus has the ability to widely modulate activity throughout the cerebral cortex. Table 1 lists the nuclei that have been implicated in wakeful arousal. These nuclei have widespread anatomical connections to the cerebral cortex, and/or project to the reticular and intralaminar thalamic nuclei, the hypothalamus, or the basal forebrain, all of which project widely to the cerebral cortex [24].
TABLE 1.
| Brain region involved in maintaing wakefulness | Main neurotransmitter/neuro modulator Involved | Name originally given to the arousal system | Other Function |
|---|---|---|---|
| Brainstem: | |||
| Mesopontine reticular formation nuclei [26]: -Mesencephalic reticular formation -Cuneiform nucleus -Pontine reticular nucleus, oral part |
Glutamate | Ascending reticular activating system | Coordination of autonomic/motor/sensory brainstem nuclei [29] |
| Pontomedullary reticular formation nuclei [24]: -Pontine reticular nucleus, caudal part- Gigantocellular reticular nucleus -Parvicellular reticular nucleus -Subnucleus reticularis dorsalis |
Serotonin Adrenaline Norepinephrine |
Ascending reticular activating system | Coordination of autonomic/motor/sensory brainstem nuclei [29] |
| Mesopontine tegmental nuclei [24,27–28]: -Pedunculotegmental nucleus -Laterodorsal tegmental nucleus |
Acetylcholine | Diffuse neuromodulatory system | Locomotor, Limbic [29] |
| Raphe nuclei [24,27–28]: -Median raphe -Dorsal raphe -Raphe pallidus -Raphe obscurus |
Serotonin | Diffuse neuromodulatory system | Nociception, Limbic, Temperature regulation, Blood pressure control, Memory, Motor [29] |
| Locus coeruleus [24,27–28] | Norepinephrine | Diffuse neuromodulatory system | Autonomic, Attention, Memory, Motivation [29] |
| Ventral tegmental area [12, 24, 44] | Dopamine | Diffuse neuromodulatory system | Attention, Memory, Reward, Drug abuse, Motivation [29] |
| Parabrachial nuclei [11,33,27,28] | Glutamate | – | Autonomic, Limbic, Viscerosensory [29] |
| Subcoeruleus area [11,28] | Glutamate | – | Limbic, Motor [29] |
| Periaqueductal gray, ventrolateral part [24,28] | Dopamine | – | Autonomic, Limbic [29] |
| Solitary nucleus [24] | Glutamate | – | Viscerosensory Autonomic [29] |
| Hypothalamus: | |||
| - Tuberomamillary nucleus [27,28] | Histamine | – | Temperature regulation Endocrine homeostasis [150] |
| - Lateral hypothalamus [27,28] | Orexin | – | Feeding behavior, Temperature regulation, Nociception, Reward [151] |
| Thalamus: | |||
| - Thalamic reticular nuclei [24] | GABA | – | Modulation of thalamic nuclei [152] |
| - Intralaminar thalamic nuclei [24] (centromedian, parafascicular, centrolateral) | Glutamate | – | Nociception, Motor [153] |
| Basal forebrain: | |||
| - Nucleus accumbens, nucleus basalis, diagonal band of Broca [24,27] | Acetylcholine | – | Memory, Attention [154] |
“Ascending” refers to the fact that several pathways of this system involve ascending fibers from the brainstem, hypothalamus, thalamus and basal forebrain to the cortex.
Note that we only list brain regions that are thought to directly increase arousal (e.g. level of wakefulness, responsiveness to stimuli). In addition, arousal could also be increased by inhibiting sleep promoting brainstem (e.g lateral pontine tegmentum) and hypothalamic (ventrolateral preoptic nucleus) nuclei.
For a review of sleep promoting regions and their involvement in sleep state switching see [28].
The search for the key subcortical regions involved in arousal began with the observation that widespread cortical EEG de-synchronization differentiated wakefulness from sleep [25], which subsequently was used as a neural signature for the awake state [3]. Much of the initial work focused on the reticular formation, a collection of nuclei in the brainstem that extends longitudinally from the medulla through the pons to the midbrain, and on nuclei surrounding the reticular formation. Landmark work in the cat by Moruzzi and Magoun [26] found that stimulating nuclei of the mesopontine (i.e. at the midbrain/pons junction) reticular formation elicited wakefulness, from which the term “ascending reticular activating system” was coined. Consistent with this notion, it was later found that some of the nuclei spanning through the pons and medulla of the reticular formation have the anatomical prerequisites to widely modulate the cerebral cortex via afferents to the thalamus and/or upper brainstem nuclei [see Table 1, 24].
It is now known, however, that many brainstem nuclei surrounding the reticular formation, rather than the reticular formation itself, are more central for generating the widespread modulation of cortical activity associated with the EEG wakefulness state [24, 27–29]. The subsequently proposed “diffuse modulatory system” [29] underlying cortical arousal comprises several distinct modulators that are each capable of exerting their influence upon a diffuse set of regions (i.e. modulate neuronal cell properties, such as excitability). These putative neuro-modulators of wakeful arousal are cholinergic and monoaminergic (i.e. serotoninergic, norepinephrinergic and dopaminergic) neuronal cell groups in the brainstem, histaminergic and orexinergic hypothalamic nuclei, and cholinergic nuclei of the basal forebrain [28, 30–32] (see Table 1 for a detailed list of arousal neuro-modulators proposed by the non-human animal literature). Note, that today the reticular formation is recognized mainly as a coordination network of brainstem nuclei (for autonomic, postural, oculomotor function) and is not the diffuse modulatory system originally proposed [29].
1.2 Autonomic and Affective Arousal
Accumulating research on the neural basis of arousal suggests that there is substantial overlap between the brain systems that control wakefulness and those that control autonomic function and affective arousal. The diffuse modulatory system for wakefulness encompasses nuclei that have also been implicated in autonomic and affective arousal [11, 24, 28, 33]. One example is the parabrachial nucleus. Extending beyond the initial focus on cholinergic and monoamingergic nuclei, Fuller et al. [11] found that glutamatergic brainstem cell populations in the parabrachial nuclear complex (and adjacent preoptic area) were also necessary to maintain a waking state in rodents [Table 1, also see 34]. Notably, the parabrachial complex is also important for autonomic function [29, 35] and phenomena that involve intense affective arousal such as pain [36, 37] and affect [38, 39]. For example, in rodents, a large proportion of lamina 1 spinal neurons that are important for pain processing project to the lateral parabrachial complex [40]. From an anatomical perspective, it is reasonable that the parabrachial complex contributes to various types of arousal: It is a major integrative center that receives interoceptive input from the solitary nucleus and intermediate reticular zone, and operates as an interface between these structures and the limbic forebrain [amygdala, hypothalamus, anterior insula, 41]. Integration across varieties of arousal is also present several other nuclei (Table 1), such as the periaqueductal gray [29, 35, 42, 43] and ventral tegmental area [12, 44].
Moreover, several limbic structures influence brainstem nuclei involved in wakefulness and sleep [45–47]. For example, the amygdala, while known for its role in affective behaviors [48–50], also plays a key role in the rapid-eye-movement (REM) sleep circuit, and is involved in sleep disorders such as narcolepsy and cataplexy [46, 51]. Limbic areas that are typically observed in studies of affective processing [e.g. 31, 52, 53, 54], are also known to influence autonomic function via the hypothalamic-pituitary-adrenocortical (HPA) axis [55]. Research on physical and psychological stressors has implicated multiple brain regions in controlling autonomic arousal, including limbic prefrontal cortex, amygdala, hippocampus (and the ventral subiculum in particular), the bed nucleus of the stria terminalis, the hypothalamus, and multiple brainstem nuclei (see Table 2 for a list). Recent work has utilized optogenetic and pharmacogenetic manipulations to link specific amygdala circuits to affective arousal behaviors [e.g. freezing, anxiety, feeding, and reinforcement learning, as reviewed in, 50].
TABLE 2.
Integrative reference neural space of arousal
| Brain region | Acronym | W.A. | Au.A. | Aff.A. |
|---|---|---|---|---|
| Brainstem: | ||||
| mesencephalic reticular nucleus | MRN | |||
| cuneiform nucleus | CUN | |||
| pontine reticular nucleus, caudal part | PRNc | |||
| gigantocellular reticular nucleus | GRN | |||
| parvicellular reticular nucleus | PARN | |||
| medullary reticular nucleus, dorsal part | MDRNd | |||
| pedunculopontine (“pedunculotegmental”) nucleus | PPN | |||
| laterodorsal tegmental nucleus | LDT | |||
| superior central nucleus (“median”) raphe, lateral part | CS1 | |||
| superior central nucleus (“median”) raphe, medial part | CSm | |||
| dorsal nucleus raphe | DR | |||
| nucleus raphe pallidus | RPA | |||
| nucleus raphe obscurus | RO | |||
| locus coeruleus | LC | |||
| ventral tegmental area | VTA | |||
| parabrachial nucleus, ventral lateral part | PBlv | |||
| parabrachial nucleus, external medial part | PBme | |||
| subcoeruleus nucleus | SLC | |||
| periaqueductal gray, ventrolateral division | PAGvl | |||
| nucleus of the solitary tract, central part | NTSce | |||
| nucleus of the solitary tract, lateral part | NTS1 | |||
| nucleus of the solitary tract, medial part | NTSm | |||
| Hvnothalamus: | ||||
| tuberomammillary nucleus, dorsal part | TMd | |||
| tuberomammillary nucleus, ventral part | TMv | |||
| lateral hypothalamic area | LHA | |||
| Thalamus: | ||||
| reticular nucleus | RT | |||
| intralaminar nuclei | ILM | |||
| Basal forebrain: | ||||
| nucleus accumbens | ACB | |||
| substantia innominata | SI | |||
| diagonal band of Broca | db | |||
| Other subcortical areas: | ||||
| basolateral nucleus amygdala, anterior part | BLAa | |||
| basolateral nucleus amygdala, posterior part | BLAp | |||
| basomedial nucleus amygdala, anterior part | BMAa | |||
| basomedial nucleus amygdala, posterior part | BMAp | |||
| central nucleus amygdala, medial part | CEAm | * | ||
| central nucleus amygdala, lateral part | CEA1 | * | ||
| medial nucleus amygdala, anterodorsal part | MEAad | |||
| medial nucleus amygdala, anteroventral part | MEAav | |||
| field CA1 hippocampus | CA1 | |||
| field CA2 hippocampus | CA2 | |||
| field CA3 hippocampus | CA3 | |||
| Cortical areas: | ||||
| entorhinal area, medial part, ventral zone | ENTmv | |||
| piriform area | PIR | |||
| agranular insular area, ventral part | AIv | |||
| anterior cingulate area, dorsal part | ACAd | |||
| anterior cingulate area, ventral part | ACAv | |||
| infralimbic area | I LA | |||
| prelimbic area | PL | |||
| primary motor area | MOp | |||
| secondary motor areas | MOs | |||
| posterior cingulate cortex | PCC | |||
| precuneus | PCUN | |||
| medial prefrontal cortex | mPFC |
Note. The table lists a set of neural nodes that that been generally implicated in wakeful arousal (W.A.), autonomic arousal (Au.A.), and affective arousal. (Aff. A.), as pooled across several references [23, 24, 27–29, 35, 49, 50, 55, 71, 77, 149–151, 155–163]. We note that the table is primarily a schematic representation of an integrative neural reference space for arousal. Figure 1 provides a graph representation of the above set of regions to illustrate how nodes may form functional networks that give rise to different varieties of arousal. Much work remains to be done to determine the precise role of many of these nodes across varieties of arousal.
For example, the amygdala is involved in sleep (REM sleep atonia), yet not strictly in promoting and maintaining wakefulness.
As we will see in the next section, the overlap in neural pathways involved in between wakeful, autonomic, and affect arousal is also apparent in brain imaging studies of humans.
2 The neuroanatomic substrate for arousal: Findings from neuroimaging studies
Studying the functionality of these key brainstem nuclei in humans has been difficult using conventional fMRI. Many of these nuclei are small and in close proximity to other nuclei, making partial volume effects a major limitation. Scanning artifacts, too, can be a larger contributor to noise in brainstem areas. Perhaps because of these concerns, research on the neural basis of arousal in humans has focused mainly on cortical areas and gross subcortical structures. As reviewed below, neuroimaging studies of humans suggest that areas of the default mode network (discussed below) and limbic areas participating in a “salience network” play a role in wakeful, autonomic, and affective arousal (Table 2). A handful of more recent studies have also begun to replicate findings from studies of non-human animal studies showing the importance of brainstem nuclei, thalamus, hypothalamus, and basal forebrain structures to arousal (Table 1).
2.1 Wakeful Arousal
Wakefulness has primarily been examined using a combination of EEG coupled with MRI or PET techniques. Findings from these studies highlight the importance of the thalamus but also wide-spread changes in the engagement and functional architecture of large-scale cortical networks, such as the default mode network [DMN, which includes prominent nodes in the precuneus and the anterior medial prefrontal cortex, among other areas, 56, 57]. Using a behavioral measure of decreasing wakefulness based on eyelid closing and EEG, Chang et al. [58] found that in non-human primates, decreased wakefulness was associated with a combination of increased thalamic activity and reduced cortical activity with particularly reliable decreases in the DMN. Other studies examined how functional connectivity (i.e. correlations in functional activity between brain regions over time) change during sleep v. wakefulness. In humans, functional connectivity between the thalamus and the DMN was reduced during sleep [59]. In a large sample study examining a combination of EEG and fMRI in humans [60], reduced connectivity was observed during sleep among nodes in the DMN [also see, 61], and also the frontoparietal network. Using a combination of PET-fMRI and anesthesia-induced unconsciousness, glucose metabolism and cerebral blood flow significantly decreased in DMN but also frontoparietal networks in unconscious v. awake states [62]. DMN areas also showed reduced functional connectivity with the thalamus during unconsciousness [62].
Most of these studies implicating the cortical areas are on the basis of correlational findings using human neuroimaging. It is important to note that in studies of comatose patients, brainstem lesions (rather than more diffuse cortical damage) seems to be the necessary component for producing complete loss of wakefulness at least in comatose states [63]. Nevertheless, consistent across the studies reviewed above is the involvement of DMN and DMN-thalamus connectivity during arousal, and potentially other large-scale functional networks like the frontoparietal network. Few neuroimaging studies have examined how these cortically oriented networks relate with the small brainstem nuclei that have been the focus of non-human animals with respect to sleep and wakefulness. However, at least in resting fMRI, some emerging work is examining how brainstem nuclei functionally integrate with large-scale networks. For example, using seed based connectivity, seeds placed in areas of the brainstem that correspond with portions of the raphe nuclei show functional connectivity with the DMN [64]. Of note, the so-called “salience” network, or limbic regions [which includes the anterior cingulate cortex and insula, and amygdala, among other regions, 65], also shows functional connectivity with portions of the thalamus and brain stem areas. These functional connectivity findings parallel structural connections with areas involved in wakeful arousal and autonomic function including the hypothalamus, thalamus, and brainstem nuclei [66, 67].
2.2 Autonomic Arousal
As in the research on non-human animals, the neural basis of autonomic nervous system (ANS) arousal [i.e., increases in heart rate, electrodermal response, etc., 68, 69, 70] suggests that there is substantial overlap between the brain systems that control wakefulness and those that control ANS arousal. Beissner and colleagues [71] conducted a comprehensive meta-analysis of dozens of functional neuroimaging studies that examine the relationship between brain activity and autonomic changes. These studies typically induce changes in autonomic function using stress inducing tasks, such as physical challenge [e.g. squeezing a pressure bulb, 72, 73] and mental challenge [e.g. cognitive and social performance tasks completed under pressure, 72, 74]. The primary areas that reliably relate with autonomic change include nodes in the DMN (the anterior medial prefrontal cortex, medial orbitofrontal cortex, precuneus, angular gyrus), nodes of the salience network (the insula and cingulate cortex), and subcortically, the amygdala, hippocampus, thalamus, and brainstem areas near the PAG.
Recent work on systems-level characterization of cardiovascular arousal and skin conductance in a social stress task has shown that they have: (a) distributed neurophysiological bases that include DMN areas (ventrolateral and medial prefrontal cortex, angular gyrus), limbic areas (insula and cingulate cortex), and subcortical areas (including hippocampus, thalamus, and cerebellum); (b) that across physiological channels, these bases overlap in DMN areas (ventromedial prefrontal cortex, lateral orbitofrontal cortex, temporoparietal junction, among others) and also the thalamus and brainstem areas (covering the locus coeruleus and PAG); and (c) but also differentiable bases, indicating that different autonomic output channels reflect activation of different central processes [75]. Activity in cingulate and insular cortices is also often associated with interoception, which involves the awareness of changes in ANS [23, 73, 76–78]. Damage to some of these areas, such as the orbitofrontal/ventromedial prefrontal cortex and amygdala can cause impairments in autonomic response [e.g. 79, 80–82]. Recent connectivity work using resting fMRI data has shown that brainstem nuclei participate in networks shared with limbic and paralimbic structures [23, 64].
2.3 Affective Arousal
Affective arousal, which is also associated with interoception [83], is considered a property of consciousness that accompanies every waking moment of life [84]. The subjective experience of arousal is experimentally manipulated by presenting participants with evocative stimuli [e.g. images of spiders, victims of injury, etc. that are well-normed for how much subjective arousal and pleasantness/unpleasantness is experienced upon view them, 85] and comparing their neural response to when they are presented with neutral stimuli [7, 86–88]. These studies routinely implicate an ensemble of cortical and subcortical areas that have also been implicated in wakefulness and autonomic function. As shown by meta-analyses of now hundreds of such studies, frequently activated brain regions include portions of the default mode network (e.g. anterior medial prefrontal cortex, portions of ventrolateral prefrontal cortex) and the salience network (e.g. the anterior cingulate cortex, insula, amygdala, hypothalamus) [see meta-analyses by, 88, 89], as well as brainstem regions [90, 91]. Using more fine-grained techniques, high resolution neuroimaging has specifically confirmed the involvement of the periaqueductal gray [92], and invasive intracranial recordings [93] and stimulation studies in humans [94] implicate the subthalamic nucleus [95].
Many of the salience network areas are differentially engaged depending on the sensory modality driving the affective experience [96]. For example, the anterior cingulate was more reliably engaged visual and somatosensory affect inductions, different induction modalities appeared to target different portions of the insula, and the amygdala, too, shows greater reliability of activation depending on the sensory modality [96, 97]. The distribution of activity within the insula and cingulate cortices appears to carry a combination of modality general and modality specific representations with respect to affective arousal [98, 99].
Intriguingly, early sensory cortical areas, too, show reliably greater activity when processing affective stimuli [see meta-analyses by, 96, 100, also, 101, 102, 103]. For example, occipital cortex exhibits greater activity when participants are viewing affectively arousing natural scene images [7, 87], including valence-neutral, high arousal images [86, 104] compared to when viewing low arousal images or control stimuli with similar amounts of visual information. Distributed activity in early sensory areas is informative for classifying arousal [99, 105, and also pleasure/displeasure, 106, 107, 108]. Even in experimental contexts that do not manipulate affect, activity in early sensory cortices is modulated by multimodal inputs depending in part on informational value [e.g., 109, 110, 111]. This suggests that the modulation of early sensory areas by affect may also depend on the informational value of affect.
The above sections foreground exemplary studies of arousal using EEG measurements for wakeful arousal, measurements such as heart rate and EDR for autonomic arousal, and subjective experience reports for affective arousal. However, these measurements are also not isolated from one another. EEG measurements of wakeful arousal (including oscillatory waves that are commonly used in sleep and wakefulness research) also relate with affective valence and autonomic function [112] including heart rate variability [113–115]. The subjective experience of arousal routinely correlates with measures of autonomic arousal [83, 116–118], and readily incorporates states of sleep and wakefulness alongside emotions into a now widely used “affective circumplex” model [119]. In fact, a small but growing body of research explicitly investigates how one variety of arousal relates to the others, hypothesizing and demonstrating relationships between neural activity during sleep, affective arousal, and emotion [120–122], sleep and autonomic activity [123, 124], and autonomic activity and affective valence [125].
3 Toward an integrated framework for the neural basis of arousal
To accommodate the variety of findings above across research areas of arousal requires outlining an integrative neural reference space for arousal (see Table 2, and Figure 1) consisting of a set of neuroanatomic structures, neurotransmitter systems, and connectivity among these neural components which are the physical ingredients that constitute arousal in its various formulations (wakeful, arousal, affective), but are separable in terms of their weighted contributions and functional interactions (i.e., their recipes). As Saper [126] notes, integration in the neural circuity across some varieties of arousal occurs as early on as the NTS and parabrachial complex, and practically at every level of the central nervous system. Our proposed reference space (Figure 1A) builds from the neural basis of wakeful arousal (as shown in Table 1), and broadens it to include other brain regions that are also affiliated with autonomic-related changes in arousal and affective arousal (Table 2). These include nuclei of the diffuse neuromodulatory system, brainstem reticular nuclei, autonomic nuclei, limbic and paralimbic structures, and sensory-systems. The state of the neural reference space for arousal is captured by the functional activity and connectivity of these nodes, which in turn relates to the quality of arousal that emerges. This is illustrated in Figure 1B, in which we show the subset of nodes that we hypothesize as being active during three different states of arousal (wakeful, autonomic, affective).
Figure 1.
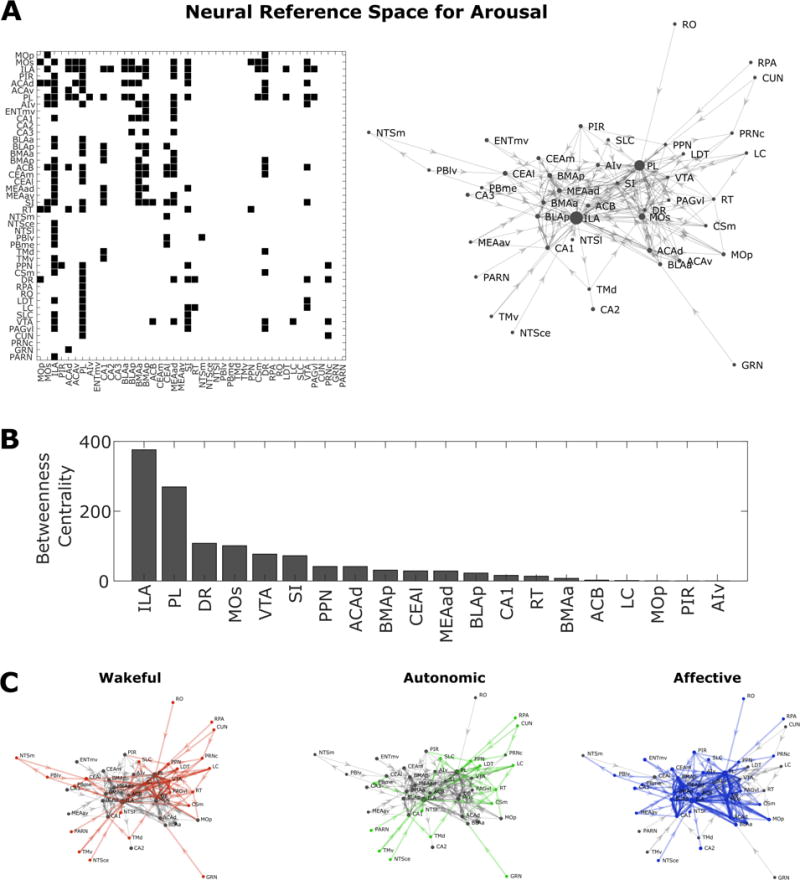
The neural reference space for arousal. (A) Left panel indicates connections between brain regions of the arousal reference space (see Table 2 for abbreviations) based on the Brain Architecture Knowledge Management System (BAMS) rat neuroanatomical macroconnectome [148]. The right panel depicts these structural connections as a network using a force-directed layout, where more connected regions are placed near each other. Node (i.e. circle) size is based on the betweenness-centrality (i.e. the number of shortest paths from all vertices to all others that pass through that node). Regions with high values of betweenness centrality, such as ILA (BC = 376) and PL (BC =270), link many different cortical, subcortical, and brainstem areas. These two regions have a larger influence on the network than other regions (e.g., the next highest region is dorsal raphe nucleus, BC = 108). The direction of each connection is indicated with an arrow, based on observations in the animal literature. Regions are identified based on Swanson’s nomenclature [149]. Note that the medullary reticular nucleus and the lateral part of the superior central nucleus raphe are excluded because their connections are absent in the macroconnectome data. (B) Bar plot of betweenness centrality for the top 20 nodes in the network. (C) Visualization of the neural reference space during instances of wakeful, autonomic, and affective arousal. These networks provide a preliminary, conceptual sense for how varieties of arousal are formulated in the integrated space. The connections are weighted on the basis of information in Table 2. Increased activation of nodes (colored circles) and heightened connectivity (colored thick lines) serve to differentiate the three forms of arousal (red, green, blue color respectively for wakeful, autonomic and affective arousal). This account proposes that the strength of activity and connectivity are differentially altered during different types of arousal.
The neural reference space and its states might be thought of as dynamic recipes for various formulations of arousal, but does not, in and of itself, reveal the mechanisms that govern the time-dependent dynamic interactions between its nodes. One possibility is that functional interactions are described well by the concept of allostasis [1] in which activity in brainstem areas is modulated by predictions of future visceromotor fluctuations sent from limbic and paralimbic circuitry [23, 127–131]. Allostatic demands also occur along different time scales, corresponding with tonic changes in neuronal activity (i.e. states such as wakefulness vs. sleep) and phasic changes in neuronal activity (i.e. transient fluctuations during wakefulness, vigilance, or as triggered by unexpected stimuli with allostatic relevance, such as evocative images and sounds). Notably, orthogonal tonic and phasic activity modes of the locus coeruleus recorded in monkeys have been associated respectively to disengagement from a task and increased task performance [132].
4 Potential of Ultra-High-Field Neuroimaging to More Fully Probe the Neural Reference Space for Arousal in Humans
Exploring this neural reference space for arousal requires the ability to measure activity simultaneously in both cortical and small subcortical areas across a variety of arousal inducing situations, in vivo. Recent advances in human neuroimaging technology (ultra-high field -i.e. 7 Tesla-scanners) and methods [e.g. simultaneous multi-slice imaging, 133] enable researchers to concurrently investigate in vivo cortical and subcortical arousal mechanisms with enhanced spatial and temporal resolution (i.e. enhanced precision and speed). These technological developments may provide a pathway to address several gaps in our understanding the neural architecture for arousal including: i) the structural organization of brainstem nuclei and connectivity pathways in humans, ii) how functional activity in these nuclei combine with activity in other subcortical and cortical areas to dynamic states in a neural reference space for arousal, and iii) how these “recipes” produce various instances of arousal with the properties of wakefulness, ANS activity, and affect (individually or in combination). Ultra-high field 7 Tesla scanning techniques may also provide further insight on how nuclei or networks affiliated with specific neurotransmitter systems play a role in arousal [for reviews of that literature, see 134, 135].
Progress in in vivo human studies of arousal has been mainly limited by the scarce knowledge of the location of brainstem, thalamic, and hypothalamic nuclei in conventional neuro-images, the limited ability to precisely and directly record behavioral/electro-physiological signatures of arousal from these small regions located deeply in the brain, and the presence of confounds in low-frequency signals which especially provide limited means of evaluating wakeful arousal. Many of these limitations may be overcome by using ultra-high field strength (≥ 7 Tesla) imaging to localize and atlas subcortical structures in humans. A remarkable feature of the human brainstem is its high density of gray matter nuclei. The brainstem is slightly larger than a human thumb, yet about 170 brainstem nuclei have been identified by ex vivo work [35], and the nuclei count reaches about 300 if the nuclei substructures are counted separately. We estimate that the location of less than 10% of brainstem nuclei has been mapped in vivo by structural neuro-imaging techniques [13–22]. This is in line with recent work, which estimates that over 90% of the subcortical annotations are missing from human MRI atlases [136].
Difficulty in localizing brainstem nuclei in conventional (e.g. 3 Tesla) imaging can be ascribed to the deep location of the brainstem (resulting in a lower MRI sensitivity compared to the cortex), the small size of brainstem nuclei, and – for most nuclei – the diminished gray-white matter contrast (e.g. relaxivity-based) compared to the cortex. Promisingly, technological advances in neuroimaging (ultra-high field –e.g. 7 Tesla– scanners, phase-array receive coils) and/or multi-contrast approaches using novel (e.g. diffusion-based, proton density, magnetization transfer) MRI contrasts for brainstem nuclei delineation have shown progress in brainstem nuclei delineation and atlasing [13, 14, 16, 21, 22]. These approaches suggest that delineation of small (volume greater or equal to 15 mm3) brainstem nuclei (including nuclei of the ascending arousal and autonomic system) can be achieved using an about 1mm-isotropic resolution MRI with adequate sensitivity and contrast. By a preliminary inspection of the brainstem nuclei size [35], we foresee that the use of multi-contrast MRI with currently achievable 0.5-1mm-isotropic resolution might enable the delineation of at least one third of the brainstem nuclei described in ex vivo human brainstem atlases. In vivo brainstem nuclei atlasing will thus greatly benefit from the tailoring of current MRI contrast or the development of novel MRI contrast for in vivo brainstem nuclei delineation. We and others have created in vivo probabilistic atlases of several brainstem nuclei involved in arousal, including the locus coeruleus [22], as well as the dorsal/median/paramedian raphe, the pedunculotegmental nucleus, and the periaqueductal gray, raphe magnus cuneiform nucleus, and pontis oralis nuclei [13, 14]. These atlases can be used to map the nuclei location in healthy subjects and patients (i.e. by coregistering conventional -e.g. 3 Tesla- MRI to the brainstem atlas space), and investigate arousal mechanisms, connectivity pathways (“connectomes”) and spatiotemporal dynamics within the arousal matrix in the living and behaving human brain. A comprehensive atlas of brainstem nuclei of the ascending arousal system would be a critical step towards the identification of the key nodes and pathways that are necessary to maintain a waking state in living humans and to promptly respond to affective stimuli. Such an atlas would also bring new insight in the understanding the possible redundancy or hierarchy of the multitude of channels assumed to be involved in arousal.
There are some general limitations in the use of ultra-high field MRI and fMRI to examine subcortical circuits. The contribution of physiological (e.g. respiration, heartbeat, brain motion) noise [137] and the presence of field inhomogeneities (both related to static magnetic field and to radiofrequency wavelength effects, [138] [139] increase with the field strength. These effects need to be properly characterized [138, 140] [141] to be able to fully exploit the improvements in detection sensitivity, contrast and spatial resolution of ultra-high field MRI [142], and to abide to statistical fMRI assumptions [143]. Finally, a 0.5-1mm isotropic spatial resolution might not be able to investigate the anatomy and function of some arousal brainstem nuclei and subnuclei that have a columnar shape and small cross-sectional area (e.g. the raphe obscurus has an in plane linear size of about 100–200 μm and extends rostro-caudally for more than 1 cm). Thus, further technological development might be needed to fully investigate in living humans the neural reference space for arousal and its dynamic mechanisms.
Despite these limitations, recent in vivo ultra-high resolution neuroimaging studies have been used successfully to examine functional activity and functional connectivity in brainstem nuclei. For instance, using submillimeter resolution, Satpute et al. [92] localized functional activity to distinct subregions of the periaqueductal gray that correlated with the presentation of images known to evoke affective arousal, and Sclocco et al. [144] examined how brain stem nuclei relate with painful stimuli. Bianciardi et al. [145] and Edlow et al. [146] delineated respectively a functional and a structural connectome of several brainstem nuclei involved in arousal and allostasis. Future high-resolution 7 Tesla MRI and EEG/MRI studies might bring clarity to arousal mechanisms in living humans by combining the resolution to identify subcortical/brainstem nuclei and by providing the means to examine cortical-brainstem connectivity.
Finally, ultra-high field imaging has the potential to improve the robustness and generalization of research findings across species, particularly for issues that are of interest to humans. Invasive studies in non-human animals have examined in much detail the subcortical circuitry involved in various forms of arousal. Ultra-high field neuroimaging may compliment this work by examining dynamic activity in these circuits in the whole brain in vivo, and how they coalesce or dissociate during different stages or varieties of arousal. Few other techniques offer the promise of examining whole-brain functional connectivity between subcortical and cortical circuitry. In humans, ultra-high field imaging may further address how well this circuitry, which has been largely defined from studies in non-human animals, generalizes to arousal-related circuitry in human thereby providing validation for work in non-human animals. It may also produce unique insights into the subjective experience of arousal, and studying how the various features of arousal relate to mental health, physical health [147], and intervening biological processes of interest (e.g. inflammation and immune system challenge).
Highlights.
We review research on wakeful, autonomic, and affective arousal.
And we propose an integrated neural reference space for arousal.
The reference space highlights coordination between brainstem and cortical areas.
The role of brainstem nuclei in arousal is poorly understood in humans.
7T imaging provides new opportunities to examine arousal in humans.
Acknowledgments
This work was supported by the following sources of funding: National Institutes of Health (NIH) National Cancer Institute U01 CA193632; NIH National Institute for Biomedical Imaging and Bioengineering K01 EB019474. National Institute on Deafness and other Communication Disorders R21 DC015888.
References
- 1.Sterling P, Laughlin S. Principles of neural design. MIT Press; 2015. [Google Scholar]
- 2.Barrett LF, Bliss-Moreau E. Affect as a Psychological Primitive. Advances in Experimental Social Psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel J. Brain mechanisms that control sleep and waking. Naturwissenschaften. 2004;91:355–365. doi: 10.1007/s00114-004-0541-9. [DOI] [PubMed] [Google Scholar]
- 4.Lacey JI. The evaluation of autonomic responses: Toward a general solution. Ann N Y Acad Sci. 1956;67:125–163. doi: 10.1111/j.1749-6632.1956.tb46040.x. [DOI] [PubMed] [Google Scholar]
- 5.Cannon WB. Bodily changes in pain, hunger, fear and rage. 1929 [Google Scholar]
- 6.Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- 7.Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- 8.Russell JA. A circumplex model of affect. J Pers Soc Psychol. 1980;39:1161–1178. [Google Scholar]
- 9.Yik MS, Russell JA, Barrett LF. Structure of self-reported current affect: Integration and beyond. J Pers Soc Psychol. 1999;77:600. [Google Scholar]
- 10.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA, USA: 1998. [Google Scholar]
- 11.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci. 2016;19:1356. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianciardi M, Toschi N, Edlow BL, Eichner C, Setsompop K, Polimeni JR, Brown E, Kinney HC, Rosen BR, Wald LL. Towards an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic and motor systems. Brain connectivity. 2015 doi: 10.1089/brain.2015.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianciardi M, Strong C, Toschi N, Edlow BL, Fischl B, Brown EN, Rosen BR, Wald LL. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7 T MRI. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keuken MC, Bazin PL, Crown L, Hootsmans J, Laufer A, Müller-Axt C, Sier R, van der Putten E, Schäfer A, Turner R. Quantifying inter-individual anatomical variability in the subcortex using 7T structural MRI. Neuroimage. 2014;94:40–46. doi: 10.1016/j.neuroimage.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, Hariz MI. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131:1588–1598. doi: 10.1093/brain/awn075. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury R, Lambert C, Dolan RJ, Düzel E. Parcellation of the human substantia nigra based on anatomical connectivity to the striatum. Neuroimage. 2013;81:191–198. doi: 10.1016/j.neuroimage.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY, Oh ES, Chi JG, Kim YB, Jeon BS, Cho ZH. Seven-tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012;71:267–277. doi: 10.1002/ana.22592. [DOI] [PubMed] [Google Scholar]
- 19.Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson’s disease. Neuroimage. 2010;52:1175–1180. doi: 10.1016/j.neuroimage.2010.05.086. [DOI] [PubMed] [Google Scholar]
- 20.Mori S, Oishi K, Faria AV. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol. 2009;22:362. doi: 10.1097/WCO.0b013e32832d954b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- 22.Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47:1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleckner I, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley K, Dickerson B, Barrett L. Evidence for a large-scale brain system supporting allostasis and interoception in humans. bioRxiv. 2017:098970. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 25.Berger H. Über das elektrenkephalogramm des menschen. Eur Arch Psychiatry Clin Neurosci. 1929;87:527–570. [Google Scholar]
- 26.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 27.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 28.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olszewski J, Baxter D. In: Cytoarchitecture of the human brainstem. Karger S, editor. JB Lippincott Company; Philadelphia and Montreal, North America: 1954. [Google Scholar]
- 30.Berridge CW. Noradrenergic modulation of arousal. Brain research reviews. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez-Peon R, Chavez-Ibarra G, Morgane P, Timo-Iaria C. Limbic cholinergic pathways involved in sleep and emotional behavior. Exp Neurol. 1963;8:93–111. [Google Scholar]
- 32.Robbins TW, Everitt BJ. Arousal systems and attention. 1995 [Google Scholar]
- 33.Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 34.Martelli D, Stanic D, Dutschmann M. The emerging role of the parabrachial complex in the generation of wakefulness drive and its implication for respiratory control. Respir Physiol Neurobiol. 2013;188:318–323. doi: 10.1016/j.resp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Huang XF, Sengul G, Watson C, Organization of brainstem nuclei . The Human Nervous System. Elsevier Academic Press; Amsterdam: 2012. pp. 260–327. [Google Scholar]
- 36.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 37.Brooks J, Tracey I. REVIEW: From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an affective pain circuit that creates a threat memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Ito M, Nagase M, Sugimura YK, Takahashi Y, Watabe AM, Kato F. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Molecular brain. 2015;8:22. doi: 10.1186/s13041-015-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neuro. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 43.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 44.Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625–646. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- 45.Vanderah TW, Gould DJ. Nolte’s the human brain: an introduction to its functional anatomy. Elsevier; Philadelphia, PA, USA: 2015. [Google Scholar]
- 46.Fraigne JJ, Torontali ZA, Snow MB, Peever JH. REM sleep at its core–circuits, neurotransmitters, and pathophysiology. Frontiers in neurology. 2015;6:123. doi: 10.3389/fneur.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatraman A, Edlow BL, Immordino-Yang MH. The Brainstem in Emotion: A Review. Frontiers in neuroanatomy. 2017;11 doi: 10.3389/fnana.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 49.Murray EA. The amygdala, reward and emotion. Trends in cognitive sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Blanco-Centurion C, Konadhode RR, Luan L, Shiromani PJ. Orexin gene transfer into the amygdala suppresses both spontaneous and emotion-induced cataplexy in orexin-knockout mice. Eur J Neurosci. 2016 doi: 10.1111/ejn.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 53.Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci Biobehav Rev. 2011;35:1791–1804. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Maclean PD. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain) Electroencephalogr Clin Neurophysiol. 1952;4:407–418. doi: 10.1016/0013-4694(52)90073-4. [DOI] [PubMed] [Google Scholar]
- 55.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang C, Leopold DA, Schölvinck ML, Mandelkow H, Picchioni D, Liu X, Frank QY, Turchi JN, Duyn JH. Tracking brain arousal fluctuations with fMRI. Proceedings of the National Academy of Sciences. 2016:201520613. doi: 10.1073/pnas.1520613113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picchioni D, Pixa ML, Fukunaga M, Carr WS, Horovitz SG, Braun AR, Duyn JH. Decreased connectivity between the thalamus and the neocortex during human nonrapid eye movement sleep. Sleep. 2014;37:387–397. doi: 10.5665/sleep.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Jahnke K, Laufs H. Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. Proceedings of the National Academy of Sciences. 2013;110:15419–15424. doi: 10.1073/pnas.1312848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akeju O, Loggia ML, Catana C, Pavone KJ, Vazquez R, Rhee J, Ramirez VC, Chonde DB, Izquierdo-Garcia D, Arabasz G. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;3:e04499. doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenblum WI. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol. 2015;74:198–202. doi: 10.1097/NEN.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 64.Bär KJ, de la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, Wagner G. Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage. 2016;134:53–63. doi: 10.1016/j.neuroimage.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 65.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 67.Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cacioppo JT, Tassinary LG, Berntson G. Handbook of psychophysiology. Cambridge University Press; 2007. [Google Scholar]
- 69.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- 70.Mendes WB. Assessing autonomic nervous system activity. Methods in social neuroscience. 2009:118–147. [Google Scholar]
- 71.Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Critchley HD, Corfield D, Chandler M, Mathias C, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. The Journal of physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 74.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Eisenbarth H, Chang LJ, Wager TD. Multivariate brain prediction of heart rate and skin conductance responses to social threat. J Neurosci. 2016;36:11987–11998. doi: 10.1523/JNEUROSCI.3672-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, affective & behavioral neuroscience. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- 77.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 78.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos T Roy Soc B. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 79.Klüver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am J Physiol. 1937;119:352–353. [Google Scholar]
- 80.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 81.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 82.Feinstein JS. Lesion studies of human emotion and feeling. Curr Opin Neurobiol. 2013;23:304–309. doi: 10.1016/j.conb.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. J Pers Soc Psychol. 2004;87:684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wundt WM. Grundriss der psychologie. A Kröner; Berlin, Germany: 1913. [Google Scholar]
- 85.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainesville, FL: 2008. (Technical Report A-8). [Google Scholar]
- 86.Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 87.Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 90.Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes BL, Kross E, Atlas LY, McRae K, Wager TD. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle J, Wald LL, Barrett LF. Identification of discrete functional subregions in the human periaqueductal grey. Proc Natl Acad Sci U S A. 2013;110:17101–17106. doi: 10.1073/pnas.1306095110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sieger T, Serranová T, Růžička F, Vostatek P, Wild J, Šťastná D, Bonnet C, Novák D, Růžička E, Urgošík D. Distinct populations of neurons respond to emotional valence and arousal in the human subthalamic nucleus. Proceedings of the National Academy of Sciences. 2015;112:3116–3121. doi: 10.1073/pnas.1410709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guillory SA, Bujarski KA. Exploring emotions using invasive methods: review of 60 years of human intracranial electrophysiology. Social cognitive and affective neuroscience. 2014:nsu002. doi: 10.1093/scan/nsu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Péron J, Frühholz S, Vérin M, Grandjean D. Subthalamic nucleus: A key structure for emotional component synchronization in humans. Neurosci Biobehav Rev. 2013;37:358–373. doi: 10.1016/j.neubiorev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Satpute AB, Kang J, Bickart KC, Wager T, Barrett LF. Involvement of sensory regions in affective experience: A meta-analysis. Frontiers in psychology. 2015;6:1860. doi: 10.3389/fpsyg.2015.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain research reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Corradi-Dell’Acqua C, Tusche A, Vuilleumier P, Singer T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nature communications. 2016;7 doi: 10.1038/ncomms10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLoS Biol. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayes DJ, Northoff G. Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front Integr Neurosci. 2011;5:49. doi: 10.3389/fnint.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 102.Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of Us Disgusted in< i>My</i> Insula: The Common Neural Basis of Seeing and Feeling Disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 104.Mourao-Miranda J, Volchan E, Moll J, de Oliveira-Souza R, Oliveira L, Bramati I, Gattass R, Pessoa L. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20:1955–1963. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Baucom LB, Wedell DH, Wang J, Blitzer DN, Shinkareva SV. Decoding the neural representation of affective states. Neuroimage. 2012;59:718–727. doi: 10.1016/j.neuroimage.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 106.Gazzola V, Spezio ML, Etzel JA, Castelli F, Adolphs R, Keysers C. Primary somatosensory cortex discriminates affective significance in social touch. Proceedings of the National Academy of Sciences. 2012;109:E1657–E1666. doi: 10.1073/pnas.1113211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ceravolo L, Frühholz S, Grandjean D. Proximal vocal threat recruits the right voice-sensitive auditory cortex. Social cognitive and affective neuroscience. 2016:nsw004. doi: 10.1093/scan/nsw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shinkareva SV, Wang J, Kim J, Facciani MJ, Baucom LB, Wedell DH. Representations of modality-specific affective processing for visual and auditory stimuli derived from functional magnetic resonance imaging data. Hum Brain Mapp. 2014;35:3558–3568. doi: 10.1002/hbm.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Werner S, Noppeney U. Distinct functional contributions of primary sensory and association areas to audiovisual integration in object categorization. J Neurosci. 2010;30:2662–2675. doi: 10.1523/JNEUROSCI.5091-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 111.Pooresmaeili A, FitzGerald TH, Bach DR, Toelch U, Ostendorf F, Dolan RJ. Cross-modal effects of value on perceptual acuity and stimulus encoding. Proceedings of the National Academy of Sciences. 2014;111:15244–15249. doi: 10.1073/pnas.1408873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Yang CC, Lai CW, Lai HY, Kuo TB. Relationship between electroencephalogram slow-wave magnitude and heart rate variability during sleep in humans. Neurosci Lett. 2002;329:213–216. doi: 10.1016/s0304-3940(02)00661-4. [DOI] [PubMed] [Google Scholar]
- 114.Kuo TB, Yang CC. Scatterplot analysis of EEG slow-wave magnitude and heart rate variability: an integrative exploration of cerebral cortical and autonomic functions. Sleep. 2004;27:648–656. doi: 10.1093/sleep/27.4.648. [DOI] [PubMed] [Google Scholar]
- 115.Jurysta F, Van De Borne P, Migeotte PF, Dumont M, Lanquart JP, Degaute JP, Linkowski P. A study of the dynamic interactions between sleep EEG and heart rate variability in healthy young men. Clin Neurophysiol. 2003;114:2146–2155. doi: 10.1016/s1388-2457(03)00215-3. [DOI] [PubMed] [Google Scholar]
- 116.Levenson RW. Autonomic nervous system differences among emotions. SAGE Publications Sage CA; Los Angeles, CA: 1992. [Google Scholar]
- 117.Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 118.Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. Oxford University Press; USA: 2001. [Google Scholar]
- 119.Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J Pers Soc Psychol. 1999;76:805. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- 120.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 122.Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration, heart rate, and systolic blood pressure in human sleep. J Appl Physiol. 1964;19:417–422. doi: 10.1152/jappl.1964.19.3.417. [DOI] [PubMed] [Google Scholar]
- 124.Bunde A, Havlin S, Kantelhardt JW, Penzel T, Peter JH, Voigt K. Correlated and uncorrelated regions in heart-rate fluctuations during sleep. Physical Review Letters. 2000;85:3736. doi: 10.1103/PhysRevLett.85.3736. [DOI] [PubMed] [Google Scholar]
- 125.Schachter S. The interaction of cognitive and physiological determinants of emotional state. Advances in experimental social psychology. 1964;1:49–80. [Google Scholar]
- 126.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 127.Barrett LF. The theory of constructed emotion: An active inference account of interoception and categorization. Social cognitive and affective neuroscience. 2016:nsw154. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neuro. 2015;16 doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chanes L, Barrett LF. Redefining the role of limbic areas in cortical processing. Trends in cognitive sciences. 2016;20:96–106. doi: 10.1016/j.tics.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17:1607–1612. doi: 10.1038/nn.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Frontiers in psychology. 2012;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 133.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hot spots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci. 2015:1–100. doi: 10.1017/S0140525X15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal, Nature reviews. Neuroscience. 2008;9:370. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 136.Forstmann BU, de Hollander G, van Maanen L, Alkemade A, Keuken MC. Towards a mechanistic understanding of the human subcortex. Nat Rev Neuro. 2017;18:57–65. doi: 10.1038/nrn.2016.163. [DOI] [PubMed] [Google Scholar]
- 137.Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 138.Duyn JH. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62:1241–1248. doi: 10.1016/j.neuroimage.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage. 2013;80:80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 T study. Magn Reson Imaging. 2009;27:1019–1029. doi: 10.1016/j.mri.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Martino F, Esposito F, van de Moortele PF, Harel N, Formisano E, Goebel R, Uğurbil K, Yacoub E. Whole brain high-resolution functional imaging at ultra high magnetic fields: an application to the analysis of resting state networks. Neuroimage. 2011;57:1031–1044. doi: 10.1016/j.neuroimage.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sladky R, Baldinger P, Kranz GS, Trostl J, Hoflich A, Lanzenberger R, Moser E, Windischberger C. High-resolution functional MRI of the human amygdala at 7 T. Eur J Radiol. 2013;82:728–733. doi: 10.1016/j.ejrad.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang X, Holmes MJ, Newton AT, Morgan VL, Landman BA. A Comparison of Distributional Considerations with Statistical Analysis of Resting State fMRI at 3T and 7T. Proceedings of SPIE–the International Society for Optical Engineering 8314. 2012 doi: 10.1117/12.911307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Kim J, Garcia RG, Renvall V, Bianchi AM. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Phil Trans R Soc A. 2016;374:20150189. doi: 10.1098/rsta.2015.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bianciardi M, Toschi N, Eichner C, Polimeni JR, Setsompop K, Brown EN, Hämäläinen MS, Rosen BR, Wald LL. In vivo functional connectome of human brainstem nuclei of the ascending arousal, autonomic, and motor systems by high spatial resolution 7-Tesla fMRI, Magnetic Resonance Materials in Physics. Biology and Medicine. 2016;29:451–462. doi: 10.1007/s10334-016-0546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Edlow BL, McNab JA, Witzel T, Kinney HC. The structural connectome of the human central homeostatic network. Brain connectivity. 2016;6:187–200. doi: 10.1089/brain.2015.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bota M, Dong HW, Swanson LW. Combining collation and annotation efforts toward completion of the rat and mouse connectomes in BAMS. Frontiers in neuroinformatics. 2012;6 doi: 10.3389/fninf.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Swanson LW. Brain maps III: structure of the rat brain: an atlas with printed and electronic templates for data, models, and schematics. Gulf Professional Publishing. 2004 [Google Scholar]
- 150.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neuro. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 151.Li J, Hu Z, Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Research Reviews. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 153.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 154.Villano I, Messina A, Valenzano A, Moscatelli F, Esposito T, Monda V, Esposito M, Precenzano F, Carotenuto M, Viggiano A. Basal forebrain cholinergic system and orexin neurons: effects on attention. Frontiers in behavioral neuroscience. 2017;11 doi: 10.3389/fnbeh.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34:3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 157.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 158.Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychol Rev. 1975;82:116. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- 159.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 160.Craig A. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 161.Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neuro. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 162.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 163.Laine CM, Spitler KM, Mosher CP, Gothard KM. Behavioral triggers of skin conductance responses and their neural correlates in the primate amygdala. J Neurophysiol. 2009;101:1749–1754. doi: 10.1152/jn.91110.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


