Abstract
Acute myeloid leukemia carries a dismal prognosis in older patients. The objective of this study was to investigate the safety and efficacy of decitabine combined with the CXCR4 antagonist plerixafor in newly diagnosed older patients with acute myeloid leukemia and to evaluate the effects of plerixafor on leukemia stem cells. Patients were treated with monthly cycles of decitabine 20 mg/m2 days 1–10 and escalating doses of plerixafor (320–810 mcg/kg) days 1–5. Sixty-nine patients were treated, with an overall response rate of 43%. Adverse karyotype did not predict response (P=0.31). Prior hypomethylating agent treatment was the strongest independent predictor of adverse overall survival (hazard ratio 3.1; 95%CI: 1.3–7.3; P=0.008) and response (14% in previously treated patients, 46% in treatment naïve; P=0.002). As expected, the most common toxicities were myelosuppression and infection. Plerixafor induced mobilization of leukemia stem and progenitor cells, but did not cause clinically significant hyperleukocytosis. Reduction in leukemia stem cells appeared to correlate with duration of response. Plerixafor can be safely added to decitabine in poor-prognosis, elderly acute myeloid leukemia patients. The maximum tolerated dose of the combination was 810 mcg/kg. While mobilization of leukemia stem cells was observed in some patients, the clinical benefit of adding plerixafor was uncertain. This trial was registered at clinicaltrials.gov identifier: 01352650.
Introduction
Acute myeloid leukemia (AML) carries a dismal prognosis in older patients, especially those with adverse cytogenetics and/or poor performance status. The median age at diagnosis is 67 years, with 5-year survival less than 10%.1 Standard induction chemotherapy with cytarabine and an anthracycline can achieve remission in selected older AML patients, but this regimen is often not feasible due to toxicity and poor tolerability. Furthermore, long-term survival after initial intensive chemotherapy is rare in these patients, even in the setting of initial complete remission (CR). Decitabine (5-aza-2’-deoxycytidine), a DNA methyltransferase inhibitor, has shown efficacy with an acceptable extramedullary toxicity profile in newly diagnosed older AML patients, with 25% CR, 7% 30-day mortality, and a median overall survival of 7.7 months when administered using a schedule of 20 mg/m2 over one hour (h) daily for five days. Of note, patients in this multicenter study received a median of 3 cycles of treatment (range 1–25 cycles).2 Blum et al. treated 53 AML patients with a median age of 74 years (range 60–85 years) with decitabine 20 mg/m2 daily for ten days every four weeks.3 The CR rate was 47% after a median of 3 cycles of therapy, with 2% 30-day and 15% 8-week induction mortality, mostly due to disease progression. Median overall and disease-free survival were 55 and 46 weeks, respectively.3 Similar results have been achieved in other single-center trials using single-agent decitabine in a 10-day schedule, including at our own center.4,5 Still, since most newly-diagnosed AML patients treated with decitabine generally relapse within 6–18 months, and overall median survival is less than one year, a variety of potentially synergistic agents are under investigation.
Acute myeloid leukemia originates from a rare population of leukemia stem cells (LSCs) that are capable of self-renewal, proliferation, and differentiation into malignant blasts. LSCs and leukemic blasts can persist after treatment and contribute to disease relapse.6 Drugs that release LSCs and blasts from their protective microenvironment may leave them more vulnerable to therapy, as they are strongly dependent on the bone marrow niche for proliferation and survival.7 CXCR4, the chemokine receptor for stromal cell-derived factor 1 (CXCL12/SDF-1), is a critical component of the bone marrow niche and is expressed on both normal stem cells and AML blasts. The CXCR4/SDF-1 axis in AML promotes leukemic cell homing to the marrow, as well as in vivo growth.8,9 Plerixafor, a small molecule antagonist of CXCR4, is commercially available as a stem cell mobilizing agent and is approved by the US Food and Drug Administration (FDA) for use in combination with granulocyte-colony stimulating factor (G-CSF) for patients with multiple myeloma or non-Hodgkin lymphoma undergoing autologous stem cell transplantation (Mozobil, Sanofi-Aventis). Plerixafor blocks CXCR4-mediated signaling and significantly decreases the survival of AML cells in vitro. Plerixafor has been safely combined with cytotoxic chemotherapy in several studies of patients with relapsed/refractory AML.10–12 While mobilization of leukemic blasts was achieved, these trials were not randomized and, thus, the impact of plerixafor on clinical outcomes was unclear. The objective of this investigator-initiated clinical trial was to investigate the safety and efficacy of adding plerixafor to decitabine in newly diagnosed older patients with AML. Extensive correlative scientific studies were performed to determine the effects of plerixafor on the mobilization of LSCs and leukemic progenitor cells.
Methods
This trial was registered at clinicaltrials.gov identifier: 01352650 and was approved by the Institutional Review Board of Weill Cornell Medical College. The study was performed in accordance with the Declaration of Helsinki, and all subjects provided written informed consent.
Patient selection and study design
The study population included patients ≥60 years old with newly diagnosed, pathologically confirmed AML, as defined by World Health Organization criteria.13 Patients with an antecedent hematologic disorder or therapy-related myeloid neoplasm were included, but those with acute promyelocytic leukemia or favorable risk cytogenetics according to the European LeukemiaNet (ELN) criteria were excluded from participation.14 Patients with a history of prior treatment with either decitabine or plerixafor, and those undergoing active treatment for a concomitant malignancy were also excluded. There were no mandatory requirements for organ system function or performance status, but patients with a calculated CrCl of ≤50 mL/min using the Cockcroft-Gault formula had a dose reduction of plerixafor by one-third during that cycle, as per the FDA package insert for plerixafor.
The trial was designed as an open-label, phase I feasibility study to optimize mobilization of leukemia stem and progenitor cells using a fixed dose and schedule of decitabine combined with escalating doses of plerixafor. Based on previous data, it was expected that patients would require between 1-4 10-day cycles of decitabine to achieve clinical response. Plerixafor was administered during alternating treatment cycles, which allowed each patient to serve as his/her own “control” for measurements of mobilization and other correlative scientific studies. Half of the patients received plerixafor during odd-numbered treatment cycles, and half during even-numbered cycles, as the optimal timing of plerixafor administration was unknown.
Treatment schedule
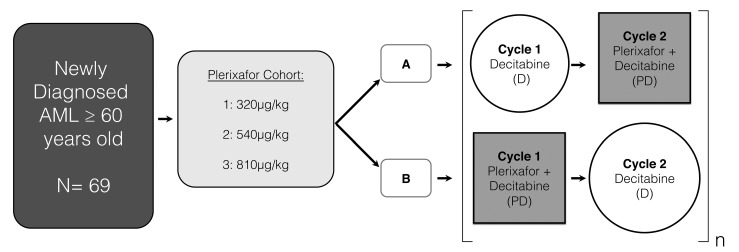
Patients were treated according to the schedule in Figure 1. Ninety-three patients were screened and 69 were enrolled onto the trial. Prior to protocol treatment, patients were treated with hydroxyurea to reduce the total white blood cell count to <30 ×109/L. Up to 4 induction cycles of decitabine, with or without the addition of plerixafor, were permitted, with 28–56 days between the starting days of each cycle. Decitabine was administered as an intravenous infusion of 20 mg/m2 over 1 hour (h) on days 1–10 of every treatment cycle. Plerixafor was administered 4 h prior to decitabine, during alternating treatment cycles: schedule A patients received plerixafor during even-numbered cycles and schedule B patients received plerixafor during odd-numbered cycles. There were three dosing cohorts of plerixafor. Cohorts 1, 2 and 3 received 320, 540, and 810 μg/kg of plerixafor intravenously on days 1–5, respectively, during alternating treatment cycles. All patients in all groups were treated with decitabine at the same dose and schedule. Patients with evidence of clinical benefit from treatment, including improved blood counts, reduced transfusion requirements, and/or improved performance status were eligible for treatment with ongoing monthly maintenance cycles of five days of decitabine, with plerixafor administered during alternate cycles according to the same dose and schedule as during induction. Patients were treated with antibiotics, transfusions, and other supportive care measures as per institutional guidelines. The use of erythropoietic growth factors was not permitted. GSCF was permitted at the discretion of the investigator, but could not be administered on the same days as plerixafor. Plerixafor was provided by Genzyme Inc., which was later acquired by Sanofi Oncology.
Figure 1.
Treatment schema.
Safety assessments
Patients were hospitalized for daily laboratory and clinical monitoring, as per institutional practice. Adverse events were reported using the National Cancer Institute (NCI) Common Terminology Criteria (CTCAE) v.4.0. A data and safety monitoring board (DSMB) was established as per the guidelines of Weill Cornell Medical College, and assessments of dose-limiting toxicity (DLT) were made in conjunction with the Data and Safety Monitoring Board.
Response assessments
Responses were determined using the International Working Group criteria.15 Complete remission (CR) was defined as a decrease in bone marrow blasts to less than 5% and absence of blasts in the peripheral blood, coupled with recovery of the absolute neutrophil count (ANC) to ≥1.0×106/mL and platelet count to ≥100×106/mL. Patients who met all criteria for CR except ANC or platelet recovery were defined as CR with incomplete peripheral blood count recovery (CRi). Partial response (PR) was defined as a ≥50% reduction in bone marrow blasts.
Statistical analysis
The primary study end point was to determine the safety and toxicity of this novel treatment regimen combining decitabine and plerixafor. The secondary clinical end points included overall response rate and overall survival. Overall survival was measured in months from cohort assignment to date of death or last followup date. The overall response rate (ORR) calculation included CR, CRi and PR. Descriptive statistics (i.e. median, range, frequency, and percent) were calculated to characterize the study population. Overall survival (OS) was assessed by Kaplan-Meier survival analysis, and univariate associations between demographic/clinical variables of interest and OS were assessed by the log-rank test. The independent effect of demographic/clinical predictors of interest on OS was assessed by multivariable Cox proportional hazards regression analysis. Adjusted hazard ratios (HR) were computed and 95% confidence intervals (95%CI) for hazard ratios and median OS time estimates are presented to assess the precision of the obtained estimates. Median follow-up time for the study group was computed based on survivors. Associations between demographic/clinical variables of interest and overall response were evaluated by the χ2 test or Fisher’s exact test, as appropriate. All P-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SPSS v.24.0 (SPSS Inc., Chicago, IL, USA) and Stata v.14.0 (StataCorp, College Station, TX, USA).
Correlative scientific studies
Details of correlative scientific studies and mutational profiling can be found in Online Supplementary Appendix 1.
Results
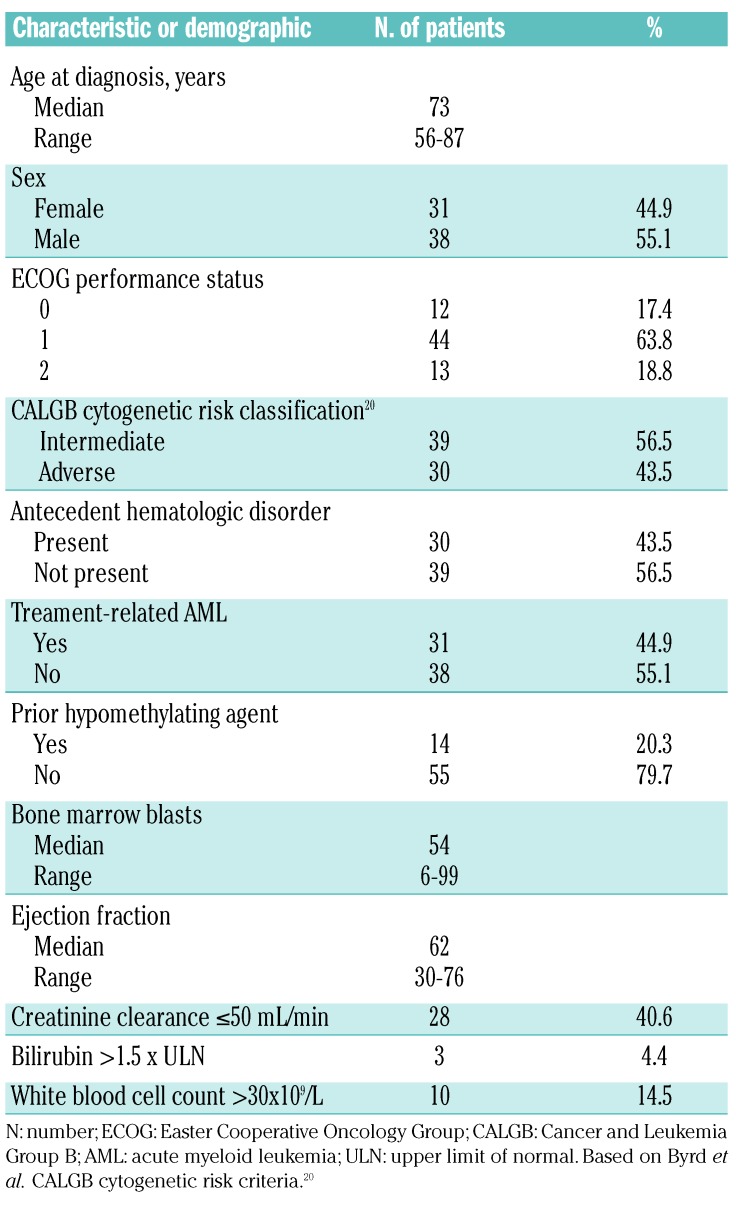
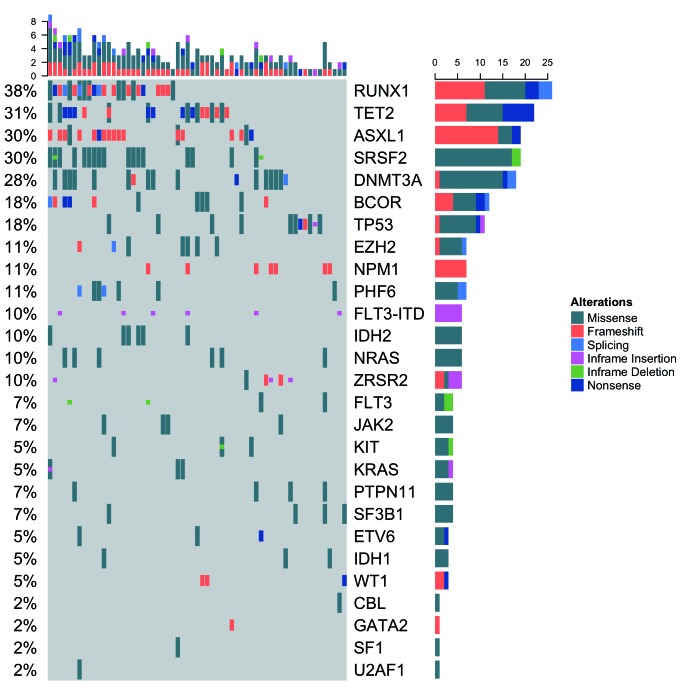
Patients’ characteristics
Ninety-five newly diagnosed AML patients were screened for eligibility and 69 patients were enrolled onto the study between June 2011 and January 2013. Of the 26 patients ineligible for study participation, 18 were reclassified as having myelodysplastic syndrome and 8 patients chose to receive standard induction chemotherapy. Baseline characteristics are presented in Table 1. The baseline mutational profile of the cohort is presented in Figure 2. The most frequently observed mutations were in RUNX1 (38%), TET2 (31%), ASXL1 (30%), SRSF2 (30%), DNMT3A (28%), BCOR (18%), and TP53 (18%). The median age at diagnosis was 73 years (range 56–87 years); 55% were male. Approximately 80% of the patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1 and the remainder had ECOG PS 2. Forty-four percent of patients had adverse cytogenetics, 44% had an antecedent hematologic disorder, and 45% had therapy-related AML. The median bone marrow blasts was 54% (range 6–99%) and 15% of patients had a baseline white blood cell count greater than 30×109/L. Forty-five percent of patients had impaired hepatic or renal function at study entry. Twenty percent of patients had received prior treatment with azacitidine.
Table 1.
Patients’ baseline characteristics.
Figure 2.
Mutational profile of patients studied. Each column indicates a patient, while each row indicates a gene tested (right label) and the percent of patients mutated for each gene (left label). Each mutation is colored according to the mutation type(s) present. Bar plots show the number of mutations per patient (top) and the total number of mutations per gene (right).
Patients were evenly distributed among the three plerixafor dosing cohorts and received a median of 3 cycles of decitabine and one cycle of plerixafor. Thirty-two percent (n=21) of patients received more than 3 cycles of decitabine and more than one cycle of plerixafor.
Safety
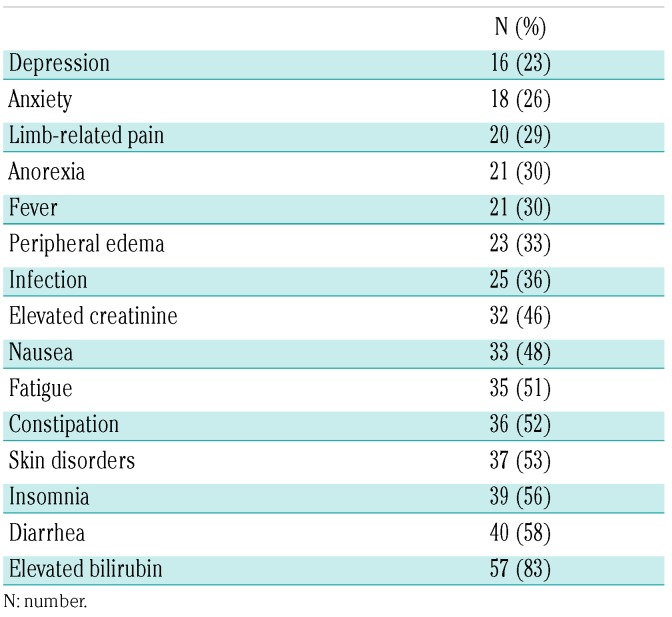
Grade 3 and 4 adverse events were as expected for older AML patients and included myelosupression in all patients, febrile neutropenia (65%), bacteremia (30%), and respiratory infections (23%). While myelosuppression was common, there were no episodes of unexpectedly prolonged myelosuppression in the absence of residual AML. Grade 1 and 2 adverse events observed in more than 20% of patients included, in ascending order of frequency: depression (23%), anxiety (26%), limb-related pain (29%), anorexia (30%), fever (30%), peripheral edema (33%), infection (36%), elevated creatinine (46%), nausea (48%), fatigue (51%), constipation (52%), skin disorders (53%), diarrhea (58%), and elevated bilirubin (83%) (Table 2). Grade 1 and 2 insomnia was unique to plerixafor-containing cycles and was experienced by 56.1% of patients. There was no clinically significant hyperleukocytosis caused by plerixafor. There was one episode of dose-limiting toxicity, renal insufficiency, in the 810 μg/kg cohort, but this was not clearly related to plerixafor. Still, this event, combined with the clinical impression of increased insomnia and increased non-serious gastrointestinal complaints in the 810 μg/kg cohort, led us to recommend 675 μg/kg as the maximum tolerated dose of plerixafor for this regimen. The 30-day induction mortality was 5.8% (n=4) and 60-day induction mortality was 13.0% (n=9).
Table 2.
Grade 1/2 adverse events observed in over 20% of subjects, regardless of drug attribution.
Efficacy
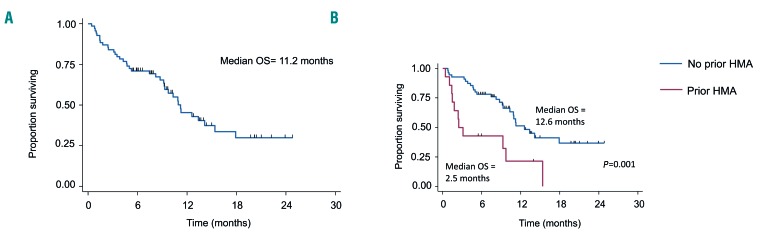
Of 69 evaluable patients, the overall response rate was 43% (35% CR, 7% CRi, 1% PR), with a median time to best response of 1.9 months (2 cycles, range 0.9–7.9 months). The median response duration was 4.5 months, with a median follow-up time for the entire study group (based on survivors) of 9.9 months (range 5.4–24.8 months). Median OS was 11.2 months (95%CI: 8.5 months, 13.9 months) (Figure 3A). As expected, the median OS for responders was significantly longer (18 months; 95%CI: 10.5–25.4 months) than for non-responders (5.0 months; 95%CI: 1.4–8.6 months) (P<0.0001). Prior treatment with azacitidine was the strongest independent predictor of OS (adjusted hazard ratio 3.1, 95%CI: 1.3–7.3; P=0.008) (Figure 3B). The median survival of patients previously treated with azacitidine was also much shorter than for previously untreated patients (2.5 months, 95%CI: 1.1–3.8 months vs. 12.6 months, 95%CI: 9.5–15.7 months; P=0.001). In addition, whereas 52% of HMA treatment-naïve patients responded to treatment (46%/6% CR/CRi), only 14% of patients previously treated with an HMA achieved a response (0%/14% CR/CRi; P=0.002). Finally, adverse karyotype did not predict overall response (P=0.31) and 53% of patients with adverse cytogenetics achieved responses (43% CR, 7% CRi, 3% PR). Median OS was 10.9 months in 59 patients without a TP53 mutation and 18.1 months in the 10 patients with TP53 mutation, but this result was not statistically significant, probably due to the small sample size. In multivariate analysis, however, adverse karyotype, as well as baseline bone marrow blasts more than 54%, were significant predictors of poor OS. Neither therapy-related AML nor the presence of an antecedent hematologic disorder was a significant predictor of response; 10 patients with therapy-related AML (32%) and 14 patients (45%) with an antecedent hematologic disorder achieved CR/CRi. There were no significant differences in ORR or OS based on plerixafor dose level (P=0.55 and P=0.19, respectively) or treatment schedule (A vs. B; P=0.71 and P=0.53, respectively), but the study was not powered for these comparisons.
Figure 3.
Overall survival (OS) for all evaluable patients in the study. (A) Kaplan-Meier OS curves for all patients (n=69) enrolled on the study. Median OS 11.2 months (95% CI: 8.6 months, 13.8 months). (B) OS for patients stratified by prior hypomethylating agent (HMA) therapy. No prior HMA (n=55), median OS 12.6 months (95%CI: 9.2, 15.9 months); prior hypomethylating agent (n=14), median OS 2.5 months (95%CI: 1.1, 3.8 months); P=0.0008 by log-rank test. PD: plerixafor + decitabine; D: decitabine.
After discontinuation of study treatment, 28 patients (42%) received further anti-leukemia therapies. Of these 28 patients, 8 achieved first or second remission with standard cytarabine/daunorubicin induction (n=5), additional decitabine (n=2), or elacytarabine (n=1). Thirteen patients (20%) underwent hematopoietic stem cell transplantation, of whom 9 were in remission, one was in partial remission, and 3 were not in remission at the time of transplant.
Correlative studies
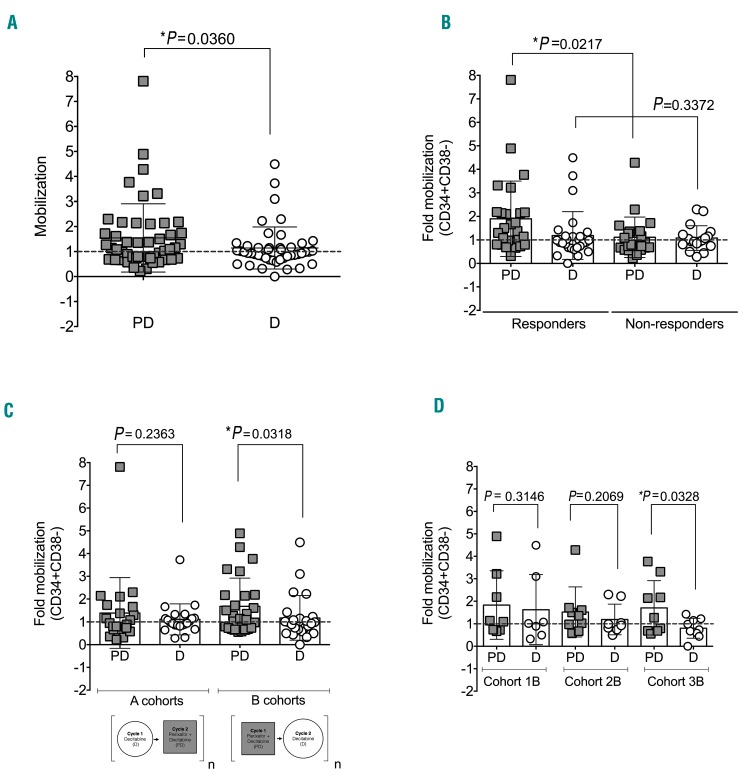
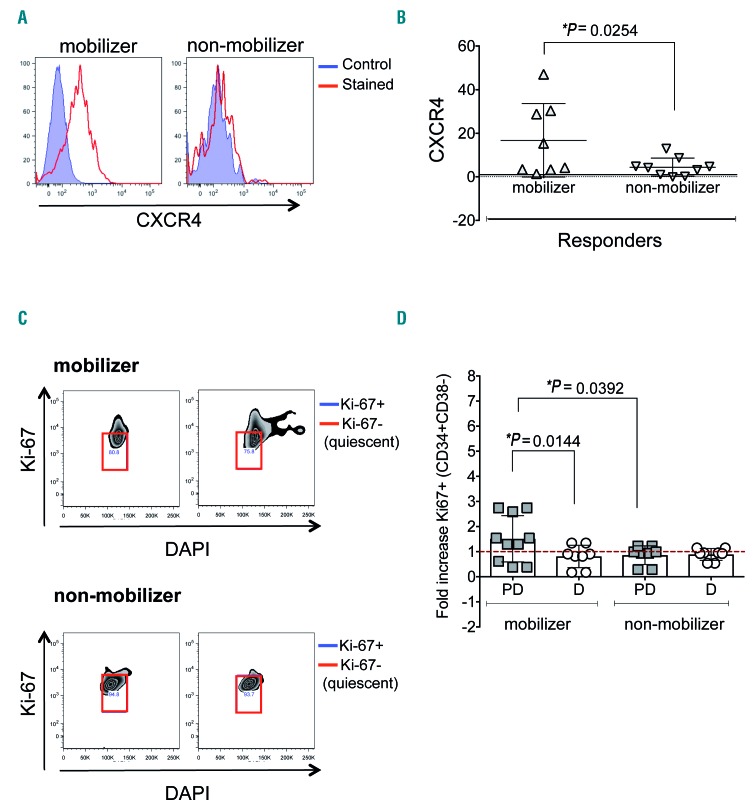
The primary goal of the correlative studies was to assess the ability of plerixafor to induce mobilization. As shown in Figure 4, treatment with plerixafor resulted in significant mobilization of leukemic stem and progenitor cells, but not as robustly and consistently as predicted. The mobilizing effect of plerixafor was highly significant (P=0.0221) among clinical responders (n=21) versus non-responders (n=19) (Figure 4B). Patients who received plerixafor combined with decitabine starting in their first cycle of treatment (B cohorts) were observed to have their most significant mobilizations during plerixafor-containing cycles (n=26; P=0.0318) (Figure 4C and D). As plerixafor is a CXCR4 antagonist that is expected to induce cell cycle entry of LSCs via loss of CXCR4-SDF1 interaction, we evaluated CXCR4 expression prior to plerixafor (Figure 5A), as well as cell cycle status before and after exposure to plerixafor (Figure 5C). As expected, we found that, within the responder group, plerixafor was more likely to mobilize CXCR4+ cells (Figure 5A and B). Interestingly, no significant differences were found for the non-responder group when evaluating mobilization with respect to CXCR4 expression (Online Supplementary Figure S1A). In addition, we found that plerixafor was more likely to increase the cycling of stem/progenitor cells, as measured by Ki-67 staining (Figure 5C and D). However, even though we observed that mobilizers tended to have increased proportions of cycling cells, we did not find differences in the duration of response or time to relapse between patients with and without increased cycling (Online Supplementary Figure S1B).
Figure 4.
Plerixafor increases mobilization of stem/progenitor cells. (A) Violin plot representing the fold change, evaluated at 4 hours after infusion, in stem/progenitor cells (CD34+CD38−) for all patients comparing cycles containing plerixafor (DP) to cycles without plerixafor (P). Scatter plots representing the average fold change in stem/progenitor cells (CD34+CD38−) comparing (B) responders and non-reponders in all cohorts, (C) cohorts A and B, and (D) all cohorts. Each symbol represents a patient, horizontal bar represents the mean, error bars represent the Standard Error of Mean. Paired t-tests between plerixafor and non-plerixafor cycles.
Figure 5.
Plerixafor is more likely to mobilize CXCR4+ cells and increase the cycling of stem/progenitor cells. (A) Representative flow cytometry histograms for CXCR4 expression in diagnostic samples from a mobilizer and a non-mobilizer. Blue filled histogram represents control, red represents CXCR4-stained. (B) Scatter plots for the expression of CXCR4 at diagnosis comparing mobilizers and non-mobilizers within the responder group. (C) Representative flow cytometry dot plots to determine cell cycle status. Red squares show quiescent cells. (D) Scatter plots representing Ki-67+ stem/progenitor cells in mobilizers and non-mobilizers during plerixafor-containing cycles (PD) and non-plerixafor cycles (D). Each symbol represents a patient, horizontal bar represents the mean, error bars represent the Standard Error of Mean.
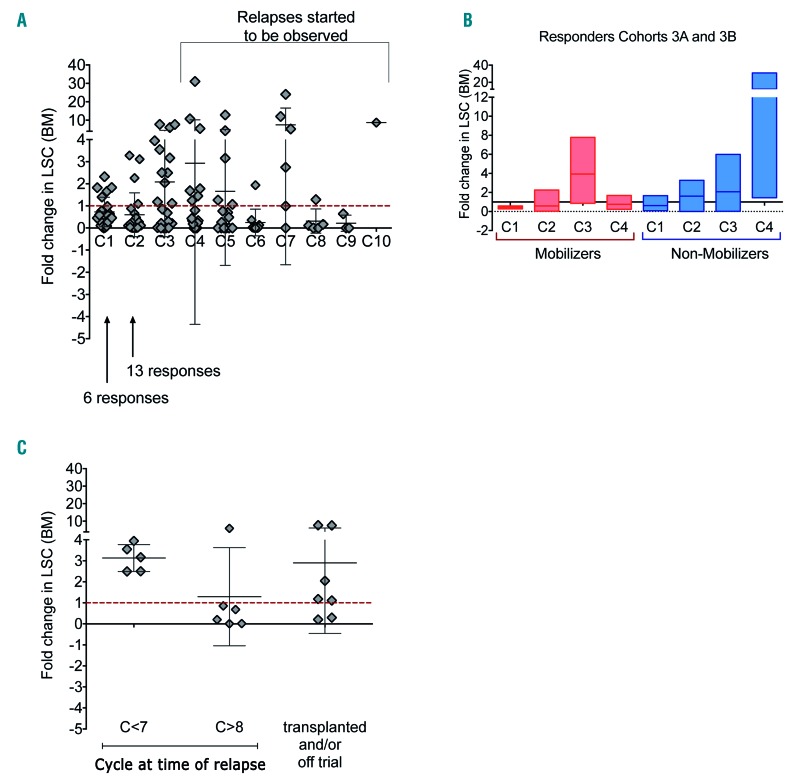
Phenotypically defined LSCs were evaluated in the bone marrow during treatment. It is clear that LSCs were shown to persist even after multiple cycles of decitabine (Figure 6). An increase in LSC frequency was noted after cycle 3, when relapses were beginning to become evident (Figure 6A). Interestingly, among non-mobilizers, there was a continuous increase in LSCs (Figure 6B), suggesting the inability of decitabine to eliminate these cells. Furthermore, among responders, the absence of an increased population of LSCs after cycle 3 correlated with prolonged remission, with relapse in most patients only after 8 cycles of therapy. In contrast, all patients with a greater than two-fold increase in LSCs at cycle 3 relative to the previous bone marrow time point replapsed before completing 7 cycles of therapy (Figure 6C).
Figure 6.
Leukemia stem/progenitor cells persist after treatment with decitabine; absence of increase in leukemia stem cells (LSCs) at cycle 3 in responders correlates with longer remissions. (A) Scatter plots representing the fold change in LSCs (CD34+CD38−CD123+CD90−) in the bone marrow at the end of the cycle indicated, relative to the previous time point. (B) Fold change in LSCs in the bone marrow for a subset of responders from cohorts 3A and 3B comparing mobilizers and non-mobilizers. (C) Fold change in LSCs in the bone marrow at cycle 3 comparing patients who relapsed before cycle 7 (C <7) to those who relapsed after cycle 8 (C >8); patients who were transplanted or off trial are shown. Each symbol represents a patient, horizontal bar represents the mean, error bars represent the Standard Error of Mean.
Discussion
This investigator-initiated clinical trial was the first time a mobilizing agent has been combined with a DNA methyltransferase inhibitor in newly diagnosed patients with AML. The study was based on the hypothesis that blockade of CXCR4/SDF-1 signaling with plerixafor would mobilize leukemic stem and progenitor cells out of their bone marrow microenvironment and make them more susceptible to the effects of decitabine. We found that adding plerixafor to decitabine was safe and tolerable. The toxicity profile was as expected for older patients with newly diagnosed AML treated with 10-day cycles of decitabine and included myelosuppression, febrile neutropenia, and infections. Importantly, the addition of plerixafor did not induce clinically significant hyperleukocytosis or tumor lysis syndrome. Grade 1 and 2 insomnia and gastrointestinal disturbances were more frequent with plerixafor, but there were no major, unexpected events. We established the maximum tolerated dose of plerixafor as 810 μg/kg and the recommended treatment dose for combination with decitabine as 675 μg/kg, based on an event of renal insufficiency, combined with increased episodes of gastrointestinal complaints and insomnia.
We designed extensive correlative scientific studies in an effort to understand the effects of plerixafor on leukemic stem and progenitor populations. Treatment with plerixafor resulted in significant mobilization of leukemic stem and progenitor cells, but not as effectively as predicted. There are several possible reasons for this, including: a) suboptimal dose and/or schedule of plerixafor, leading to inadequate CXCR4 blockade; b) suboptimal CXCR4 blockade despite adequate dosing; c) adequate CXCR4 blockade, but presence of additional factors influencing the dependency of LSCs on their microenvironment, such as VLA-4 and/or E-selectin. Also, CXCR4-expressing LSCs were more effectively mobilized than those without CXCR4 expression and plerixafor effectively increased the cycling of stem/progenitor cells, but we did not measure the effect of increased cycling on the ability of decitabine to incorporate into these cells. Our data show that leukemia stem and progenitor cells persist after treatment with decitabine. Persistence of LSCs has been associated with disease progression in larger AML studies using chemotherapy,16 but the significance of phenotypically-defined LSCs in disease progression or clinical out comes for HMA-based therapies requires further investigation in larger studies. We did not observe consistent data indicating that CXCR4 blockade with plerixafor sensitizes LSCs and progenitors to decitabine, but depletion of LSC populations by cycle 3 among responders seemed to be predictive of longer remission duration.
While this trial was designed to optimize acquisition of clinically relevant, correlative scientific data, the design may not have optimized the antileukemic effects of the plerixafor/decitabine combination, since patients received plerixafor only every other cycle and for only half of the decitabine doses (5 of 10 doses). At the trial outset, we were uncertain as to the optimal dose and schedule of plerixafor, and concerned about its potential for inducing leukocytosis. These issues led to a treatment design in which plerixafor was administered either during even numbered or odd-numbered cycles for each dosing cohort, with the objective of using each patient as his/her own control. Mobilization may have been enhanced by additional days of dosing, or by concomitant administration of G-CSF, as recommended for plerixafor’s FDA-approved indication.
The overall response rate of 43% and overall survival of 11 months are consistent with, but not significantly better, than the results from single-agent, 10-day schedules of decitabine reported by our center and others. Responses were seen in poor-prognosis, older patients with AML, including in those with particularly aggressive biological features, such as adverse karyotype, TP53 mutation, or therapy-related disease. Of interest, as has been previously reported with decitabine,17 OS was longer in patients with TP53 mutations, though the small sample size precluded statistical significance. While our data did not confirm a robust clinical benefit for the addition of plerixafor to decitabine in the doses and schedules investigated, the strong scientific rationale for the combination, the sugges tive correlative scientific data, and the safety and feasibility of the approach argue that the concept of CXCR4 blockade in AML therapy should not be abandoned. Strategies for further investigation could include mobilization with alternative CXCR4 antagonists, such as novel small molecule inhibitors and antibodies, as well as combination strategies with G-CSF, E-selectin antagonists, and/or BCL2-inhibitors.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Genzyme/Sanofi Oncology for research support and providing plerixafor, the investigational product for this trial. The authors are grateful to Wen Xie for expert technical assistance. The authors also gratefully acknowledge contributors to Leukemia Fighters. MG and GR were partially supported by LLS 6427-13, DP2 OD007399, R01 CA102031. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (UL1-TR000457-06).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/8/1308
References
- 1.Institute NC. Cancer Stat Facts: Acute Myeloid Leukemia (AML). 2014. Available from: http://seer.cancer.gov/statfacts/html/amyl.html
- 2.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–561. [DOI] [PubMed] [Google Scholar]
- 3.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107(16):7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014;55(7):1533–1537. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2009;2(6):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29(5):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. [DOI] [PubMed] [Google Scholar]
- 9.Konopleva M, Tabe Y, Zeng Z, Andreeff M. Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug Resist Updat. 2009;12(4–5):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper TM, Sison EAR, Baker SD, et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study (POE 10-03). Pediatr Blood Cancer. 2017;64(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Cuadron D, Boluda B, Martinez P, et al. A phase I-II study of plerixafor in combination with fludarabine, idarubicin, cytarabine, and G-CSF (PLERIFLAG regimen) for the treatment of patients with the first early-relapsed or refractory acute myeloid leukemia. Ann Hematol. 2018;97(5):763–772. [DOI] [PubMed] [Google Scholar]
- 12.Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. [DOI] [PubMed] [Google Scholar]
- 14.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 16.van Rhenen A, Moshaver B, Kelder A, et al. Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21(8):1700–1707. [DOI] [PubMed] [Google Scholar]
- 17.Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med. 2016;375(21):2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.