Abstract
This phase II, single-arm, multicenter study examined the efficacy and safety of coltuximab ravtansine (an anti-CD19 antibody drug conjugate) in 61 patients with histologically documented (de novo or transformed) relapsed or refractory diffuse large B-cell lymphoma who had previously received rituximab-containing immuno-chemotherapy. Patients had received a median of 2.0 (range 0-9) prior treatment regimens for diffuse large B-cell lymphoma and almost half (45.9%) had bulky disease (≥1 lesion >5 cm) at trial entry. Patients received coltuximab ravtansine (55 mg/m2) in 4 weekly and 4 biweekly administrations until disease progression or unacceptable toxicity. Forty-one patients were eligible for inclusion in the per protocol population. Overall response rate (International Working Group criteria) in the per protocol population, the primary end point, was 18/41 [43.9%; 90% confidence interval (CI:) 30.6-57.9%]. Median duration of response, progression-free survival, and overall survival (all treated patients) were 4.7 (range 0.0-8.8) months, 4.4 (90%CI: 3.02-5.78) months, and 9.2 (90%CI: 6.57-12.09) months, respectively. Common non-hematologic adverse events included asthenia/fatigue (30%), nausea (23%), and diarrhea (20%). Grade 3-4 adverse events were reported in 23 patients (38%), the most frequent being hepatotoxicity (3%) and abdominal pain (3%). Eye disorders occurred in 15 patients (25%); all were grade 1-2 and none required a dose modification. Coltuximab ravtansine monotherapy was well tolerated and resulted in moderate clinical responses in pre-treated patients with relapsed/refractory diffuse large B-cell lymphoma. (Registered at: clinicaltrials.gov identifier: 01472887)
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent form of non-Hodgkin lymphoma, representing approximately 30-58% of cases.1 The majority of cases of DLBCL occur de novo, although some develop from indolent lymphoma.2 DLBCL is subclassified as germinal center B-cell-like (GCB) or activated B-cell-like (ABC) subtypes based on gene expression profiling. The ABC subtype has a worse prognosis than the GCB subtype.3 In addition, concurrent deregulation of MYC and BCL2 has been associated with poor outcomes,4,5 however the prognostic significance of these rearrangements remains controversial.6–8
Standard first-line therapy for DLBCL is cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone, combined with rituximab (R-CHOP). Five-year overall survival (OS) in patients treated with this regimen is over 70%.9,10 Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), showed promise as an alternative first-line regimen to R-CHOP in a phase II study,11 but failed to demonstrate superior event-free survival or OS in a phase III trial which directly compared the two regimens.12 The majority of patients in the phase III study had good prognostic features, and therefore it is possible that DA-EPOCH-R may provide an advantage in patients with an adverse prognosis (such as MYC/BCL2 double-hit lymphoma) or rare subtypes (such as primary mediastinal lymphoma). However, the phase III study was not designed to answer this question, and R-CHOP remains the standard of care for the majority of unselected patients with DLBCL.12–15 Salvage treatment with autologous stem cell transplantation (ASCT) is the most effective approach at first relapse. However, it can only be offered to young, fit patients, and long-term survival is only 40%.16 There are limited treatment options with unsatisfying results for patients relapsing after, or ineligible for, ASCT.17 New therapeutic strategies are essential for these patients.
Coltuximab ravtansine (SAR3419) is an anti-CD19 monoclonal antibody conjugated to a potent cytotoxic maytansinoid, DM4, via an optimized, hindered, disulfide bond. The antibody selectively binds to the CD19 antigen present on the majority of B cells, resulting in internalization of the receptor-drug complex and intracellular release of DM4. DM4 is a potent inhibitor of tubulin polymerization and microtubule assembly, functioning by similar mechanisms to vincristine and vindesine.18,19
Coltuximab ravtansine has been evaluated in patients with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma. A first-in-human phase I study examined several dose levels in 3-weekly administrations. At the maximum tolerated dose (160 mg/m2) few clinical responses and high levels of treatment-related ocular toxicity were observed.20 A further phase I, dose-escalation study examined once-weekly dosing and a modified schedule consisting of 4 weekly doses followed by 4 doses given once every 2 weeks. Both schedules showed anti-lymphoma activity in approximately 30% of patients with either indolent or aggressive disease. The maximum tolerated dose was 55 mg/m2, and the modified dosing schedule was found to limit drug accumulation, reduce toxicity, and improve response rates.19
To confirm the clinical benefit observed in the phase I setting in a population with aggressive lymphoma, we conducted a phase II, open-label, multicenter study evaluating coltuximab ravtansine monotherapy in transplant-ineligible patients with CD19-positive, R/R DLBCL.
Methods
Study design
In this phase II, open-label, single-arm study patients received 4 weekly doses of intravenous (iv) coltuximab ravtansine 55 mg/m2, followed by a 1-week rest period, then biweekly doses until disease progression (PD), unacceptable toxicity, or discontinuation of treatment. One cycle was 4 weeks, except for cycle 1 (5 weeks). At the investigator’s discretion, patients received premedication consisting of iv diphenhydramine 50 mg and oral acetaminophen 650 mg 30-45 minutes before each infusion. Dose reductions were permitted (see Online Supplementary Methods).
Patients
Adult patients with de novo or transformed histologically confirmed DLBCL and more than 30% of cells expressing CD19 (local assessment) were enrolled. Patients had relapsed (progression ≥6 months after completion of last line of therapy) or refractory (progression during or within 6 months of a prior therapy) disease and had previously received standard chemotherapy (including rituximab). Patients with primary refractory disease (refractory to first-line therapy) were ineligible. However, some primary refractory patients were wrongly enrolled (see Results section). Full inclusion and exclusion criteria are included in the Online Supplementary Appendix.
All patients provided written informed consent. The protocol and subsequent amendments were approved by independent ethics committees and/or institutional review boards at each center. The study was conducted according to the Declaration of Helsinki.
Outcomes
The primary end point was overall response rate [ORR; proportion of patients achieving a partial response (PR) or complete response (CR) (International Working Group criteria21)]. Secondary end points included duration of response (DOR; time from first PR or CR until PD or death), progression-free survival (PFS; time from first study treatment until PD or death), OS (time from first study treatment until death), and safety. Assessment of biomarkers was an exploratory end point.
Assessments
Assessment of clinical response involved physical examination, bone marrow biopsy, and computerized tomography (CT) every 12 weeks until PD or treatment discontinuation. Positron-emission tomography (PET) was performed at baseline and, if positive, repeated to confirm a CR. Patients with a negative CT but positive PET were classified as PR.
Adverse events (AEs) were classified using National Cancer Institute Common Terminology Criteria for Adverse Events (v.4.03). Pre-specified AEs of special interest were eye disorders, neuropathy, and infusion-related reactions (all drug hypersensitivity reactions and treatment-related AEs occurring on the day of infusion).
Details of biomarker assessments are included in the Online Supplementary Methods.
Statistical analysis
The predicted beneficial ORR was 40% or over. Assuming 44 patients were evaluable for response, the study had 90% power to reject the null hypothesis of an ORR of 20% with a one-sided α=0.05. An ORR of less than 20% was considered clinically uninteresting based on available observations from coltuximab ravtansine and new agents in relapsed/refractory non-Hodgkin lymphoma and/or DLBCL, for which activity ranged between 15% and 30% in phase II studies.22–28 The primary end point (ORR), was assessed in the per protocol (PP) population (all treated patients who had an evaluable response assessment during or at the end of treatment or who died due to PD before response assessment, without any important protocol deviations affecting efficacy at study entry). ORR was also assessed in the biomarker-evaluable population (all patients with results of biomarker analysis from a fresh or archival sample). DOR and PFS were assessed in the PP population, and OS and safety were assessed in all treated patients (safety population).
Statistical analyses of biomarkers are detailed in the Online Supplementary Appendix.
Results
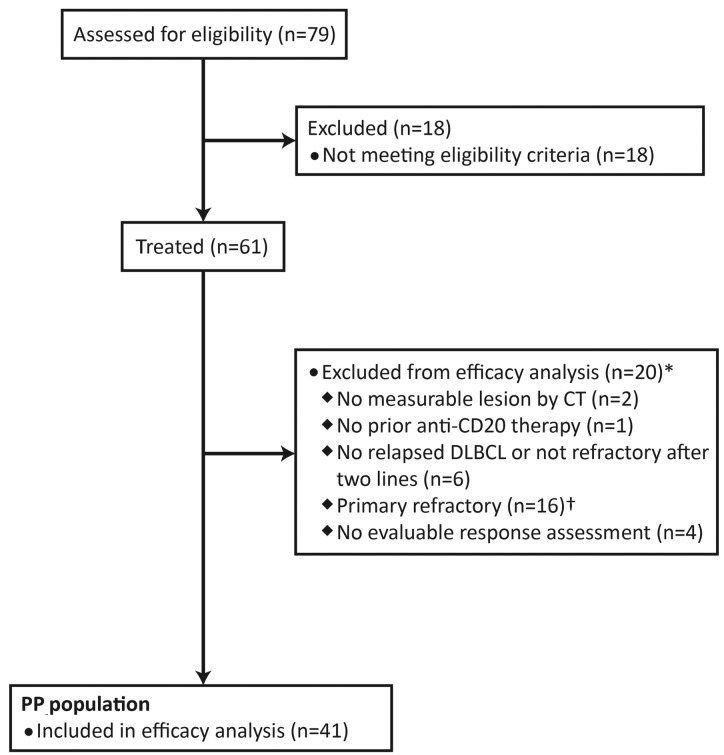
Overall, 61 patients were enrolled (January 20, 2012 to July 23, 2013) and received at least one dose of study drug (safety population). Twenty patients were excluded from the PP population (Figure 1), of whom 16 were wrongly enrolled in the study due to misclassification of their prior treatment history. Of these 16 patients, primary refractory disease was the sole important deviation at study entry in 14. The primary end point (ORR) was analyzed separately in this subgroup.
Figure 1.
Inclusion of patients in the per protocol (PP) efficacy analysis. Patients were recruited at 28 sites in the USA, Belgium, Czech Republic, Israel, Italy, Poland, Spain, Turkey, and UK. The PP population consisted of all treated patients who had an evaluable response assessment during or at the end of the treatment protocol or who died due to progressive disease before response assessment, without any important protocol deviations affecting efficacy at study entry. CT: computed tomography; DLBCL: diffuse large B-cell lymphoma. *Some patients met multiple exclusion criteria. †Fourteen patients had primary refractory disease as their only protocol deviation.
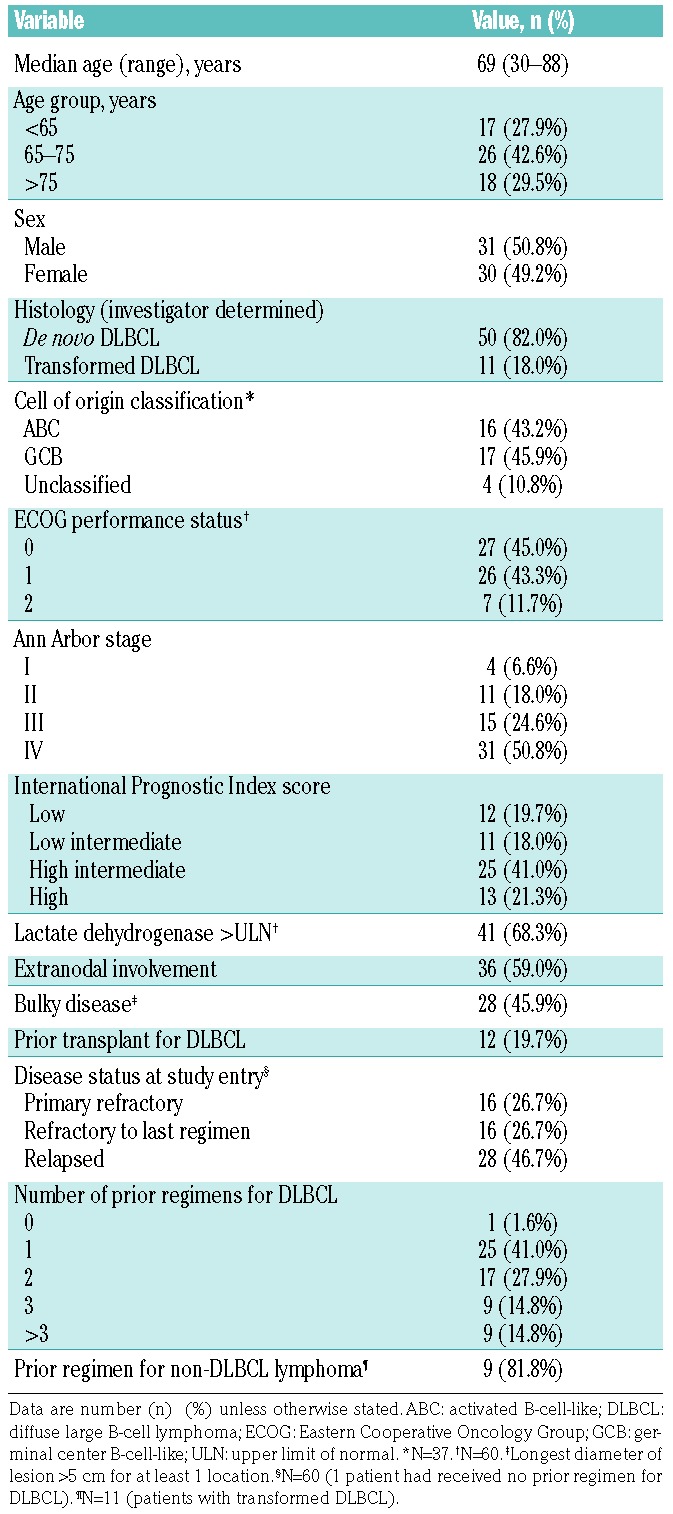
Baseline characteristics of the safety population are summarized in Table 1. Most patients (50 of 61 patients; 82.0%) presented with DLBCL at initial diagnosis. Of those patients with transformed lymphoma (n=11), 7 were initially diagnosed with follicular lymphoma, and 9 had received prior anticancer therapy for non-DLBCL lymphoma (6 patients received ≥1 prior anti-CD20-containing regimen). Almost half of the patients (45.9%) had bulky disease (defined as longest diameter of the lesion >5 cm for at least one location). Patients had received a median of 2.0 (range 0-9) prior treatment regimens for DLBCL, with 18 patients (29.5%) having received 3 or more prior regimens.
Table 1.
Baseline characteristics (safety population; n=61).
Patients received a median of 3 (range 1-10) cycles of therapy [median duration of treatment 13.3 (range 5-41) weeks]. Thirty-nine of 61 treated patients (63.9%) received 3 or more treatment cycles, including 16 patients who received 6 cycles or more. Overall, 56 patients discontinued treatment due to: PD (n=47), AEs (n=6), or investigator’s decision (n=3). At the time of analysis (May 6, 2014), 5 patients were continuing on therapy.
The ORR (primary end point), analyzed in the PP population (n=41), was 43.9% (18 of 41; 90%CI: 30.6-57.9%); therefore, the null hypothesis was rejected (P<0.0001). Among the 18 responders, 6 achieved CR (PET negative) and 12 achieved PR [PET positive (n=8) or not examined (n=4)] (Table 2). Seven patients (7 of 41; 17.1%) had stable disease, and the remaining patients (16 of 41; 39.0%) had progressive disease. Higher response rates were observed among patients with relapsed DLBCL (14 of 26; 53.8%, 90%CI: 36.2-70.8%) compared with patients refractory to their last regimen (4 of 15; 26.7%, 90%CI: 9.7-51.1%). A higher ORR (56.3%) was also observed in patients who received only one prior therapeutic regimen (n=16). At the time of analysis, 6 patients with relapsed disease were still responding to therapy (3 CRs and 3 PRs).
Table 2.
Summary of best response to treatment by subgroup based on International Working Group criteria.
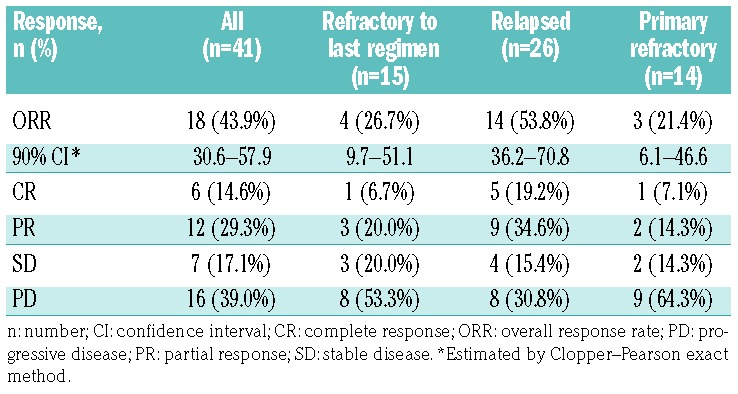
Overall response rate was also assessed in 14 patients with primary refractory disease (sole important deviation affecting efficacy) who were excluded from the PP population. Among these patients, the ORR was 21.4% (3 of 14; 90%CI: 6.1-46.6%), with the majority having PD (9 of 14; 64.3%) and only one patient achieving CR.
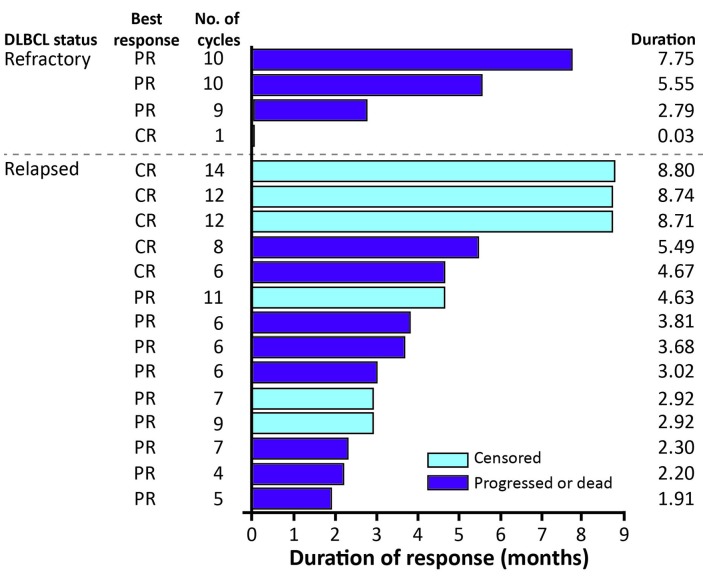
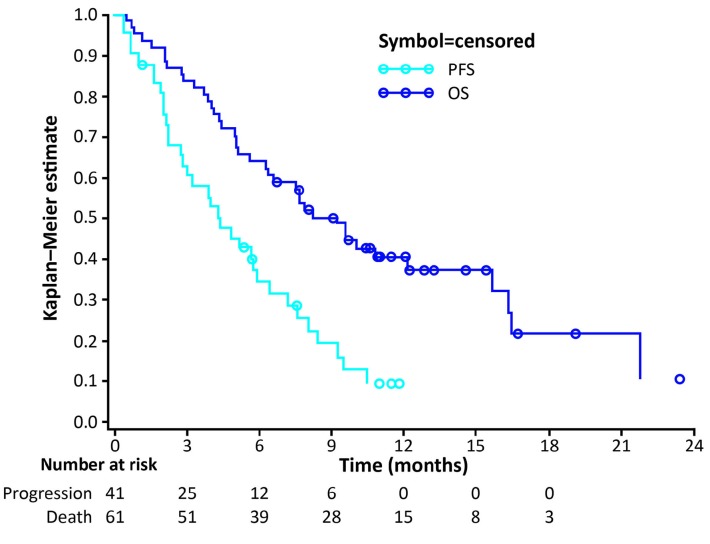
Figure 2 shows the DOR in individual patients in the PP population according to initial responses. The median DOR was 4.7 (range 0-8.8) months. Of 18 patients who responded to coltuximab ravtansine treatment (PR or better), 4 achieved a DOR of >6 months (one of 4 patients with refractory disease and 3 of 14 patients with relapsed disease). At the time of analysis, 34 of 41 patients (82.9%) in the PP population had experienced PD and the median PFS was 4.4 (90%CI: 3.02-5.78) months. Forty-one of the 61 patients in the safety population had died at the analysis cut-off date. Estimated median OS was 9.2 (90%CI: 6.57-12.09) months (Figure 3).
Figure 2.
Duration of response for individual patients in the per protocol population. Patients with a duration of response of 0.03 months were censored to the first documentation of the response, in the absence of another evaluable assessment before the cut-off date. CR: complete response; DLBCL: diffuse large B-cell lymphoma; PR: partial response.
Figure 3.
Kaplan–Meier curve of progression-free survival (PFS) (per protocol population) and overall survival (OS) (safety population).
CD19 was locally assessed in all patients (n=41) during enrollment, and centrally assessed in 37 of 41 PP patients (90.2%) during biomarker analysis. Overall, 35 patients had 30% or more CD19-positive cells (range 30-100%). Variable levels of expression were recorded, with 11, 16, and 8 samples having a mean intensity of 1+, 2+, and 3+, respectively. The median H-score (see Online Supplementary Methods) was 162 (range 0-270). There was no relationship between levels of expression of CD19 and response; some patients with high CD19 expression had PD as their best response whereas some patients with lower expression experienced a PR (Online Supplementary Figure S1). Two patients with absent CD19 staining had progressive disease. For each measure of CD19 expression, the receiver operating characteristic curve AUC values varied between 0.42 and 0.65, indicating that none of the CD19 expression measures showed good predictive accuracy for distinguishing between responders and non-responders (Online Supplementary Table S1). No significant optimal cut-off point for CD19 expression was identified. In addition, there was no apparent correlation between cell of origin classification or MYC/BCL2 expression and response rate (data not shown).
All 61 patients in the safety population (Table 3) experienced at least one AE, including 33 of 61 patients (54%) who experienced at least one treatment-related AE. Grade 3-4 AEs were reported in 23 of 61 patients (38%), the most frequent being hepatotoxicity (2 of 61, 3%) and abdominal pain (2 of 61, 3%). Serious AEs (SAEs) were reported in 24 of 61 patients (39%). Six SAEs (occurring in 3 patients) were considered related to treatment: hepatotoxicity (n=2), pneumonia, abdominal pain, nausea, and grade 5 febrile neutropenia (n=1).
Table 3.
Adverse events (AEs) occurring in ≥10% of patients (safety population; n=61).
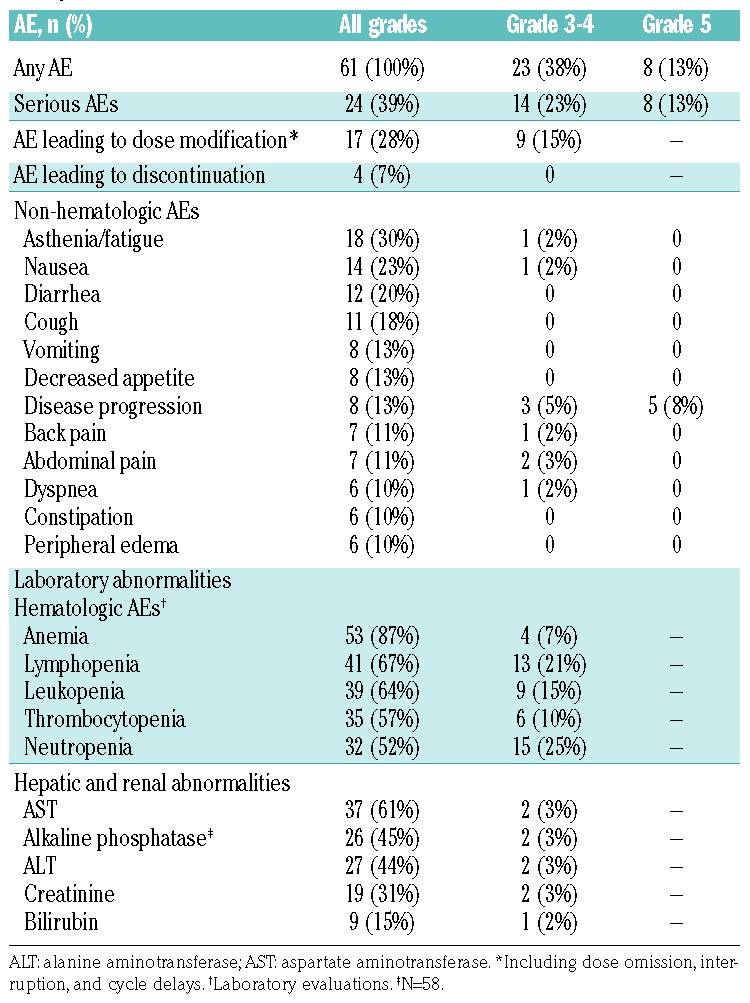
The most common grade 3-4 hematologic laboratory abnormalities were neutropenia (25%), lymphopenia (21%), and leukopenia (15%) (Table 3). Grade 3-4 non-hematologic laboratory abnormalities were rare, with elevated levels of aspartate aminotransferase, alkaline phosphatase, alanine aminotransferase, and creatinine each occurring in 2 patients. Grade 3-4 febrile neutropenia was also observed in one patient, but this did not require growth factor administration.
Eye disorders occurred in 15 patients (25%); all were grade 1-2 and none required a dose modification. Nineteen extracorneal eye disorders were observed in 13 patients (21.3%), with the first occurrence during cycle 1 (6 patients), cycle 2 (n=2), cycle 3 (n=3), cycle 7 (n=1), and cycle 9 (n=1). Fourteen of these events had resolved at the time of data cut-off, with a median recovery time of 12.5 days (range 1-47). One patient experienced a corneal event (grade 2 keratitis during cycle 4), which resolved within 9 days. A further 2 patients experienced dry eyes, occurring during cycle 1 and resolving after 13 and 17 days, respectively. Neuropathy was observed in 7 patients (11%). Five patients (8%) reported peripheral neuropathy (PN) occurring during cycle 1 (n=3) or cycle 2 (n=2), including one case of grade 3 PN (unrelated to study treatment) in a patient with a history of the condition. Dose modifications were not required in any of the patients with PN, although none of these events had resolved at the time of analysis. A further 2 patients presented with events compatible with optic neuropathy (grade 1); this diagnosis was not confirmed, but could not be confidently excluded. Neither of these patients required a dose modification and both events resolved within a median of 9 days (range 4-14). Overall, infusion-related reactions occurred in 10 patients (16%), and were most commonly gastrointestinal in nature (nausea 10%, vomiting 3%). Drug hypersensitivity was observed in one patient.
Dose modifications (dose omission, interruption, or cycle delay) due to AEs were required in 17 patients (28%), including 9 patients (15%) who experienced a grade 3-4 AE. Nine patients (15%) had at least one cycle delayed by >3 days, and 9 patients (15%) had one dose omitted. One patient (2%) required a dose interruption due to grade 1 hypotension, which was considered to be unrelated to treatment.
Of 8 patients (13%) who experienced AEs leading to death, 7 were due to PD. The other patient who died developed febrile neutropenia 34 days after the last dose of coltuximab ravtansine while receiving further anticancer therapy (gemcitabine-cisplatin); the investigator could not exclude the possibility that the event was due to a delayed effect of coltuximab ravtansine treatment.
Discussion
The results of this phase II trial indicate that treatment with coltuximab ravtansine as monotherapy is associated with moderate clinical responses in a proportion of DLBCL patients previously treated with rituximab-based chemotherapy, and has a favorable toxicity profile.
The responses described here are numerically higher than those reported in a phase II study of coltuximab ravtansine in combination with rituximab [ORR 44% (90%CI: 30.6-57.9%) vs. 31% (90%CI: 22.0-41.6%), respectively].29 However, patients enrolled in the combination study were limited to 3 cycles of treatment, whereas in the current study patients continued on therapy until disease progression or discontinuation due to an AE or investigator’s decision. Additionally, the patients in the combination therapy study could be described as a more refractory population (60% of patients had primary refractory disease), whereas the primary analysis population for the current study excluded patients with primary refractory disease. It should be noted that some patients with primary refractory disease were wrongly included in this study due to a misclassification of their prior treatment history. The response rates described here are in line with other antibody-drug conjugates, when tested as monotherapy (44-56%)30,31 or in combination with rituximab (29-54%).32,33 Interestingly, the anti-CD30 antibody-drug conjugate brentuximab vedotin achieved an ORR of 44% among patients with R/R DLBCL, most of whom were refractory to their first (76%) and last (82%) line of therapy.30 The response rates were also similar to anti-CD19 monoclonal antibodies, such as MEDI-551, MOR208, and blinatumomab, currently in phase II development for DLBCL.34–36 In comparison, in a recent multicenter, randomized study of the azaanthracenedione pixantrone in patients with aggressive B-cell lymphoma (DLBCL, transformed indolent lymphoma, or follicular lymphoma),37 the ORR was 26% (CR, 15%), with a median PFS of 5.7 months (95%CI: 2.4-6.5).
The response rates among patients refractory to their first or last line of therapy were numerically lower than those observed among the relapsed patients included in the study (21.4% and 26.7% vs. 53.8%, respectively). However, given the limited numbers of patients in each group it is difficult to draw firm conclusions.
Biomarker analysis revealed no apparent correlation between cell of origin classification or MYC/BCL2 expression and clinical response. In addition, none of the CD19 expression measures analyzed showed good predictive accuracy for distinguishing responders and non-responders, and no significant optimal cut-off point for CD19 expression could be identified. This lack of correlation between CD19 expression and efficacy is counterintuitive, but may represent an effect of coltuximab ravtansine on the tumor microenvironment that is important for lymphoma cell growth and survival.38 Additional ad hoc analyses would be required to investigate this further. Interestingly, pre-clinical studies have also demonstrated that low levels of CD22 or CD79B expression on target cells does not reduce the antitumor activities of pinatuzumab vedotin or polatuzumab vedotin, respectively.39 Similar findings have also been reported in a brentuximab vedotin phase II study in DLBCL, in which responses were not dependent on CD30 expression.30
Overall, coltuximab ravtansine exhibited a favorable safety profile, with the majority of the most common AEs reported at grade 1-2. The most frequent grade 3-4 AEs were hematologic or gastrointestinal in nature. No study-onset occurrences of grade 3-4 PN or ocular toxicity were observed, and grade 1-2 toxicities were reversible and manageable. In addition, the majority of these events occurred during cycles 1-2 suggesting that they may result from the more intensive dosing of coltuximab ravtansine during the first cycle of the study, rather than drug accumulation. Indeed Ribrag et al.19 demonstrated a reduced incidence of ocular toxicities and PN with the optimized schedule used here versus a weekly dosing schedule. Dose modifications were required in 28% of patients due to AEs, approximately half of which were grade 3-4. No dose reductions were required during the study. SAEs considered related to study treatment were uncommon.
In conclusion, the results of this phase II study indicate that the optimized dosing regimen of coltuximab ravtansine may have some efficacy in patients with relapsed or refractory DLBCL, previously treated with rituximab.
Supplementary Material
Acknowledgments
The authors would like to thank Amy-Leigh Johnson, PhD, of Adelphi Communications Ltd. (Bollington, UK) for editorial support. This study and the editorial support were funded by Sanofi.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/8/1351
References
- 1.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–3734. [DOI] [PubMed] [Google Scholar]
- 2.Montoto S, Fitzgibbon J. Transformation of indolent B-cell lymphomas. J Clin Oncol. 2011;29(14):1827–1834. [DOI] [PubMed] [Google Scholar]
- 3.Dunleavy K, Wilson WH. Appropriate management of molecular subtypes of diffuse large B-cell lymphoma. Oncology (Williston Park). 2014;28(4):326–334. [PMC free article] [PubMed] [Google Scholar]
- 4.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–3467. [DOI] [PubMed] [Google Scholar]
- 5.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilly H, Gomes da SM, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v116–v125. [DOI] [PubMed] [Google Scholar]
- 7.Staiger AM, Ziepert M, Horn H, et al. Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35(22):2515–2526. [DOI] [PubMed] [Google Scholar]
- 8.Petrella T, Copie-Bergman C, Briere J, et al. BCL2 expression but not MYC and BCL2 coexpression predicts survival in elderly patients with diffuse large B-cell lymphoma independently of cell of origin in the phase 3 LNH03-6B trial. Ann Oncol. 2017;28(5): 1042–1049. [DOI] [PubMed] [Google Scholar]
- 9.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunleavy K, Fanale M, Lacasce A, et al. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma. 56th American Society of Hematology (ASH) Annual Meeting and Exposition, San Francisco, CA, USA, December 6–9, 2014. [Google Scholar]
- 12.Wilson WH, Jung SH, Pitcher BN, et al. Phase III randomized Study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood. 2016;128(22):469. [Google Scholar]
- 13.Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincri stine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017:JCO2017733402. [DOI] [PubMed] [Google Scholar]
- 14.Arakaki H, Nakazato T, Osada Y, Ito C, Aisa Y, Mori T. Comparison of R-CVP with R-CHOP for very elderly patients aged 80 or over with diffuse large B cell lymphoma. Ann Hematol. 2017;96(7):1225–1226. [DOI] [PubMed] [Google Scholar]
- 15.Nolasco-Medina D, Reynoso-Noveron N, Mohar-Betancourt A, Aviles-Salas A, Garcia-Perez O, Candelaria M. Comparison of three chemotherapy regimens in elderly patients with diffuse large B cell lymphoma: experience at a single national reference center in Mexico. Biomed Res Int. 2016;2016: 9817606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52(2):216–221. [DOI] [PubMed] [Google Scholar]
- 18.Raufi A, Ebrahim AS, Al-Katib A. Targeting CD19 in B-cell lymphoma: emerging role of SAR3419. Cancer Manag Res. 2013;5:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribrag V, Dupuis J, Tilly H, et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2014;20(1):213–220. [DOI] [PubMed] [Google Scholar]
- 20.Younes A, Kim S, Romaguera J, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol. 2012;30(22):2776–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 22.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol. 2010;28(12):2085–2093. [DOI] [PubMed] [Google Scholar]
- 23.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(30):4952–4957. [DOI] [PubMed] [Google Scholar]
- 24.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25(2):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–1627. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117(22):5058–5066. [DOI] [PubMed] [Google Scholar]
- 27.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib dis-odium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13): 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coiffier B, Thieblemont C, de GS, et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173(5):722–730. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–1402. [DOI] [PubMed] [Google Scholar]
- 31.Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16(6):704–715. [DOI] [PubMed] [Google Scholar]
- 32.Morschhauser F, Flinn I, Advani RH, et al. Preliminary results of a phase II randomized study (ROMULUS) of polatuzumab vedotin (PoV) or pinatuzumab vedotin (PiV) plus rituximab (RTX) in patients (Pts) with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL). American Society of Clinical Oncology (ASCO), Chicago, IL, USA, May 30-June 3, 2014. Abstract 8519. [Google Scholar]
- 33.Wagner-Johnston ND, Goy A, Rodriguez MA, et al. A phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplant in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(10): 2863–2869. [DOI] [PubMed] [Google Scholar]
- 34.Viardot A, Goebeler M, Hess G, et al. Treatment of relapsed/refractory diffuse large B-cell lymphoma with the bispecific T-cell engager (TiTE) antibody construct blinatumomab: primary analysis results from an open-label, Phase 2 study. 56th Amercian Society of Hematology (ASH) Annual Meeting and Exposition, San Francisco, CA, USA, December 6–9, 2014. Abstract 4460. [Google Scholar]
- 35.Goswami T, Forero A, Hamadani M, et al. Phase I/II study of MEDI-551, a humanized monoclonal antibody targeting CD19, in subjects with relapsed or refractory advanced B-cell malignancies. American Society of Clinical Oncology (ASCO), Chicago, IL, USA, June 1-5, 2012. Abstract 8065. [Google Scholar]
- 36.Jurczak W, Zinzani PL, Goy A, et al. Phase IIa study of single-agents MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma (NHL). ASCO Annual Meeting, Chicago, IL, USA, May 29–June 2, 2015. Abstract 8500. [Google Scholar]
- 37.Pettengell R, Coiffier B, Narayanan G, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13(7): 696–706. [DOI] [PubMed] [Google Scholar]
- 38.Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeifer M, Zheng B, Erdmann T, et al. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia. 2015;29(7):1578–1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.