Abstract
Diseases with clonal hematopoiesis such as myelodysplastic syndrome and acute myeloid leukemia have high rates of relapse. Only a small subset of acute myeloid leukemia patients are cured with chemotherapy alone. Relapse in these diseases occurs at least in part due to the failure to eradicate leukemic stem cells or hematopoietic stem cells in myelodysplastic syndrome. CD123, the alpha chain of the interleukin-3 receptor heterodimer, is expressed on the majority of leukemic stem cells and myelodysplastic syndrome hematopoietic stem cells and in 80% of acute myeloid leukemia. Here, we report indiscriminate killing of CD123+ normal and acute myeloid leukemia / myelodysplastic syndrome cells by SL-401, a diphtheria toxin interleukin-3 fusion protein. SL-401 induced cytotoxicity of CD123+ primary cells/blasts from acute myeloid leukemia and myelodysplastic syndrome patients but not CD123− lymphoid cells. Importantly, SL-401 was highly active even in cells expressing low levels of CD123, with minimal effect on modulation of the CD123 target in acute myeloid leukemia. SL-401 significantly prolonged survival of leukemic mice in acute myeloid leukemia patient-derived xenograft mouse models. In addition to primary samples, studies on normal cord blood and healthy marrow show that SL-401 has activity against normal hematopoietic progenitors. These findings indicate potential use of SL-401 as a “bridge-to-transplant” before allogeneic hematopoietic cell transplantation in acute myeloid leukemia / myelodysplastic syndrome patients.
Introduction
Acute myeloid leukemia (AML) incidence increases with age, and about 21,000 new cases are expected in 2017.1,2 Significant heterogeneity exists in AML as shown by diversity of karyotype, genetic mutations and epigenetic aberrations. Standard chemotherapies and immunotherapies have only limited efficacy, and most AML patients relapse partly due to failure to eradicate AML leukemic stem cells (LSC) which undergo clonal evolution and serve as a reservoir for relapse.3 Up to 47% of patients older than 60 years who undergo allogeneic transplantation for AML will relapse.4 Myelodysplastic syndrome (MDS) incidence also increases with age with an expected incidence of 15,000 cases annually.5 Upon transformation to AML, MDS patients have a poor prognosis as compared to AML cases that occur de novo.6 Patients with MDS who are refractory to hypomethylating agents also have very limited therapeutic options.7
CD33 is a widely expressed myeloid marker present on the majority of AML cells, and CD33-targeted immunotherapies have shown promising results.8,9 However, LSC of immature AML lack CD33 expression and are not eliminated by CD33 targeted agents.8 CD123 is the α chain of the interleukin-3-receptor (IL-3R) heterodimer that has affinity and specificity for interleukin-3 (IL-3).10 LSC are known to express CD12311,12 and several efforts to target CD123 to eradicate these cells have emerged.13–18 To date, however, each of these agents exhibited shortcomings that limited their development. While a prototype monoclonal antibody against CD123 showed no promising response in AML, new clones of antibodies/single chain fragment variable (ScFv) are currently under investigation for antibody-drug conjugates (ADC) and antibody-dependent cellular cytotoxicity (ADCC) functions.13–15 CD123 targeted chimeric antigen receptor T cells (CART cells) are now being evaluated in early phase clinical trials and fatalities from cytokine release syndrome in patients receiving CD123-CART therapy were reported as of this writing. Recently, preclinical effects of a dual affinity retargeting (DART) molecule, generated from CD3 and CD123 antibodies, were described in AML.18
SL-401 is a recombinant fusion protein consisting of human IL-3 and truncated diphtheria toxin, previously reported to be active in CD123+ neoplasms.19,20 The IL-3 domain dictates the specificity for CD123 expressing cells, and the catalytic unit of diphtheria toxin upon internalization inhibits the translational machinery to initiate cell death. Although, most chemotherapy regimens are effective in eradicating tumor bulk in the periphery, progenitor tumor cells in the marrow are less exposed to treatments and receive protective support from the niche. Bone marrow stromal cells are a part of the bone marrow microenvironment and play an important role in the proliferation of AML by offering various forms of protection mediated through soluble factors and contact-dependent survival signals to leukemic cells.21–24 Co-culturing of AML cells on human bone marrow stromal cell line HS-5 monolayers increases proliferation, viability, and colony formation of the AML cells, while diminishing chemotherapy-induced apoptosis.25 Furthermore, bone marrow-derived mesenchymal stromal cells (MSC) from AML patients exhibit overlapping as well as distinct cytogenetic abnormalities versus AML blasts and provide a conducive cytokine and growth milieu for leukemia cells.24,26 Therefore, it is critical to assess potential AML therapies in the context of the bone marrow microenvironment with autologous MSCs, which might be unique for each patient. In the present study, we report the potent activity of SL-401 in CD123 expressing primary AML blasts and MDS samples and its relation to CD123 levels. We also show in vitro studies of SL-401 when AML cells are co-cultured with MSCs and in vivo studies using patient derived xenograft (PDX) mouse models.
Whether CD123 is sufficiently specific for leukemic stem cells is controversial. We show here definitively that CD123 targeted SL-401 is cytotoxic to both normal cord blood-derived hematopoietic stem cells and CD123+ blasts in AML and MDS. These findings suggest that CD123 targeting may cause pancytopenia as a consequence of on-target off-tumor effects and have translational relevance for use of CD123 targeting as a “bridge to transplant” in AML and MDS. Whether MDS may be less likely to develop on-target and off-tumor side effects is being explored in combination studies of SL-401 and hypomethylating agents in early phase clinical trials (clinicaltrials.gov identifier 03113643).
Methods
Cells
AML cell lines, MV4-11 and MOLM-13 were obtained from DSMZ in 2015, confirmed to have FLT3-ITD mutation by DNA sequencing, and routinely tested for mycoplasma using MycoAlert™ Mycoplasma Detection Kit (Lonza). Primary AML and MDS cells were obtained from patient apheresis products or bone marrow aspirates following written informed consent under an institutional review board (IRB) approved protocol, according to the Declaration of Helsinki. Umbilical cord blood samples were received through the Leukemia tissue bank and collected under an IRB-approved protocol. AML primary cells were cultured in RPMI 1640 (Life Technologies, Grand Island, NY) with 10–20% fetal bovine serum (FBS) (Sigma-Aldrich, St Louis, MO); 2mM L-glutamine; penicillin (100 U/ml); streptomycin (100 μg/ml) (Life Technologies) and supplemented with 10ng/ml GM-CSF and SCF (R&D Systems) overnight before treatment with SL-401. MDS samples were cultured similarly, with additional IL-3 (10ng/ml; R&D Systems). AML subtype and mutational characteristics of AML patient samples used in this study are listed in Online Supplementary Table S1.
Bone marrow-derived mesenchymal stromal cell expansion
For AML-stromal cell co-culture experiments, AML cells were cultured in RPMI 1640 base medium with 10% FBS without any growth factors on the monolayer of the HS-5 human fibroblast cell line expressing green fluorescent protein (GFP) or with autologous mesenchymal stromal cells (MSC) derived from bone marrow of AML patients. To derive MSC for each AML, bone marrow cells were incubated for 24–36 hours in DMEM (Life Technologies) with 10% FBS and antibiotics. The plastic-adherent, elongated spindle- to stellate-shaped cells were expanded following aspiration of non-adherent cells and weekly exchange of media and passaging when confluent. MSC in passage 3 or 4 were trypsinized and stained for CD45, CD73, CD90 and CD105 with a viability stain and assessed via flow cytometry for purity.
Chemicals and reagents
SL-401 was provided by Stemline Therapeutics Inc. and is now in clinical trials20 (clinicaltrials.gov identifier 02270463). Additional information in Online Supplementary Methods.
Cell viability and staining
Apoptosis was measured by annexin-V-FITC and propidium iodide as described previously.27 For co-culture experiments, stromal cells were gated out based on GFP+ for HS-5 and CFSE+ for MSC, and the viability of leukemic cells was determined by annexin-V PE and 7-AAD staining. Cell counts were measured using a Tali cytometer (Invitrogen) according to the manufacturer’s instruction. LIVE/DEAD stain (Invitrogen) was used in combination with surface markers for gating live cells in flow cytometric experiments. CD123 Molecules of Equivalent Soluble Fluorochrome (MESF) on AML cells was calculated using Quantum™ MESF microsphere kit (Bangs Laboratories, Fishers, IN) and CD123 antibody (Clone: SSDCLY107D2; Beckman Coulter) after establishing calibration curve for each experiment per the manufacturer’s instructions. These CD123-MESF experiments were performed at the OSU Wexner Medical Center Clinical Cytometry Facility. Flow cytometric data were analyzed using Kaluza software (Beckman-Coulter).
AML Patient-Derived Xenograft (PDX) models
Animal experiments were performed under a protocol approved by the OSU Institutional Animal Care and Use Committee (IACUC) in NRG-SGM3 (NRGS) mice. Detailed in Online Supplementary Methods.
Statistics
Detailed in Online Supplementary Methods.
Results
AML cells express varying levels of CD123 and are sensitive to SL-401
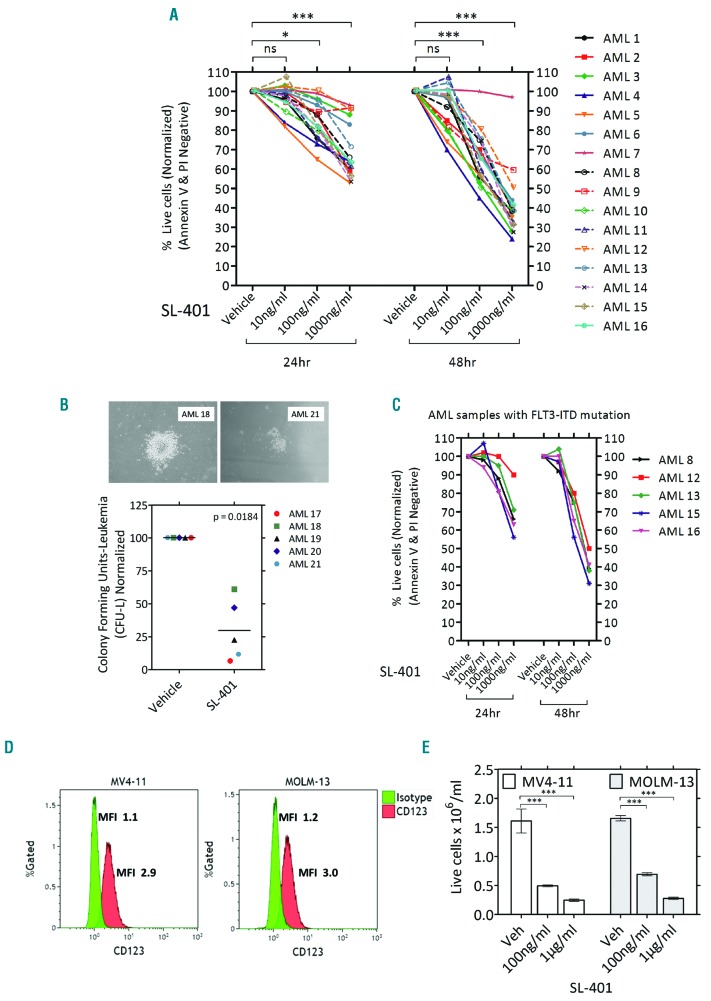
While more than 80% of AML cases show CD123 expression on blasts,28 the levels of expression are variable. Therefore, we tested the cell surface expression of CD123 on primary AML blasts. As expected, CD123 was found on the majority of AML samples tested (Table 1, and Online Supplementary Figure S1). Rarely, CD33-/CD123+ AML cell populations were also observed (Online Supplementary Figure S2A). SL-401 induced potent cytotoxicity on AML primary cells as seen by dose-dependent reduction in viability of AML cells (Figure 1A) and absolute cell numbers (Online Supplementary Figure S2B). The clonogenicity of AML samples was also significantly reduced by SL-401 as seen in colony forming assays (Figure 1B). We next determined if the activity of SL-401 was diminished in the presence of IL-3. In AML cell cultures containing 10ng/ml IL-3, a concentration > 100 fold of physiological levels, SL-401 was effective in inducing cytotoxicity and reducing the cell counts (Online Supplementary Figure S2 B–C). However, SL-401 (100ng/ml) cytotoxicity was inhibited by IL-3 in dose dependent manner, especially at doses >1000 fold of physiological levels, proving target specificity (Online Supplementary Figure S2D). Blasts derived from high-risk FLT3-ITD mutated AML often express high levels of CD123,28 so we next tested the activity of SL-401 on AML primary cells and cell lines with this mutation. Importantly, high risk FLT3-ITD+ AML tend to express similar or higher than median CD123-MESF (22825) and responded to SL-401 (Figure 1C). The FLT3-ITD+AML cell lines MV4-11 and MOLM-13 express CD123 with other myeloid markers CD45, CD33 (Figure 1D) and SL-401 strongly inhibited the growth of these cell lines (P<0.0001 for dose trends of MOLM-13 and MV4-11) (Figure 1E). However, SL-401 did not induce cytotoxicity in CD123 cell line K562 proving target specificity (Online Supplementary Figure S3A). Interestingly, cell density did not affect cytotoxicity in CD123+ MV4-11 cells (Online Supplementary Figure S3B).
Table 1.
Acute myeloid leukemia blasts express varying levels of cell surface CD123.
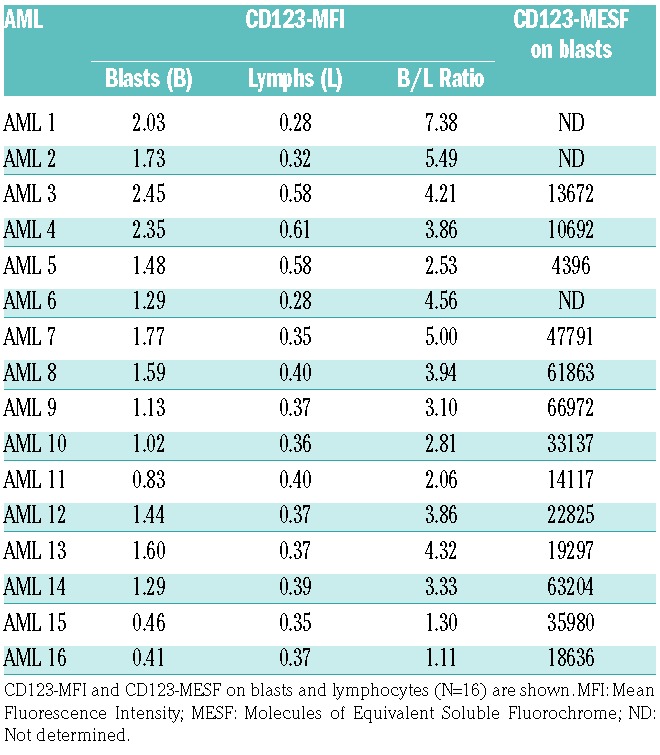
Figure 1.
AML cells express CD123 and can be targeted with SL-401. (A) SL-401 induces cytotoxicity in patient-derived AML blasts. AML blasts were cultured with varying doses of SL-401 and viability was measured at 24 and 48 hr (N=16; Trend: 24hr difference = −42.13, P<0.0001 and 48hr difference =−91.59, P<0.0001). Only AML samples >50% viable by PI staining were used for the analysis. (B) SL-401 inhibits clonogenicity of AML cells. Representative images of AML colony from vehicle treated plates are shown (PH 4X EVOS® XL Core imaging system). Leukemic colonies were counted 10–14 days after plating AML cells with continuous presence of vehicle or SL-401(1 μg/ml) in duplicates. Only AML samples that formed at least 15 colonies (more than 20 cells per colony) or clusters (5–20 cells per cluster) in vehicle treated plates were used for the analysis. Each dot represents average of duplicate plates for an AML sample (N=5 AML). (C) FLT3-ITD+ AML are sensitive to SL-401(same samples in Figure 1A). (D) Expression of CD123 in AML cell lines MV4-11 and MOLM-13 as determined by flow cytometry. (E) Growth inhibition by SL-401 in MV4-11 and MOLM-13 cells. Cells (0.5×106/ml) were treated with vehicle or SL-401 (100ng/ml and 1 μg/ml) and cell viability was measured after 72 hrs.
SL-401 overcomes autologous stromal cell protection in co-cultures
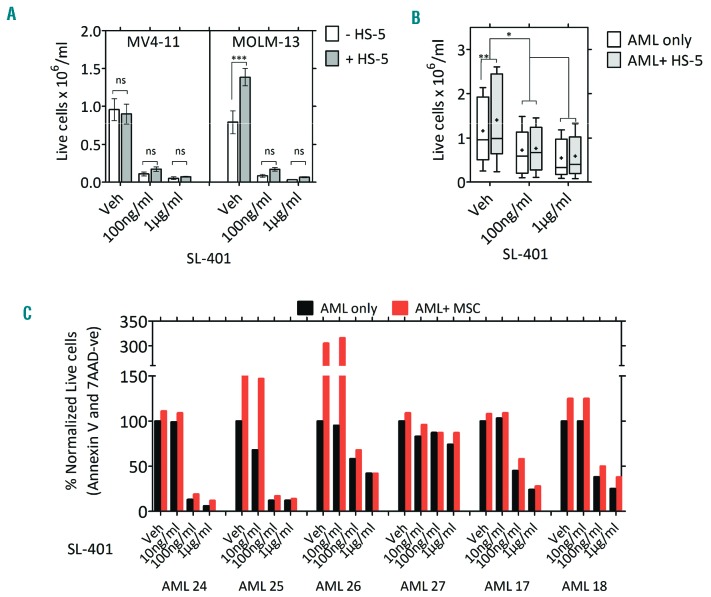
As AML cells and their progenitors derive growth and survival support from the bone marrow stromal cells and microenvironment,22,23 we evaluated the effect of stromal protection on SL-401-induced cytotoxicity. Although MV4-11 cells did not receive additional protection, the protective effect of stromal HS-5 on MOLM-13 and AML cells was reduced by SL-401 treatment (Figure 2 A-B). We next tested the effect of SL-401 on AML cells cultured on primary autologous bone marrow stromal cells from AML patients. For this purpose, we derived MSC from individual AML patient marrow samples. MSCs grown in flasks exhibited stellate- to spindle-shaped large cells and were adherent. Purity and phenotype were confirmed by multi-color flow cytometry (Online Supplementary Figure S4A). Importantly, SL-401 was able to reduce the growth of AML regardless of culture with autologous MSC (Figure 2C and Online Supplementary Figure S4B).
Figure 2.
SL-401 is active despite the presence of stroma in co-cultures. (A-B) Anti-leukemic activity of SL-401 in AML-HS5 stromal co-cultures. Cell lines (0.25×106/ml) (A) or patient-derived AML blasts (1×106/ml) (N=7) (B) were seeded onto pre-cultured GFP+ HS5 stromal cells and treated with vehicle or SL-401 (100 ng/ml; 1 μg/ml). Viable AML cell counts were measured at 48 hr by annexin-V-PE and 7-AAD staining after exclusion of GFP+ stromal cells. (C) SL-401 abrogates AML growth in autologous bone marrow-derived MSC co-cultures. AML cells were seeded onto CFSE-labeled marrow-derived autologous MSC and cultured with varying concentration of SL-401 for 120 hrs. Viability and cell counts were measured using a flow cytometric panel after staining with annexin-V-PE, 7-AAD and adding Countbrite beads (N=6; protective effect of MSC P=0.0107). Presence of MSC had no significant inhibitory effect on drug (P=0.7297, SL-401 100 ng/ml ± MSC).
Low levels of CD123 are sufficient for SL-401 mediated cytotoxicity
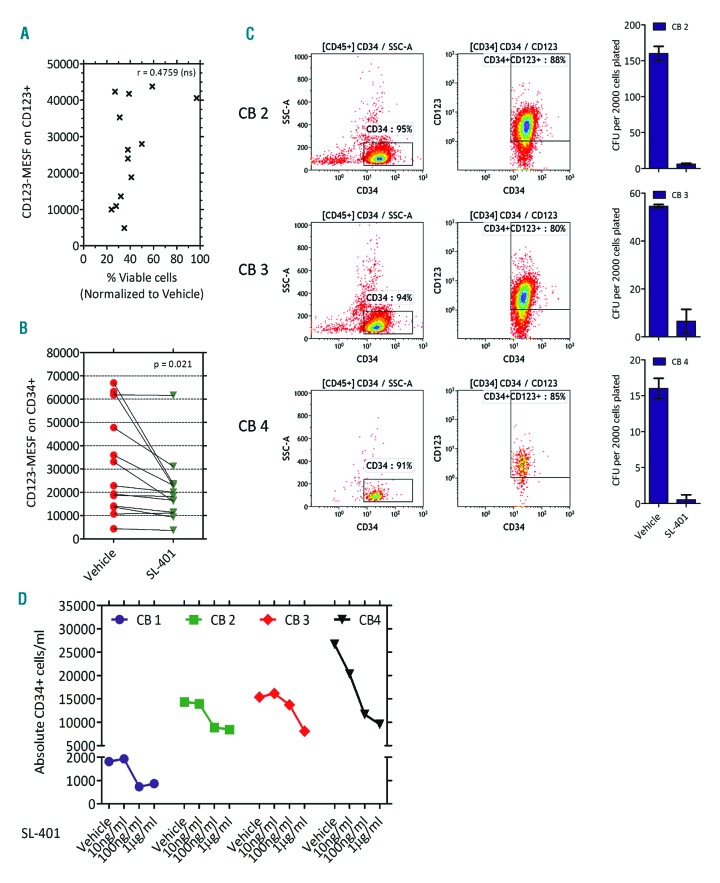
Although earlier studies utilizing variant/wild type IL-3-diphtheria toxin have reported direct correlation of cytotoxicity to the levels of IL-3R subunits, the clinical trials have shown intriguing results with lack of correlation between pharmacokinetics and pharmacodynamics.19,20,29–34 To study this relationship between SL-401 and its target, we compared the sensitivity of AML cells to SL-401 vs. their expression of CD123. As shown in Figure 3A, there was no significant correlation between the relative viability of AML samples treated with SL-401 for 48 hours with their CD123 expression (CD123-MESF on AML cells). Interestingly, AML cells expressing as few as 10,000 CD123 molecules were still sensitive to SL-401-mediated cytotoxicity. As leukemic cells can acquire resistance through target down regulation during the course of treatment, we sought to determine if SL-401 modulated cell surface CD123 levels after exposure. Although SL-401 treated samples had slightly reduced CD123 (CD123 ΔMFI= 0.689 Vehicle vs. SL-401 at 24 hr; N=16 AML; P=0.001), it should be noted that a proportion of the leukemic cells were killed at this time point and CD123 levels were calculated from the viable cells. Moreover, the leukemic cells still maintained CD123 expression >10,000 MESF (Figure 3B) and showed further decrease in viability at 48 hr (same samples as in Figure 1A). Together, these data suggest a lack of an SL-401 escape mechanism for malignant cells via target down-modulation.
Figure 3.
Low levels of CD123 are sufficient for SL-401-mediated cytotoxicity. (A) Correlation between CD123 levels and SL-401 induced cytotoxicity in patient AML cells. CD123-MESF on vehicle-treated AML samples (N=13) and viability after 48 hr of SL-401 (1 μg/ml) treatment were used for correlation analysis. (B) Changes in CD123 molecules in AML blasts after SL-401 treatment. CD123-MESF was determined on viable AML blasts after 24 hours of treatment with vehicle or SL-401 (N=13). Mean difference = 11086 (95% CI for mean difference: 2006, 20166), P=0.021. (C-D) CD123 expression and effect of SL-401 on umbilical cord blood derived CD34+ cells. (C) CD34 positive selected cells were used for multicolor flow cytometry analysis and colony formation assays. Colonies were counted 10–14 days after plating CD34+ cells with continuous presence of vehicle or SL-401(1 μg/ml) P=0.019. (D) Effect of SL-401 on umbilical cord blood liquid cultures. Non-enriched Cord blood samples (N=4 CB) were ficoll processed to obtain mononuclear cells and cultured in RPMI media with 20% FBS and GM-CSF, SCF and IL-3 (10 ng/ml) in presence of SL-401 and the cells were counted and immunophenotyped after 48 hours. Live CD34+ cell counts per ml of culture are shown in the graph. Vehicle vs. SL-401 10ng/ml not significant; Vehicle vs. SL-401 100ng/ml; P=0.0356 and Vehicle vs. SL-401 1 μg/ml; P=0.0089.
Effects of SL-401 on normal CD123+ cells derived from cord blood
To further assess the effect of SL-401 on normal CD123+ cells, we selected CD34+ cells from umbilical cord blood samples. Cord blood derived CD34+ cells expressed dim to high expression of CD123 (Figure 3C). In long-term cultures using semisolid methylcellulose media and myeloid growth factors, SL-401 completely abrogated myeloid colonies suggesting that SL-401 can compromise hematopoiesis of normal CD34+ cells (Figure 3C and Online Supplementary Figure S5A). Moreover, in short-term liquid culture of cord blood samples, SL-401 mediated moderate toxicity of CD34+ cells (Figure 3D). This was also confirmed using CD34+CD38−Lineage− sorted cells from normal donor bone marrow where SL-401 compromised the clonogenicity of hematopoietic stem cells (Online Supplementary Figure S5B).
Blasts from high risk MDS express CD123 and are sensitive to SL-401
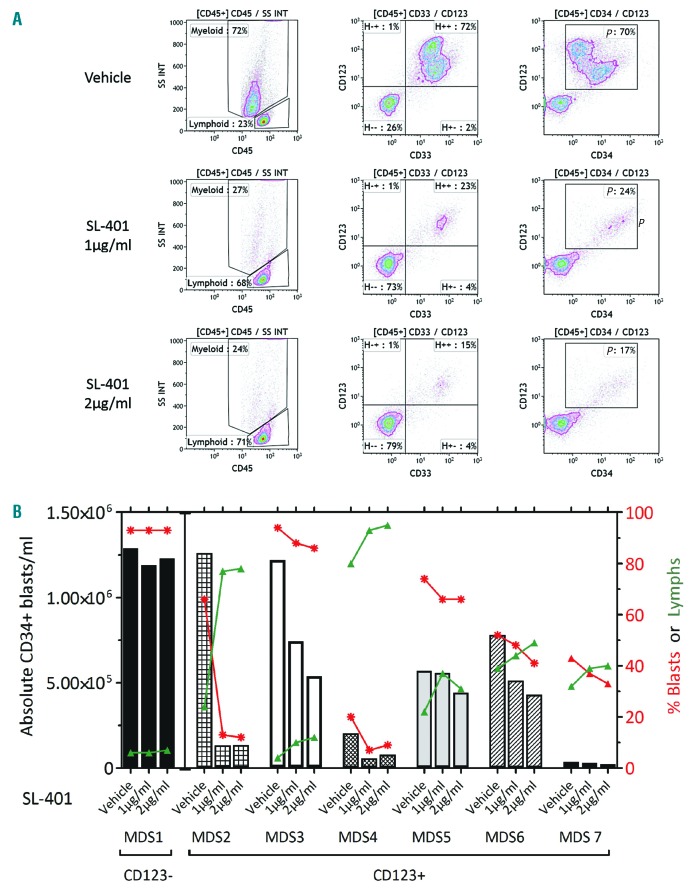
As SL-401 exhibited cytotoxic effects in AML blasts expressing low levels of CD123, we evaluated if SL-401 is active in high-risk MDS, where blasts % is < 20%. We first tested CD123 expression in MDS with refractory anemia and excess blasts (RAEB)/(MDS-EB) samples by flow cytometry. The majority of these samples (6/7) tested positive for CD123 expression, as determined by MFI. Importantly, CD123+ MDS blasts were sensitive to SL-401 mediated cytotoxicity, while CD123− lymphoid cells in the same cultures were spared (Figure 4 A-B and Online Supplementary Figure S6).
Figure 4.
Blasts from high-risk MDS express CD123 and are sensitive to SL401. (A-B) SL-401 depletes CD123+ MDS blasts. MDS samples cultured with vehicle or SL-401 (1 μg/ml, 2 μg/ml) for 120 hours were stained for various myeloid markers (CD45, CD33, CD34, CD123, and viability stain) and live cells were counted. (A) Representative MDS-RAEB cells cultured with varying doses of SL-401 for 120 hours. Density plots gated on live CD45+ population are shown. (B) Live CD34+ blast concentration (bars), live CD34+ blast % (red lines) and live lymphoid % (green lines) are shown for each sample (N=6 CD123+ MDS) P=0.0183 for live CD34+ blast concentration between SL401 1 μg/ml vs. vehicle, and P=0.0063 for live CD34+ blast concentration between SL401 2 μg/ml vs. vehicle. CD123− MDS sample (MDS 1) was included as a control.
SL-401 prolongs survival in AML PDX model
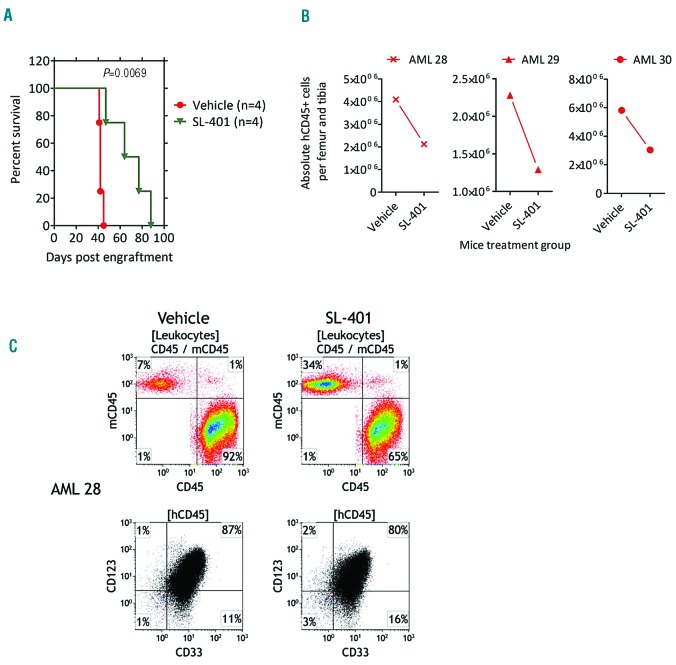
To demonstrate the therapeutic effect of SL-401 in vivo, we used AML patient-derived xenograft (PDX) models. As described in the methods, cells from CD123+ AML patients (n=3) pre-tested for engraftment potential were used to engraft NRGS mice (one AML donor per mouse) and randomized to treatment cohorts. Despite the selection of patient samples containing a high percentage of CD123+AML cells (Online Supplementary Figure S7), we initially encountered T-cell expansion in vivo due to contaminating T cells in our preliminary studies (data not shown). We therefore tested the effect of anti-CD3 antibody (OKT3) on AML cultures in vitro in ablating T cells, and confirmed that OKT3 reduced both absolute T-cell numbers and CD3 expression (data not shown). Engraftment of AML was confirmed in the peripheral blood of mice by week 4. Treatment of (AML 28/AML29) xenografted mice with SL-401 significantly increased survival compared to the vehicle control (Figure 5A). In the absence of busulfan preconditioning (AML 30), the development of AML was delayed and the mouse reached removal criteria at 102 days after engraftment (versus with busulfan 48days; data not shown). In this group, SL-401 treatment increased the survival time in the treated mouse (survival: vehicle, 102 days; SL401, 154 days; not shown). Further, evaluation of bone marrow after treatment with SL-401 in AML PDX mice revealed reduced leukemic burden compared to vehicle treated control mice (Figure 5B). The presence of AML in the animals was confirmed in spleen, bone marrow and blood using a multi-color flow cytometric panel (CD45/CD33/CD34/CD123/CD19/CD3/HLA-DR/murineCD45/viability stain) and CD123 expression was found to be maintained in vivo in engrafted mice (Figure 5C and Online Supplementary Figure S8-S9).
Figure 5.
In vivo activity of SL-401 in AML PDX models. (A) Survival curves of treatment groups from busulfan preconditioned NRGS mice engrafted with primary AML (AML 28 and AML 29). AML 28 was used to engraft three animal in each group and AML 29 was used to engraft one animal in each group. Total mice used are four per group. Ten days after engraftment, mice were randomized and treated with vehicle or SL-401 (50 μg/kg administered intraperitoneally, 3 doses: M/W/F per week for 5 weeks). (B) AML burden in bone marrow of NRGS mice engrafted with AML and treated with vehicle or SL-401 during week 3–6. Mice were treated blindly and sacrificed on week 7. Bone marrow was harvested, counted and immunophenotyped by multicolor flow cytometry. P=0.004 for Vehicle vs. SL-401 treatment. (C) Representative flow cytometric dot plots of bone marrow showing tumor burden of Vehicle and SL-401 treated PDX mice, engrafted with AML 28, at week 7.
Discussion
Relapse-free survival is low in AML and MDS in the absence of allogeneic hematopoietic stem cell transplantation. Current therapies, even if targeted for specific subtypes and mutational groups, provide only transient remissions, likely due in part to persistent stem cells.3 The expression of CD123, the alpha chain of IL-3 receptor, is limited to a sub-population of normal stem cells, few lymphoid progenitors, basophils and AML LSCs.35–37 Moreover, blasts in about 10% of AML cases express CD123 without concurrent CD33 expression, and overall about 80% of AML patients express CD123.28 Furthermore, high numbers of CD34+CD38low/−CD123+ blasts predict poor outcome in AML patients and CD34+CD38−CD123+ population is increased in AML patients at relapse.37 While favorable and intermediate risk AML subtypes have comparable levels of CD123 expression, high-risk AML sub-groups harboring a FLT3-ITD mutation tend to have higher CD123 expression.38 It was recently shown that CD123 targeted chimeric antigen receptor (CAR) T-cell therapy leads to myeloablation in primary AML xenografts.16 Therefore, CD123 serves as an important target for treating AML.
Here, we have shown that AML cells express varying levels of cell surface CD123. Importantly, we show that SL-401 is potent in killing AML cells that express even low levels of CD123 and activity of SL-401 is not dependent on surface density of CD123. Earlier reports have used IL-3 or variant IL-3-fused toxins to investigate the CD123 targeted killing of leukemic cells.29–34 These studies did not include a detailed analysis on AML cell survival or activity using in vivo PDX survival models. Previous studies involving IL-3-protein-toxin have used fluorescence intensities or transcript levels to compare the CD123 expression on different cell types and colony forming ability of AML as a prime read out. To control for variability between experiments, we used microspheres with a standardized fluorochrome to derive the CD123-MESF, thus minimizing variations due to instrument or time points. In our study, we saw no correlation between CD123-MESF and sensitivity to SL-401 cytotoxicity. It is important to note that the previous studies utilized a different clone of antibody, assay for receptor subunits, AML culture methods and cytotoxicity assays and end points. The use of high serum containing medium to culture AML in our studies may have affected CD123 expression less likely (Online Supplementary Figure S10) and possible induction of LSC differentiation.
The fact that AMLs expressing less than 10,000 CD123-MESF are sensitive to SL-401 underscores the potency of this agent. This is corroborated by a clinical trial with SL-401 that showed no correlation between robust responses and SL-401 pharmacokinetics.19,20 Interestingly, SL-401 mediated cytotoxicity in the presence of exogenous IL-3 (10ng/ml) in in vitro AML/MDS cultures. We think this may be due to high potency of diphtheria toxin, as few molecules are sufficient for cytotoxicity and/or independent binding sites of IL-3 and SL-401. However, the results we saw with decreased myeloid colony formation ability of cord blood derived CD34+ cells and normal bone marrow derived CD34+CD38− cells with SL-401 suggests that SL-401 can impact normal stem cells and myeloid progenitors. These findings suggest that SL-401 could be used to target leukemic progenitors and decrease leukemic burden with potential use as a “bridge-to-transplant” before allogeneic hematopoietic cell transplantation.39 These results are consistent with previous studies showing reduction in normal hematopoiesis when CD123-targeted CAR-T cells were used in mouse models.16
Bone marrow MSC play a critical role in leukemic cell survival and drug resistance. Recently, bone marrow-derived MSC from primary AML samples were used to establish faithful PDX models by coating ossicle/bioscaffold with MSC before inoculation into the mice by investigators.40–42 We therefore evaluated SL-401 in AML co-cultures using HS5 stromal cell lines and autologous MSC derived from bone marrow aspirates. Despite variabilities and inconsistencies in culturing patient marrow-derived MSCs, we were able to successfully propagate six AML MSC and co-cultured AML blasts on these monolayers. This method accounts for the unique MSC cytogenetic/mutational aberration-dependent survival of AML blasts. Despite this MSC protection, SL-401 retained its activity, supporting its therapeutic potential in patients.
MDS are a group of myeloid disorders characterized by dysplastic changes in the bone marrow and peripheral cytopenias, which are manifestations of ineffective hematopoiesis.6 The MDS-RAEB / MDS-EB groups represent high risk MDS and often show high levels of CD34+ cells. Thus, we interrogated the expression of CD123 in these groups. Almost all MDS samples tested (6/7) were positive for CD123 expression. Due to limited number of cells, we did not perform CD123 MESF analysis on MDS samples. However, MDS blasts responded to SL-401 as shown by decreases in both the blast percentage and the absolute count. Moreover, we showed that SL-401 reduced the numbers of CD123+ myeloid cells but not CD123− lymphoid cells in long-term cultures, supporting the selectivity of SL-401.
Our evaluation of SL-401 in mice engrafted with primary AML cells is particularly relevant to the therapeutic potential of this agent in AML. Although difficult to establish, AML PDX models capture the patient heterogeneity and genomic landscape of patients and form an important tool for evaluation of therapies.43 Consistent with previously described variability in engraftment capabilities of primary AML samples, only 3 of 7 AML samples engrafted successfully in NRGS mice, although the inclusion of busulfan as conditioning regimen allowed accelerated leukemic engraftment. Recently it was shown that inclusion of OKT3 either in in vitro cultures or in mice prevented T-cell mediated GvHD and improved human hematopoietic cell engraftment. Thus, for our in vivo studies, we cultured AML with growth factors and OKT3 prior to engraftment to eliminate CD3+ cells. SL-401 treated mice had significantly longer mean survival compared to the vehicle-treated controls. However, there was no difference in the proportion of human leukemic cells in the spleen, bone marrow or peripheral blood of mice treated with vehicle and SL-401 at end points in the survival study, presumably due to discontinuation of treatment in SL-401 group and repopulation of leukemic cells in subsequent accruing days and late time points the SL-401 group mice died. To assess the effect of SL-401 on tumor burden, we designed another study using same AML donors, but sacrificed both the groups on week 7 where we found that SL-401 treated mice had reduced human AML cells in marrow.
A first-in-human clinical trial with SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) noted that patients’ humoral immune responses against SL-401 limited the treatment cycles.20 However, in myeloid neoplasms where memory immune responses are poor, this is less likely to be a limitation. Furthermore, SL-401 is not a substrate of any known drug efflux pumps, making resistance due to this mechanism unlikely.44 Capillary leak syndrome is a known, severe side effect of SL-401 and needs to be managed for successful utilization of this agent in MDS and AML.
Together, these data indicate that SL-401 is active against CD123+ AML/MDS and normal hematopoietic progenitors. These findings have translational relevance in regards to management of potential side effects such as marrow aplasia. This agent is currently being evaluated in a Phase 2 trial of AML patients in remission with minimal residual disease, a setting associated with the persistence of CD123+ LSCs (clinicaltrials.gov identifier 02270463) and a phase I trial of azacitidine and SL-401 in MDS (clinicaltrials.gov identifier 03113643).
Supplementary Material
Acknowledgments
The authors are grateful to the AML and MDS patients who contributed to these studies, the OSU Comprehensive Cancer Center Leukemia Tissue Bank Shared Resource (P30 CA016058), the Clinical Flow Cytometry facility of the OSU Wexner Medical Center, R01 CA197844, R35 CA197734-01, D Warren Brown Foundation and the Lauber AML fund.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/8/1288
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Estey EH. Acute myeloid leukemia: 2014 update on risk-stratification and management. Am J Hematol. 2014;89(11):1063–1081. [DOI] [PubMed] [Google Scholar]
- 3.Shlush LI, Mitchell A, Heisler L, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547(7661):104–108. [DOI] [PubMed] [Google Scholar]
- 4.Devine SM, Owzar K, Blum W, et al. Phase II Study of allogeneic transplantation for older Patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33(35):4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogle CR. Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep. 2015;10(3):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383(9936):2239–2252. [DOI] [PubMed] [Google Scholar]
- 7.Shastri A, Will B, Steidl U, Verma A. Stem and progenitor cell alterations in myelodysplastic syndromes. Blood. 2017; 129(12): 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119(26):6198–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasu S, He S, Cheney C, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127(23): 2879–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato N, Caux C, Kitamura T, et al. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood. 1993;82(3):752–761. [PubMed] [Google Scholar]
- 11.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000; 14(10):1777–1784. [DOI] [PubMed] [Google Scholar]
- 12.Munoz L, Nomdedeu JF, Lopez O, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86(12):1261–1269. [PubMed] [Google Scholar]
- 13.Busfield SJ, Biondo M, Wong M, et al. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia. 2014;28(11):2213–2221. [DOI] [PubMed] [Google Scholar]
- 14.Du X, Ho M, Pastan I. New immunotoxins targeting CD123, a stem cell antigen on acute myeloid leukemia cells. J Immunother. 2007;30(6):607–613. [DOI] [PubMed] [Google Scholar]
- 15.Stein C, Kellner C, Kugler M, et al. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol. 2010; 148(6):879–889. [DOI] [PubMed] [Google Scholar]
- 16.Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014; 123(15):2343–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardiros A, Dos Santos C, McDonald T, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hussaini M, Rettig MP, Ritchey JK, et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49(3):543–553. [DOI] [PubMed] [Google Scholar]
- 20.Frankel AE, Woo JH, Ahn C, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macanas-Pirard P, Leisewitz A, Broekhuizen R, et al. Bone marrow stromal cells modulate mouse ENT1 activity and protect leukemia cells from cytarabine induced apoptosis. PLoS One. 2012;7(5): e37203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–1724. [DOI] [PubMed] [Google Scholar]
- 23.Binato R, de Almeida Oliveira NC, Du Rocher B, Abdelhay E. The molecular signature of AML mesenchymal stromal cells reveals candidate genes related to the leukemogenic process. Cancer Lett. 2015; 369(1):134–143. [DOI] [PubMed] [Google Scholar]
- 24.Huang JC, Basu SK, Zhao X, et al. Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J. 2015;5:e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp Hematol. 2001;29(4):448–457. [DOI] [PubMed] [Google Scholar]
- 26.Blau O, Baldus CD, Hofmann WK, et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood. 2011;118(20):5583–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani R, Mao Y, Frissora FW, et al. Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia. Leukemia. 2015;29(2):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehninger A, Kramer M, Rollig C, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander RL, Kucera GL, Klein B, Frankel AE. In vitro interleukin-3 binding to leukemia cells predicts cytotoxicity of a diphtheria toxin/IL-3 fusion protein. Bioconjug Chem. 2000;11(4):564–568. [DOI] [PubMed] [Google Scholar]
- 30.Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62(6):1730–1736. [PubMed] [Google Scholar]
- 31.Black JH, McCubrey JA, Willingham MC, Ramage J, Hogge DE, Frankel AE. Diphtheria toxin-interleukin-3 fusion protein (DT(388)IL3) prolongs disease-free survival of leukemic immunocompromised mice. Leukemia. 2003;17(1):155–159. [DOI] [PubMed] [Google Scholar]
- 32.Testa U, Riccioni R, Biffoni M, et al. Diphtheria toxin fused to variant human interleukin-3 induces cytotoxicity of blasts from patients with acute myeloid leukemia according to the level of interleukin-3 receptor expression. Blood. 2005;106(7): 2527–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogge DE, Yalcintepe L, Wong SH, Gerhard B, Frankel AE. Variant diphtheria toxin-interleukin-3 fusion proteins with increased receptor affinity have enhanced cytotoxicity against acute myeloid leukemia progenitors. Clinical Cancer Res. 2006;12(4):1284–1291. [DOI] [PubMed] [Google Scholar]
- 34.Yalcintepe L, Frankel AE, Hogge DE. Expression of interleukin-3 receptor subunits on defined subpopulations of acute myeloid leukemia blasts predicts the cytotoxicity of diphtheria toxin interleukin-3 fusion protein against malignant progenitors that engraft in immunodeficient mice. Blood. 2006;108(10):3530–3537. [DOI] [PubMed] [Google Scholar]
- 35.Hassanein NM, Alcancia F, Perkinson KR, Buckley PJ, Lagoo AS. Distinct expression patterns of CD123 and CD34 on normal bone marrow B-cell precursors (“hematogones”) and B lymphoblastic leukemia blasts. Am J Clin Pathol. 2009;132(4):573–580. [DOI] [PubMed] [Google Scholar]
- 36.Hwang K, Park CJ, Jang S, et al. Flow cyto-metric quantification and immunopheno-typing of leukemic stem cells in acute myeloid leukemia. Ann Hematol. 2012;91(10):1541–1546. [DOI] [PubMed] [Google Scholar]
- 37.Ho TC, LaMere M, Stevens BM, et al. Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood. 2016;128(13):1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riccioni R, Pelosi E, Riti V, Castelli G, Lo-Coco F, Testa U. Immunophenotypic features of acute myeloid leukaemia patients exhibiting high FLT3 expression not associated with mutations. Br J Haematol. 2011; 153(1):33–42. [DOI] [PubMed] [Google Scholar]
- 39.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15(1):30–38. [DOI] [PubMed] [Google Scholar]
- 40.Antonelli A, Noort WA, Jaques J, et al. Establishing human leukemia xenograft mouse models by implanting human bone marrow-like scaffold-based niches. Blood. 2016;128(25):2949–2959. [DOI] [PubMed] [Google Scholar]
- 41.Reinisch A, Thomas D, Corces MR, et al. A humanized bone marrow ossicle xeno-transplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med. 2016; 22(7):812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abarrategi A, Foster K, Hamilton A, et al. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. The Journal of clinical investigation. 2017;127(2):543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Sanchez-Martin M, Wang X, et al. Patient-derived xenotransplants can recapitulate the genetic driver landscape of acute leukemias. Leukemia. 2017; 31(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frankel AE, Hall PD, McLain C, Safa AR, Tagge EP, Kreitman RJ. Cell-specific modulation of drug resistance in acute myeloid leukemic blasts by diphtheria fusion toxin, DT388-GMCSF. Bioconjugate chemistry. 1998;9(4):490–496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.