Multiple myeloma (MM) patients who become refractory to anti-MM drugs have a very poor prognosis. Therefore, it is important to gain insight into the mechanisms of resistance to these drugs. Immunomodulatory drugs (IMiDs) have immune-stimulatory and anti-angiogenic properties as well as direct anti-MM activity. Binding of IMiDs to Cereblon promotes ubiquitination and subsequent proteasomal degradation of the substrate proteins IKZF1 (Ikaros) and IKZF3 (Aiolos), which is followed by downregulation of interferon regulatory factor 4 (IRF-4) and c-Myc leading to growth inhibition and apoptosis of MM cells.1,2 A low expression of Cereblon at baseline is associated with a decreased response to IMiD therapy.3 Furthermore, in MM cell lines, acquired resistance to IMiDs is accompanied by a decrease in Cereblon expression.4
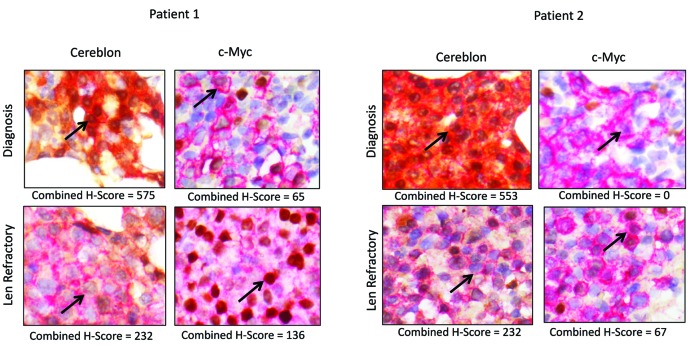
To gain insight into the expression levels of lenalidomide targets in lenalidomide-refractory patients, we analyzed the protein expression of Cereblon and downstream targets in BM-localized plasma cells using immunohistochemistry (Figure 1 and Online Supplementary Figure S1). BM samples from 55 patients were obtained at the time of diagnosis and at the time of development of lenalidomide-refractory disease. Furthermore, BM biopsies taken at earlier time points during the disease course (after first line treatment (lenalidomide naïve), after second line treatment (lenalidomide naïve) and during lenalidomide treatment (responsive)) were analyzed.
Figure 1.
Immunohistochemical staining of Cereblon and c-Myc in CD138+ bone-marrow plasma cells of MM patients. Multiple myeloma BM core biopsies stained with dual CD138 (red membrane) and Cereblon (brown cytoplasm and nucleus) or c-Myc (brown nucleus). Shown are two representative patients at the time of diagnosis (upper panel) and lenalidomide-refractory disease (lower panel). Decreased Cereblon and increased c-Myc expression are noted upon development of lenalidomide resistance. Arrows point to tumor cells. H-scores range from 0 to 600 for Cereblon and from 0–300 for c-Myc. Len: lenalidomide.
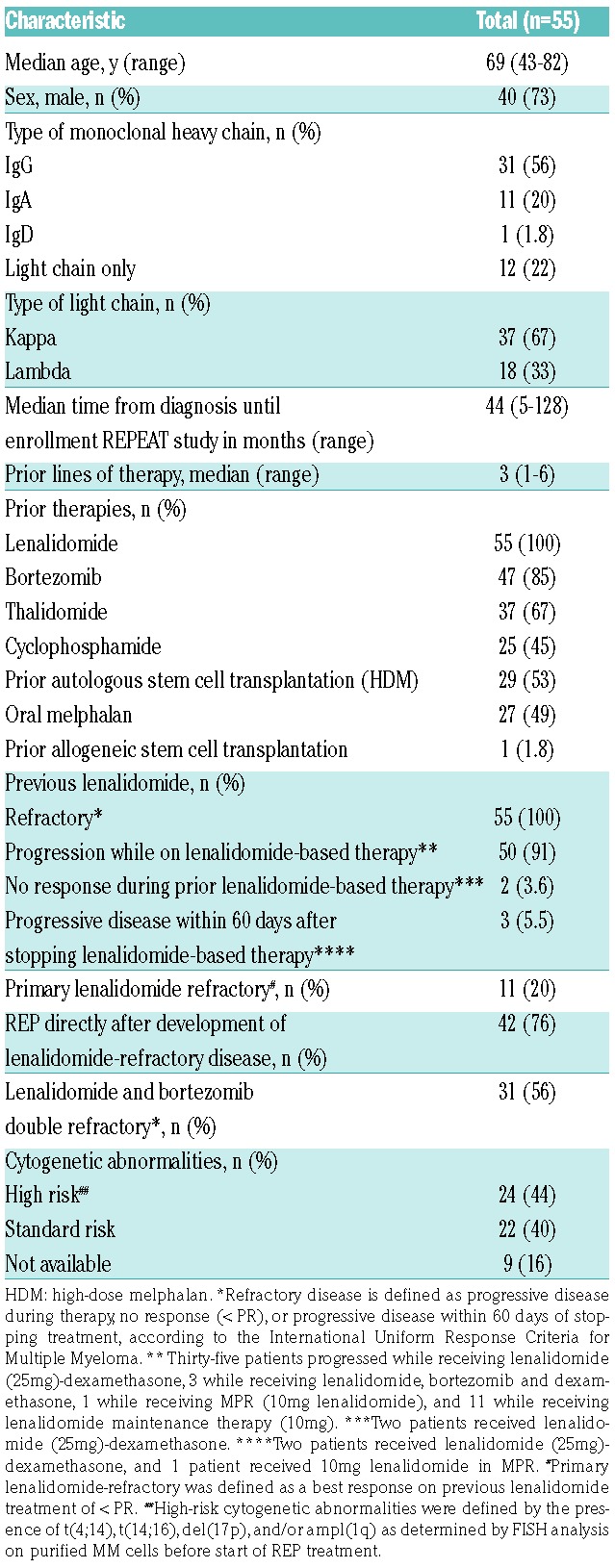
The study population consisted of patients included in the REPEAT study,5 a prospective, phase 1 dose-finding trial, followed by a phase 2 expansion at the recommended dose level to evaluate the safety, tolerability and efficacy of lenalidomide, low-dose oral cyclophosphamide and prednisone (REP) in lenalidomide-refractory MM patients. Patient characteristics are shown in Table 1 and details on the protocol are presented in the Online Supplementary Methods.
Table 1.
Patient characteristics.
Sequential dual color immunohistochemistry (IHC) assays were performed as described in the Online Supplementary Data.6 Briefly, the target markers Cereblon, Aiolos, Ikaros, IRF4, and c-Myc were evaluated in CD138-positive plasma cells in the BM to generate an H-score. H-scores range from 0 to 300 and take into account frequency and intensity of staining [H-score = (% at 1+) × 1 + (% at 2+) × 2 + (% at 3+) × 3]. For Cereblon, cytoplasmic and nuclear compartments were scored separately. The sum of cytoplasmic and nuclear H-Scores accounted for the total H-Score (0-600), which was generated for each sample.
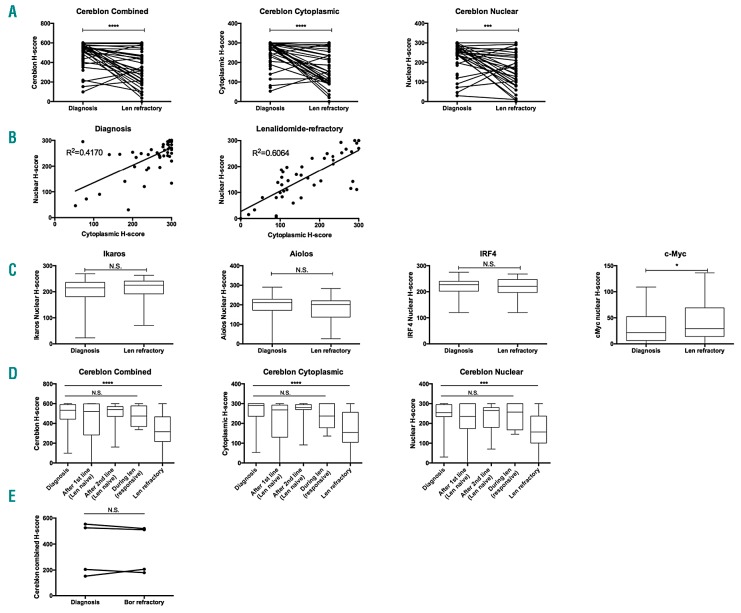
There was marked heterogeneity in the intensity of Cereblon expression across patients. However, a significant reduction in Cereblon protein levels was observed, both nuclear and cytoplasmic, at the time patients developed lenalidomide-refractory disease as compared to time of diagnosis (Figure 2A). Seventy-seven percent of patients showed a reduction in overall Cereblon expression (combined H-score), with a median decrease in Cereblon in these patients of 53.1% (range: 6.6–99.2%). In 23% of patients, no decrease in Cereblon expression was observed (Figure 2A). Levels of cytoplasmic and nuclear Cereblon H-scores were highly correlated, both at diagnosis as well as at the time of lenalidomide-refractory disease (Figure 2B). The expression levels of Ikaros, Aiolos and IRF4 did not change from diagnosis to development of lenalidomide-refractory disease, while there was a small, but significant, increase in c-Myc expression at the time of lenalidomide-resistant disease (Figure 2C). There was no correlation between c-Myc and Cereblon levels, or between change in c-Myc and Cereblon levels over time. To investigate the possibility that diminished Cereblon levels may also occur during other treatment lines before development of lenalidomide-refractory disease, we analyzed Cereblon expression of BM plasma cells in samples obtained at additional time points during the patient’s disease course (Figure 2D). There was no significant change in Cereblon expression at these other time points. Furthermore, we obtained paired samples from 4 patients both before start of bortezomib treatment, and at the time of development of bortezomib-refractory disease. The four patients were lenalidomide-naïve when these samples were taken. In contrast to lenalidomide treatment, bortezomib therapy did not alter Cereblon expression levels (Figure 2E). Altogether, these results indicate that the decrease in Cereblon protein levels is specifically associated with lenalidomide resistance.
Figure 2.
Longitudinal analysis of bone-marrow biopsies shows reduced Cereblon and increased c-Myc expression in MM cells from lenalidomide-refractory patients. Plasma cell protein expression levels of Cereblon and downstream targets were determined in 55 patients at the time of diagnosis and at the time of lenalidomide-refractory disease. (A) Cereblon expression (cytoplasmic, nuclear and combined expression [sum of the cytoplasmic and nuclear score]). (B) Correlations between nuclear and cytoplasmic H-scores at diagnosis (left panel) and at the time of lenalidomide-refractory disease (right panel). (C) Expression of Ikaros, Aiolos, IRF4, and c-Myc. (D) Cereblon expression was also analyzed after first line treatment (lenalidomide naive) (n=18), after second line treatment (lenalidomide naive) (n=11), and during lenalidomide treatment (lenalidomide sensitive myeloma, n=6). These additional time-points do not contain data from all patients, because of a limited number of available bone marrow biopsies. (E) Combined Cereblon H-score for 4 patients treated with bortezomib while lenalidomide-naïve, showing no change in Cereblon expression by bortezomib. Boxes represent first and third quartile with median value, and error bars represent minimum to maximum. Bars represent mean, and error bars represent the SEM. P-values were calculated by using Wilcoxon matched pairs signed rank test. Len: lenalidomide; Bor: bortezomib; N.S.: not significant. ****: P<0.0001; ***: P<0.001; *: P<0.05.
There was no association between Cereblon expression at the time of inclusion in the REPEAT study and response (defined as PR or better), PFS or OS with REP treatment. The extent of change in Cereblon expression from diagnosis to inclusion in the REPEAT study was also not correlated with outcome. For c-Myc expression, no significant association with response or OS after REP treatment was observed. However, there was a trend for decreased PFS with high c-Myc expression levels at the time of inclusion in the REPEAT study (HR 1.011, 95% CI: 1.000–1.022, P=0.053). Ikaros, Aiolos, and IRF4 were not associated with clinical outcome following REP. Furthermore, differential expression of Cereblon, c-Myc, Ikaros, Aiolos and IRF4 at the time of diagnosis did not affect OS. No correlative studies were done on other lines of treatment because of the small numbers of BM samples from the patients at the corresponding lines of treatment.
To our knowledge, this is the first study analyzing plasma cell expression levels of the Cereblon pathway proteins in paired samples at diagnosis and at the time of lenalidomide-refractory disease using a validated assay.6 Our correlative analysis showed that development of lenalidomide-resistance is associated with reduced Cereblon protein levels in the majority of patients. Our observation that Cereblon expression levels did not show a significant change during other treatment lines indicates that the decrease in Cereblon is specifically associated with acquired lenalidomide resistance.
The underlying mechanisms of Cereblon downregulation remain to be determined. Epigenetic modifications of the Cereblon promotor region were previously described as a mechanism of Cereblon downregulation.7 Other possible mechanisms may involve Cereblon gene mutations or chromosomal deletions although these mechanisms need to be addressed in future studies.
The median reduction in Cereblon of 53% can be considered modest. However, in preclinical studies, a similar degree of shRNA-mediated Cereblon depletion induced lenalidomide resistance in MM cell lines.4,8 In fact, an absolute threshold of Cereblon protein expression for induction of lenalidomide resistance is currently unknown, especially not in patients. Therefore, we expect that the Cereblon reduction that we observed in the MM cells, contributes to the development of lenalidomide-refractory disease. However, Cereblon expression was not reduced in a subset of patients in our current analysis. Interestingly, 50% of these patients were primary refractory to lenalidomide-based treatment, versus only 13% in patients with a decrease in Cereblon expression (P=0.03), while there was no difference in baseline Cereblon expression between primary refractory patients and patients with an initial response to lenalidomide-based treatment. Other mechanisms of lenalidomide-resistance are likely involved in these patients, such as Cereblon pathway mutations, Cereblon splice variants, or increased activity of the MEK/ERK pathway.9,10 Given the limited availability of patient samples from our current study, no additional analysis of mRNA levels or analysis of Cereblon pathway mutations could be performed to further characterize these potential mechanisms.
The observed increase in c-Myc protein expression at the time of lenalidomide-refractory disease, as compared to levels at diagnosis, suggests that c-Myc increase may also contribute to development of lenalidomide resistance. Although c-Myc expression levels had not been studied in the setting of acquired lenalidomide resistance, c-Myc upregulation has been reported in the evolution from MGUS to MM.11 Furthermore, c-Myc aberrations and/or overexpression are generally associated with adverse clinical features and poor survival.11
The lack of association between Cereblon levels and outcome of REP treatment indicates that the anti-myeloma effect of lenalidomide as part of the REP regimen may not be mediated via plasma cell Cereblon. Indeed, there is evidence that lenalidomide can still have immunomodulatory activity in the setting of lenalidomide-refractory disease,12 which might be complementary to the previously described immune-activating effects of metronomically dosed cyclophosphamide.13 Alternatively, modulation of residual Cereblon by lenalidomide may still be able to synergize with the other components of the REP regimen. In addition, downregulation of Cereblon may affect its recently described chaperon-like function that promotes maturation of CD147 and MCT-1 proteins, which regulate proliferation and survival of MM cells.14
In our analysis, the expression levels of Cereblon and its downstream substrates and effector proteins at diagnosis did not affect OS. We were not able to assess the effect of Cereblon expression at diagnosis on PFS or response after first-line treatment due to lack of clinical data. The association of Cereblon expression and survival in newly diagnosed MM patients has been assessed in several studies with conflicting results. When interpreting these studies, it is important to note that there is a lack of correlation between Cereblon mRNA and protein levels.15 Furthermore, different techniques and scoring methods have been used for assessment of protein and mRNA expression in these studies.
In conclusion, we demonstrated in a longitudinal analysis of BM biopsy samples obtained from the REPEAT study, that a decrease in Cereblon protein levels is one of the characteristics of acquired resistance to lenalidomide. Other mechanisms of resistance, such as acquired Cereblon pathway mutations or defects in other components of the E3 ligase complex, may play a role in patients without reduction in Cereblon expression.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broyl A, Kuiper R, Van Duin M, et al. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood. 2013;121(4):624–627. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijhof IS, Franssen LE, Levin M-D, et al. Phase 1/2 study of lenalidomide combined with low-dose cyclophosphamide and prednisone in lenalidomide-refractory multiple myeloma. Blood. 2016; 128(19):2297–2307. [DOI] [PubMed] [Google Scholar]
- 6.Ren Y, Wang M, Couto S, et al. A dual color immunohistochemistry assay for measurement of cereblon in multiple myeloma patient samples. Appl Immunohistochem Mol Morphol. 2015;0(0):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimopoulos K, Søgaard Helbo A, Fibiger Munch-Petersen H, et al. Dual inhibition of DNMTs and EZH2 can overcome both intrinsic and acquired resistance of myeloma cells to IMiDs in a Cereblon-independent manner. Mol Oncol. 2018;12(2):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YX, Braggio E, Shi C-X, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kortüm KM, Mai EK, Hanafiah NH, et al. Targeted sequencing of refractory myeloma reveals a high incidence of mutations in CRBN and Ras pathway genes. Blood. 2016;128(9):1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neri P, Maity R, Keats JJ, et al. Cereblon splicing of exon 10 mediates IMiDs resistance in multiple myeloma: clinical validation in the CoMMpass trial. Blood. 2016;128(22):120.27162243 [Google Scholar]
- 11.Chng W-J, Huang GF, Chung TH, et al. Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia. 2011; 25(6):1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.San Miguel J, Mateos M-V, Shah JJ, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): Keynote-023. Blood. 2015;126(23):505. [Google Scholar]
- 13.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichner R, Heider M, Fernández-Sáiz V, et al. Immunomodulatory drugs disrupt the cereblon–CD147–MCT1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016;22(7):735–743. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi AK, Mendy D, Waldman M, et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol. 2014;164(2):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.