Abstract
Histone deacetylases are promising therapeutic targets in hematological malignancies. In the work herein, we investigated the effect of chidamide, a new subtype-selective histone deacetylase inhibitor that was independently produced in China, on multiple myeloma and its associated bone diseases using different models. The cytotoxicity of chidamide toward myeloma is due to its induction of cell apoptosis and cell cycle arrest by increasing the levels of caspase family proteins p21 and p27, among others. Furthermore, chidamide exhibited significant cytotoxicity against myeloma cells co-cultured with bone mesenchymal stromal cells and chidamide-pretreated osteoclasts. Importantly, chidamide suppressed osteoclast differentiation and resorption in vitro by dephosphorylating p-ERK, p-p38, p-AKT and p-JNK and inhibiting the expression of Cathepsin K, NFATc1 and c-fos. Finally, chidamide not only prevented tumor-associated bone loss in a disseminated murine model by partially decreasing the tumor burden but also prevented rapid receptor activator of nuclear factor κ-β ligand (RANKL)-induced bone loss in a non-tumor-bearing mouse model. Based on our results, chidamide exerted dual anti-myeloma and bone-protective effects in vitro and in vivo. These findings strongly support the potential clinical use of this drug as a treatment for multiple myeloma in the near future.
Introduction
Multiple myeloma (MM) is an incurable plasma malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow (BM), the secretion of high levels of monoclonal immunoglobulins and osteolytic bone lesions.1 Myeloma cells interact with different cell types in the BM microenvironment, such as osteoclasts (OCs) and mesenchymal stromal cells, which supports their growth, drug resistance and the development of bone disease through cell–cell adhesion and the release of growth factors, such as interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF).1 Osteolytic bone lesions, which result from increased bone resorption by OCs and reduced osteoblastic bone formation, are the most common complication of MM and often decrease the quality of life and survival time of patients with MM.2,3 New active drugs, including proteasome inhibitors and immunomodulatory agents, have improved the outcomes of patients with MM and alleviated bone damage.1 However, disease progression is still inevitable. Therefore, the identification of new targets and development of new drugs that focus on MM cells and their BM microenvironment are crucial to the development of more effective treatments.
Chidamide is a novel oral histone deacetylase inhibitor (HDACi) designed to inhibit the activity of HDAC1, 2, 3 and 10 which is produced independently in China and is now undergoing phase I clinical trials in America and Japan.4,5 Chidamide has been shown to induce cell apoptosis and cell cycle arrest in a variety of cancers, such as lung cancer, pancreatic cancer, leukemia and lymphoma.4–7 Moreover, HDAC3 was recently shown to be overexpressed in MM and was a key factor that propelled myeloma cell proliferation.8,9 Additionally, HDAC1 and HDAC2 are important positive regulators of osteoclastogenesis.10,11 Based on these data, we hypothesize that chidamide may exert dual anti-myeloma and bone-protective effects.
In the work herein, we investigated the dual anti-myeloma and bone-protective effects on myeloma cells and preclinical models. To the best of our knowledge, this compound is the first HDACi that was independently developed in China and shows both anti-myeloma and anti-osteolytic bone disease activity in vitro and in vivo, supporting the future clinical application of this drug as a treatment for MM.
Methods
Drugs
Chidamide was a generous gift from the Chipscreen Company, Shenzhen, China. Additional information regarding drugs and reagents is provided in the Online Supplementary Materials.
Human myeloma cell lines, primary myeloma samples, bone marrow stromal cells (BMSCs), healthy peripheral blood mononuclear cells (PBMCs) and bone marrow-derived mononuclear cells (BMMCs)
The human myeloma cell lines RPMI-8226, MM.1s, and U266B1 were purchased from the cell bank of the Chinese Academy of Science; H929, CAG, ARP-1, LP-1, and OPM2 cells were generous gifts from Dr. Qing Yi (Department of Cancer Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA). ANBL-6 cells were a generous gift from Professor Zhiqiang Liu, Tianjin Medical University, China. The luciferase-expressing myeloma cell line RPMI-8226-luc was constructed by infecting RPMI-8226 cells with a lentivirus encoding the luciferase gene, and infected cells were selected with puromycin. Primary myeloma samples and BMSCs were collected from newly diagnosed patients, and PBMCs and BMMCs were collected from healthy donors. Primary CD138(+) cells were sorted using CD138 microbeads (Miltenyi Biotech, CA, USA).
Cell culture
Myeloma cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% L-glutamine at 37°C in a 5% CO2 atmosphere. BMSCs were cultured in minimum essential medium α (MEM-α) supplemented with 10% fetal bovine serum and 1% L-glutamine. BMSCs were cultured as premature osteoblasts for seven days and as mature osteoblasts for 21 days in the human mesenchymal stem cell (MSC) osteogenic differentiation basal medium.
Cell proliferation, cell cycle, and cell apoptosis assays
Cell counting kit-8 (CCK-8) assays were used to detect MM cell viability. Flow cytometry was used to assess the cell cycle and apoptosis. A detailed description of each procedure is provided in the Online Supplementary Materials.
In vitro OC differentiation, pit formation and F-actin ring formation
Osteoclasts were differentiated from PBMCs in osteoclastogenic medium as previously described.12 Briefly, MEM-α containing 50ng/ml RANKL and 25ng/ml monocyte colony stimulating factor (M-CSF) supplemented with 10% fetal bovine serum and 1% L-glutamine was used as osteoclastogenic medium for cells cultured in the presence or absence of chidamide. PBMCs from healthy donors were cultured as pre-OCs for 14 days and as mature OCs for 21 days. The F-actin ring formation assay, assessment of the resorption ability of OCs and tartrate-resistant acid phosphatase (TRAP)+ staining were performed as described in a previous report.12
Drug treatments in different mouse models
Two tumor-bearing murine models and a non-tumor-bearing model were employed to investigate the anti-tumor and bone-protective effects of chidamide.4,13–15 Micro computed tomography (CT) was used to evaluate MM bone disease. Further details are provided in the Online Supplementary Materials.16,17
Statistical analysis
All data are expressed as means ± SEM and are representative of at least three experiments with similar results performed in triplicate, unless indicated otherwise. A two-tailed Student’s t-test was used to determine the significance of differences between two groups, and one-way analysis of variance was used to estimate the differences between three or more groups. GraphPad Prism 5.0 software (GraphPad Software, CA, USA) was used for the analysis.
Ethical approval
Primary myeloma samples, BMSCs, PBMCs, and BMMCs were collected after obtaining informed consent from the patients and approval from the Ethics Committee of the First Affiliated Hospital of Zhejiang University. The animal experiments were carried out after obtaining approval from the Ethics Committee of the First Affiliated Hospital of Zhejiang University.
Results
Chidamide exhibits HDAC inhibitory activity in MM cell lines
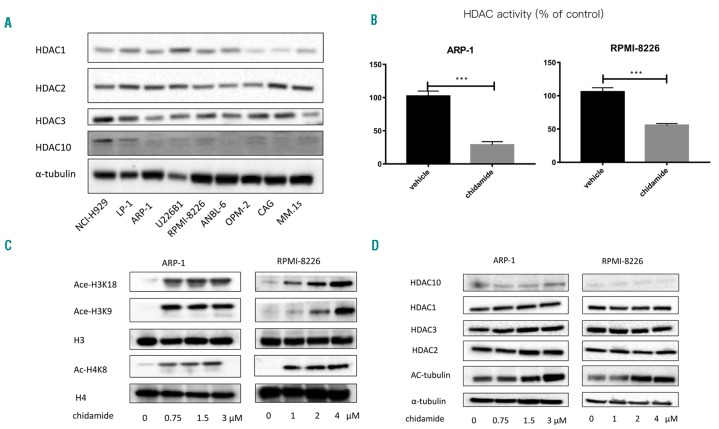
We first investigated the basal expression levels of HDAC1, 2, 3, and 10, which are chidamide targets, in MM cell lines. As shown in Figure 1A, HDAC1, HDAC2 and HDAC3 were all expressed in the nine myeloma cell lines (H929, LP-1, ARP-1, U266B1, RPMI-8226, ANBL-6, OPM2, CAG, and MM.1s), and both HDAC2 and HDAC3 were expressed at slightly higher levels than HDAC1. HDAC10 was barely detectable in human myeloma cell lines. Next, we measured HDAC activity and the acetylation of lysine residues on histones H3 and H4 to determine the inhibitory effect of chidamide on HDAC. As shown in Figure 1B,C, chidamide inhibited HDAC activity and significantly increased the acetylation of H3K8, H3K18 and H4K8. However, the expression of HDAC1, HDAC2, HDAC3 and HDAC10 was not affected (Figure 1D).
Figure 1.
Chidamide exhibits HDAC inhibitory activity in MM cell lines. (A) Western blotting was used to detect the protein levels of HDAC1, HDAC2, HDAC3 and HDAC10. (B) HDAC activity assay showed that chidamide could significantly inhibit HDAC activity (Both in ARP-1 and RPMI-8226, ***P<0.001). (C) Western blot analysis of H3 acetylation at Lys18 and Lys9 and H4 acetylation at Lys8. H3 and H4 expression levels were used as loading controls. (D) Western blot analysis of HDAC1, HDAC2, HDAC3, and HDAC10 levels. Acetylation of α-tubulin at K40 was also examined, and α-tubulin expression was used as the loading control. HDAC: histone deacetylase.
Chidamide not only exerts anti-myeloma effects but also overcomes the resistance conferred by the BM microenvironment
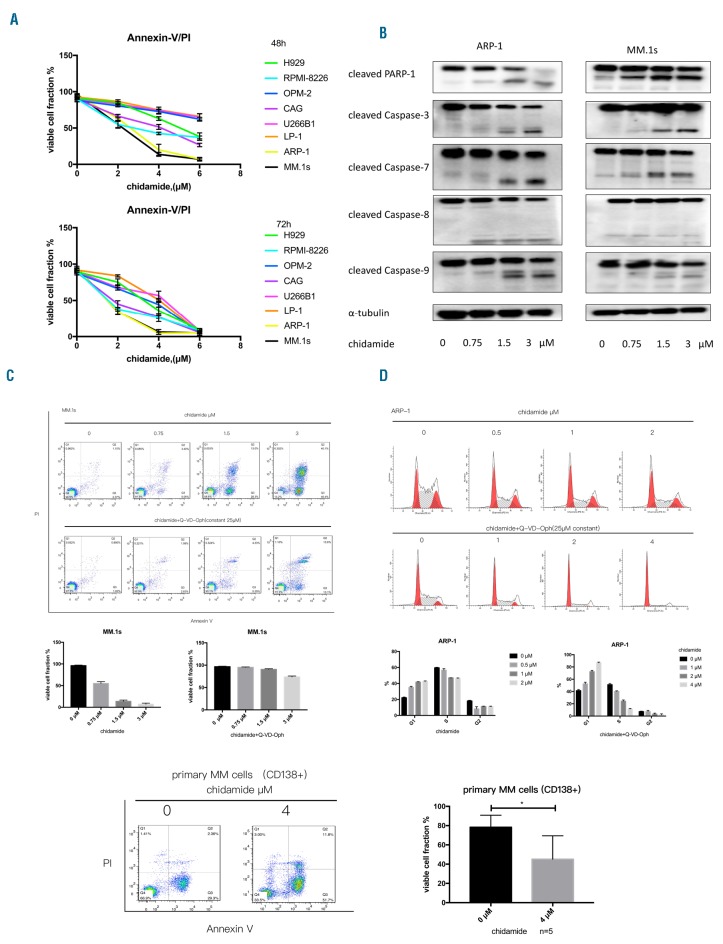
Subsequently, we evaluated the anti-myeloma effect of chidamide. Cell lines were treated with increasing concentrations of chidamide (0.5–8μM) for different times (24, 48, or 72h). CCK-8 assays revealed a dose-dependent and time-dependent pattern of chidamide cytotoxicity (Online Supplementary Figure S1A). Flow cytometry (Annexin V and propidium iodide [PI] staining) showed that chidamide significantly induced MM cell apoptosis at 48h and 72h (Figure 2A and Online Supplementary Figure S1B). ARP-1, MM.1s and CAG cells were more sensitive to chidamide, with IC50 values of approximately 2–4μM, whereas the IC50 values were approximately 4–6μM for the other six cell lines at 48h. When the incubation time was extended to 72h, the IC50 values of most of the cell lines were 1.5–4μM. Furthermore, incubation of ARP-1 and MM.1s cells with an optimal concentration of chidamide increased the levels of cleaved caspases 3, 7, 8, and 9 and cleaved PARP-1 cleavage (Figure 2B). To evaluate whether apoptosis was induced in a caspase-dependent or caspase-independent manner, we incubated MM.1s and ARP-1 cells with chidamide in the presence of a pan-caspase inhibitor, Q-VD-Oph, for 48h. After co-incubating chidamide with Q-VD-Oph, the apoptotic cells were significantly reduced (Figure 2C and Online Supplementary Figure 2A), with down-regulated active caspase-3 and caspase-9 expression (Online Supplementary Figure 3). Additionally, the cell cycle arrest effect of chidamide was evaluated by flow cytometry. ARP-1, OPM-2, LP-1, and RPMI-8226 cells were treated with increasing doses of chidamide (0.25–6μM) for 48h; chidamide induced ARP-1 cell cycle arrest in the G0/G1 phase and reduced the percentage of cells in the proliferation phases (S and G2) regardless of co-incubation with Q-VD-Oph (***P<0.001, Figure 2D). OPM-2, RPMI-8226, and LP-1 cells showed similar results after incubation with chidamide (Online Supplementary Figure 4).
Figure 2.
MM cells are vulnerable to chidamide. (A) The cytotoxicity of chidamide toward MM cell lines was examined using flow cytometry (Annexin V/PI), and all data are summarized as the means±SEM (n=3). (B) The expression of apoptosis-associated proteins, including PARP-1 and cleaved caspases 3, 7, 8, and 9, in chidamide-treated ARP-1 and MM.1s cells was detected. (C) The effect of chidamide on apoptosis in MM.1s cells after 48h with or without co-incubation with the pan-caspase inhibitor Q-VD-Oph. After co-incubation with Q-VD-Oph, chidamide-induced apoptosis was inhibited. (D) Cell cycle arrest was examined using flow cytometry, and ARP-1 cells underwent G1 phase arrest in response to incubation with chidamide regardless of co-incubation with Q-VD-Oph. (E) Chidamide could significantly induce + primary CD138 cell apoptosis after incubation for 48h (n=5, *P<0.05). (E) Chidamide could significantly induce primary CD138+ cell apoptosis after incubation for 48h (n=5). PI: propidium iodide; MM: multiple myeloma.
In addition, as shown in Figure 2E, chidamide induced the apoptosis of CD138-positive cells from patients with MM (n=5, 48h). Moreover, the BMMCs from four healthy donors were incubated with 4μM chidamide for 48h to examine the cytotoxicity of chidamide toward non-tumor cells. Although approximately 10% of monocytes underwent apoptosis, the numbers of apoptosis cells were only slightly higher than the vehicle group and far less than MM cells (Online Supplementary Figure 2B).
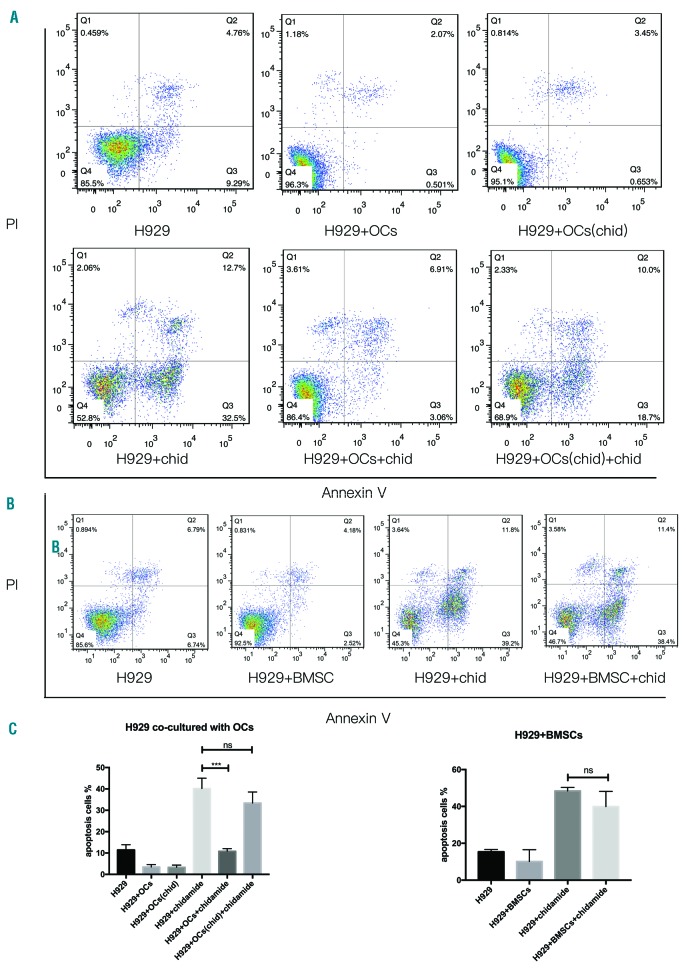
Furthermore, we investigated the cytotoxicity of chidamide in the presence of the BM microenvironment. For this purpose, H929 cells were co-cultured with BMSCs and OCs in the presence of 6μM chidamide for 48h. OCs showed a significant anti-apoptotic effect, but pretreatment with chidamide during osteoclastogenesis suppressed the protective effect of OCs on myeloma cell apoptosis (Figure 3A, Figure 3C). Chidamide-induced apoptosis in approximately 30% of the MM cells, even after co-culture with BMSCs, which usually exert a protective effect on MM cells (Figure 3B,C).
Figure 3.
Chidamide overcame the resistance conferred by chidamide-pretreated OCs and BMSCs. (A) and (C) H929 cells were co-cultured with OCs and chidamide-pretreated OCs for 48h or with 6μM chidamide, and cell apoptosis was detected by flow cytometry after staining with Annexin V and PI. (B) and (C) H929 cells were co-cultured with BMSCs or with 6μM chidamide for 48h, and cell apoptosis was detected by flow cytometry after staining with Annexin V and PI (H929+chidamide vs. H929+OCs+chidamide, ***P<0.001; H929+chidamide vs. H929+OCs(chida)+chidamide, P>0.05, non-significant; H929+chidamide vs. H929+BMSCs+chidamide, P>0.05 non-significant). PI: propidium iodide; OC: osteoclasts; BMSC: bone marrow stromal cell.
The molecular mechanisms underlying chidamide activity in myeloma cells
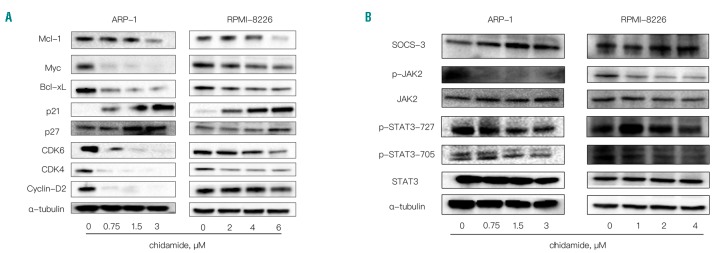
When ARP-1 and RPMI-8226 cells were treated with an increasing dose of chidamide (0–6μM) (Figure 4A), the expression of Myc, Mcl-1, and Bcl-xL decreased in a dose-dependent manner. Additionally, in the presence of chidamide, levels of cell cycle-related proteins, including p27, p21, Cyclin-D2, CDK4, and CDK6, were all reduced (Figure 4A). Finally, as shown in Figure 4B, SOCS3 levels increased and p-JAK2 and p-STAT3 levels decreased in RPMI-8226 and ARP-1 cells treated with different concentrations of chidamide. Additionally, a pan-caspase inhibitor, Q-VD-Oph, was used to treat MM cells together with chidamide to prevent inhibitory effects of activated caspases on the levels of signaling and apoptosis-related proteins. As shown in Figure 4 and Online Supplementary Figure S5, most of these proteins showed the same changes in the presence and absence of Q-VD-Oph.
Figure 4.
The molecular mechanisms underlying chidamide activity in myeloma cells. (A) Western blot analysis of Mcl-1, Myc, Bcl-xL, Bcl-2, p21, p27, CDK4, CDK6, and Cyclin-D2 levels; α-tubulin was used as the loading control. (B) SOCS-3, p-JAK2, JAK2, p-STAT3-727, p-STAT3-705, and STAT3 levels were analyzed by Western blotting; α-tubulin was used as the loading control.
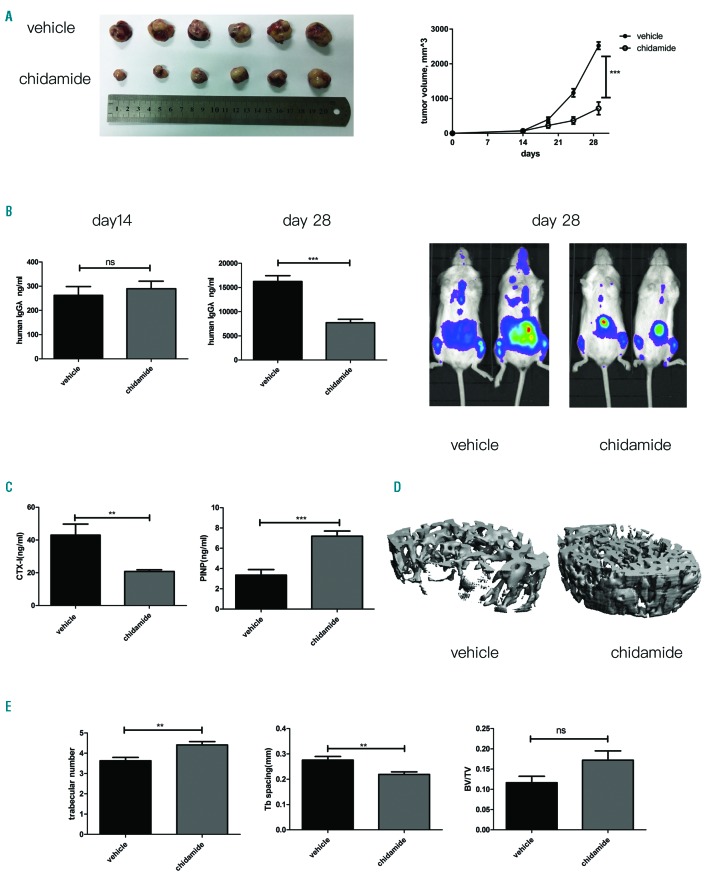
Chidamide decreases the MM tumor burden and prevents myeloma-associated bone loss in a mouse model of disseminated myeloma
Two murine models were employed to examine the dual anti-tumor and bone-protective effects of chidamide. First, as shown in Figure 5A, a significant decrease in tumor volume was observed in chidamide-treated NOD/SCID mice compared with that observed in the vehicle group. Consistent with the Western blot results from the cell lines, immunohistochemical staining revealed decreased expression of Bcl-xL, CDK4, CDK6 and Myc and increased levels of cleaved caspase-3 (Online Supplementary Figure S6). Next, NCG mice were intravenously injected with RPMI-8226-luc cells to establish the murine model of myeloma-induced bone destruction. Compared with the vehicle group, chidamide clearly controlled the tumor burden, as measured by serum levels of human Ig λ light chain secreted by RPMI-8226-luc cells (***P<0.001, Figure 5B) and the bioluminescence in vivo imaging (Figure 5B). Then, serum levels of the bone resorption marker Carboxy-terminal telopeptide 1 (CTX-I) were measured and were found to be significantly diminished in chidamide-treated mice (**P<0.01, Figure 5C); in addition, a significantly increased serum level of amino-terminal propeptide (PINP), the bone formation marker, was observed (***P<0.001, Figure 5C). Consistent with these findings, micro-CT three-dimensional reconstructed images at the metaphysis of distal femurs revealed greater myeloma-associated bone loss in the vehicle group as compared with the chidamide-treated group (Figure 5D). Moreover, based on the bone morphometric parameters, chidamide increased the trabecular number (**P<0.01) and bone volume density (P=0.0755) and reduced trabecular separation (**P<0.01) compared to the control group (Figure 5E).
Figure 5.
Dual anti-myeloma and bone-protective effects of chidamide on a disseminated myeloma murine model. (A) Chidamide suppresses the growth of MM xenografts, ***P<0.001. (B) Tumor volumes, serum human Igλ levels in each group and in vivo bioluminescence imaging were used to compare the tumor burdens between the two groups. (C) Serum levels of CTX-I (**P<0.01) and PINP (***P<0.001) in each group. (D) Micro-CT three-dimensional reconstructed images from the vehicle and chidamide groups. (E) Micro-CT parameters of the bone in the vehicle and chidamide groups. Trabecular numbers, ***P<0.001; trabecular space, **P<0.01; BV/TV, non-significant (ns).
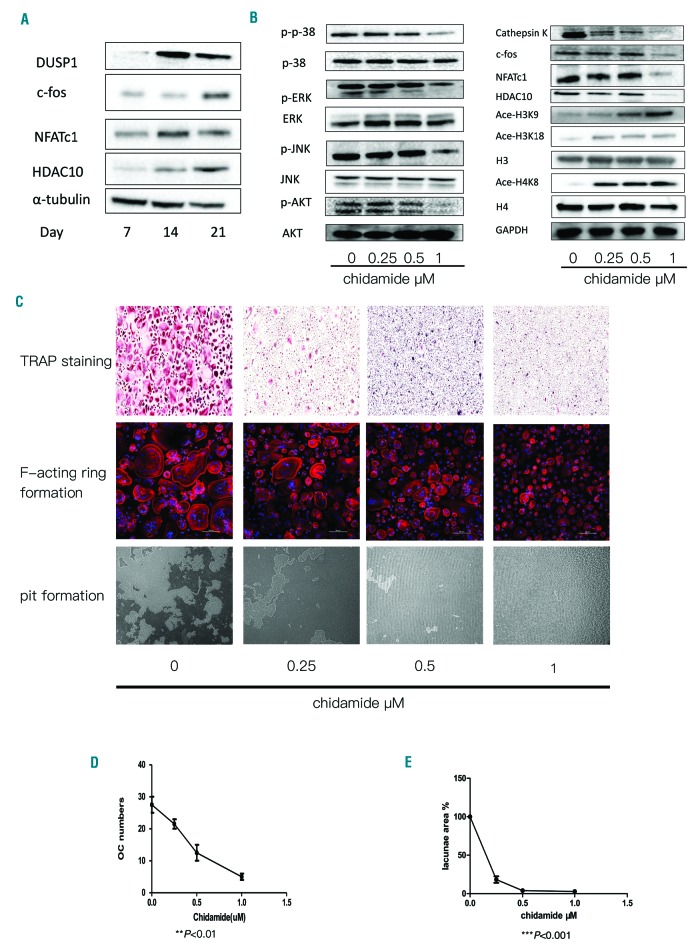
Chidamide inhibits osteoclastogenesis and bone resorption in vitro
As the in vivo study revealed that chidamide exerts anti-myeloma effects, we investigated the effect of chidamide on the formation and function of OCs of human origin. The expression of DUSP1, c-fos, NFATc1 and HDAC10 increased during RANKL-induced OC formation (Figure 6A). We then tested several key factors and signaling pathways that mediate osteoclastogenesis in order to identify the mechanisms underlying the aforementioned effects. The levels of p-p38, p-ERK1/2, p-JNK and p-AKT were also reduced, indicating that chidamide suppressed the classical pathways of OC activation. Cathepsin K, c-fos, HDAC10 and NFATc1 expression levels were all down-regulated after chidamide treatment in a dose-dependent manner. In addition, acetylation of H3K9, H3K18 and H4K8 was increased after chidamide treatment (Figure 6B). PBMCs cultured in osteoclastogenic medium were incubated with different concentrations of chidamide to evaluate its effects on OC formation. At a low concentration (0-1μM) of the drug, the number of TRAP+ multinucleated cells derived from healthy donors was reduced, but the density of cells was not significantly reduced (**P<0.01, Figure 6C,D). Additionally, F-actin ring formation was inhibited in the presence of chidamide during osteoclastogenesis (Figure 6C). Next, we evaluated functional changes in OCs cultured on calcium substrate-coated slides. Chidamide treatment induced a dose-dependent reduction in the area of resorption pits. With increasing drug doses, the resorption area was substantially reduced (Figure 6C, Figure 6E ***P<0.001).
Figure 6.
Chidamide inhibited osteoclast differentiation in vitro. (A) Western blotting was used to detect the expression levels of key factors (DUSP1, c-fos, NFATc1, and HDAC10) during OC maturation; α-tubulin was used as the loading control. (B) Western blot analysis of the expression levels of pathway proteins including pp38, p38, p-ERK, ERK, p-JNK, JNK p-AKT, and AKT; levels of key factors, including Cathepsin K, c-fos, NFATc1 and HDAC10, in OCs treated with different concentrations of chidamide. GAPDH was used as the loading control. Acetylation of H3K18 and H4K8 was also evaluated; H3 and H4 were used as loading controls. (C) TRAP staining of OCs treated with different concentrations of chidamide. F-actin ring formation. Rhodamine-conjugated phalloidin was used to visualize F-actin (red), and nuclei were stained with DAPI (blue), 200×. Pit formation assay of OCs treated with different concentrations of chidamide. (D) The number of TRAP-positive cells was reduced in cultures treated with increasing doses of chidamide, **P<0.01. (E) Calcium resorption was reduced in the presence of chidamide (0–1μM). Resorption in the lacunae area (%) diminished with increasing doses of chidamide, ***P<0.001. OC: osteoclasts; TRAP: tartrate-resistant acid phosphatase.
Effect of chidamide on osteoblasts
HDAC1 and HDAC3 are regarded as negative regulators of osteoblastogenesis during bone formation, thus we also evaluated the effect of chidamide on osteoblasts. As shown in Online Supplementary Figure S7, primary BMSCs from patients with MM (n=6) were cultured in human MSC osteogenic differentiation basal medium in the presence of different chidamide concentrations. Alkaline phosphatase (ALP) was measured as a positive marker of osteoblast differentiation, and its expression was slightly increased by chidamide treatment. Additionally, Alizarin red staining (ARS) of calcium deposits showed that chidamide had no promotion or inhibitory effect on osteoblast differentiation (Online Supplementary Figure S7A,B). Finally, the expression of osteocalcin (OCN), Activin A and semaphorins at the messenger ribonucleic acid (mRNA) level was also examined; OCN (day 21) was increased, while Activin A (day 21) was down-regulated (Online Supplementary Figure S7C).
Chidamide exerts bone-protective effects on non-tumor-bearing mice
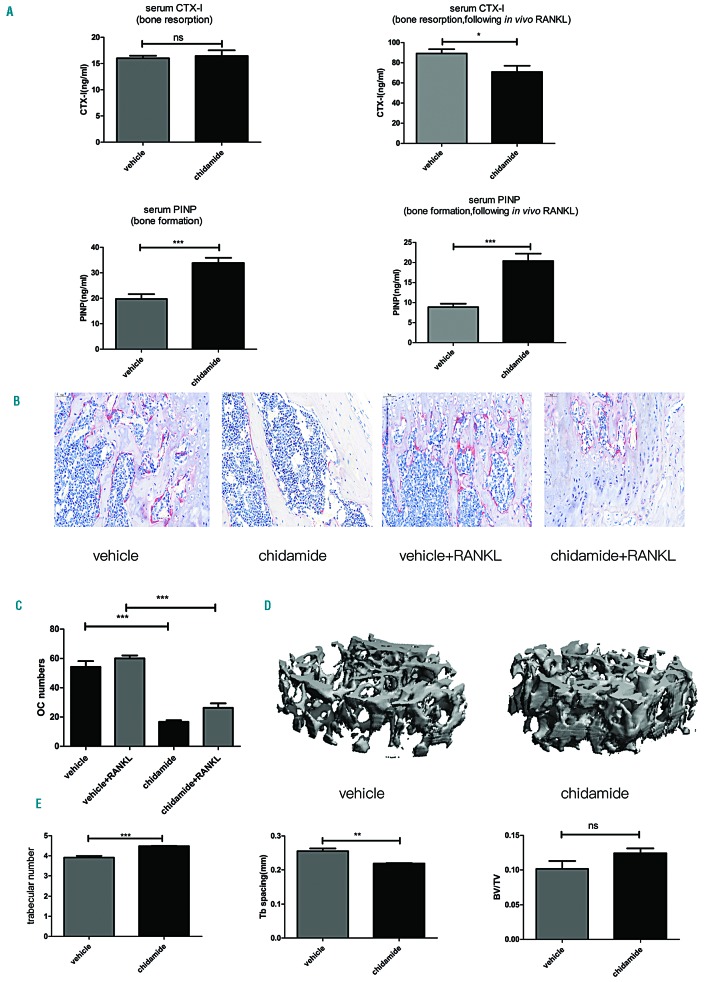
Chidamide was administered via oral gavage to non-tumor-bearing C57BL/6 mice at a dose of 25mg/kg for 21 days to establish whether chidamide directly exerts its bone-preserving effect on the bone tissue or exerts an indirect effect by decreasing the tumor burden. As shown in Figure 7A, serum CTX-I levels were not significantly different between the vehicle (n=5) and chidamide (n=5) groups, whereas the serum PINP level was clearly increased after treatment with chidamide (***P<0.001). Thereafter, in vivo intraperitoneal injections of soluble receptor activator of nuclear factor-κB ligand (sRANKL; three doses) were administered within 50h followed by gavage with chidamide for 21 days to mimic OC stimulation by myeloma-derived sRANKL. Serum CTX-I levels increased in both the vehicle group (n=5) and the chidamide group (n=5) after sRANKL injections, but the level in the chidamide-treated group was increased to a lesser extent than the level in the vehicle group (*P<0.05). Serum PINP levels were reduced in both groups; however, chidamide attenuated the reduced PINP level in the chidamide-treated group (***P<0.001). TRAP+ staining of bone showed a reduced number of OCs (Figure 7B,C) in the chidamide-treated group (***P<0.001). Moreover, the bone morphometric parameters evaluated by micro-CT (Figure 7D,E) indicated that chidamide increased the trabecular number (***P<0.001) and reduced trabecular separation compared to the control group (**P<0.01). Although bone volume density over total volume (BV/TV, P>0.05) showed an increasing trend in the chidamide-treated group, the difference between the two groups was not significant. Based on these results, chidamide increased the bone volume of healthy mice to some extent and directly prevented RANKL-induced OC activation.
Figure 7.
Chidamide inhibited osteoclast differentiation and the function of chidamide in non-tumor-bearing mice was examined. (A) Serum CTX-I and PINP levels before (CTX-1, P>0.05, non-significant; PINP, ***P<0.001) and after intraperitoneal injections of sRANKL following chidamide treatment (CTX-1, *P<0.05; PINP, ***P<0.001). (B) TRAP staining of mouse femur bone tissues. (C) Chidamide treatment decreased the number of OCs (TRAP-positive cells, dark red staining) in the mouse femur compared with the vehicle group; ***P<0.001. (D) Micro-CT three-dimensional reconstructed images of the vehicle and chidamide groups. (E) Micro-CT parameters of the bone in the vehicle and chidamide groups. Trabecular numbers, ***P<0.001; trabecular space, **P<0.01; BV/TV, non-significant (ns). RANKL: Receptor activator of nuclear factor κ-Β ligand; CTX: carboxy-terminal telopeptide; PINP: amino-terminal propeptide.
Discussion
HDACs are an important family of enzymes with crucial roles in carcinogenesis through their repressive effects on tumor suppressor gene transcription and are proposed as therapeutic targets in oncology.18,19 HDAC inhibitors induce cancer cell apoptosis, cell cycle arrest and promote differentiation, particularly in hematological malignancies. However, the anti-cancer effects of HDAC inhibitors differ and their pharmacological effects vary, depending on the cancer cell types, HDAC targets and doses. Chidamide, a novel HDACi which is currently being widely used in China to treat patients with T-cell lymphoma, shows good efficacy and tolerability.5 In our investigation herein, we evaluated and explored the efficacy of chidamide in treating myeloma and its associated osteolytic bone disease.
Chidamide reduced myeloma cell viability in both primary MM cells and MM cell lines, even in the presence of BMSCs or chidamide-pretreated OCs. As the microenvironment is crucial for myeloma drug resistance and relapse, our data has shown that chidamide overcomes the drug resistance mediated by the interaction between MM cells and the microenvironment. Moreover, the good anti-myeloma effect of chidamide on the disseminated MM mouse model reveals the efficacy of chidamide in the presence of the BM microenvironment.
HDACs regulate a variety of targets involved in different cell functions and processes, such as apoptosis, the cell cycle and differentiation.20 Chidamide inhibited HDAC activity and induced cell cycle arrest in G1 phase, accompanied by cell apoptosis. Notably, cell cycle arrest and the alterations in levels of related proteins were not reversed by the pan-caspase inhibitor. HDAC inhibitors cause cell cycle arrest though several pathways, the most important of which seems to be increasing the expression of cell cycle genes. p21 plays a crucial role in chidamide-induced G1 cell cycle arrest. HDAC1 regulates p21 expression to some extent. HDAC1 interacts with the p21 promoter by competing with the p53 protein to decrease the transcription of p21.21 Based on our results from MM cell lines, in chidamide-treated MM cells HDAC1 was released from the p21 promotor, subsequently increasing p21 expression. Additionally, chidamide inhibited the expression of the Cyclin-D2 gene, thus inhibiting the functions of CDK4 and CDK6. Aberrant activation of the JAK2/STAT3 pathway is required for the pathogenesis of a variety of cancers.22,23 Inappropriate STAT3 activation protects cells from apoptosis. HDAC3 targets STAT3, and inhibition of HDAC3 induces STAT3 de-phosphorylation, thus reducing MM cell growth.8,9 Based on our results and findings from previous studies, we speculated that chidamide promoted MM cell apoptosis by inhibiting the JAK2/STAT3 signaling pathway. Furthermore, platelet factor 4 up-regulates SOSC3 expression to induce MM cell apoptosis by inhibiting STAT3.24 The expression of SOCS3, which acts as a tumor suppressor gene and is always silenced by epigenetic modulation in hematological malignancies, was up-regulated in chidamide-treated cells, which explains the decrease in p-JAK2 and p-STAT3 levels.22,25 Bcl-2 family members, including Bcl-xL and Myc, are downstream targets of the JAK2/STAT3 signaling pathway.23 Moreover, active caspase-3 is a negative regulator of the Bcl-2 family which represses the expression of Bcl-xL and Mcl-1 and subsequently induces cell apoptosis.26 Chidamide inhibited the constitutive activation of the JAK2/STAT3 pathway and caspase-3 activation, thus down-regulating Bcl-xL and Myc.
Myeloma-associated bone disease is a major complication of MM and decreases patients’ quality of life.2,27 Interactions between myeloma cells and OCs not only support myeloma survival but also increase osteoclastogenesis and inhibit osteoblastogenesis, leading to osteolytic bone lesions and myeloma progression.28,29 An important finding of our study is that chidamide not only suppresses the formation and function of OCs in vitro, but also prevents myeloma-associated bone disease in vivo. Bone remodeling is a balance between bone resorption and bone formation.30 Active OCs, together with myeloma cells, increase bone resorption and inhibit osteoblast differentiation, leading to myeloma-associated bone disease. Chidamide-induced myeloma cell apoptosis prevented osteoclast maturation and showed no inhibitory effect on osteoblast differentiation. HDACs, which are targets of chidamide, are involved in OC formation.10,11,31 HDAC2 is reported to play an important role in OC maturation by activating the AKT pathway.11 Additionally, HDAC3 knockdown decreases the expression of NFATc1, Cathepsin K and DC-STAMP, thus inhibiting OC formation.31 Based on our data, chidamide repressed the expression of key factors, such as NFATc1, c-fos and Cathepsin K, during OC maturation, suggesting that chidamide suppresses OC differentiation by inhibiting the function of its targets (such as HDAC2 and HDAC3). A previous study reported the effect of HDAC10 on osteoclast differentiation. They showed that during OC differentiation, HDAC10 levels gradually increased, which is consistent with our study. However, when they knocked down HDAC10 in monocytes, OC differentiation was promoted, indicating that HDAC10 may have a negative effect on OC differentiation.32 However, in our study, following chidamide treatment and inhibition of OC formation, HDAC10 was down-regulated at the protein level. Since chidamide can inhibit other HDACs in addition to HDAC10, its inhibitory effect on OC differentiation may not have been caused by HDAC10 down-regulation. Both MM cells and OCs can suppress the differentiation of osteoblasts. As a previous study reported, OCs inhibited osteoblast differentiation though exosomes containing micro ribonucleic acids (miRNAs) and some cytokines.33 In our study, chidamide could induce MM cell apoptosis and abrupt OCs maturation, indicating that chidamide may attenuate the inhibitory effect of MM cells and OCs on osteoblasts. When BMSCs were treated with chidamide during osteoblast differentiation, chidamide increased the gene expression levels of ALP and OCN while reducing the gene expression level of Activin A, which acted as a negative regulator in osteoblast differentiation via SMAD2-mediated DLX5 down-regulation.34 The ARS experiment showed neither promotion nor an inhibitory effect on osteoblast differentiation. These results may explain the direct bone-protective effect of chidamide in the mouse models.
Our work reveals the dual anti-myeloma and bone-protective effects of chidamide in vitro and in vivo. These findings strongly support the potential clinical use of this drug as a treatment for MM in the near future.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/8/1369
Funding
This work was supported by the Funds for the National Natural Science Foundation of China (Grant No. 91742110 and 81471532) and the Funds for the Natural Science Foundation of Zhejiang province, China (LY17H080001).
References
- 1.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. [DOI] [PubMed] [Google Scholar]
- 2.Silbermann R, Roodman GD. Myeloma bone disease: Pathophysiology and management. J Bone Oncol. 2013;2(2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christoulas D, Terpos E, Dimopoulos MA. Pathogenesis and management of myeloma bone disease. Expert Rev Hematol. 2009;2(4):385–398. [DOI] [PubMed] [Google Scholar]
- 4.Gong K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J. 2012;443(3):735–746. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Jia B, Xu W, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He M, Qiao Z, Wang Y, et al. Chidamide inhibits aerobic metabolism to induce pancreatic cancer cell growth arrest by promoting Mcl-1 degradation. PLoS One. 2016; 11(11):e0166896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X, Wang L, Lin L, et al. A phase I trial of an oral subtype-selective histone deacetylase inhibitor, chidamide, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer. Chin J Cancer Res. 2016;28(4):444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minami J, Suzuki R, Mazitschek R, et al. Histone deacetylase 3 as a novel therapeutic target in multiple myeloma. Leukemia. 2014;28(3):680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada T, Ohguchi H, Grondin Y, et al. HDAC3 regulates DNMT1 expression in multiple myeloma: therapeutic implications. Leukemia. 2017;31(12):2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantley MD, Fairlie DP, Bartold PM, Marino V, Gupta PK, Haynes DR. Inhibiting histone deacetylase 1 suppresses both inflammation and bone loss in arthritis. Rheumatology (Oxford). 2015; 54(9):1713–1723. [DOI] [PubMed] [Google Scholar]
- 11.Dou C, Li N, Ding N, et al. HDAC2 regulates FoxO1 during RANKL-induced osteoclastogenesis. Am J Physiol Cell Physiol. 2016;310(10):C780–787. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Gomez A, Ocio EM, Crusoe E, et al. Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One. 2012;7(4):e34914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomimori Y, Mori K, Koide M, et al. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res. 2009;24(7):1194–1205. [DOI] [PubMed] [Google Scholar]
- 14.Paton-Hough J, Chantry AD, Lawson MA. A review of current murine models of multiple myeloma used to assess the efficacy of therapeutic agents on tumour growth and bone disease. Bone. 2015;77:57–68. [DOI] [PubMed] [Google Scholar]
- 15.Hurchla MA, Garcia-Gomez A, Hornick MC, et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 2013; 27(2):430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane NE, Yao W, Nakamura MC, et al. Mice lacking the integrin beta5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state. J Bone Miner Res. 2005;20(1):58–66. [DOI] [PubMed] [Google Scholar]
- 17.Paino T, Garcia-Gomez A, Gonzalez-Mendez L, et al. The Novel Pan-PIM Kinase Inhibitor, PIM447, Displays Dual Antimyeloma and Bone-Protective Effects, and Potently Synergizes with Current Standards of Care. Clin Cancer Res. 2017;23(1):225–238. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Yan H, Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci (Lond). 2013;124(11):651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laubach JP, San-Miguel JF, Hungria V, et al. Deacetylase inhibitors: an advance in myeloma therapy? Expert Rev Hematol. 2017;10(3):229–237. [DOI] [PubMed] [Google Scholar]
- 20.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4):a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavithra L, Mukherjee S, Sreenath K, et al. SMAR1 forms a ternary complex with p53-MDM2 and negatively regulates p53-mediated transcription. J Mol Biol. 2009; 388(4):691–702. [DOI] [PubMed] [Google Scholar]
- 22.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. [DOI] [PubMed] [Google Scholar]
- 23.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014; 1845(2):136–154. [DOI] [PubMed] [Google Scholar]
- 24.Liang P, Cheng SH, Cheng CK, et al. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98(2):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med. 2015;21(10):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci USA. 1998;95(8):4386–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan E, Weiss BM, Mena E, Korde N, Choyke PL, Landgren O. Current and future imaging modalities for multiple myeloma and its precursor states. Leuk Lymphoma. 2011;52(9): 1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dotterweich J, Schlegelmilch K, Keller A, et al. Contact of myeloma cells induces a characteristic transcriptome signature in skeletal precursor cells. Implications for myeloma bone disease. Bone. 2016;93:155–166. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Moschetta M, Manier S, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160–172. [DOI] [PubMed] [Google Scholar]
- 30.Cantley MD, Zannettino ACW, Bartold PM, Fairlie DP, Haynes DR. Histone deacetylases (HDAC) in physiological and pathological bone remodelling. Bone. 2017; 95:162–174. [DOI] [PubMed] [Google Scholar]
- 31.Pham L, Kaiser B, Romsa A, et al. HDAC3 and HDAC7 have opposite effects on osteoclast differentiation. J Biol Chem. 2011;286(14):12056–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blixt NC, Faulkner BK, Astleford K, et al. Class II and IV HDACs function as inhibitors of osteoclast differentiation. PLoS One. 2017;12(9):e0185441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun W, Zhao C, Li Y, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallet S, Mukherjee S, Vaghela N, et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci USA. 2010;107(11):5124–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.