Abstract
Background
Based on the notion that full activation of platelets is required for a growth factor release, in regenerative dentistry, platelet-rich plasma (PRP) in liquid form is usually clotted by addition of CaCl2 in glassware before topical implantation. However, there has been no evidence as to which is better, full or partial activation of platelets, for minimizing the loss of growth factors and improving the controlled release of growth factors from coagulated PRP. To address this matter, here, we primarily examined direct effects of CaCl2 on platelets in PBS and on coagulation in citrated PRP.
Methods
PRP was prepared from healthy volunteers’ blood. Platelets’ actions were monitored by scanning electron microscopy, flow cytometry, digital holographic microscopy, and immunofluorescent staining. Clot formation was examined in plasma.
Results
In plasma-free PBS, 0.1% CaCl2 immediately upregulated CD62P and CD63, causing a release of microparticles and fibrinogen/fibrin; consequently, platelets aggregated and adhered to polystyrene culture dishes with enlargement of their attachment area. In a clot formation assay in plasma, CaCl2 initially induced platelet aggregation, which triggered loop-like matrix formation and subsequently induced coagulation on a watch glass. Such changes were not clearly observed either with PRP in a plastic dish or in platelet-poor plasma on a watch glass: coagulation was delayed in both conditions.
Conclusions
These findings indicate that besides the well-known coagulation pathway, which activates platelets via thrombin conversion in a coagulation cascade, CaCl2 directly activates platelets, which then facilitate clot formation independently and in cooperation with the coagulation pathway.
Keywords: Platelet, Activation, Coagulation, Fibrin, Flow cytometry, Calcium
Background
Since Marx’s report [1], platelet-rich plasma (PRP) and subsequently modified PRP derivatives have been widely applied in regenerative dentistry. Unlike self-clotted platelet-rich fibrin (PRF), for better handling efficiency and minimizing the loss of growth factors to diffusion, PRP and some other derivatives in liquid form are usually clotted by addition of exogenous coagulation factors, such as thrombin and/or CaCl2. For example, in the case of plasma-rich in growth factors (PRGF) (the most successful PRP derivative) [2], venipuncture is performed with anticoagulants, usually citrate or acid citrate dextrose (ACD), to chelate plasma Ca2+ [3]. Somewhat excessive amounts of Ca2+ are recommended for addition to citrated PRGF preparations to reconstitute plasma by recovering free Ca2+ levels on a watch glass at 37 °C [4, 5].
Behind this clot formation, there is the intrinsic coagulation pathway, which is activated at the level of factor XII by the glass surface and proceeds in the presence of Ca2+ to convert prothrombin to thrombin, subsequently fibrinogen to fibrin, and consequently facilitates fibrin polymerization and cross-linking [6]. In this process, thrombin converted from prothrombin is known to activate platelets via specific subtypes of protease-activated receptors [7, 8]. Therefore, it is likely that added CaCl2 indirectly activates platelets through activation of a coagulation pathway in citrated PRP in glassware. The resulting fibrin fibers are thick and well cross-linked and are almost identical to those formed in a preparation of PRF [9].
In contrast to Ca2+, when an alternative coagulation factor, e.g., thrombin, is added to citrated PRP, the resulting fibrin fibers are thin and often fused together turning into a sheet-like matrix. Because this thrombin-induced fibrin matrix is relatively easily degradable [9], to stabilize its existence at an implantation site and to retain its growth factors longer, it is better to use Ca2+ as a coagulation factor and to employ glassware for activation of the intrinsic coagulation pathway. This approach is not limited to PRP and PRGF and can be extended to PRF preparation from stored whole-blood samples. Nonetheless, direct effects of exogenously added Ca2+ on platelets in vitro have been poorly investigated and understood.
In this study, we attempted to dissociate platelets from the coagulation pathway and to evaluate possible direct action of Ca2+ on platelet functions in citrated whole-blood samples. In addition, in response to recent increasingly frequent requests for scheduled or outsourced, but not immediate on-site, preparation of various platelet concentrate types [4, 5], we examined time course changes in platelet responsiveness to added CaCl2 along with coagulation time in stored whole-blood samples.
Methods
Preparation of the PRP fraction and clotting
Blood samples were collected from eight nonsmoking healthy male volunteers at ages from 32 to 68 years. The study design and consent forms of all the procedures performed were approved by the ethics committee for human participants of the Niigata University School of Medicine (Niigata, Japan) in accordance with the Helsinki Declaration of 1964 as revised in 2013.
Peripheral blood (~ 9 mL) was collected into plastic vacuum plain blood collection tubes (Neotube; NIPRO, Osaka, Japan) containing 1 mL of the A-formulation of ACD (ACD-A; Terumo, Tokyo, Japan) and was immediately centrifuged at 530×g for 10 min. The upper plasma fraction was collected, transferred to fresh tubes, and served as a PRP fraction [10]. The numbers of platelets and other blood cells in whole-blood samples and PRP preparations were determined on an automated hematology analyzer (pocH 100iV, Sysmex, Kobe, Japan).
Evaluation of platelet surface antigen expression by immunofluorescence (IT) staining
Platelet concentrates were prepared from citrated whole-blood samples, pretreated with 5 μg/mL prostaglandin E1 (PGE1; Cayman Chemical Co., Ann Arbor, MI, USA) for 5 min, rinsed, and resuspended in PBS in sample tubes. Washed platelets were then treated with CaCl2 at a final concentration of 0.1% and incubated in polystyrene culture dishes for up to 30 min at ambient temperature. At the end of the incubation, the reaction was stopped by adding 10% of neutralized formaldehyde. Platelets were washed twice, blocked with 0.1% Block-Ace (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) in 0.1% Tween 20-containing Tris-buffered saline (TBS) (T-TBS) for 1 h, and treated with a mouse monoclonal anti-human CD41, anti-CD62P, or anti-CD63 antibody (1:20 dilution; BioLegend, San Diego, CA, USA) overnight at 4 °C. At the end of treatment, the platelets were again washed twice with T-TBS and were then probed with a secondary antibody, i.e., a goat anti-mouse IgG H&L antibody (conjugated with Alexa Flour 555; 1:50 dilution; Abcam, Cambridge, MA, USA), for 30 min at ambient temperature. Finally, after a subsequent PBS wash, the platelets were mounted with an antifade mounting medium (Vectashield; Vector laboratories, Burlingame, CA, USA), and CD41, CD62P, and CD63 expression levels were examined under a fluorescence microscope equipped with a cooled charge-coupled device (CCD) camera (Nikon, Tokyo, Japan).
Flow cytometric analysis
As described in the subsection above, the platelet fractions were prepared and treated with CaCl2 in polypropylene sample tubes. At the end of incubation, the platelets were fixed with an equal volume of a commercial fixative, ThromboFix (Beckman-Coulter, Brea, CA, USA), for 30 min, washed twice with PBS, and probed simultaneously with phycoerythrin (PE)-conjugated mouse monoclonal anti-CD41 and FITC-conjugated anti-CD62P or FITC-conjugated CD63 antibodies (5 μL per 100 μL of a sample) (BioLegend) for 40 min at ambient temperature. After two washes with PBS, the platelets were analyzed on a flow cytometer (Cell Lab Quanta SC; Beckman-Coulter Inc., Brea, CA, USA) as described before [5]. For isotype controls, mouse IgG1 (BioLegend) was employed. The data were analyzed in the FlowJo software (FlowJo, LLC, Ashland, Oregon, USA).
Scanning electron microscopy (SEM)
As described above, to observe changes in platelet appearance, washed platelets were treated with 0.1% CaCl2 in polypropylene sample tubes for up to 20 min and placed in polystyrene cell culture dishes where they were incubated for the last 5 min of treatment. Alternatively, to examine microparticles and platelet-derived fibrin fibers, washed platelets were treated with 0.1% CaCl2 in polypropylene sample tubes, were immediately transferred onto specific filters (Sem Pore; JOEL, Akishima, Japan), and incubated for up to 15 min. At the end of treatment, the platelets were washed with PBS, fixed with 2.5% neutralized glutaraldehyde, serially dehydrated in ethanol and t-butanol solutions, and freeze-dried.
Individual fibrin clots were compressed in a stainless steel compressor (JMR, Niigata, Japan) to eliminate abundant serum proteins with the exudate [11], washed three times with PBS, fixed with 2.5% neutralized glutaraldehyde, serially dehydrated in ethanol and t-butanol solutions, and freeze-dried.
After that, these samples were examined under a scanning electron microscope (TM-1000; Hitachi, Tokyo, Japan) at an accelerating voltage of 15 kV, as described previously [9].
Quantitative assessment of cell morphology by digital holographic microscopy (DHM)
As described in the section on SEM examination of platelets in cell culture dishes, washed platelets were treated with 0.1% Ca in sample tubes and plated in cell culture dishes (or in flasks) where they were incubated for the last 5 min of treatment. After fixation with 2.5% neutralized glutaraldehyde, the platelets were washed with PBS and stored in PBS until DHM examination.
Imaging by DHM (HoloMonitor M4; Phase Holographic Imaging AB, Lund, Sweden) was performed as described elsewhere [12]. The data were analyzed using specialized software, HoloStudio M4 (Phase Holographic Imaging AB). For surface roughness and area analysis, after a series of images were captured, the grayscale images were converted to the black-and-white format by the Otsu method, and the cell identification and segmentation in the images were adjusted either automatically or manually.
Based on the accumulated data on the cell refractive index, the average refractive index for cultured monolayer cells was fixed at 1.38 (Phase Holographic Imaging AB, personal communication). This value was applied to platelets. The refractive index of the surrounding medium is 1.34 and should not excessively deviate (± 0.08) from the cell refractive index.
On the basis of our preliminary data, we focused on the parameters related to the cell area and roughness and examined at least 4000–8000 platelets in each platelet population.
Determination of prothrombin time
This parameter was determined by means of a Coaguchek XS system (Roche Diagnostics International Ltd., Basel, Switzerland). For citrated samples, 500 μL of whole-blood samples was pre-warmed at 37 °C for 5 min, mixed well with 10 μL of 10% CaCl2 by gentle inverting, and incubated for 5 min prior to the analysis.
A clot formation assay
Next, PRP fractions were centrifuged at 1060×g for 5 min to fractionate platelet-poor plasma (PPP). PRP or PPP (1.5 mL) was mixed with 10% CaCl2 in the ratio mentioned for whole-blood samples on a watch glass or in a plastic dish. To activate platelets, small cut pieces of a collagen sponge composed of collagen microfibers (Integran®; Koken, Tokyo, Japan) were added to PRP in the plastic dish. Time for complete clot formation was determined.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). For multigroup comparisons, statistical analyses were conducted to compare the mean values by one-way analysis of variance (ANOVA), followed by Dunn’s multiple-comparison test (SigmaPlot 12.5; Systat Software, Inc., San Jose, CA, USA). Differences with P values of < 0.05 were considered statistically significant.
Results
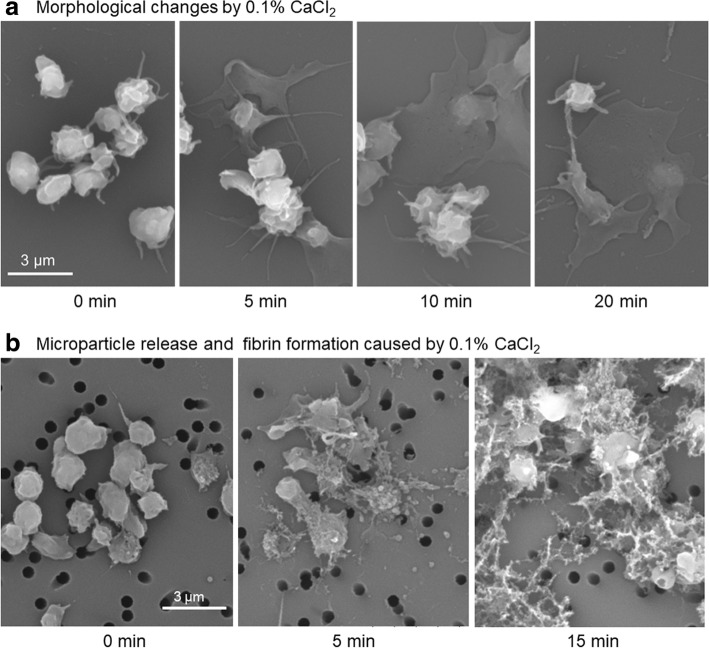
Morphological changes of (and microparticle release and fibrin formation by) Ca2+-stimulated platelets are shown in Fig. 1. When washed platelets were treated with 0.1% CaCl2 in sample tubes and plated in culture dishes for the last 5 min of treatment (control, 2 min), platelets’ ability to adhere to the dish bottom surface increased with the duration of Ca2+ treatment (Fig. 1a). On the other hand, when washed platelets were treated with 0.1% CaCl2 in sample tubes, immediately transferred onto percolated filters, and incubated for up to 15 min, the platelets were found to release microparticles and fibrinogen, which was converted to fibrin even in the absence of plasma components (Fig. 1b).
Fig. 1.
Morphological changes of (and microparticle release and fibrin formation by) Ca2+-stimulated platelets. a Washed platelets were treated with 0.1% CaCl2 in sample tubes and then in culture dishes for 5, 10, and 20 min in total. b Washed platelets were treated with 0.1% CaCl2 in sample tubes, immediately transferred onto filters, and incubated for 5 and 15 min in total. Control platelets were directly placed in culture dishes or filters and fixed. Similar results were obtained from samples of three other donors
Changes in attachment area, optical thickness on average, and surface roughness of Ca2+-simulated platelets are shown in Fig. 2. In the control, i.e., resting platelets, the average thickness of almost all platelets was within 2 μm. Among the platelets stimulated by 0.1% Ca2+ for 15 min, approximately 50% of the cells increased their apparent thickness beyond 2 μm. Attachment area was also significantly enlarged by added Ca2+, but roughness was not changed. In this case, enlarged platelets in terms of both thickness and area definitely represent aggregated platelets, but, probably, thicker platelets also represent aggregated platelets.
Fig. 2.
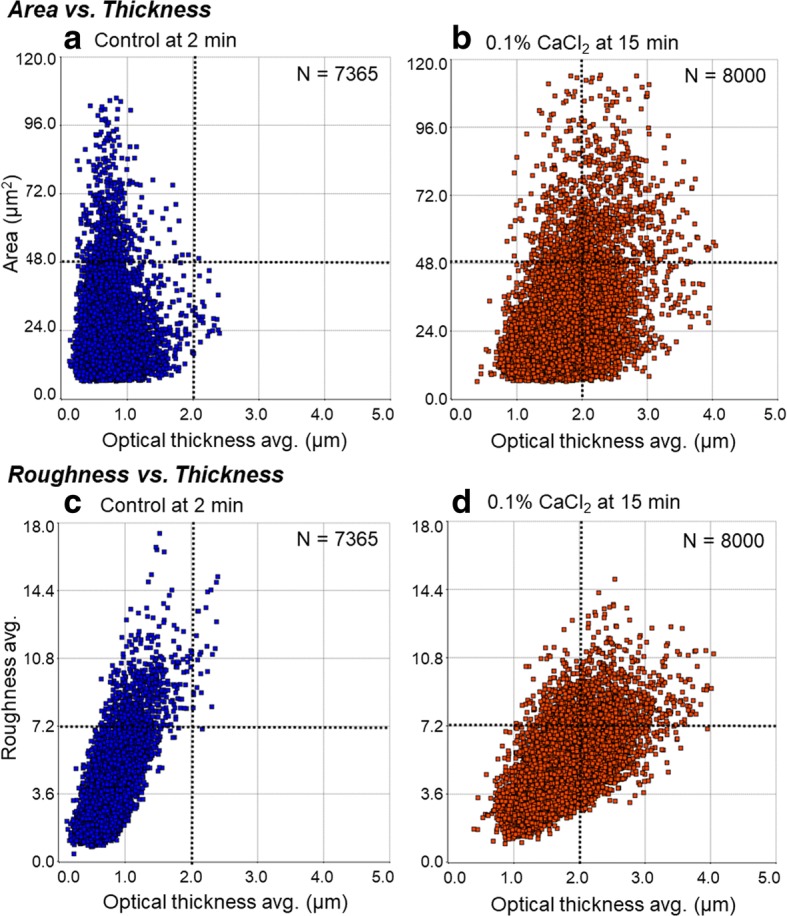
DHM examination of changes in attachment area, optical thickness, and surface roughness of Ca2+-simulated platelets. Washed platelets were treated with 0.1% CaCl2 for 15 min (b, d) on culture flasks, fixed, and subjected to DHM examination. Control was no treatment at 2 min (a, c). Each platelet was plotted in the scatter plots of “Area vs. Thickness” (a, b) or “Roughness vs. Thickness”. Similar results were obtained from samples of two other donors
Immunofluorescent (IF) evaluation of changes in surface marker expression in Ca2+-stimulated platelets is shown in Fig. 3. When washed platelets were treated with 0.1% CaCl2 in sample tubes, immediately placed in culture dishes, and incubated for 15 min, CD62P and CD63, but not CD41, were substantially upregulated.
Fig. 3.
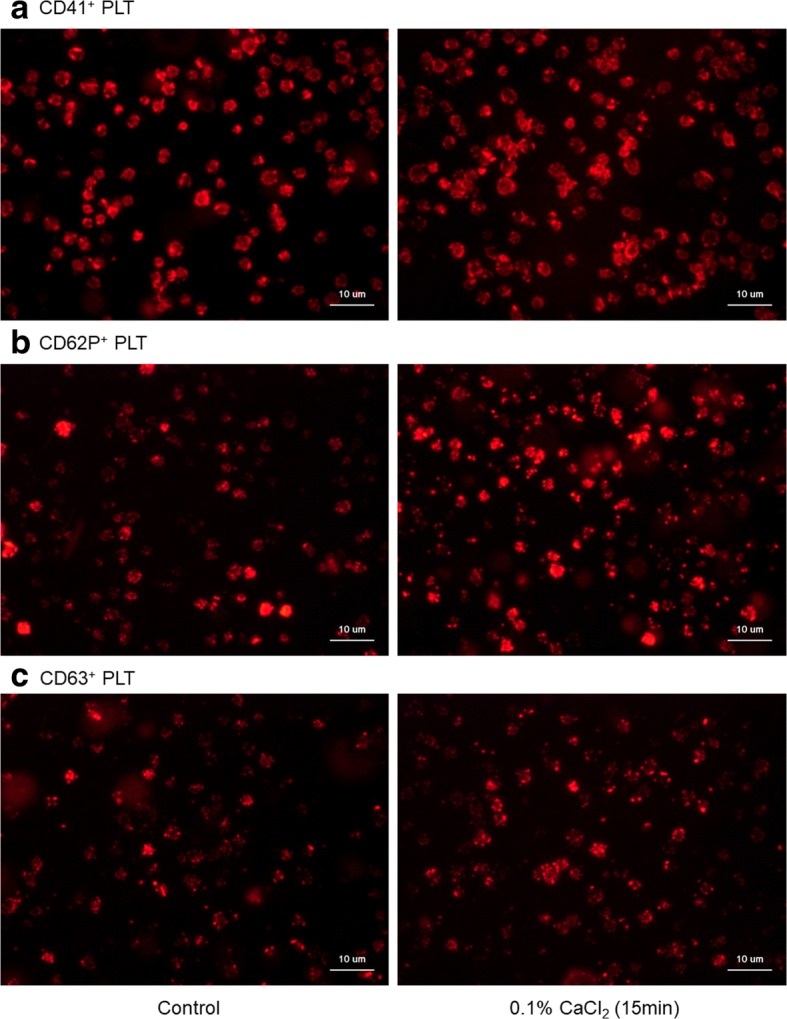
Immunofluorescent (IF) evaluation of changes in surface marker expression—CD41 (a), CD62P (b), and CD63 (c)—in Ca2+-stimulated platelets. PLT platelets. Washed platelets were treated with 0.1% CaCl2 in culture dishes and subjected to IF staining. Similar results were obtained from samples of three other donors
Flow cytometric analysis of changes in surface marker expression in Ca2+-stimulated platelets is presented in Fig. 4. When washed platelets were treated with 0.1% CaCl2 in sample tubes for up to 30 min, CD62P+ platelet counts in CD41+ platelet populations increased time-dependently. Similarly, platelet volume, as assessed by impedance, increased with the duration of Ca2+ treatment.
Fig. 4.
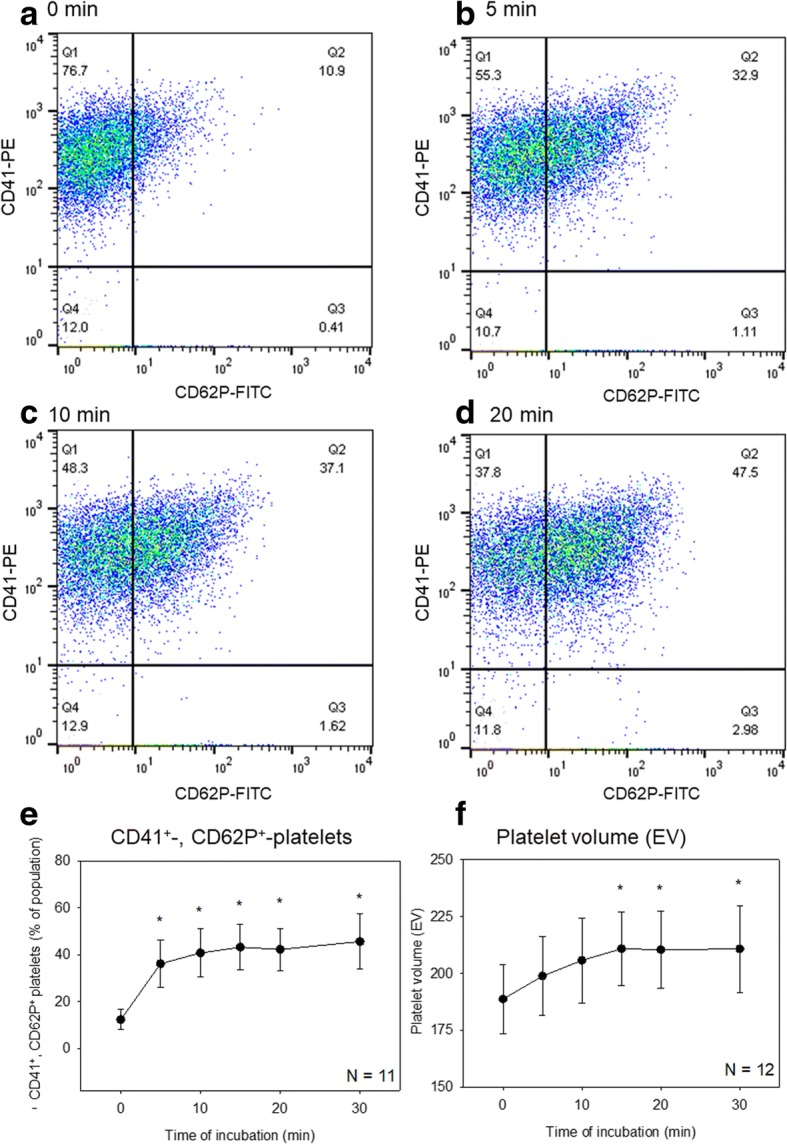
Flow cytometric analysis of changes in surface marker expression in Ca2+-stimulated platelets. Washed platelets were prepared and treated as described in the caption of Fig. 1a. a–d Representative scatter plots of CD41+ and CD62P+ platelets. e Effects of Ca2+ on percentages of double-positive platelets in whole platelet populations (N = 11). f Effects of Ca2+ on platelet volume (N = 12). Asterisks indicate statistically significant differences between the control data and treatment data
Because these findings taken together implied that exogenously added Ca2+ beyond physiological in vivo levels (approximately 9.0 vs. 2.5 mM) directly activates platelets in the absence of plasma components, platelets’ direct involvement in coagulation was then examined using platelet-rich and platelet-poor plasma. Effects of CaCl2 on fibrin clot formation on watch glasses and in polystyrene culture dishes are shown in Fig. 5. On watch glasses (panel a, control), addition of 0.1% CaCl2 most rapidly formed loop-like substances (in a dashed-line circle) and subsequently fibrin clots in PRP at 8 min of treatment (Fig. 5a). Polystyrene culture dishes, whose surface is optimized for adherent cells, significantly delayed Ca2+-induced clot formation in PRP from 8 to 19 min (Fig. 5b). When the treatment was carried out in PPP, clot formation was also significantly delayed (Fig. 5c). Nonetheless, in the presence of the collagen sponge, Ca2+ addition caused formation of a fibrin clot in PRP in culture dishes as rapidly as in the control (Fig. 5d vs. a).
Fig. 5.
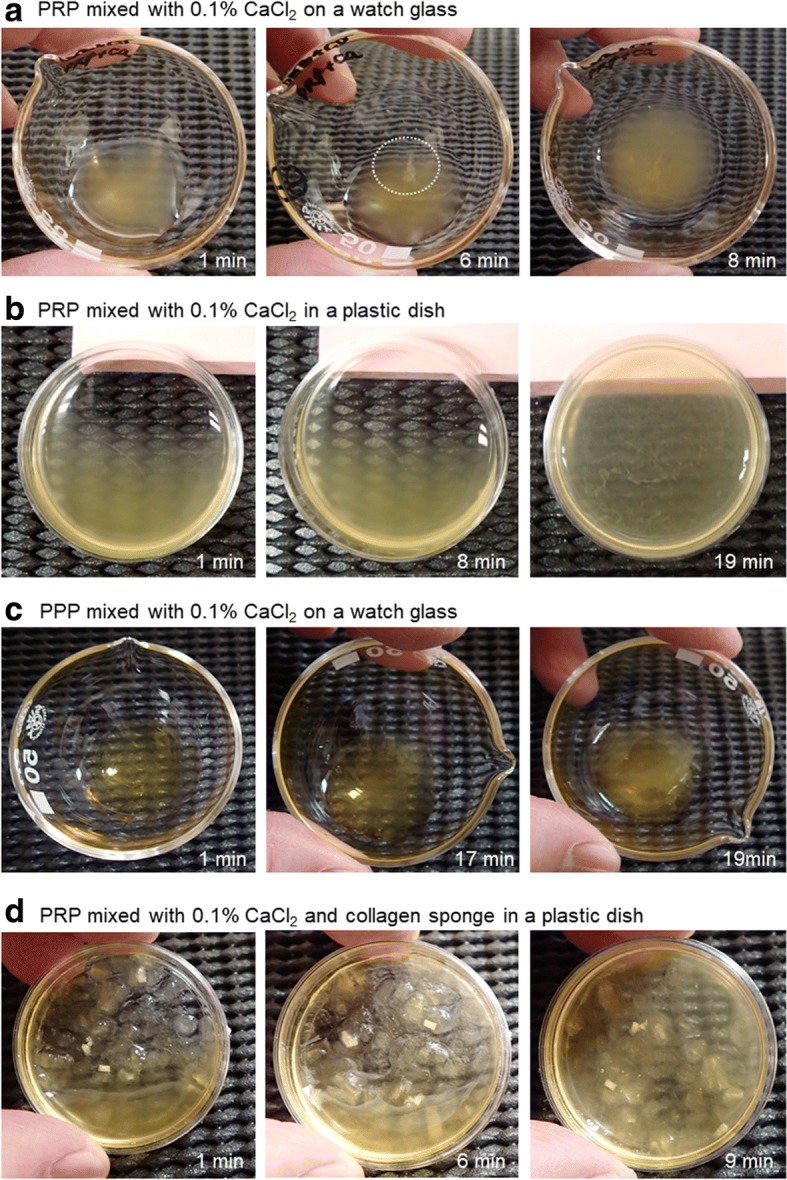
Effects of CaCl2 on fibrin clot formation on watch glasses and polystyrene culture dishes. a PRP mixed with 0.1% CaCl2 on a watch glass. b PRP mixed with 0.1% CaCl2 in a plastic dish. c PPP mixed with 0.1% CaCl2 on a watch glass. d PRP mixed with 0.1% CaCl2 and a collagen sponge in a plastic dish. Similar results were obtained from three other donors
The surface microstructures of the formed fibrin clots are depicted in Fig. 6. The loop-like substances that initially formed in PRP on watch glasses were composed of abundant aggregated platelets, and relatively smaller amounts of fibrin fibers were deposited around platelet aggregates (Fig. 6a). By contrast, in the final version of fibrin clots, platelet aggregates were hardly detectable and most parts consisted of fibrin fibers (Fig. 6b). Compared with the control clots, those formed in culture dishes were enriched in platelets although either thickness or cross-link density of fibrin fibers was apparently similar to that in the control clots (Fig. 6c). Fibrin clots formed from PPP on watch glasses and from PRP in culture dishes with collagen sponges were similar to the control clots.
Fig. 6.
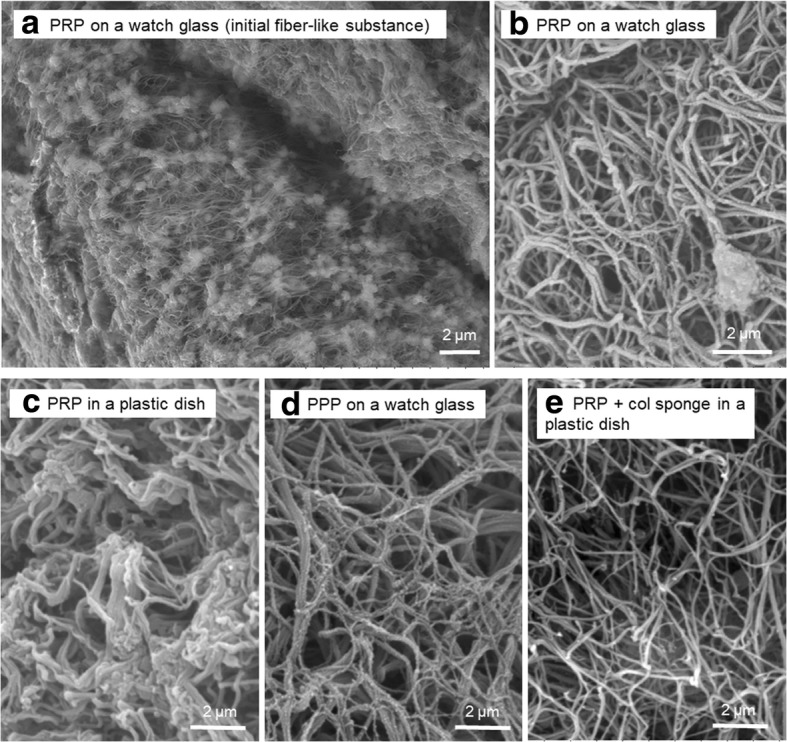
SEM analysis of the surface microstructures of formed fibrin clots. a A fiber-like substance initially formed in Ca2+-treated PRP on a watch glass. b The fibrin clot formed in Ca2+-treated PRP on a watch glass. c The fibrin clot formed in Ca2+-treated PRP in a plastic culture dish. d The fibrin clot formed in PPP on a watch glass. e The fibrin clot formed in PRP in the presence of a collagen sponge in a plastic dish. Similar results were obtained from three other donors
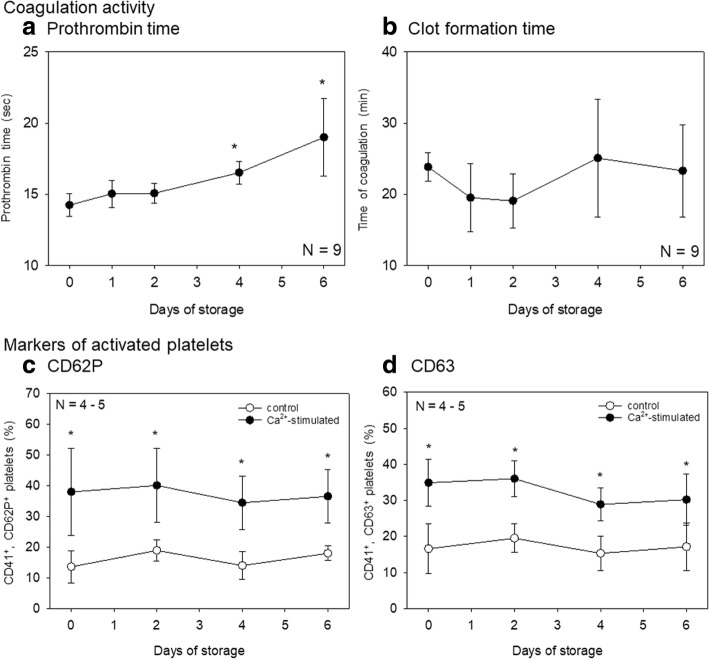
Finally, effects of storage time on coagulation activity and platelet functions were examined. Effects of Ca2+ on prothrombin time and clot formation of stored whole-blood samples are shown in Fig. 7a, b. Because we preliminarily confirmed that reconstitution of citrated blood with CaCl2 can recover the conditions applicable to the prothrombin time assay, we evaluated prothrombin time of citrated whole-blood samples stored for up to 6 days. Prothrombin time increased with the duration of storage. Effects of Ca2+ on CD62P and CD63 expression in platelets isolated from citrated whole-blood samples stored for up to 6 days are presented in Fig. 7c, d. The responsiveness to Ca2+ in terms of both CD62P and CD63 at 15 min seemed to somewhat decrease with storage time; however, even after 6-day storage, platelets maintained their response to added Ca2+: upregulation of CD62P and CD63 (vs. control levels).
Fig. 7.
Effects of storage time on the coagulation pathway and platelets. Prothrombin time (a) and clot formation time (b) of citrated whole-blood samples stored for up to 6 days were examined simultaneously. Platelets’ responsiveness to Ca2+ was assessed by upregulation of CD62P (c) and CD63 (d) in CD41+ platelets
Discussion
It is well known that platelets are activated by adenosine diphosphate (ADP), thrombin, epinephrine, thromboxane A2, collagen, and many other compounds and thus aggregate through binding of fibrinogen and glycoprotein IIb/IIIa receptors and upregulate surface antigens known as “platelet activation markers”: CD62P and CD63 [13, 14]. During activation, platelet morphology generally changes from a disc-shaped appearance (resting) to a rolling ball-shaped appearance, hemisphere-shaped appearance, and finally spreading adhesion appearance [15]. To our knowledge, however, increased extracellular free Ca2+ levels have not been sufficiently studied as an activator of platelets probably because free Ca2+ levels are maintained within some range, but not depleted, under pathological conditions of plasma and tissues.
Compared with the in vivo settings, citrated whole blood provides totally different conditions to platelets: extracellular free Ca2+ is chelated by citrate. The primary purpose of addition of Ca2+-chelating anticoagulants is to inhibit serial reactions of the coagulation pathway. Nevertheless, it is known that this chelation also suppresses various platelet functions related to aggregation [16]. Platelet activators mentioned above elevate intracellular Ca2+ concentrations, either by releasing Ca2+ from intracellular stores or by increasing Ca2+ influx across the plasma membrane [17]. Conversely, acute chelating of extracellular Ca2+ by EGTA inhibits the platelet ability to adhere to glass and to aggregate in response to ADP or other platelet activators [16].
The mechanism of Ca2+-induced platelet activation is discussed below. We preliminarily postulated expression of Ca2+-sensing receptors (CaSR) but failed to detect this type of receptor by the methods of flow cytometry and IF staining with a mouse monoclonal anti-CaSR antibody (cat. # ab19347; Abcam). No appreciable specific binding was observed in IF staining, and fewer than 5% of platelets tested positive in the flow cytometric analysis. Therefore, because this CaSR antigen is known to be expressed in the brain, kidneys, lungs, liver, heart, skeletal muscle, and placenta [18], but not in platelets, it is reasonable to rule out the participation of CaSR in Ca2+-induced platelet activation in our study.
An alternative possibility may be related to the status of platelets stored in a low-Ca2+ environment: platelets constantly increase thromboxane A2 (TXA2) production and tend to easily aggregate under the influence of the TXA2 autocrine loop [19]. Therefore, it is thought that platelets prepared from citrated whole-blood samples continuously repeat Ca2+ discharge from intracellular Ca2+ stores and Ca2+ efflux across the plasma membrane [20] in response to endogenously produced TXA2. In addition, even though the autocrine loop of TXA2 does not function actively, intracellular free Ca2+ levels of platelets (~ 100 nM at actual resting levels) are maintained also by a balance between the “passive” leak of Ca2+ into platelets and the concurrent efflux of Ca2+ across the plasma membrane and accumulation in intracellular stores [21]. Therefore, in a citrated medium, it is plausible that intracellular free Ca2+ in platelets is depleted gradually by repeated Ca2+ discharge from intracellular stores and passive and active Ca2+ flux across the plasma membrane. We can speculate that Ca2+ addition may promptly enable Ca2+ entry via the Ca2+ leak pathway and subsequently activate platelets by the autocrine pathway of TXA2 or activators stored in α-granules, such as ADP and thrombin. In support of this possibility, Aoki et al. demonstrated that added Ca2+ increases thrombin production by washed platelets [22]. Further investigation is needed to clarify the mechanism of Ca2+-induced platelet activation.
The mechanism of Ca2+-induced clot formation is worthier of discussion. In the human body, two types of thrombosis are known: white and red thrombi [23]. According to this definition, a fibrin mesh is deposited on platelet aggregates in a white thrombus, whereas platelets (and red blood cells) are trapped and aggregated by the fibrin mesh in a red thrombus. In this study, we found that platelet aggregates function like nuclei of clot formation. Therefore, platelets are located mainly near the center or in a deep region of a clot, and this clot may be classified essentially as a “white thrombus.” In this case, growth factors stored in platelets can be assumed to be retained for a relatively long time. It is possible that this type of clot functions as a long-lasting carrier with a better regenerative potential.
On the other hand, in PRF prepared from fresh whole-blood without anticoagulants, it is thought that the centrifugal force increases the contact of factor XII with a glass surface, thereby primarily forming fibrin clots. Although platelets can also be activated by a glass surface, the centrifugal force accumulates platelets at and just below the interface between the red blood cell fraction and plasma fraction as well as in the surface area of PRF preparations [11]. Even though only a few red blood cells are embedded in the clot, in terms of the formation mechanism, this clot could be classified as a “red thrombus.” Therefore, it is plausible that growth factors that are stored in the platelets trapped in fibrin mesh may be released at early phases of degradation.
As described previously [24], glass tube production is no longer practiced by major manufacturers of medical equipment in Japan and in major Western countries. One of the possible solutions (when glassware is needed) is to coat the inner wall of plastic tubes with micronized silica particles, as in Greiner Bio-One Serum Clot Activator Tubes (Greiner Bio-One North America Inc., Monroe, NC, USA). Likewise, various types of plastic vacuum blood collection tubes containing a coagulation-activating film are produced by several manufacturers. Nevertheless, these products are intended for routine clinical examination, and their quality, especially safety, has never been ensured for preparation and implantation of platelet concentrates.
If platelet activity can be controlled in well-qualified plastic tubes, it is expected that safer PRF will be provided to patients without activation of the intrinsic coagulation pathway. In this study, we demonstrated that a collagen sponge can rapidly facilitate fibrin clot formation (without the aid of glassware) probably via activation of platelets. On the basis of this concept, we recently developed a PRF kit composed of plastic tubes containing a synthetic, RGD motif-enriched collagen-like peptide (RCP; FUJIFILM, Tokyo, Japan) and validated its utility in PRF preparation and its efficacy in bone regeneration [Tsukioka et al., manuscript submitted]. This fibrin clot formation is triggered by activated platelets.
Conclusions
In addition to the well-known intrinsic coagulation pathway, which activates platelets via thrombin conversion, added Ca2+ may directly activate washed platelets and promote clot formation alone and in cooperation with the coagulation pathway as illustrated in Fig. 8. Therefore, it is possible to control platelet activation levels and the consequent growth factor release by modulating not only indirect but also direct activation pathways.
Fig. 8.
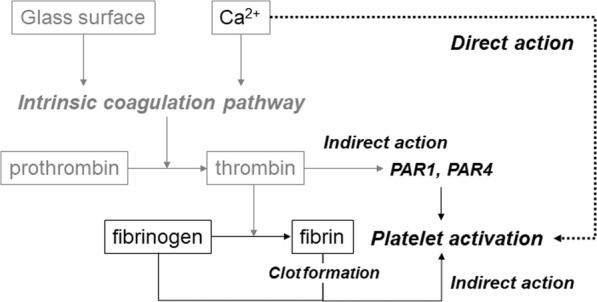
Mechanisms of Ca2+-induced fibrin clot formation in citrated plasma. As a major mechanism, Ca2+ activates the coagulation pathway in cooperation with a glass surface and thus subsequently activates platelets through production of thrombin and fibrin. As an additional mechanism, Ca2+ directly stimulates platelets to promote coagulation
Acknowledgements
The authors appreciate Shoshin EM (Okazaki, Japan) for giving an opportunity to use DHM.
Availability of data and materials
Because an article performed and prepared in parallel is now submitted elsewhere for publication, the authors do not wish to share their data at present time.
Abbreviations
- ACD
Acid citrate dextrose solution
- ADP
Adenosine diphosphate
- CaSR
Calcium-sensing receptor
- DHM
Digital holographic microscopy
- PBS
Phosphate-buffered saline
- PPP
Platelet-poor plasma
- PRF
Platelet-rich fibrin
- PRP
Platelet-rich plasma
- SEM
Scanning electron microscope
- TBS
Tris-buffered saline
- TXA2
Thromboxane A2
Authors’ contributions
TT, KI, TT, and TK conceived and designed the study, performed the experiments and data analysis, and wrote the manuscript. YK, FO, TW, MN, and YK designed and performed the experiments and data analysis. HO and KN conceived the study and participated in the discussion of the results and manuscript preparation. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study design and consent forms of all the procedures performed were approved by the ethics committee for human participants of the Niigata University School of Medicine (Niigata, Japan) in accordance with the Helsinki Declaration of 1964 as revised in 2013. Written informed consents to participate in the study were obtained from all the participants.
Consent for publication
Written informed consent for the publication of these were obtained from all the participants.
Competing interests
Toshihisa Toyoda, Kazushige Isobe, Tetsuhiro Tsujino, Yasuo Koyata, Fumitaka Ohyagi, Taisuke Watanabe, Masayuki Nakamura, Yutaka Kitamura, Hajime Okudera, Koh Nakata, and Tomoyuki Kawase declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 2.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–535. [PubMed] [Google Scholar]
- 3.Masuki H, Okudera T, Watanabe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T. Growth factor and pro-inflammatory cytokine contents in PRP, plasma rich in growth factors (PRGF), advanced-platelet-rich fibrin (A-PRF) and concentrated growth factors (CGF) Int J Implant Dent. 2016;2:19. doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isobe K, Suzuki M, Watanabe T, Kitamura Y, Suzuki T, Kawabata H, Nakamura M, Okudera T, Okudera H, Uematsu K, Nakata K, Tanaka T, Kawase T. Platelet-rich fibrin prepared from stored whole-blood samples. Int J Implant Dent. 2017;3:6. doi: 10.1186/s40729-017-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata H, Isobe K, Watanabe T, Okudera T, Nakamura M, Suzuki M, Ryu J, Kitamura Y, Okudera H, Okuda K, Nakata K, Kawase T. Quality assessment of platelet-rich fibrin-like matrix prepared from whole blood samples after extended storage. Biomedicine. 2017;5(57). http://www.mdpi.com/2227-9059/5/3/57. [DOI] [PMC free article] [PubMed]
- 6.Margolis J. Glass surface and blood coagulation. Nature. 1956;178:805–806. doi: 10.1038/178805b0. [DOI] [PubMed] [Google Scholar]
- 7.Goldsack NR, Chambers RC, Dabbagh K, Laurent GJ. Molecules in focus thrombin. Int J Biochem Cell Biol. 1998;30:641–646. doi: 10.1016/S1357-2725(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 8.Posma JJ, Posthuma JJ, Spronk HM. Coagulation and non-coagulation effects of thrombin. J Thromb Haemost. 2016;14:1908–1916. doi: 10.1111/jth.13441. [DOI] [PubMed] [Google Scholar]
- 9.Isobe K, Watanebe T, Kawabata H, Kitamura Y, Okudera T, Okudera H, Uematsu K, Okuda K, Nakata K, Tanaka T, Kawase T. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF) Int J Implant Dent. 2017;3:17. doi: 10.1186/s40729-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Isobe K, Suzuki T, Kawabata H, Nakamura M, Tsukioka T, Okudera T, Okudera H, Uematsu K, Okuda K, Nakata K, Kawase T. An evaluation of the accuracy of the subtraction method used for determining platelet counts in advanced platelet-rich fibrin and concentrated growth factor preparations. Dentistry Journal. 2017;5:7. doi: 10.3390/dj5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Kawase T, Horimizu M, Okuda K, Wolff LF, Yoshie H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012;40:323–329. doi: 10.1016/j.biologicals.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kawase T, Okuda K, Nagata M, Tsuchimochi M, Yoshie H, Nakata K. Non-invasive, quantitative assessment of the morphology of gamma-irradiated human mesenchymal stem cells and periosteal cells using digital holographic microscopy. Int J Radiat Biol. 2016;92:796–805. doi: 10.1080/09553002.2016.1230242. [DOI] [PubMed] [Google Scholar]
- 13.Taylor ML, Misso NL, Stewart GA, Thompson PJ. Differential expression of platelet activation markers CD62P and CD63 following stimulation with PAF, arachidonic acid and collagen. Platelets. 1995;6:394–401. doi: 10.3109/09537109509078478. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury A, Chung I, Blann AD, Lip GY. Platelet surface CD62P and CD63, mean platelet volume, and soluble/platelet P-selectin as indexes of platelet function in atrial fibrillation: a comparison of “healthy control subjects” and “disease control subjects” in sinus rhythm. J Am Coll Cardiol. 2007;49:1957–1964. doi: 10.1016/j.jacc.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara M, Sugimoto M, Tsuji S, Matsui H, Mizuno T, Miyata S, Yoshioka A. Platelet shape changes and adhesion under high shear flow. Arterioscler Thromb Vasc Biol. 2002;22:329–334. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- 16.Zucker MB, Grant RA. Nonreversible loss of platelet aggregability induced by calcium deprivation. Blood. 1978;52:505–513. [PubMed] [Google Scholar]
- 17.Jy W, Haynes DH. Intracellular calcium storage and release in the human platelet. Chlorotetracycline as a continuous monitor. Circ Res. 1984;55:595–608. doi: 10.1161/01.RES.55.5.595. [DOI] [PubMed] [Google Scholar]
- 18.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Forslund M, Li N. Influence of extracellular calcium on single platelet activation as measured by whole blood flow cytometry. Thromb Res. 2005;116:241–247. doi: 10.1016/j.thromres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 21.Roberts DE, McNicol A, Bose R. Mechanism of collagen activation in human platelets. J Biol Chem. 2004;279:19421–19430. doi: 10.1074/jbc.M308864200. [DOI] [PubMed] [Google Scholar]
- 22.Aoki I, Aoki N, Kawano K, Shimoyama K, Maki A, Homori M, Yanagisawa A, Yamamoto M, Kawai Y, Ishikawa K. Platelet-dependent thrombin generation in patients with hyperlipidemia. J Am Coll Cardiol. 1997;30:91–96. doi: 10.1016/S0735-1097(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Blitz A. Pump thrombosis—a riddle wrapped in a mystery inside an enigma. Ann Cardiothor Surg. 2014;3:450–471. doi: 10.3978/j.issn.2225-319X.2014.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawase T, Watanabe T, Okuda K. Platelet-rich plasma and its derived platelet concentrates: what dentists involved in cell-based regenerative therapy should know. Nihon Shishubyou Gakkai Kaishi. 2017;59:68–76. doi: 10.2329/perio.59.68. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because an article performed and prepared in parallel is now submitted elsewhere for publication, the authors do not wish to share their data at present time.