Abstract
Avian trichomonosis is an upper digestive tract disease of birds typically caused by the protozoan parasite Trichomonas gallinae. In California (U.S.A), trichomonosis is known to cause periodic epidemics in the Pacific Coast band-tailed pigeon (Patagioenas fasciata monolis), a migratory upland game bird. We summarize the mortality events that occurred during winter 2014–2015 including the duration, estimated mortality, pathology, and genetic identity of infecting parasites. Increased mortality was reported from locations in 25 counties between November 2014 and June 2015. Based on reports, carcasses received, wildlife rehabilitation center admissions, site visits, and regular monitoring by local personnel, total mortality was estimated at 18,440. At necropsy, birds had multiple coalescing lesions in the oral cavity involving the upper palate and/or around the tongue and glottis, esophagus, crop, and/or proventriculus. Birds collected from Contra Costa (63.9%; 30/47); Marin (75.0%; 6/8), San Mateo (46.7%; 14/30), and Santa Clara (35.0%; 37/106) counties were more likely to have lesions extending into their head involving muscle, sinuses, ear canals, eye sockets, and bone (χ2 = 62.9; df = 11; P < 0.001). Histopathologic findings included pharyngitis, esophagitis, myositis, and air sacculitis of the pneumatic bone of the skull. Mixed bacterial colonies were found multifocally at the fronts of the necrosis in six of the eleven birds examined histologically. Infecting trichomonads included T. gallinae subtype A2 (n = 5), un-typed T. gallinae (n = 4), mixed infection with T. gallinae subtype A2 and T. stableri (n = 1), and mixed infection with un-typed T. gallinae and T. stableri (n = 1). The winter 2014–2015 epidemic was the largest on record in terms of duration, locations, and birds affected. Infection dynamics may have been exacerbated by the drought in California. Increased monitoring of band-tailed pigeons is needed to understand the long-term impacts of large-scale mortality events on their population.
Keywords: Avian trichomonosis, Band-tailed pigeon, Epidemic, Trichomonas gallinae, ITS1/5.8S rRNA/ITS2, Fe-hydrogenase
Graphical abstract

Highlights
-
•
Avian trichomonosis mortality events in winter 2014–15 involving band-tailed pigeons.
-
•
Summary of range, duration, numbers affected, and characterization of trichomonads.
-
•
Total mortality exceeded an estimated 18,000 birds with over 90% being subadults.
-
•
Trichomonas gallinae subtype A2 was predominant parasite in band-tailed pigeons.
-
•
Timing of mortality events has serious consequences for future recruitment potential.
1. Introduction
Avian trichomonosis is a disease caused by a protozoan parasite affecting a diverse array of species with columbids being especially susceptible (Forrester and Foster, 2008). The parasite most frequently isolated from infected birds is Trichomonas gallinae of which 15 different subtypes have been identified based on the internal transcribed spacer (ITS) region (Gerhold et al., 2008; Grabensteiner et al., 2010). Further genotyping of T. gallinae strains can be accomplished by sequencing the hydrogenosomal Fe-hydrogenase gene (Lawson et al., 2011; Chi et al., 2013). Infection can range from subclinical in carrier birds, to the development of caseonecrotic lesions in the upper digestive tract and death. Birds become infected by ingesting contaminated water or food while adult columbids can directly infect their chicks when feeding them crop milk (Forrester and Foster, 2008). Once infected, the parasite multiplies rapidly in the oral cavity invading the mucosa leading to parasite-mediated desquamation and development of lesions (Kietzmann, 1993). Infected birds typically become emaciated as the lesions block the passage of food and eventually die from starvation or suffocation if the lesions block the airway (Forrester and Foster, 2008). As such, sick birds are often weak and reluctant to fly, have difficulty swallowing and exhibit labored or open-mouth breathing with death occurring typically 10–14 days post-infection (Stabler, 1947; Perez-Mesa et al., 1961). While the death of an individual bird from trichomonosis may occur any time of the year, epidemics or mortality events tend to have a distinct seasonality within an avian species, typically coinciding with the period of closest contact between conspecifics often including an influx of immunologically naïve juveniles. In California, these events occur most frequently in mourning doves (Zeneida macroura) during the spring and summer (Stabler and Herman, 1951) and band-tailed pigeons (Patagioenas fasciata) during the winter (Rogers et al., 2016a).
Whereas mourning doves range across the continental United States, band-tailed pigeons have a more limited range with two distinct populations inhabiting the western U.S., the Interior (P. f. fasciata) and Pacific Coast (P. f. monolis) subspecies (Keppie and Braun, 2000). Interior band-tailed pigeons occur in the southwestern U.S. and Mexico, while Pacific Coast band-tailed pigeons occur primarily from British Columbia south through southern California. While once an important game bird in the western U.S., both populations have undergone drastic declines resulting in reduced hunting opportunities (Seamans, 2016). Periodic trichomonosis epidemics have been documented since the mid-1940s within the Pacific Coast population and are an important factor in their decline, with near annual mortality events occurring since the early 2000s (Rogers et al., 2016a). Estimated mortality during these events is highly variable from year to year (Stromberg et al., 2008; Rogers et al., 2016a), but has the potential to be more than 2–3 times higher than the annual harvest, which typically ranges between 6000–10,000 pigeons (Seamans, 2016). In contrast to most other game birds, such as doves, turkeys, and waterfowl, the reproductive rate for band-tailed pigeons is relatively low. A pair of band-tailed pigeons produce, on average, one chick per year (Keppie and Braun, 2000) which may result in slow recovery when the population experiences large losses of individual birds through trichomonosis epidemics and harvest.
In previous studies, Trichomonas gallinae Fe-hydrogenase subtype A2, was the only T. gallinae subtype isolated from band-tailed pigeons during epidemics in California (Girard et al., 2014b). Subtype A1 has also been isolated from band-tailed pigeons in California, however, at a much lower prevalence and only during non-epidemic time periods (Girard et al., 2014b). In Europe and Canada, subtype A1 is the most common subtype isolated from avian species including songbirds, raptors, and columbids (Lawson et al., 2011; Chi et al., 2013; McBurney et al., 2015; Stockdale et al., 2015). In addition to T. gallinae, band-tailed pigeons were also found to be infected with a newly described species, T. stableri, which is more closely related to the human pathogen T. vaginalis than to T. gallinae (Girard et al., 2014a). Trichomonas stableri has been isolated from band-tailed pigeons both during epidemics and non-epidemics and occurs at a lower prevalence than T. gallinae (Girard et al., 2014a); however, both parasites cause similar disease, highlighting the importance of molecular characterization of parasites when attributing mortality events to a specific etiology.
Historically, trichomonosis epidemics involving Pacific Coast band-tailed pigeons have only been documented during the winter when the majority of the population is overwintering in central and southern California (Rogers et al., 2016a). During this time, pigeons form large flocks increasing contact between individuals at communal water sources and facilitating parasite transmission between susceptible birds. Similar to infections in other columbids (Bunbury et al., 2007) the likelihood of an epidemic occurring during a given winter in band-tailed pigeons is correlated with weather conditions, namely warmer temperatures and lower precipitation, which may improve parasite viability while increasing contact between individual birds at fewer available water sources (Rogers et al., 2016a).
This pattern of winter-only trichomonosis mortality events changed in 2014, when events were documented for the first time during the summer in at least three central California locations (Rogers et al., 2016b). During summer, band-tailed pigeons are more dispersed for breeding activities compared to winter (Keppie and Braun, 2000) presumably leading to reduced contact between pigeons and reduced opportunity for parasite transmission. However, 2014 was the warmest year on record in California, followed by 2015, the second warmest year on record (http://ca.water.usgs.gov/data/drought/). By early 2015, California was entering a fifth year of drought with roughly 94% of the state classified in severe drought according to the National Drought Mitigation Center (http://droughtmonitor.unl.edu).
Here we describe the avian trichomonosis mortality events involving Pacific Coast band-tailed pigeons that took place during winter 2014–2015 in California. Our analysis includes a summary of the geographic range and duration of events, numbers of birds affected, histopathological analysis of diseased birds, and molecular characterization of infecting parasites. By evaluating the characteristics of these epidemics, we improve our understanding of the impacts of large-scale disease events on the population health of this declining upland game bird.
2. Materials and methods
2.1. Mortality reports
The California Department of Fish and Wildlife's (CDFW) Wildlife Investigations Laboratory (WIL; Rancho Cordova, CA) is responsible for investigating causes of mortality in the state's wildlife. Incidents of sick and dead band-tailed pigeons were reported to WIL by department staff, the public, wildlife rehabilitation centers, and other government agencies by phone, e-mail, and online mortality reporting form (www.wildlife.ca.gov). Information compiled from these reports included date reported, location, number of sick and dead pigeons observed, and start and end dates of mortality, if applicable. When possible, a site visit was made to locations with reported mortality to confirm the bird species involved and to record numbers of sick and dead birds, habitat characteristics, and food and water sources.
Reports of avian mortality are also provided to WIL by the California Department of Public Health (CDPH; Richmond, CA) through their Dead Bird Hotline. The Dead Bird Hotline was established in 2003 for West Nile virus surveillance and enables the public to report dead birds to CDPH via a toll-free telephone number or online form (www.westnile.ca.gov). From these reports, incidences of band-tailed pigeon mortality were compiled.
Finally, wildlife rehabilitation centers in California are required to submit an annual report to CDFW of all wildlife admitted into the center under their permit. These reports total the number and final disposition (released, transferred, pending, euthanized, died, or DOA) of each species admitted to the center between January 1 and December 31 in a given year. While these reports do not denote date, location, or reason for intake for each individual animal, they do provide intake numbers for each species for the geographic area (e.g. county) in which the center is located. Band-tailed pigeon intake numbers were compiled by county from the 2015 annual reports for the 41 wildlife rehabilitation centers reporting band-tailed pigeon intake.
2.2. Post-mortem examination
Avian trichomonosis mortality events were defined as ≥5 birds dying in the same geographic location over several days to weeks (Rogers et al., 2016a). Once alerted to mortality, an effort was made to collect a subset of carcasses from outbreak locations for post-mortem examination and sampling for trichomonad isolation. Carcasses were received at WIL and stored in a freezer (−20 °C) until the post-mortem examination. Prior to the examination, the carcass was thawed at 4 °C for 24–48 h and gross findings were recorded including age, sex, presence and location of lesions, adipose deposition, condition of organs, and abnormalities (e.g. injuries). Birds were aged by plumage as juveniles (hatch year), subadults (second year), or adults (after second year) (Sanders and Braun, 2014). Nutritional condition was assessed by degree of adipose deposition and mass (grams). Adipose deposition was rated as none (no subcutaneous or internal adipose), trace (no subcutaneous and minimal internal adipose), and moderate to heavy (adequate subcutaneous and internal adipose).
2.3. Histopathology and trichomonad characterization
Of the carcasses received, eleven were selected for histopathology at the California Animal Health and Food Safety Laboratory (CAHFS; Davis, CA) and molecular characterization of infecting parasites was conducted at the One Health Institute, University of California, Davis. Tissue samples of oral mucosa with lesions, skull including the eyes and brain, muscle, thyroid/parathyroid glands, peripheral nerves, trachea, lung, heart, esophagus, crop, proventriculus, gizzard, pancreas, intestines, liver, spleen, adrenals, kidneys, and gonads were collected and immersed in 10% neutral buffered formalin, paraffin-embedded, sectioned at 4 μm, and stained with hematoxylin and eosin for histologic examination by light microscopy (Girard et al., 2014a). Lesion tissue was sampled by excision, placed in a cryovial, and stored at −80 °C for molecular analysis. DNA was amplified and sequenced using primers targeting the ITS1/5.8S rRNA/ITS2 and Fe-hydrogenase loci and sequence analysis was performed by alignment to published trichomonad sequences in GenBank (Girard et al., 2014b). Amplicons whose sequences were difficult to interpret using Geneious Pro v. 5.34 sequence analysis software (http://www.geneious.com) (Kearse et al., 2012) were cloned using the TOPO® TA cloning kit (Thermo Fisher Scientific, Waltham, MA), and 10 colonies per DNA isolate were chosen for further sequence analysis. All nucleotide sequences generated from isolates in this study were identical to isolates previously deposited in GenBank (Girard et al., 2014a, Girard et al., 2014b).
Cause of death was assigned based on case history, clinical signs, if known, post-mortem findings (e.g. presence of lesions, trauma), and diagnostic testing for the eleven birds that underwent histopathology and trichomonad isolation.
2.4. Statistical analysis
We analyzed differences in prevalence using the chi-square (χ2) test of independence and mean body mass was compared between event locations using the Mann-Whitney U test. Values reported are mean ± SE. Statistical analyses were performed using NCSS (Hintze, 2007) and P ≤ 0.05 were considered statistically significant. Maps were prepared using ArcMap (ESRI, Inc., 2014).
3. Results
3.1. Mortality reports
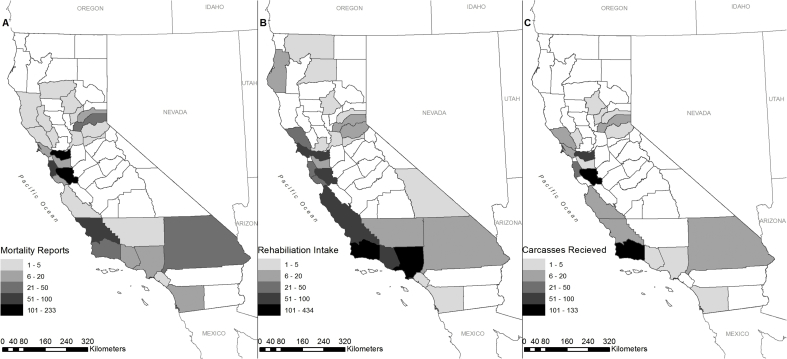
Band-tailed pigeon mortality was reported between late November 2014 and June 2015. A total of 339 reports from locations in 22 counties were received by WIL between 27 Nov 2014 and 22 Jun 2015 while 295 reports from locations in 25 counties were received by CDPH between 02 Jan and 20 Jun 2015. Most reports were received from Santa Clara (n = 233), Contra Costa (n = 111), San Mateo (n = 71), San Luis Obispo (n = 53), Placer (n = 23), Santa Barbara (n = 22), San Bernardino (n = 21), Los Angeles (n = 20), and San Diego (n = 19) counties; <10 reports were received from each of the remaining counties (Fig. 1A). Reports were received in November (0.2%; 1/634), December (0.2%; 1/634), January (48.3%; 306/634), February (33.6%; 213/634), March (9.8%; 62/634), April (5.8%; 37/634), May (1.3%; 8/634), and June (0.9%; 6/634). In total, these reports identified 2084 confirmed dead pigeons.
Fig. 1.
Number of band-tailed pigeon (Patagioenas fasciata monolis) mortality reports from phone, email, and online form received by county by the California Department of Fish and Wildlife (CDFW; Rancho Cordova, CA) and the California Department of Public Health (Richmond, CA) between November 2014 and June 2015 in California, U.S.A. (A). Number of band-tailed pigeons admitted to wildlife rehabilitation centers in California, U.S.A. and compiled by county between January and December 2015 (B). Number of band-tailed pigeon carcasses collected by county and received by CDFW between November 2014 and June 2015 in California, U.S.A. (C).
3.2. Wildlife rehabilitation center intake
Band-tailed pigeon intake was reported by 41 wildlife rehabilitation centers in 27 counties between 01 Jan and 31 Dec 2015. In total, 1215 pigeons were admitted to wildlife rehabilitation centers during 2015 (Fig. 1B). During the mortality event, rehabilitation centers reported increased intake of pigeons with visible lesions in the oral cavity or palpable lesions in the throat or crop, agonal breathing, exophthalmos often with discharge, otitis, and/or neurological symptoms including ataxia and torticollis.
3.3. Post-mortem examination
In total, 407 carcasses from 19 counties were submitted to WIL by the public and wildlife rehabilitation centers (n = 290) or collected during site visits (n = 117) between 27 Dec 2014 and 28 May 2015. Submitted pigeons were found dead (n = 271) or were euthanized (n = 116); no disposition was noted for 20 carcasses received from rehabilitation centers. Most carcasses were received from Santa Barbara (n = 133), Santa Clara (n = 115), Contra Costa (n = 58), San Mateo (n = 30), Monterey (n = 15), and San Luis Obispo (n = 14) counties; <10 carcasses were received from each of the remaining counties (Fig. 1C). A gross necropsy was performed on 376 pigeons. Avian trichomonosis, identified by the presence of caseous oral lesions, was the main finding for 93.1% (350/376) of the pigeons and prevalence was similar for males (89.6%; 112/125) and females (94.2%; 113/120) (χ2 = 1.70, df = 1, P = 0.19). For birds for which age could be determined by plumage, prevalence of infection was higher in juveniles (100.0%; 3/3) and subadults (95.2%; 258/271) than adults (87.0%; 87/100) (χ2 = 7.82, df = 2, P = 0.02).
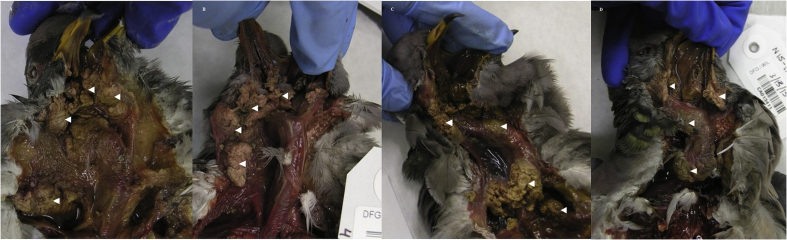
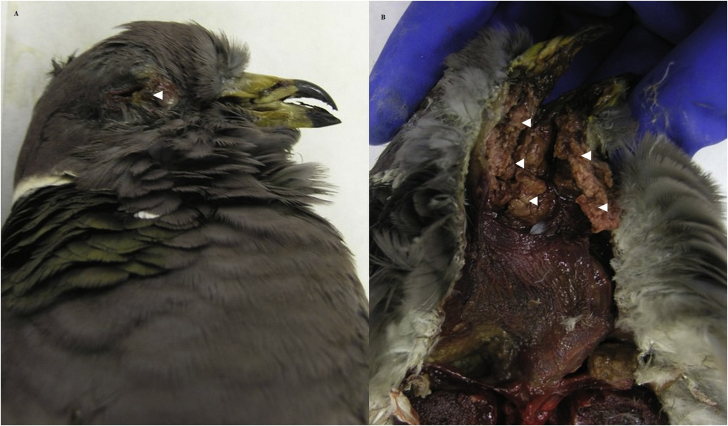
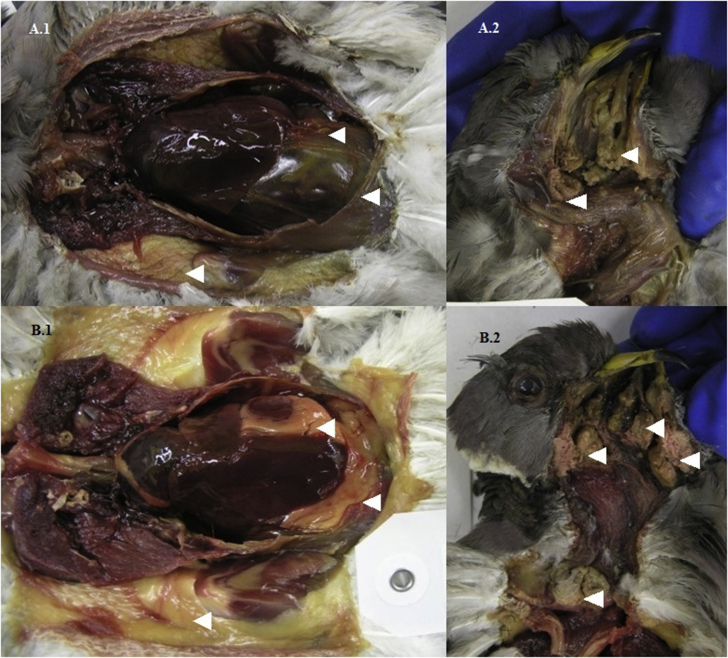
Generally, birds at necropsy had multiple coalescing lesions in the oral cavity, involving the upper palate and/or around the tongue and glottis, esophagus, crop, and/or proventriculus (80.3%; 281/350) (Fig. 2). Lesions often extended into the head involving the underlying muscle, sinuses, ear canals, and bone of the skull (30.0%; 105/350) and occasionally the eye sockets (9.1%; 32/350) (Fig. 3). Birds with lesions had varying amounts of adipose including none (46.9%; 150/320), trace (22.2%; 71/320), and moderate to heavy (30.6%; 98/320). Mean body mass for birds with an adipose deposition score of none was 257 g (180–360 g; n = 148), trace 281 g (215–395 g; n = 70), and moderate to heavy 315 g (215–425 g; n = 97). Birds with observed adipose stores, versus those with none, were more likely collected from Contra Costa (66.0%; 31/47); Marin (75%; 6/8), San Mateo (70%; 21/30), and Santa Clara (66.0%; 70/106) counties (χ2 = 49.0; df = 16; P < 0.001). Mean body mass for birds from these 4 counties was significantly higher (286 g ± 3.25, n = 196) than birds from the remaining 13 counties (275 g ± 3.99, n = 128; Mann-Whitney U test: Z = −2.14, P = 0.03) (Fig. 4). Birds from these 4 counties also were more likely to have lesions extending into their head: Contra Costa (63.8%; 30/47); Marin (75.0%; 6/8), San Mateo (46.7%; 14/30), and Santa Clara (35.0%; 37/106) (χ2 = 62.9; df = 11; P < 0.001).
Fig. 2.
Examples of caseonecrotic lesions (white arrowheads) in the oral cavity and upper digestive tracts of band-tailed pigeons (Patagioenas fasciata monolis) collected during an avian trichomonosis mortality event in California, U.S.A., between November 2014 and June 2015. Birds collected from Contra Costa County (A), Marin County (B), and Monterey County (D) in January 2015 and Placer County (E) in February 2015.
Fig. 3.
Caseonecrotic lesions (white arrowheads) in the right eye socket (A) and oral cavity (B) of a band-tailed pigeon (Patagioenas fasciata monolis) collected during an avian trichomonosis mortality event in Santa Clara County, California, U.S.A., in January 2015.
Fig. 4.
Body cavity with no adipose (white arrowheads) reserves (A.1) and the caseonecrotic lesions (white arrowheads) in the oral cavity (A.2) of a band-tailed pigeon (Patagioenas fasciata monolis) collected during an avian trichomonosis mortality event in Ventura County, California, U.S.A., in January 2015. Body cavity with abundant adipose (white arrowheads) reserves (B.1) and the caseonecrotic lesions (white arrowheads) in the oral cavity and upper digestive tract (B.2) of a band-tailed pigeon collected during an avian trichomonosis mortality event in Santa Clara County, California, U.S.A., in January 2015.
Other causes of death for pigeons collected during mortality events included trauma (4.2%; 16/376) and intestinal blockage (0.5%; 2/376). Cause of death could not be determined for 8 (2.1%; 8/376) pigeons.
Heteromorphic deutonymphs (hypopi) of hypoderatid mites (presumptive Hypodectes spp.) (El-Dakhly et al., 2013; Fain and Laurence, 1974) were observed in the subcutaneous adipose of the abdomen and inguinal areas of 19.8% (37/186) of birds evaluated including birds from Contra Costa (32.0%; 8/25); Marin (42.9%; 3/7), Monterey (22.2%; 2/9), San Mateo (18.5%; 5/27), Santa Clara (21.2%; 14/66), and Sonoma (66.7%; 2/3) counties.
Based on reports, carcasses received, rehabilitation center intake, site visits, and regular monitoring by local personnel during the duration of the events, the number of pigeons confirmed dead during these mortality events was 3467. Across all mortality event locations, the total estimated mortality was 18,440 birds, after considering the geographic range, duration of events, numbers of susceptible live pigeons present, and predator and scavenger activity. In total, deaths were reported from 33 of 58 California counties, with ≥5 deaths reported from locations in 25 counties between November 2014 and June 2015.
3.4. Histopathology
Due to the variation in lesion presentation for birds from certain geographic areas, histopathology was conducted on select birds collected from Santa Clara (n = 9) and San Mateo (n = 2) counties in January 2015 and included eight males and three females; all birds were aged as subadults with the exception of two males which were aged as adults. Histopathologic findings were similar among the birds and included pharyngitis (n = 10), esophagitis (n = 10), myositis (n = 10), cellulitis (n = 9), air sacculitis of the pneumatic bone of the skull (n = 6), sinusitis (n = 6), osteomyelitis (n = 4), tracheitis (n = 3), otitis (n = 2), hepatitis (n = 2) and/or splenitis (n = 1). Two birds had pneumonia due to aspiration of necrotic debris. A mixture of bacterial colonies including both Gram negative rods and Gram positive cocci were found multifocally at the fronts of the necrosis in six of the birds; culture and identification of bacteria was nonproductive. Large numbers of trichomonads were observed at the front of the lesions and in the necrotic debris (Fig. 5).
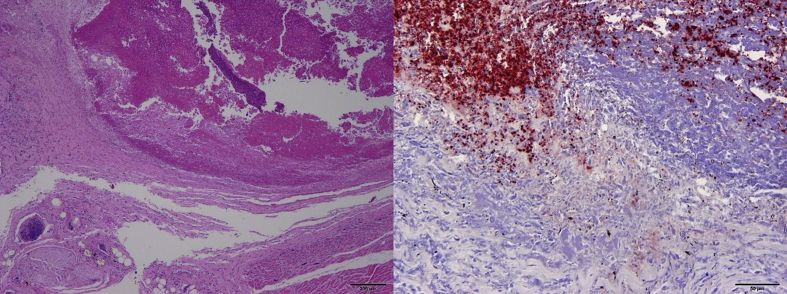
Fig. 5.
Haemotoxylin and eosin staining (left) of the oral tissue of a band-tailed pigeon (Patagioenas fasciata monilis) recovered during an avian trichomonosis mortality event showing a diffuse thick layer of necrosis extending through the submucosa and multifocally into the deeper soft tissue layers and skeletal muscle; scale bar is 200 μm. Immunohistochemical staining (right) of trichomonad antigen (red) of the same bird demonstrating large numbers of trichomonads in the oral tissue; scale bar is 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Trichomonad characterization
Trichomonad infection was confirmed by PCR in all eleven birds. Trichomonas gallinae subtype A2 (n = 5) was isolated in 4 birds from Santa Clara County and 1 bird from San Mateo County. Trichomonas gallinae that could not be fine-typed (n = 4) was isolated in 3 birds from Santa Clara County and 1 bird from San Mateo County. Mixed infection with T. gallinae subtype A2 and T. stableri (n = 1) and mixed infection with un-typed T. gallinae and T. stableri (n = 1) where isolated in birds from Santa Clara County.
4. Discussion
In this study, we document the most widespread and longest duration of an avian trichomonosis epidemic ever recorded for Pacific Coast band-tailed pigeons. Mortality events with ≥5 deaths were reported in 25 counties in California. This outbreak also was of the longest duration ever recorded, beginning in November 2014 and continuing until June 2015. The largest mortality event prior to this time was observed during the winter of 2011–2012 and involved locations in 8 counties and occurred between December 2011 and March 2012 (Girard et al., 2014b). Total mortality for the 2011–2012 events was estimated around 9000 birds, while the total mortality for the 2014–2015 events reported here, likely exceeded 18,000 birds. These findings are significant given that the Pacific Coast band-tailed pigeon population has been declining since the 1960s (Seamans, 2016). Although legal harvest has been maintained at a restrictive level since 1990 because of population decline, low recruitment, and the potential for overharvest, the average annual harvest was still 9760 birds between 2011 and 2015 based on the Migratory Bird Harvest Information Program administered by the U.S. Fish and Wildlife Service (Seamans, 2016). As such, the estimated losses of band-tailed pigeons from this mortality event was approximately two times the amount removed during annual harvest.
Over 90% of infected birds submitted for necropsy were subadults, in their first winter, and adults, suggesting the timing of these events has serious consequences for future recruitment potential. Even when a pair of band-tailed pigeons breeds successfully, they produce on average only one chick per year (Keppie and Braun, 2000), as such, if disease events are frequent and/or severe, mortality will exceed recruitment resulting in population decline. Given the low reproductive rate of this species, high adult survivorship is required to grow the population. With a potentially large number of breeding birds dying due to disease, combined with losses during subsequent fall hunting seasons (Smith, 1968; Silovsky, 1969), adult survivorship likely drops well below management expectations during event years, hampering long-term recruitment potential (Jarvis and Passmore, 1992).
Altering or periodically withholding hunting seasons has been effectively utilized in the past when band-tailed pigeon populations dropped below sustainable levels (Pacific Flyway Council, 2010). Historically, a hunting moratorium was enforced between 1913 and 1931 as a result of overharvest that took place in the late 1800s to the early 1900s prior to the establishment of the Migratory Bird Treaty Act (Grinnell, 1913; Pacific Flyway Council, 2010). More recently, Washington (1991–2001) and British Columbia (1994–2001) have closed hunting seasons in response to the declining population (Seamans, 2016). These and other approaches may need to be considered range-wide to help recover the Pacific Coast population during consecutive years with high disease mortality to sustain the population.
During the 2014–2015 mortality events the clinical symptoms and the pathology of infected birds from Contra Costa, Marin, San Mateo, and Santa Clara counties were different than birds from other locations and during previously investigated events (Girard et al., 2014b; Stromberg et al., 2008). Pigeons admitted to rehabilitation centers within these counties were characterized as having neurological deficits including vision and balance problems. The post-mortem examination of these birds often revealed caseous lesions involving the mucosa extending deep into underlying muscle and bone of the upper palate and into the choana to the sinuses, eye sockets and ear canals causing vestibular disease; manifestations that were not reported in previous studies (Girard et al., 2014b; Stromberg et al., 2008). In addition, roughly 60% of birds examined were in good nutritional condition at the time of death. In fact, birds from Contra Costa, Marin, San Mateo, and Santa Clara counties were on average 10 g heavier than birds collected from the remaining counties. This contrasts sharply with the typical pathology observed in band-tailed pigeons infected by Trichomonas spp., in which individuals become emaciated and die of starvation after lesions blocked the passage of food (Girard et al., 2014b; Rogers et al., 2016b Stromberg et al., 2008). Birds with notable adipose stores at time of death likely succumbed to disease more quickly, perhaps corresponding to infection with a particular parasite strain with high virulence.
Additionally, bacteria were observed multifocally at the fronts of necrosis in six of eleven birds examined histologically. Unfortunately, due to the volume of carcasses received, the carcasses had been frozen prior to necropsy and bacterial cultures were not productive limiting our ability to identify the bacteria present in the lesions. However, the bacteria was identified as a mixture of Gram negative rods and Gram positive cocci indicating their presence was likely secondary to the trichomonosis (Girard et al., 2014b; Stoute et al., 2009).
Consistent with previous surveillance data from the 2011–2012 mortality events (Girard et al., 2014b), the primary trichomonad identified in band-tailed pigeons in this study was T. gallinae subtype A2. While T. gallinae subtype A2 has occasionally been isolated from apparently healthy band-tailed pigeons (Girard et al., 2014b), it has been the only T. gallinae subtype isolated from band-tailed pigeons during mortality events. Interestingly, apart from band-tailed pigeons, the A2 subtype has only rarely been isolated, including from a mourning dove in California (Girard et al., 2014b) and a captive budgerigar (Melopsittacus undulates) in Scotland (Chi et al., 2013), both with oral lesions, and a lesion-free Madagascar turtle dove (Streptopelia picturata) in Seychelles (Lawson et al., 2011). Given the high pathogenicity of T. gallinae subtype A2 in band-tailed pigeons, and susceptibility of other bird species for the parasite (Rogers et al., 2016b), continued surveillance among band-tailed pigeons and conspecifics is warranted. Although fewer band-tailed pigeons in the present study were infected with the newly described parasite T. stableri (Girard et al., 2014a), its isolation during the 2014–2015 mortality events confirms this parasite continues to be an important pathogen for Pacific Coast band-tailed pigeons. Also consistent with the 2011–2012 mortality events (Girard et al., 2014b), some pigeons during the 2014–2015 mortality events were co-infected with T. gallinae and T. stableri.
Warmer temperatures and lower precipitation have been linked to increased Trichomonas spp. infection prevalence in endangered pink pigeons (Nesoenas mayeri) in Mauritius (Bunbury et al., 2007) and winter epidemics in band-tailed pigeons in California (Rogers et al., 2016a). Whether these conditions favor improved viability of trichomonads and/or increase transmission rates is unknown. However, the reduced availability of natural water sources during the drought in California (Hall et al., 2016, California Executive Order B-29-15), meant pigeons were relying more on artificial water sources such as bird baths, garden fountains, retention ponds, and horse troughs. Given the drought and statewide water restrictions (California Executive Order B-29-15), these sources of water may have contained higher amounts of organic debris possibly contributing to parasite persistence (Purple et al., 2015; Purple and Gerhold, 2015). In combination with warmer temperatures, pigeons may have increased their frequency of water consumption putting individuals at higher risk of infection (Swinnerton et al., 2005). In addition to influencing infection dynamics, the drought conditions may also have acted to increase an individual band-tailed pigeon's susceptibility to infection through physiological stress (Fair and Whitaker, 2008). Temperature extremes and drought are predicted to become more frequent in California as the climate changes (Diffenbaugh et al., 2015; Swain et al., 2016), in which case, large-scale mortality events may also become more common, threatening the long-term persistence of the band-tailed pigeon.
Given the increasing frequency of trichomonosis mortality events in combination with other sources of mortality (e.g. harvest, poaching, trauma), more research is needed to fully understand the dynamics of this disease in band-tailed pigeons and the future sustainability of the population. Evaluating pigeon migration and within-season movements in relation to food availability and habitat use may highlight risk factors for parasite transmission at communal sites and/or interaction with other animals. Continued studies of parasite molecular biology and virulence, coupled with host susceptibility data, will improve understanding of risk factors for widespread mortality in Pacific Coast band-tailed pigeons, and pave the way for species management interventions that may mitigate further population decline.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We thank the staff of Animal Rescue Team (Santa Ynez, CA), Bird Rescue Center (Santa Rosa, CA), California Wildlife Center (Malibu, CA), Gold Country Wildlife Rescue (Auburn, CA), Injured and Orphaned Wildlife (Campbell, CA), Lindsay Wildlife Museum (Walnut Creek, CA), Ojai Raptor Center (Oakview, CA), Pacific Wildlife Care (Morro Bay, CA), Peninsula Humane Society and SPCA (San Mateo, CA), Project Wildlife (San Diego, CA), Santa Barbara Wildlife Care Network (Santa Barbara, CA), Sierra Wildlife Rescue (Placerville, CA), SPCA for Monterey County (Salinas, CA), Sulphur Creek Nature Center (Hayward, CA), WildCare (Marin, CA), Wildlife Education and Rehabilitation Center (Morgan Hill, CA), Wildlife Center of Silicon Valley (San Jose, CA), and Wildlife Rehabilitation and Release (Penn Valley, CA) for contributing band-tailed pigeon carcasses for this project. We also thank D. Chudy with Wildlands Conservancy (Yucaipa, CA), L. Foss with CDPH (Richmond, CA), San Jose Animal Control, and members of the public for contributing reports and carcasses. We appreciate the support of CDFW staff for assisting with the mortality investigation including C. Del Signore, N. Carion, N. Clipperton, T. Kasteen, A. Lundberg, R. Mizardi, D. Mollel, J. Ober, S. Pirtle, N. Shirkey, L. Souza, C. Thompson, and S. Torres as well as S. Carter and B. Sieber with University of California, Davis. We also thank B. Smith and T. Goldstein with the One Health Institute, University of California, Davis for assistance with sample processing and molecular analysis. This work was funded by the California Department of Fish and Wildlife. The authors declare no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.06.006.
Contributor Information
Krysta H. Rogers, Email: Krysta.Rogers@wildlife.ca.gov.
Yvette A. Girard, Email: ygirard@cerus.com.
Leslie W. Woods, Email: lwwoods@ucdavis.edu.
Christine K. Johnson, Email: ckjohnson@ucdavis.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Bunbury N., Jones C.G., Greenwood A.G., Bell D.J. Trichomonas gallinae in Mauritian columbids: implications for an endangered endemic. J. Wildl. Dis. 2007;43:399–407. doi: 10.7589/0090-3558-43.3.399. [DOI] [PubMed] [Google Scholar]
- California Executive Order B-29-15. https://www.gov.ca.gov/docs/4.1.15_Executive_Order.pdf. Accessed: 6/29/2017.
- Chi J.F., Lawson B., Durrant C., Beckmann K., John S., Alrefaei A.F., Kirkbride K., Bell D.J., Cunningham A.A., Tyler K.M. The finch epidemic strain of Trichomonas gallinae is predominant in British non-passerines. Parasitology. 2013;140:1234–1245. doi: 10.1017/S0031182013000930. [DOI] [PubMed] [Google Scholar]
- Diffenbaugh N.S., Swain D.L., Touma D. Anthropogenic warming has increased drought risk in California. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:3931–3936. doi: 10.1073/pnas.1422385112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dakhly K.M., El-Nahass E.-S., Inui K., Kimura J., Sakai H., Yanai T. Hypodectes propus (Acarina: Hypoderatidae) in a rufous turtle dove, Streptopelia orientalis (Aves: Columbiformes), in Japan. J. Vet. Sci. 2013;14:421–424. doi: 10.4142/jvs.2013.14.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI, Inc . ESRI, Inc; Redlands, California: 2014. ArcMap. Version 10.3. [Google Scholar]
- Fain A., Laurence B.R. A guide to the heteromorphic deutonymphs or hypopi (Acarina: Hypoderidae) living under the skin of birds, with the description of Ibisidectes debilis gen. and sp. nov. from the scarlet ibis. J. Nat. Hist. 1974;8:223–230. [Google Scholar]
- Fair J.M., Whitaker S.J. Avian cell-mediated immune response to drought. Wilson J. Ornithol. 2008;120 713–819. [Google Scholar]
- Forrester D.J., Foster G.W. Trichomonosis. In: Atkinson C.T., Thomas J.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell; Ames, IA, USA: 2008. pp. 120–153. [Google Scholar]
- Gerhold R.W., Yabsley M.J., Smith A.J., Ostergaard E., Mannan W., Cann J.D., Fischer J.R. Molecular characterization of the Trichomonas gallinae morphologic complex in the United States. J. Parasitol. 2008;94:1335–1341. doi: 10.1645/GE-1585.1. [DOI] [PubMed] [Google Scholar]
- Girard Y.G., Rogers K.H., Gerhold R., Land K.M., Lenaghan S.C., Woods L.W., Haberkern N., Hopper M., Cann J.D., Johnson C.K. Trichomonas stableri n. sp., an agent of trichomonosis in Pacific Coast band-tailed pigeons (Patagioenas fasciata monilis) Int. J. Parasitol. Parasites Wildl. 2014;3:32–40. doi: 10.1016/j.ijppaw.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard Y.G., Rogers K.H., Woods L.W., Chouicha N., Miller W.A., Johnson C.K. Dual-pathogen etiology of avian trichomonosis in a declining band-tailed pigeon population. Infect. Genet. Evol. 2014;24:146–156. doi: 10.1016/j.meegid.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Grabensteiner E., Bilic I., Kolbe T., Hess M. Molecular analysis of clonal trichomonad isolates indicate the existence of heterogenic species present in different birds and within the same host. Vet. Parasitol. 2010;172:53–64. doi: 10.1016/j.vetpar.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Grinnell J. The outlook for conserving the band-tailed pigeon as a game bird of California. Condor. 1913;15:25–40. [Google Scholar]
- Hall L.W., Jr., Anderson R.D., Killen W.D. Spatiotemporal trends analysis of benthic communities and physical habitat during non-severe drought and severe-drought years in a residential creek in California. Calif. Fish Game. 2016;102:55–70. [Google Scholar]
- Hintze J. NCSS, LLC; Kaysville, Utah: 2007. NCSS. Statistical Software. Version 07.1.21. [Google Scholar]
- Jarvis R.L., Passmore M.F. U.S. Fish and Wildlife Service Biological Report; 1992. Ecology of Band-tailed Pigeons in Oregon; p. 6. [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Mentjies P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppie D.M., Braun C.E. Band-tailed pigeon (Patagioenas fasciata) In: Poole A., editor. The Birds of North America Online. Cornell Lab of Ornithology; Ithaca, NY, USA: 2000. http://bna.birds.cornell.edu/bna/species/530 Available online: [Google Scholar]
- Kietzmann G.E., Jr. Relationships of Trichomonas gallinae to the palatal-esophageal junction of ring doves (Streptopelia risoria) as revealed by scanning electron microscopy. J. Parasitol. 1993;79:408–415. [PubMed] [Google Scholar]
- Lawson B., Cunningham A.A., Chantrey J., Hughes L.A., John S.K., Bunbury N., Bell D.J., Tyler K.M. A clonal strain of Trichomonas gallinae is the aetiologic agent of an emerging avian epidemic disease. Infect. Genet. Evol. 2011;11:1638–1645. doi: 10.1016/j.meegid.2011.06.007. [DOI] [PubMed] [Google Scholar]
- McBurney S., Kelly-Clark W.K., Forzan M.J., Lawson B., Tyler K.M., Greenwood S.J. Molecular characterization of Trichomonas gallinae isolates recovered from the Canadian Maritime provinces' wild avifauna reveals the presence of the genotype responsible for the European finch trichomonosis epidemic and additional strains. Parasitology. 2015;142:1053–1062. doi: 10.1017/S0031182015000281. [DOI] [PubMed] [Google Scholar]
- Pacific Flyway Council . U. S. Fish and Wildlife Service; Portland, OR, USA: 2010. Pacific Flyway Management Plan for the Pacific Coast Population of Band-tailed Pigeons. Pacific Coast Band-tailed Pigeon Subcommittee, Pacific Flyway Study Committee; p. 19. Unpublished Report. [Google Scholar]
- Perez-Mesa C., Stabler R.M., Berthrong M. Histological changes in the domestic pigeon infected with Trichomonas gallinae (Jones' Barn strain) Avian Dis. 1961;5:48–60. [Google Scholar]
- Purple K.E., Gerhold R.W. Persistence of two isolates of Trichomonas gallinae in simulated bird baths with and without organic material. Avian Dis. 2015;59:472–474. doi: 10.1637/11089-041115-Reg.1. [DOI] [PubMed] [Google Scholar]
- Purple K.E., Humm J.M., Kirby R.B., Saidak C.G., Gerhold R. Trichomonas gallinae persistence in four water treatments. J. Wildl. Dis. 2015;51:739–742. doi: 10.7589/2014-05-137. [DOI] [PubMed] [Google Scholar]
- Rogers K.H., Girard Y.A., Koenig W.D., Johnson C.K. Ecological drivers and population impacts of avian trichomonosis mortality events in band-tailed pigeons (Patagioenas fasciata), California, USA. J. Wildl. Dis. 2016;52:484–494. doi: 10.7589/2015-02-029. [DOI] [PubMed] [Google Scholar]
- Rogers K.H., Girard Y.A., Woods L., Johnson C.K. Avian trichomonosis in spotted owls (Strix occidentalis): indication of opportunistic spillover from prey. Int. J. Parasitol. Parasites Wildl. 2016;5 doi: 10.1016/j.ijppaw.2016.10.002. 3005–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T.A., Braun C.E. Reevaluation of band-tailed pigeon age classification criteria using wing attributes. Wildl. Soc. Bull. 2014;38:273–278. [Google Scholar]
- Seamans M.E. Division of Migratory Bird Management; Washington D.C., USA: 2016. Band-tailed pigeon Population Status, 2016. U. S. Fish and Wildlife Service; p. 23. [Google Scholar]
- Silovsky G.D. Oregon State University; Corvallis: 1969. Distribution and Mortality the Pacific Coast Band-tailed pigeon; p. 88. M.S. Thesis. [Google Scholar]
- Smith W.A. The band-tailed pigeon in California. Calif. Fish Game. 1968;54:4–16. [Google Scholar]
- Stabler R.M. Trichomonas gallinae, pathogenic trichomonad of birds. J. Parasitol. 1947;33:207–213. [PubMed] [Google Scholar]
- Stabler R.M., Herman C.M. Transactions of the Sixteenth North American Wildlife Conference, March 5-7. Wildlife Management Institute; Washington, D.C., USA: 1951. Upper digestive tract trichomoniasis in Mourning Doves and other birds; pp. 145–163. [Google Scholar]
- Stockdale J.E., Dunn J.C., Goodman S.J., Morris A.J., Sheehan D.K., Grice P.V., Hammer K.C. The protozoan parasite Trichomonas gallinae causes adult and nestling mortality in a declining population of European turtle doves, Streptopelia turtur. Parasitology. 2015;142:490–498. doi: 10.1017/S0031182014001474. [DOI] [PubMed] [Google Scholar]
- Stoute S.T., Charlton B.R., Bickford A.A., Bland M.C. Respiratory tract trichomoniasis in breeder squab candidates in Northern California. Avian Dis. 2009;53:139–142. doi: 10.1637/8389-070108-Case.1. [DOI] [PubMed] [Google Scholar]
- Stromberg M.R., Koenig W.D., Walters E.L., Schweisinger J. Estimate of Trichomonas gallinae-induced mortality in band-tailed pigeons, upper Carmel valley, California, winter 2006-2007. Wilson J. Ornithol. 2008;120:603–606. [Google Scholar]
- Swain D.L., Horton D.E., Singh D., Diffenbaugh N.S. Trends in atmospheric patterns conducive to seasonal precipitation and temperature extremes in California. Science Advances. 2016;2 doi: 10.1126/sciadv.1501344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnerton K.J., Greenwood A.G., Chapman R.E., Jones C.G. The incidence of the parasitic disease trichomoniasis and its treatment in reintroduced and wild pink pigeons Columba mayeri. Ibis. 2005;147:772–782. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.