Highlights
-
•
In locally advanced HNSCC, baseline leukocytosis predicts OS and PFS.
-
•
Respectively 24% and 20% patients displayed baseline leukocytosis or neutrophilia.
-
•
This independent biomarker could help identifying patients with high risk of tumor relapse.
Keywords: Head and neck cancer, Squamous cell carcinoma, Concurrent chemoradiation, Prognostic factor, Biomarkers, Neutrophilia, Leukocytosis
Abstract
Objective
To study the prognostic value of leukocyte increase in a retrospective cohort of locally advanced head and neck squamous cell carcinoma (HNSCC) patients receiving definitive concurrent cisplatin and radiation.
Materials and methods
Clinical records of consecutive previously untreated locally advanced HNSCC patients treated in our Institution between March 2006 and October 2012 by concurrent cisplatin (100 mg/m2, every 3 weeks) and radiation (70 Gy in 7 weeks) were collected. The prognostic value of pretreatment leukocyte increase was examined, with focus on patterns of relapse and survival. Leukocytosis and neutrophilia were defined as a leukocyte count or a neutrophils count exceeding 10 and 7.5 G/L, respectively.
Results
We identified 193 patients, all treated with concurrent cisplatin-based chemoradiotherapy. Respectively 24% and 20% patients displayed baseline leukocytosis or neutrophilia. Mean leukocyte count were significantly more elevated in current smokers, patients with performance status (PS) >0, T4 and less in HPV + tumor. The 5-year actuarial overall survival (OS) and progression-free survival (PFS) were 56% and 51% respectively. In univariate analysis, both leukocytosis and neutrophilia were strongly associated with worse OS and PFS (p < 0.001). In multivariate analysis, N classification, HPV/p16, smoking status and leukocytosis were associated with worse OS and PFS. Patients with <3 cycles of cisplatin had worse survival.
Conclusion
In locally advanced HNSCC treated with concurrent cisplatin and radiation, baseline leukocytosis predicts OS and PFS. In addition with HPV status, this independent biomarker could help identifying patients with high risk of tumor relapse.
Introduction
Standard treatment for non-operated or unresectable locally advanced stage III/IV head and neck squamous cell carcinoma (HNSCC) is concurrent chemoradiotherapy [1]. Standard concomitant chemotherapy is platinum-based, and the most widely used is 3 cycles of cisplatin 100 mg/m2 every 3 weeks [2]. We recently reported a better locoregional control (LRC) in locally advanced HNSCC treated by cisplatin-based chemoradiation than cetuximab-based bioradiotherapy, and a nonsignificant trend towards an improved OS [3]. In addition to HPV-status, the strongest prognosis factors were current smoker status, T4 tumor stage, N3 nodal stage, and use of concomitant cisplatin over cetuximab [4].
Inflammation is a recognized hallmark of tumor progression, and infection, chronic irritation and inflammation have been involved in various steps of oncogenesis [5]. The tumor microenvironment, partially composed of inflammatory cells, orchestrates the neoplastic process, promoting tumor proliferation, survival and migration. In the field of radiotherapy, inflammation is also a key component [6]. Increased numbers of granulocytes have been observed both in the peripheral blood and in tumor tissues of patients with different types of cancer, and a number of functional in vitro and in vivo studies demonstrated that tumors stimulated neutrophils to promote angiogenesis, immunosuppression, invasion, migration and metastasis of the tumor cells [7].
Tumor HPV-status is an independent prognostic factor for overall survival (OS) and progression-free survival (PFS) in HNSCC [8]. Leukocytosis and neutrophilia may be associated with patients’ outcome in HPV-related tumors (e.g. anal, uterine cervical squamous cell carcinoma) [9], [10]. High neutrophil to lymphocytes ratio (NLR) may also correlate with poor prognosis in HNSCC, and predict resistance to first-line platinum based chemotherapy [11], [12]. Yet, more powerful biomarkers are needed to improve patients’ stratification and outcome.
In the current study, the prognostic significance of systemic leukocytosis and neutrophilia on survival and disease control was examined in a single center cohort of locally advanced HNSCC patients homogeneously treated with concurrent cisplatin (100 mg/m2, every 3 weeks) and radiation.
Materials and methods
Patients and tumors
We examined clinical records of consecutive previously untreated and histologically confirmed locally advanced head and neck cancer patients registered in our institution between March 2006 and October 2012. We excluded patients with surgical resection or induction CT prior to RT, patients treated with concurrent carboplatin or other cisplatin schemas than 100 mg/m2 every 3 weeks during radiotherapy, patients treated with concurrent cetuximab, patients with acute or chronic infection or inflammation (such as chronic obstructive pulmonary disease), and 1 patient with pre-treatment immune disorder (auto-immune thrombocytopenia), leaving 193 patients for analysis.
All patients had been referred to a multidisciplinary head and neck tumor board prior to treatment initiation. Explorations at diagnosis included physical examination, endoscopy with biopsy, computed tomography (CT) exploring cervical and thoracic regions, with or without cervical magnetic-resonance imaging (MRI) and positron-emission tomography (PET-CT). Disease staging was defined according to the UICC’s head and neck cancer TNM staging classification, 7th edition. HPV status was determined by p16 expression staining with immunohistochemistry.
Among the 193 patients, median age was 58 years (range: 36–79 years). Of the 135 patients with oropharyngeal localizations (70% of our population), HPV status was “positive” in 38 patients (28%) of oropharyngeal localisation, “negative” in 23 (17%) and “unknown” in 72 patients (53%). Total 111 patients (58%) had T3-T4 disease, and 153 patients (79%) had cervical involved nodes (Table 1a, Table 1b). Risk of recurrence regarding N stage was defined as low risk in N0-N1 patients and high risk in N2a-N3 patients in HPV negative, HPV positive or unknown groups, because of high rates of active smokers and drinkers in French population treated for HNSCC.
Table 1a.
Patients characteristics.
| Characteristics | Overall population | Leukocytes > 10 G/L (baseline) |
|||
|---|---|---|---|---|---|
| No | Yes | p | |||
| Number of patients | 193 (100%) | 145 (75%) | 48 (25%) | ||
| Median [min-max] or Number (%) | |||||
| Gender | Female | 39 (20.2) | 31 (21.4) | 8 (16.7) | 0.619 |
| Male | 154 (79.8) | 114 (78.6) | 40 (83.3) | ||
| Age (years) | 58 [36,79] | 58 [36,79] | 57 [41, 71] | 0.268 | |
| Drinking | Yes | 67 (34.7) | 40 (27.6) | 27 (56.2) | 0.001 |
| No | 126 (65.3) | 105 (72.4) | 21 (43.8) | ||
| Tobacco | Current | 72 (37.3) | 42 (29.0) | 30 (62.5) | <0.001 |
| Former | 82 (42.5) | 68 (46.9) | 14 (29.2) | ||
| No | 39 (20.2) | 35 (24.1) | 4 (8.3) | ||
| WHO Performance Status | PS0 | 148 (76.7) | 117 (80.7) | 31 (64.6) | 0.036 |
| PS1 | 42 (21.8) | 27 (18.6) | 15 (31.2) | ||
| PS2 | 3 (1.6) | 1 (0.7) | 2 (4.2) | ||
| Histology | SCC | 193 (100) | 145 (100) | 48 (100) | NA |
| HPV (p16) status | Pos | 39 (20.2) | 37 (25.5) | 2 (4.2) | 0.005 |
| No | 44 (22.8) | 32 (22.1) | 12 (25) | ||
| NA | 110 (57) | 76 (52.4) | 34 (70.8) | ||
| Localization | Oropharyngeal | 135 (69.9) | 103 (71.0) | 32 (66.7) | 0.010 |
| Oral cavity | 8 (4.1) | 3 (2.1) | 5 (10.4) | ||
| Hypopharyngeal | 21 (10.9) | 13 (9) | 8 (16.7) | ||
| Laryngeal | 29 (15.0) | 26 (17.9) | 3 (6.2) | ||
| T stage over T4 | <T4 | 145 (75.1) | 118 (81.4) | 27 (56.2) | 0.001 |
| T4 | 48 (24.9) | 27 (18.6) | 21 (43.8) | ||
| N stage (UICC 6th) | N0-1 | 73 (37.8) | 59 (40.7) | 14 (29.2) | 0.209 |
| N2-3 | 120 (62.2) | 86 (59.3) | 34 (70.8) | ||
| Metastatic | No | 193 (100) | 145 (100) | 48 (100) | NA |
HPV: Human Papilloma Virus; NA: Not Applicable; NLR: Neutrophil Lymphocyte Ratio.
Table 1b.
Patients treatment characteristics.
| Characteristics | Overall population | Leukocytes >10 G/L (baseline) |
|||
|---|---|---|---|---|---|
| No | Yes | p | |||
| Number of patients | 193 (100%) | 145 (75%) | 48 (25%) | ||
| Median [min–max] or Number. (%) | |||||
| Prior treatments | |||||
| Induction CT | No | 193 (100) | 145 (100) | 48 (100) | NA |
| Prior surgery | No | 192 (99.5) | 144 (99.3) | 48 (100) | 1.000 |
| Yes | 1 (0.5) | 1 (0.7) | 0 (0) | ||
| Following surgery | No | 193 (100) | 145 (100) | 48 (100) | NA |
| Radiotherapy | |||||
| Duration (days) | 49 [4, 70] | 49.0 [31, 70] | 49.50 [4, 64] | 0.539 | |
| >50 days | No | 104 (53.9) | 80 (55.2) | 24 (50.0) | 0.648 |
| Yes | 89 (46.1) | 65 (44.8) | 24 (50.0) | ||
| GTV Tumor dose (Gy) | 70 [12, 75] | 70 [58, 75] | 70 [12, 75] | 0.769 | |
| Fractions delivered | 35 [6, 35] | 35 [25, 35] | 35 [6, 35] | 0.565 | |
| Dose per fraction (Gy) | 2.07 (0.14) | 2.07 (0.14) | 2.07 (0.15) | 0.945 | |
| IMRT | No | 150 (77.7) | 112 (77.2) | 38 (79.2) | 0.938 |
| Yes | 43 (22.3) | 33 (22.8) | 10 (20.8) | ||
| Concurrent chemotherapy | |||||
| CDDP | 193 (100) | 145 (100) | 48 (100) | NA | |
| Number of delivered | 1–2 cycles | 85 (44.0) | 62 (42.8) | 23 (47.9) | 0.648 |
| 3 cycles | 108 (56.0) | 83 (57.2) | 25 (52.1) | ||
CDDP: cisplatin; CT: chemotherapy; GTV: Gross Tumor Volume; NA: Not Applicable;
Treatment characteristics and follow-up
Continuous-course external beam radiotherapy (EBRT) was delivered, 70 Gy in 35 fractions of 2 Gy to the gross tumor volume (GTV. A dose of 60 Gy and 50–54 Gy were delivered to the high- and low-risk clinical target volume (CTV) with 3D-conformational technique for 150 patients (78%) and IMRT for 43 patients (22%). The CTVs were each expanded using 5 mm margins to generate their respective planned target volumes (PTV). Cisplatin was administered at a planned dose of 100 mg/m2, every 3 weeks on days 1, 22, and 43. Eighty-five patients (44%) had 1–2 cycles, 108 patients (56%) had 3 cycles (Table 1a, Table 1b). Optimal cisplatin (CDDP) dose was defined as 3 delivered cycles in both HPV negative, positive or unknown patients because of higher proportion of smokers among our population compared with studies defining optimal dose as 2 delivered cycles in HPV positive patients [13]. Patients were assessed 3 months after the completion of treatment with physical examination and imaging studies and then with physical examination every 3 months for 2 years, every 6 months until 5 years, and every year after 5 years.
Complete blood count analysis
Patients underwent systematic complete white blood cell counts (WBC) weekly during chemoradiation. Pretreatment blood samples taken in the week preceding the first chemotherapy cycle were used for the current analysis. Leukocytosis and neutrophilia, defining biological inflammation, were defined as blood count over 10 G/L and 7.5 G/L respectively, while anemia was defined as hemoglobin count below 12.0 g/dL in female population, and 13.0 g/dL in male population. Thrombocytosis, lymphopenia and monocytosis were defined as platelets count over 400 G/L, lymphocytes count below 1 G/L, and monocytes count over 1 G/L respectively. These cut-off points were chosen because they have been recognized as standard pathological definitions. A study reported worse OS associated with patients with the highest NLR tertile in cohort [11]. We therefore used this 3rd tertile cutoff to define the NLR pathological threshold in our cohort. We tested these parameters for statistical correlation with OS, PFS, LRC (Locoregional Control), and DMC (Distant Metastasis Control).
Statistical analysis
Differences in patient characteristics regarding baseline leukocytosis were compared with Fisher test, Wilcoxon Mann and student-t test, and by variance analysis. Factors associated with tumor relapse were examined. Survival times were defined as the time between the diagnosis and the first event (time of death for OS, time of recurrence or death for PFS, time of loco-regional recurrence LRC and time of distant metastasis for DMC) estimated by the Kaplan Meier method. Patients were censored at the time of the most recent follow-up visit. Survival curves were compared using the log-rank test for the univariate analysis. Multivariate analyses were performed for variables with p value < 0.1 in univariate analysis, according to the Cox proportional hazards model. In the Cox model, neutrophilia and monocytosis were not tested in the same model with leukocytosis, they are subpopulation of leukocytes. Statistical analyses were performed using R (version 3.3.2).
Results
Patients and blood count
On initial blood count, before the first week of EBRT, median leukocytes and neutrophils counts were 8.3 G/L (3.1–39.1) and 5.4 G/L (1.5–33.6), respectively. The NLR 3rd tertile was 4.5 in our cohort. Leukocytosis and neutrophilia were found in 48 patients (25%) and 38 (20%) patients respectively. NLR > 4.5 was the highest tertile in the present population (Table 1a, Table 1b & Supplementary Table S1).
Mean leukocyte count were comparable in patients that achieved 3 cycles of cisplatin vs. 1 or 2 cycles between those with baseline leukocytosis and others (p = 0.500). Current smokers had significantly higher leukocyte, neutrophil, monocyte and platelet counts (p = 0.001, p = 0.013, p < 0.001 and p = 0.004 respectively).
Survival and disease control
With median follow-up of 47 months (4.7–144.4 months), relapses were reported in 93 patients (48%). Loco-regional relapses occurred in 46 patients (24%), metastatic relapses in 35 patients (18%). At last follow-up, 80 patients (41%) had died, all from tumor progression. Estimated 5-years OS was 56% (95%CI: 52–60%), 5-year PFS was 51% (95%CI: 47–55%).
Prognostic value of leukocytosis and neutrophilia
We analyzed in univariate and multivariate analysis prognostic value of leukocytosis, neutrophilia, and significant prognostic factors as described in our previous publications (i.e. PS, tumor T1-3 vs. ≥T4, N0-1 vs. N2-3, current smoker status, and HPV-positive status) [3], [4]. We added the number of cisplatin cycles received, 3 vs 0–2.
In univariate analysis factors significantly associated with worse OS were leukocytosis (p < 0.001), neutrophilia (p < 0.001), anemia (p < 0.001), HPV-negative (vs. positive) status (p < 0.001), T4 (p < 0.001), N2-3 (p = 0.001), current smoker status (p < 0.001), PS ≥1 (p = 0.001), NLR >4.5 (highest tertile; p = 0.019), thrombocytosis (p < 0.001), monocytosis (p = 0.018) and the receipt of less than 3 cycles of cisplatin achieved (p < 0.001). Patients with HPV-negative or HPV-unknown status had similar outcome (p = 0.424).
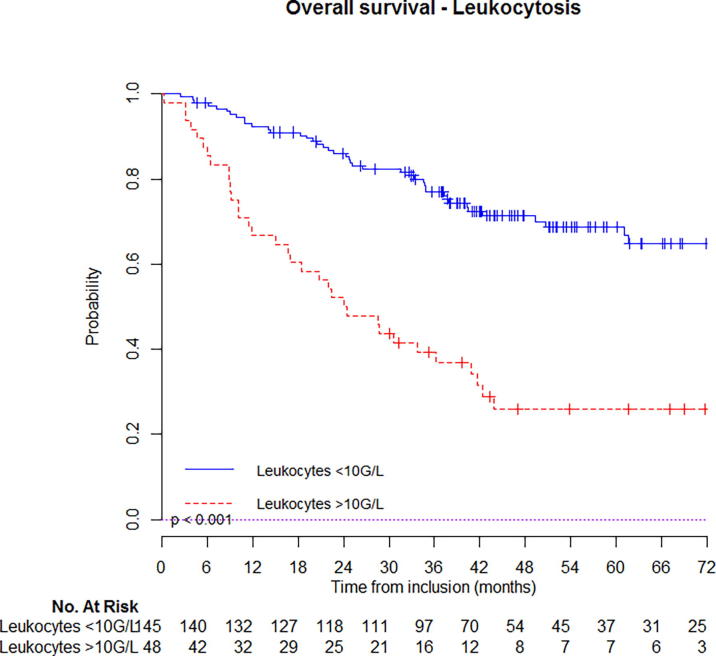
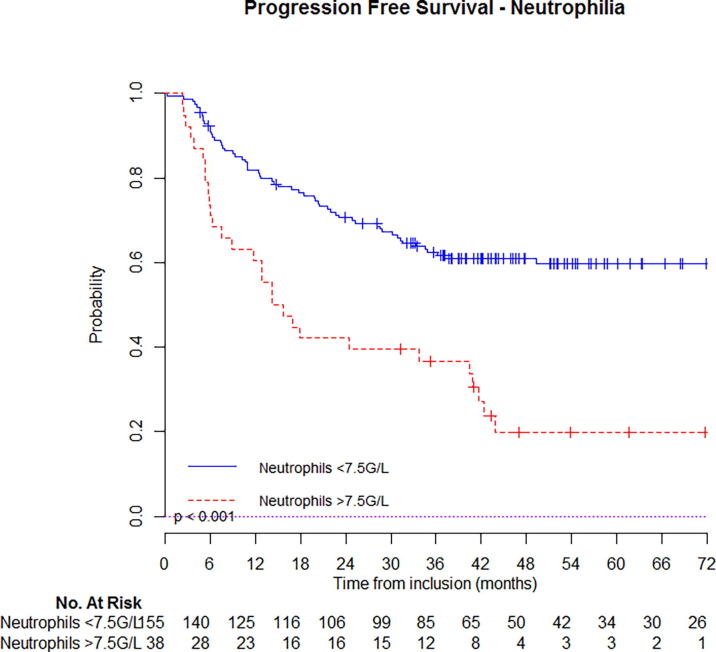
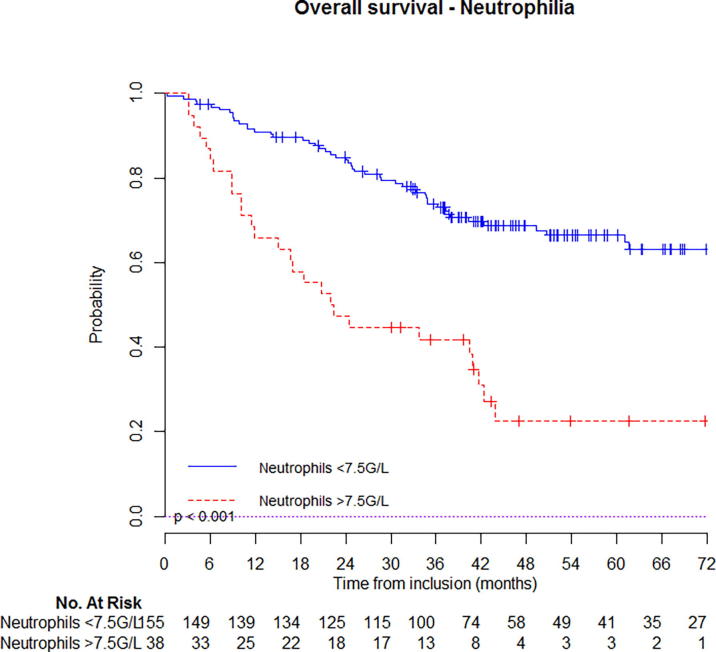
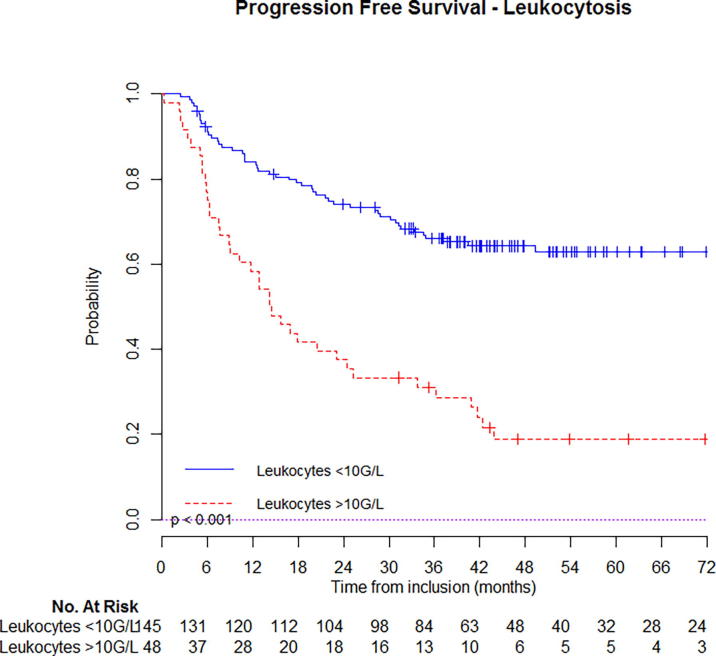
At 5-year follow-up, estimated OS was 67% (95%CI: 63–71%) for patients that had not initial leukocytosis vs. 26% (95%CI: 19–33%) if they had (Fig. 1a); PFS was 62% (95%CI: 58–66%) for patients that had not initial leukocytosis vs. 18% (95%CI: 12–24%) if they had (Fig. 2b). Similar, estimated OS was 66% (95%CI: 59–75%) for patients that had not initial neutrophilia vs. 23% (95%CI: 12–44%) if they had (Fig. 1b); PFS was 60% (95%CI: 52–68%) for patients that had not initial neutrophilia vs. 20% (95%CI: 10–40%) if they had (Fig. 2b). Kaplan-Meier curves with univariate analysis regarding leukocytosis or neutrophilia for locoregional control, local control, regional control, and distant metastasis control are displayed in Supplementary appendix (Figs. S1 & S2) (Fig. 2a).
Fig. 1a.
Estimated overall survival in patients with or without leukocytosis.
Fig. 2b.
Estimated progression free survival in patients with or without neutrophilia.
Fig. 1b.
Estimated overall survival in patients with or without neutrophilia.
Fig. 2a.
Estimated progression free survival in patients with or without leukocytosis.
Using multivariate analysis, apart from T and N classification, HPV/p16, smoking status, leukocytosis was independently associated with worse OS with hazard ratio (HR) of 2.02 (95% confidence interval (CI): 1.23–3.32, p = 0.006) and worse PFS with HR of 1.87 (95% CI: 1.17–2.97, p = 0.008). Patients who did not receive 3 cycles of cisplatin had also worse OS (p = 0.001), PFS (p < 0.001), and LRC (p = 0.006) (Table 2a). Leukocytosis correlated with OS in subgroups of patients that had <3 cisplatin cycles (p < 0.001) as well as in subgroup that achieved 3 cisplatin cycles (p < 0.001). Similar prognostic factors were found for PFS, especially for leukocytosis (p = 0.008). In a separate multivariate analysis, using NLR >4.5 (highest tertile) instead of leukocytosis or neutrophilia, NLR wasn’t related with OS or PFS. Similar, monocytosis was not independently associated with patients’ OS or PFS.
Table 2a.
Results of univariate and multivariate (Cox) analyses (significant factors in bold).
| Variable | Overall Survival n = 193 patients; events = 80 |
Progression Free Survival n = 193 patients; events = 93 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox analysis |
Multivariate Cox analysis |
Univariate Cox analysis |
Multivariate Cox analysis |
|||||||||
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Leukocytosis (absence) | 3.46 | 2.21–5.41 | <0.001 | 2.02 | 1.23–3.32 | 0.006 | 3.19 | 2.10–4.85 | <0.001 | 1.87 | 1.17–2.97 | 0.008 |
| Anemia (absence) | 3.08 | 1.98–4.79 | <0.001 | 1.54 | 0.90–2.64 | 0.115 | 2.94 | 1.95–4.43 | <0.001 | 1.54 | 0.94–2.53 | 0.097 |
| PS 1–2 (vs. 0) | 2.23 | 1.41–3.53 | 0.001 | 1.22 | 0.72–2.04 | 0.460 | 2.19 | 1.43–3.36 | <0.001 | 1.1 | 0.68–1.76 | 0.716 |
| Current smoker (non current smokers) | 2.78 | 1.79–4.33 | <0.001 | 2.45 | 1.47–4.08 | 0.001 | 3.00 | 1.98–4.53 | <0.001 | 2.66 | 1.68–4.23 | <0.001 |
| HPV - (vs. HPV +) (vs HPV unknown) |
2.26 0.82 |
1.50–3.05 0.51–1.32 |
<0.001 0.424 |
5.19 0.81 |
4.22–6.21 0.48–1.37 |
0.008 0.433 |
1.78 0.75 |
1.22–3.04 0.48–1.17 |
<0.001 0.200 |
3.19 0.78 |
2.09–4.58 0.48–1.25 |
0.013 0.170 |
| T4 (vs. ≤T3) | 2.45 | 1.54–3.90 | <0.001 | 1.47 | 0.85–2.55 | 0.167 | 2.27 | 1.48–3.51 | <0.001 | 1.49 | 0.90–2.47 | 0.195 |
| N2-3 (vs. N0-1) | 2.27 | 1.35–3.80 | 0.002 | 2.03 | 1.15–3.57 | 0.014 | 2.24 | 1.39–3.59 | 0.001 | 1.90 | 1.15–3.16 | 0.013 |
| CDDP x3 (vs. <3) | 0.51 | 0.37–0.70 | <0.001 | 0.56 | 0.40–0.78 | 0.001 | 0.54 | 0.41–0.73 | <0.001 | 0.58 | 0.43–0.79 | <0.001 |
Leukocytosis was also significantly associated with worse LRC (p = 0.001) and DMC in univariate analysis (p < 0.001) but not in multivariate analysis (Table 2b). Anemia predicted for lower DMC in the multivariate analysis (p = 0.038).
Table 2b.
Results of univariate and multivariate (Cox) analyses (significant factors in bold).
| Locoregional Control n = 193 patients; events = 46 |
Distant Free Metastasis n = 193 patients; events = 35 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox analysis |
Multivariate Cox analysis |
Univariate Cox analysis |
Multivariate Cox analysis |
|||||||||
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Leukocytosis (absence) | 2.66 | 1.46–4.82 | 0.001 | 1.37 | 0.70–2.66 | 0.348 | 3.12 | 1.59–6.11 | 0.001 | 1.95 | 0.92–4.12 | 0.081 |
| Anemia (absence) | 2.15 | 1.19–3.87 | 0.011 | 1.26 | 0.64–2.52 | 0.496 | 4.15 | 2.13–8.13 | <0.001 | 2.23 | 1.01–4.91 | 0.047 |
| PS 1–2 (vs. 0) | 1.32 | 0.62–2.83 | 0.899 | – | – | – | 3.00 | 1.53–5.86 | 0.001 | 1.63 | 0.77–3.44 | 0.199 |
| Current smoker (non current smokers) | 3.21 | 1.78–5.79 | <0.001 | 3.13 | 1.64–5.96 | 0.001 | 2.75 | 1.41–5.38 | 0.003 | 2.47 | 1.14–5.35 | 0.022 |
| *HPV − (vs. HPV +) (vs HPV unknown) |
2.79 1.18 |
2.10–5.03 0.55–2.53 |
0.007 0.605 |
8.1 0.69 |
4.71–11.70 0.36–1.34 |
0.021 0.277 |
1.37 0.65 |
1.04–2.89 0.32–1.35 |
0.043 0.250 |
1.53 1.60 |
0.19–2.21 0.28–1.37 |
0.494 0.238 |
| T4 (vs. ≤T3) | 1.93 | 1.04–3.57 | 0.038 | 1.42 | 0.70–2.89 | 0.334 | 2.10 | 1.04–4.23 | 0.037 | 1.05 | 0.43–2.14 | 0.911 |
| N2-3 (vs. N0-1) | 2.88 | 1.39–5.98 | 0.004 | 2.70 | 1.26–5.78 | 0.011 | 3.46 | 1.43–8.33 | 0.006 | 2.72 | 1.06–6.93 | 0.037 |
| CDDP x3 (vs. <3) | 0.54 | 0.36–0.82 | 0.003 | 0.56 | 0.37–0.85 | 0.006 | 0.62 | 0.39–0.99 | 0.047 | 1.44 | 0.42–1.15 | 0.154 |
Anemia: hemoglobin <13 g/dL in male or <12 g/dL in female population; CDDP: cisplatin; HPV: Human Papilloma Virus; Leukocytosis: leukocyte count >10 G/L; PS: Performance status.
Discussion
Leukocytosis is easily obtainable and cheap marker of systemic inflammation that may assist in clinical decisions regarding recurrence and survival among locally advanced HNSCC patients undergoing CRT.
Leukocytosis is common in patients with progressive oral squamous cell carcinoma, is related with T-classification, lymphovascular permeation, and recurrence or metastasis, therefore could decrease survival [14]. Previous studies evidenced that the burden of solid tumours (either large, bulky, locoregional or disseminated tumours) correlated with the degree of leukocytosis [15]. In HNSCC, pretreatment lymphocyte to monocyte ratio, neutrophils and monocyte absolute count has also been related with prognosis in previous studies [16], [17]. In previous cohort, neutrophils count as a continuous value was an independent prognosis factor associated with worse OS and LRC in both HPV-positive and negative HNSCC, with higher mean neutrophil count in HPV-negative patients (5.9 G/L) vs. HPV-positive patients (5.0 G/L) [18]. In our study, leukocytosis and neutrophilia were found in 24% and 20% patients, respectively. The large number of smokers in our population could explain these elevated proportions. Still, current smoking status and leukocytosis were both independent prognosis factors for survival. As mean leukocyte count was higher with patients with PS >0, T4 tumors and lower in HPV-positive patients, leukocytosis appears to be connected with established prognosis factors. Leukocytosis, reflecting elevated neutrophil and monocyte counts in our population, independently predicted OS and PFS from these parameters. Leukocytosis was not associated with patients able to receive 3 cycles of cisplatin (p = 0.500), which could have been a bias in patients with inflammatory cancers.
The anticancer activity of cisplatin does not only inhibit mitosis, it also stimulate the innate and adaptive immune system by promoting specific rearrangements on death tumor [19]. In our analysis, leukocytosis independently predicted PFS and LRC from number of achieved cisplatin cycles. 3 cycles of cisplatin achieved better PFS or LRC than 1 or 2 cycles in patients with leukocytosis as well as in patients with normal leukocyte count. This illustrate similar impacts on patient’s outcome and suggesting that leukocytes count couldn't predict treatment by different cycles of cispaltin and radiotherapy. As evidenced recently, HPV-positive HNSCC may not require a total of 3 concurrent cisplatin 100 mg/m2 cycles; yet we considered 3 cycles as optimal both in HPV positive and negative or unknown patients because of large proportion of heavy smokers and drinkers in French HNSCC patients, confirmed in our analysis [13].
NLR may predicts outcome in HNSCC [21], [22]. In different cohorts of HNSCC with NRL analysis, cutoff ratio is still debated, between 1.9 and 5.0, highest tertile or quartile for others [11], [22], [23]. Variances between cutoffs in previously published NLR ratios illustrate 3 different immune conditions: (1) isolated increased neutrophils count, (2) decreased lymphocytes count, (3) association of both [24]. In our study, lymphocytes count was similar in patients that experienced death or recurrence during follow-up compared with others, and NLR >4.5 (highest tertile) wasn’t related with OS or PFS in multivariate analysis.
Tumor-related leukocytosis results from hematopoietic colony-stimulating factors and inflammatory cytokines from solid tumors [9], [25]. Production of cytokines, chemokines and granule proteins promotes, which promotes tumor growth, angiogenesis, and increase its metastatic potential [26]. They are also recruited to tumor microenvironment during radiotherapy, inducing angiogenesis that could offset treatment’s effectiveness [27], [28]. In our study, leukocytosis in recurrent vs. non-recurrent population was related with both increased neutrophil and monocyte count, and isolated neutrophilia wasn’t independently associated with outcome.
In head and neck cancers, tumor oxygenation independently affects tumor evolution, and baseline anemia decreases survival [30], [31]. Previous study showed independent relationships between high-risk patients presenting inflammatory phenotypes and baseline anemia, thrombocytosis and monocytosis [32]. In our study, anemia was associated with inferior OS and PFS in non adjusted analysis but still, anemia independently predicts DMC.
The strength of this study is the robust association between leukocytosis and poor prognosis in a homogenous cohort of HNSCC, treated with concurrent cisplatin. This study is consistent with previously published and biological recent findings, notably the negative impact of a cumulative cisplatin dose below 300 mg/m2 [33].
The main limitations of this study include its retrospective design, the high-rate of HPV-unknown status patients (77%), and proportion of patients with baseline leukocytosis (24%) or neutrophilia (20%) compared to literature [11], [18], [22], [32]. High rate of HPV-undetermined status has to be balanced by the French particularity with heavy smokers rates: 60% patients with history of smoking, 32% of PCR DNA positivity, 26.7% of RT-PCR RNA E6E7 positivity, and 24.5% of p16 false positivity (vs. DNA or RNA) [34]. In our institution during the inclusion period (2006–2012), HPV/P16 status was mostly achieved in the non-smoker and non-drinker population. In this study, patients with HPV/P16 negative or unknown status had relative similar outcome, compared with those with positive status (Supplementary Fig. S3). Inherent limitation in identifying patients with high risk of recurrence from leukocytosis is that such patients have multiple adverse prognosis factors and might not be suited for more aggressive treatment.
Conclusion
Pretreatment hematologic profile with initial leukocytosis is a clinically relevant biomarker for OS and PFS in patients with locally advanced HNSCC treated with concomitant cisplatin and radiation. In addition with HPV status, this independent biomarker could help identifying patients with high risk of tumor relapse.
Acknowledgments
Acknowledgements
None.
Conflict of interest
None.
Financial disclosure
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.07.002.
Appendix A. Supplementary data
References
- 1.Tao Y., Daly-Schveitzer N., Lusinchi A., Bourhis J. Advances in radiotherapy of head and neck cancers. Curr Opin Oncol. 2010;22:194–199. doi: 10.1097/cco.0b013e3283388906. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein D.J. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Levy A., Blanchard P., Bellefqih S., Brahimi N., Guigay J., Janot F. Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlenther Onkol. 2014;190:823–831. doi: 10.1007/s00066-014-0626-0. [DOI] [PubMed] [Google Scholar]
- 4.Ou D., Levy A., Blanchard P., Nguyen F., Garberis I., Casiraghi O. Concurrent chemoradiotherapy with cisplatin or cetuximab for locally advanced head and neck squamous cell carcinomas: does human papilloma virus play a role? Oral Oncol. 2016;59:50–57. doi: 10.1016/j.oraloncology.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy A., Chargari C., Marabelle A., Perfettini J.-L., Magné N., Deutsch E. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur J Cancer Oxf Engl. 1990;2016(62):36–45. doi: 10.1016/j.ejca.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Dumitru C.A., Lang S., Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F. Human Papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi S., Matsumoto Y., Isohashi F., Yoshioka Y., Ohashi H., Morii E. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol Oncol. 2011;122:25–32. doi: 10.1016/j.ygyno.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee R., Roxin G., Eliasziw M., Joseph K., MacLean A., Buie W.D. The prognostic significance of pretreatment leukocytosis in patients with anal cancer treated with radical chemoradiotherapy or radiotherapy. Dis Colon Rectum. 2013;56:1036–1042. doi: 10.1097/DCR.0b013e31829ab0d4. [DOI] [PubMed] [Google Scholar]
- 11.Rachidi S., Wallace K., Wrangle J.M., Day T.A., Alberg A.J., Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma: neutrophils and lymphocytes: prognostic value in HNSCC. Head Neck. 2016;38:E1068–74. doi: 10.1002/hed.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., Yuan D., Liu H., Gu X., Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spreafico A., Huang S.H., Xu W., Granata R., Liu C.-S., Waldron J.N. Impact of cisplatin dose intensity on human papillomavirus-related and -unrelated locally advanced head and neck squamous cell carcinoma. Eur J Cancer. 2016;67:174–182. doi: 10.1016/j.ejca.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.-W., Chen I.-L., Lin I.-C., Kao S.-Y. Prognostic value of hypercalcaemia and leucocytosis in resected oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2014;52:425–431. doi: 10.1016/j.bjoms.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Granger J.M., Kontoyiannis D.P. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115:3919–3923. doi: 10.1002/cncr.24480. [DOI] [PubMed] [Google Scholar]
- 16.Kano S., Homma A., Hatakeyama H., Mizumachi T., Sakashita T., Kakizaki T. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2016 doi: 10.1002/hed.24576. [DOI] [PubMed] [Google Scholar]
- 17.Valero C., Pardo L., López M., García J., Camacho M., Quer M. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer: peripheral neutrophils, monocytes, and lymphocytes in HNSCC. Head Neck. 2016 doi: 10.1002/hed.24561. [DOI] [PubMed] [Google Scholar]
- 18.Huang S.H., Waldron J.N., Milosevic M., Shen X., Ringash J., Su J. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status: leukocyte and oropharyngeal cancer outcomes. Cancer. 2015;121:545–555. doi: 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 19.Bracci L., Schiavoni G., Sistigu A., Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon H., Roh J.-L., Lee S., Kim S.-B., Choi S.-H., Nam S.Y. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol. 2016;118:330–334. doi: 10.1016/j.radonc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Charles K.A., Harris B.D.W., Haddad C.R., Clarke S.J., Guminski A., Stevens M. Systemic inflammation is an independent predictive marker of clinical outcomes in mucosal squamous cell carcinoma of the head and neck in oropharyngeal and non-oropharyngeal patients. BMC Cancer. 2016;16:124. doi: 10.1186/s12885-016-2089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong B.Y.W., Stafford N.D., Green V.L., Greenman J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma: neutrophil-to-lymphocyte ratio in laryngeal cancer. Head Neck. 2016;38:E1903–8. doi: 10.1002/hed.24346. [DOI] [PubMed] [Google Scholar]
- 24.Guthrie G.J.K., Charles K.A., Roxburgh C.S.D., Horgan P.G., McMillan D.C., Clarke S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Kojima K., Nakashima F., Boku A., Muroishi Y., Nakanishi I., Oda Y. Clinicopathological study of involvement of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor in non-lymphohematopoietic malignant tumors accompanied by leukocytosis. Histol Histopathol. 2002;17:1005–1016. doi: 10.14670/HH-17.1005. [DOI] [PubMed] [Google Scholar]
- 26.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 27.Kozin S.V., Kamoun W.S., Huang Y., Dawson M.R., Jain R.K., Duda D.G. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn G.-O., Brown J.M. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle. 2009;8:970–976. doi: 10.4161/cc.8.7.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker A., Stadler P., Lavey R.S., Hänsgen G., Kuhnt T., Lautenschläger C. Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2000;46:459–466. doi: 10.1016/s0360-3016(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 31.Brizel D.M., Dodge R.K., Clough R.W., Dewhirst M.W. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen M.-H., Chang P.M.-H., Chen P.-M., Tzeng C.-H., Chu P.-Y., Chang S.-Y. Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J Cancer Res Clin Oncol. 2009;135:1783–1790. doi: 10.1007/s00432-009-0625-1. [DOI] [PubMed] [Google Scholar]
- 33.Strojan P., Vermorken J.B., Beitler J.J., Saba N.F., Haigentz M., Bossi P. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: a systematic review: cumulative cisplatin dose in head and neck cancer chemoradiotherapy. Head Neck. 2016;38:E2151–8. doi: 10.1002/hed.24026. [DOI] [PubMed] [Google Scholar]
- 34.Lacau Saint Guily J., Pretet J., Agius A., Rousseau A., Barry B., Dufour X. PAPILLOPHAR: impact of the human papillomavirus (HPV) status in the prognosis of oropharynx squamous cell carcinoma (OSCC) J Clin Oncol. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.