Abstract
We estimated national-level trends in the prevalence of probable active syphilis in adult women using the Spectrum Sexually Transmitted Infections (STI) model to inform program planning, target-setting, and progress evaluation in STI control. The model fitted smoothed-splines polynomial regressions to data from antenatal clinic surveys and screening and representative household surveys, adjusted for diagnostic test performance and weighted by national coverage. Eligible countries had ≥1 data point from 2010 or later and ≥3 from 2000 or later from adult populations considered representative of the general female population (pregnant women or community-based studies). Between 2012 and 2016, the prevalence of probable active syphilis in women decreased in 54 (41%) of 132 eligible countries; this decrease was substantive (≥10% proportionally, ≥0.10% percentage-point absolute difference and non-overlapping 95% confidence intervals in 2012 and 2016) in 5 countries. Restricting eligible data to prevalence measurements of dual treponemal and non-treponemal testing limited estimates to 85 countries; of these, 45 countries (53%) showed a decrease. These standardized trend estimates highlight the need for increased investment in national syphilis surveillance and control efforts if the World Health Organization target of a 90% reduction in the incidence of syphilis between 2018 and 2030 is to be met.
Introduction
Syphilis is an infection caused by the spirochete Treponema pallidum. It can be transmitted through sexual activity and from a mother to fetus vertically during pregnancy or newborn during childbirth. When untreated, syphilis causes substantial morbidity and mortality, not only in adults, but also in infants and young children as congenital syphilis.
Many countries have committed to reducing rates of adult syphilis, and to eliminating congenital syphilis. The World Health Organization (WHO) “Global Health Sector Strategy on Sexually Transmitted Infections (STIs) 2016–2021” has two syphilis related targets1,2: a 90% reduction in Treponema pallidum incidence globally between 2018 and 2030, and 50 or fewer cases of congenital syphilis per 100,000 live births in 80% of countries. Monitoring progress toward these targets is hampered by the quality and quantity of national data. Many national programs lack an estimate of their current burdens of adult and congenital syphilis, compromising not only progress evaluation but also target-setting, program planning, and costing3.
The WHO produces global and regional syphilis prevalence and incidence estimates for adult women roughly every four years. The most recent estimates were for 2012, when WHO estimated that the global prevalence of syphilis in adult men and women were 0.49% (0.4–0.6%) and 0.48 (0.3–0.7%) and there were 350,000 adverse pregnancy outcomes in infected pregnant women4. The Institute of Health Metrics and Evaluation (IHME) also estimated syphilis burdens5. However, to date, there has been no systematic exploration of country-level trends.
The Spectrum Sexually Transmitted Infections (STI) model (Spectrum-STI) was incorporated into the Spectrum suite of health policy models in 2016 as a tool that countries can use to estimate trends in the prevalence and incidence of syphilis, gonorrhea and chlamydia6–8. The model estimates national adult prevalence trends by fitting statistical models to prevalence data, adjusted for diagnostic test performance and weighted by national coverage.
In this paper, the Spectrum-STI model and the Spectrum syphilis database were used to examine trends over time in the prevalence of probable active syphilis in women 15 to 49 years of age between 2012 and 2016. The focus of the paper is on the methods and estimates of trends across countries and cross-country patterns in data availability, but not on the results for individual countries. The paper also explores how data eligibility criteria and statistical methods influence the estimates.
Methods
Spectrum-STI model
The basic structure of the Spectrum-STI model has been described6. Some refinements have been made to the model since the initial country applications:
Prevalence data are fitted to calendar year using segmented, second-order polynomial spline regressions9–11, with a maximum of two knots rather than a simple logistic regression (Supplementary Information 1(SI1)). This allows for up to three historic phases or trends in a country’s prevalence of syphilis.
Prevalence is fitted through the corresponding incidence rate (SI1). The constraint that incidence must be ≥0 in any year ensures that there are no unrealistic sharp falls in prevalence from one year to the next, which would be inconsistent with epidemiologically valid incidence rates and average duration of infection.
A random effects component has been included to account for heterogeneity across prevalence measurements within a country owing to the variability in prevalence measures due to variations in the sampled study population, other than sampling variation. Prevalence estimates were resampled following beta distributions, to which noise was added in the logit scale and simulated as a function of the population sampled to account for uncertainty and possible biases associated with pooling prevalence data across study populations (surveys in Antenatal Care clinics (ANC), routine ANC screening, adult women not in antenatal care, adult men, and adult men and women without sex disaggregation). This guards against over-interpreting fluctuations between successive data points reflecting unaccounted sources of variation, including reporting errors, in the data.
Prevalence time trends were extrapolated to two years after the latest national data point, and to two years before the first available national data point. For earlier and later years, prevalence was assumed to be constant at the earliest and latest estimates.
Infection episode durations, used to inform the relationship between incidence and prevalence, were taken as assumed in the WHO’s 2012 global syphilis estimations4: Weighted between treated and untreated episodes, the weighted duration was set at 1.28 years in countries with good STI treatment access (high income countries), 2.42 years in countries with moderate access (South and Central Americas and the Caribbean, Oceania, East and Central Asia, Central Europe, Eastern Mediterranean) and 4.13 years in countries with poor access (sub-Saharan Africa, and South and South-East Asia). Each of these values was assumed to have a standard error of ±50%.
As in the original 2016 model, estimated prevalence was constrained to a maximum value of 20% for all years and all countries to avoid extrapolations to overly high (and unrealistic) prevalence values. The 20% maximum value was based on available data from low- and middle-income countries since 19904,12.
Statistical analyses were carried out in R version 3.4.013. Bootstrapping (400 replications) was used to generate uncertainty bounds6. In each bootstrap iteration, three sets of variables were resampled: prevalence data, diagnostic test adjustment values, and duration of infection. The fitting procedure was applied to the simulated/bootstrapped data, and confidence intervals (CIs) were derived using the percentile method.
Spectrum syphilis database
The Spectrum-STI global syphilis database is a comprehensive compilation of syphilis prevalence data from a number of sources including the Global AIDS Monitoring system (GAM) database14, databases compiled by WHO and by IHME, data identified from other literature reviews12,14–21 and data shared during country applications of the Spectrum-STI tool22–27 (see details in SI2). The version used in the present analysis includes data identified and compiled as of 2nd May 2018.
The analysis in this paper is based on the subset of the studies in the database that met the following criteria: (i) the population could be considered representative of the general population (e.g. pregnant women, women at delivery, women attending family planning clinics, and individuals selected for participation in a Demographic and Health Survey); (ii) specimens were collected between 1990 and 2016, and (iii) syphilis was diagnosed using either a non-treponemal or treponemal serological test, or both. Studies conducted among the following non-representative groups were excluded: patients seeking care for an STI or genital symptoms, women with abnormal Papanicolaou smears, women attending gynaecology or sexual health clinics, remote or indigenous populations, men who have sex with men (MSM), and female sex workers (FSW).
All duplicate data points were removed. Studies with a reported prevalence of 0% were approximated as 1 case divided by 100 times the sample size in order to facilitate the estimation of uncertainty intervals. For studies that provided data for two or more years the data were entered separately by calendar year and each year was counted as one data point. When separation by calendar year was not possible, the study was entered as one data point at the mid-point of the study period.
In addition, to generate estimates for overall national women’s populations, we increased the weighted prevalence estimates by 10% to reflect under-sampling of higher-risk populations in ANC and general population surveys, as was done by WHO in their regional and global estimates4.
Adjusting for diagnostic test
All eligible data were adjusted to account for the diagnostic test used in the study. We defined probable active syphilis as concurrent positivity on a non treponemal and treponemal test. This is the definition recommended and used by the WHO4 and by the IHME5. Prevalence data from studies that used either a treponemal or non-treponemal test alone were adjusted using a method described in previous meta-analyses12,28–30: prevalence values for studies using only treponemal or non-treponemal tests without confirmatory tests were multiplied by 0.53 and values from studies where the diagnostic test was unknown by 0.7515,26–28. Values based on Rapid treponemal-based test were multiplied by 0.70, as these tests are believed to be relatively specific compared to conventional treponemal tests6. Each adjustment multiplier was assumed to have a standard error of ±25%.
Country eligibility
Prevalence trends were generated for the subset of countries in the Spectrum syphilis database that met the study inclusion criteria and had at least one data point post-2010 and three or more data points from 2000 or later.
Generating national estimates
All of the data from a country were pooled after adjusting for diagnostic tests. When pooling data, the prevalence in pregnant women attending ANC and in adult women in the general population4 was assumed to be the same, and the male-to-female ratio was set at 1:1, in keeping with WHO’s 2012 global estimates4 and supported by a recent global meta-analysis29 of national household and other general population surveys31–33. In addition, it was assumed that all qualifying data were representative, i.e. no adjustments were made for possible over-sampling of urban or rural sites, or for urban/rural prevalence differences.
Each data point in a country was assigned a weight to reflect how representative it was of the national population (see Table SI3). The weighted prevalence data were scaled within each country by dividing by sample size, so that a study’s sample size did not influence the estimated national prevalence level or trend. Sample size, however, did influence the uncertainty ranges obtained using bootstrap resampling, as described above.
Time trend analysis
The time trend analysis focused on changes in the median prevalence of active syphilis between 2012 and 2016 based on 400 bootstraps per country and on the average annual proportional decline between 2012 and 2016. Countries were grouped into four categories according to the change in their median estimated prevalence from 2012 to 2016 (proportional as well as absolute, so as to cover the public health significance of the change) combined with the precision of the median estimates in those two years:
Substantive decrease: the prevalence decreased, the absolute difference between the median estimated prevalence at 2012 and 2016 was ≥10% proportionally, ≥0.05% as a percentage point difference, and the lower-bound of the 2012 estimate was above the upper-bound of the 2016 estimate;
Non-substantive decrease: the median estimated prevalence decreased from 2012 to 2016, but the country did not meet the criteria above to be classified as substantive decrease;
Substantive increase: the prevalence increased, the absolute difference between the median estimated prevalence at 2012 and 2016 was ≥10% proportionally, ≥0.05% as a percentage point, and the upper-bound of the 2012 estimate was below the lower-bound of the 2016 estimate;
Non-substantive increase: the median estimated prevalence increased from 2012 to 2016, but the country did not meet the criteria above to be classified as substantive increase.
We also assessed the average annual proportional decline over 2012 to 2016, relative to the annual decline rate that would be expected to meet the WHO global strategy target of 90% reduction from 2018 to 20301.
Sensitivity analysis
Univariate sensitivity analyses were performed to evaluate the influence of key parameters, choices and uncertainties, on the estimated median prevalence across the modelled countries in 2012 and 2016, and on the number of countries in each of the time trend categories. Parameters and choices explored included tightening the eligibility criteria for the trend analysis to those studies conducted only in ANC women (Scenario A), or requiring studies to have one or more data points from 2012 or later rather than 2011 or later (Scenario B). In addition, we explored the effect of reducing the number of years of prevalence data included in the analysis to data from 2005 and later (Scenario C) and to restricting the analysis to only those studies with results from both a treponemal and non-treponemal test (Scenario D). We also explored a variant statistical model where trends were extrapolated to one rather than two years after and before the latest and earliest national data point (Scenario E).
Lastly, we looked at the impact of expanding the study entry criteria to include countries with prevalence data from routine blood donor screening34–46 (Scenario F). In this scenario, we assessed time trends in countries that did not meet the default country eligibility criteria, but that could be estimated if the eligibility criteria were expanded to include data from the screening of blood donations from 2011 or later.
Results
Syphilis prevalence data
The Spectrum-STI syphilis database, as of 02 May 2018, contained one or more data points for 186 countries (range per country: 1 to 61 data points; median 6 data points; SI5) that met the study entry criteria. Most data were from routine ANC screening (645 of 1,576 data points; 161 million tests out of 268 million total tests; Table 1), followed by ANC surveys (605 data points, 8.4 million tests). Most studies used dual Rapid Plasma Reagin (RPR) and Treponema pallidum hemagglutination assay (TPHA) testing (819 data points, 109 million tests), followed by tests of unknown type and RPR testing alone.
Table 1.
Adult syphilis prevalence data (1990–2016), available in the Spectrum-STI global database.
| Average year | Number of studies, surveys, and years of routine ANC screening | Number of positive samples | Number of samples tested | Unweighted average prevalence (%) | |
|---|---|---|---|---|---|
| Data type | |||||
| ANC routine | 2012 | 645 | 1,530,591 | 161,090,586 | 0.95 |
| ANC survey | 2005 | 605 | 113,813 | 8,422,062 | 1.35 |
| Women survey | 2003 | 93 | 13,006 | 2,333,441 | 0.56 |
| Men survey | 2003 | 78 | 5,184 | 247,945 | 2.09 |
| Men + Women survey | 2005 | 11 | 64,659 | 3,039,211 | 2.13 |
| Blood donors, Women | 2007 | 2 | 3 | 693 | 0.43 |
| Blood donors, Men | 2010 | 12 | 1,628 | 250,398 | 0.65 |
| Blood donors, Men + Women | 2012 | 130 | 147,715 | 92,247,011 | 0.16 |
| Diagnostic test | |||||
| RPR + TPHA | 2008 | 819 | 652,567 | 108,529,201 | 0.60 |
| TPHA | 2006 | 71 | 107,382 | 12,299,956 | 0.87 |
| RPR | 2007 | 353 | 592,635 | 42,606,321 | 1.39 |
| Rapid treponemal-based assay | 2013 | 79 | 143,180 | 10,107,457 | 1.42 |
| Test unknown | 2011 | 254 | 380,835 | 94,088,412 | 0.40 |
| WHO world region | |||||
| African Region | 2006 | 633 | 1,231,508 | 41,757,009 | 2.95 |
| Region of the Americas | 2011 | 276 | 186,176 | 25,142,548 | 0.74 |
| Eastern Mediterranean Region | 2010 | 130 | 93,804 | 16,899,247 | 0.56 |
| European Region | 2011 | 193 | 72,373 | 65,822,040 | 0.11 |
| South-East Asia Region | 2008 | 133 | 106,639 | 27,208,629 | 0.39 |
| Western Pacific Region | 2009 | 211 | 186,099 | 90,801,873 | 0.20 |
| All data points | 2,008 | 1,576 | 1,876,599 | 267,631,346 | 0.70 |
Notes to Table 1: Data available as of 02 May 2018.
For WHO world regions, see: (http://www.who.int/about/regions/en/). ANC = Antenatal Care. RPR = Rapid plasma reagin. TPHA = Treponema pallidum hemagglutination assay.
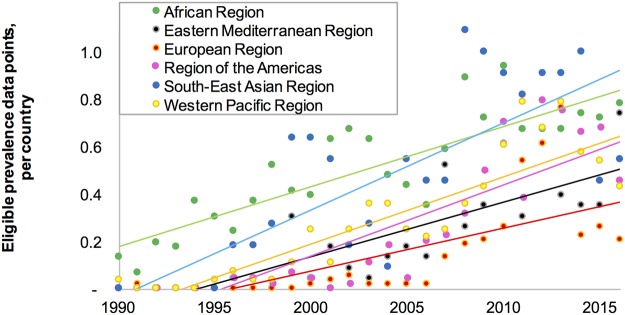
In all world regions, the number of eligible data points per country had generally increased over time (Fig. 1). Most data points were from the WHO African region, while most tests were conducted in WHO Western Pacific region (with fewer data points, of a larger sample size on average).
Figure 1.
Average number of eligible syphilis prevalence data points, recorded in the Spectrum STI syphilis database, grouped by WHO region. Note to Fig. 1: Lines represent linear trend lines fitted through the data points for each region.
The data included 60 studies with a reported prevalence of 0%, covering 1.88 million samples tested, i.e. 3.8% and 0.25% of total data points and samples tested.
National estimates
132 countries met the criteria for time trend analysis. These 132 countries accounted for 78% of the world’s population in 2016.
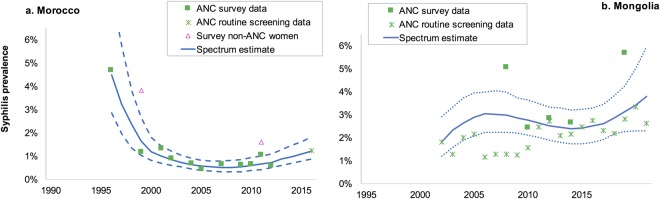
Figure 2 shows the Spectrum-STI trend estimates for two of the countries where national Spectrum-STI workshops have been held22–25. In Morocco (Fig. 2a) prevalence fell between 2000 and 2007, when Morocco rolled-out syndromic STI management, and has been increasing slowly since 2007. In Mongolia (Fig. 2b), the prevalence increased to 2002, fell slightly between 2002 and 2010 and has been increasing since 2012.
Figure 2.
Spectrum-STI estimations of adult female syphilis prevalence (a) Morocco and (b) Mongolia. Notes to Fig. 2: Data shown are after adjustment for diagnostic test performance. Neither country had any data included from years 1990–1995; both estimations used data from ANC and general populations only; both countries were classified as having a non-substantive prevalence increase over 2012–2016. Solid line = is the best estimate (median of 400 bootstraps), dashed lines are the 95% confidence interval.
Among the 132 countries, the median national prevalence estimated for 2016 ranged from 0% to 10.0% (as median of 400 bootstraps within each country), with an interquartile range of 0.16% to 1.62% and a cross-country median (of the 132 country medians) of 0.88%. In 2012 the median national prevalence ranged from 0% to 10.0%, the interquartile range was 0.16% to 1.62% and the cross country median was 0.57%.
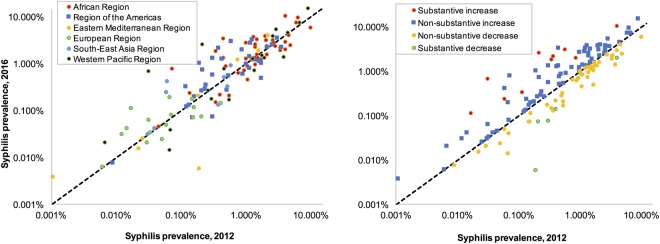
Figure 3a shows the median national prevalence of active syphilis in 2012 and 2016 for each country by geographical region. In general, the median prevalence was lowest for countries in the European Region and highest in the African Region. However, there was also considerable variability within regions, especially in the Western Pacific Region. Figure 3b shows the same results, grouped by the time trend category into which each country fell. The prevalence of probable active syphilis between 2012 and 2016 decreased in 54 of the 132 (41%) countries and in 5 of these the decrease was substantive. In the 78 countries where prevalence increased, it was substantive in 10 (Fig. 3b).
Figure 3.
Spectrum-estimated national syphilis prevalence in 2016 as a function of estimated prevalence in 2012, and each country’s time trend classification, for 132 countries: (a) Grouped by WHO region. (b) Grouped by time trend category. Notes to Fig. 3: The black dotted line indicates equality of prevalence within a country at 2012 and 2016.
Of the 54 countries with a decrease, 12 countries (including 4 of the 5 countries where the decrease was substantive) had a proportional decrease between 2012 and 2016 greater than 54%, the rate corresponding to the average decrease required to achieve the WHO-target of a 90% reduction in the incidence of syphilis globally between 2018 to 20301.
The precision of the prevalence estimates improved as the number of country data points between 2011 and 2016 increased (Fig. 4; Pearson R2 = 0.05, p = 0.011). Countries with fewer than three data points between 2011 and 2016 had considerably wider 95% CIs, which reduced the statistical significance of any difference in the 2012 to 2016 period.
Figure 4.
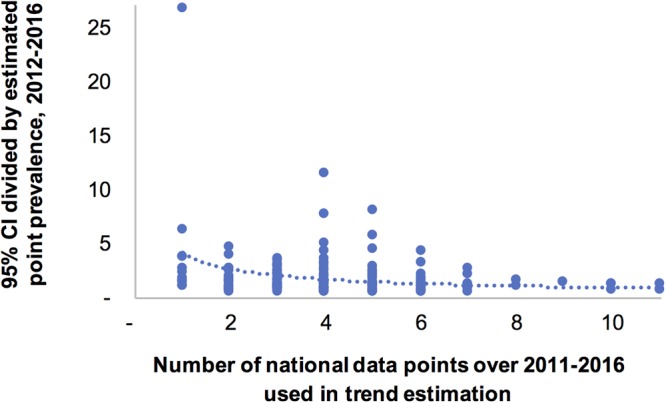
Relation between number of national prevalence data points, and precision of point prevalence estimates. Notes to Fig. 4: Precision expressed, on the y-axis, as the width of the 95% CI (averaged between 2012 & 2016) divided by the point estimate. Each dot represents a country with a Spectrum national trend estimate based on data from ANC and general populations.
Sensitivity analysis
Altering the eligibility of countries in the trend analysis by restricting the analysis to countries with one or more data point post-2011 (Scenario A) had almost no effect on the cross-country median prevalences or on the proportion of countries where prevalence decreased (Table 2). Similarly, changing the statistical model to constrain the extrapolation past the last data point to only one year instead of two years (Scenario B) had no discernible impact on the key outcomes.
Table 2.
Sensitivity analysis: Spectrum-estimated syphilis prevalence in 132 countries, under varying scenarios of data included and modelling assumptions.
| Scenario | Countries included | Median prevalence, 2012 | Median prevalence, 2016 | Countries with prevalence trend from 2012 to 2016 | |||
|---|---|---|---|---|---|---|---|
| Substantive increase | Non-substantive increase | Non-substantive decrease | Substantive decrease | ||||
| Default estimates | 132 | 0.57% | 0.88% | 14 (11%) | 64 (48%) | 48 (36%) | 6 (5%) |
| A. At least one data point from 2012 or later | 129 | 0.55% | 0.87% | 14 (11%) | 62 (48%) | 47 (36%) | 6 (5%) |
| B. More restrained time trends: extrapolate past the last year with national data, for 1 year instead of 2 years | 132 | 0.57% | 0.92% | 13 (10%) | 65 (49%) | 48 (36%) | 6 (5%) |
| C. Restrict data to 2005 and later (instead of 1990) | 130 | 0.46% | 0.93% | 17 (13%) | 61 (47%) | 44 (34%) | 8 (6%) |
| D. ANC (survey & routine screening) data only | 131 | 0.56% | 0.90% | 18 (14%) | 62 (47%) | 43 (33%) | 8 (6%) |
| E. Syphilis infections positive on both treponemal and non-treponemal tests | 85 | 0.94% | 0.86% | 8 (9%) | 32 (38%) | 42 (49%) | 3 (4%) |
| F. Countries not included in default but made eligible for trend analysis by adding blood donor data | 34* | 0.04% | 0.05% | 3 (9%) | 21 (62%) | 9 (26%) | 1 (3%) |
Notes to Table 2. The presented medians, which are unweighted, should not be interpreted as indicative of global burden trends, as global trends depend on national population sizes. Scenario F presents 34 countries that were not included in the default analysis.
In none of the 7 scenarios, none of the countries and none of the years between 2012–2016 was the 20% maximum value imposed on estimated prevalence ever reached, neither in the best estimate (i.e. the median of 400 bootstraps) nor in the upper-bound limit of each corresponding 95% confidence interval.
Reducing the number of years of prevalence data included in the analysis (Scenario C) lowered the cross-country median prevalence in 2012 from 0.57% to 0.46% and slightly increased it in 2016 (0.88% to 0.93%). The total number of countries with increasing and decreasing trend was similar, although the proportion of countries where this increase or decrease was substantive became larger. Similarly, constraining the data used to ANC populations, increased the proportion of countries where the trend of increase or decrease was substantive (Scenario D).
Trend results were sensitive to restricting the entry criteria to only surveys where blood samples were dual test positive (i.e. positive on both treponemal and non-treponemal tests; Scenario E). The total number of countries in this analysis was 85 and the cross-country median prevalence in 2012 increased from 0.57% (Default Scenario) to 0.94%, whilst the median prevalence in 2016 increased slightly from 0.88% to 0.86%. This subset of higher-prevalence countries had a larger proportion with a decreasing trend (53%, compared to 41% in the default).
Blood donors were not included in the analysis because of their likely non-representativeness of overall populations; transfusion services often exclude donors with self-reported risks or observed infections. We did, however, examine the subset of countries where there were insufficient data for estimation according to the default eligibility criteria, but where a time trend could be estimated if we included prevalence data from routine blood donor screening34–46 (Scenario F). Thirty-four countries met this criteria and their cross country median prevalence was much lower (0.04–0.05% from 2012 to 2016), and the proportion of countries with a decreasing prevalence was also lower (29%) than in the default (41%). These were mainly high-income and higher-middle-income countries, mostly in the European Region, and secondarily in the Western Pacific and Eastern Mediterranean Regions; they were also mostly smaller countries with smaller adult populations somewhat smaller countries with adult populations of on average also mainly smaller-populations (SI4 Table).
Discussion
The Spectrum-STI estimation model is a useful tool, developed for national health officials to explore historic trends in adult syphilis prevalence to inform and improve their program planning, target-setting and progress evaluation in syphilis control and congenital syphilis elimination. The present study is the first attempt to examine systematically recent trends in the prevalence of syphilis at the national level, and collectively for much of the world’s population. The analysis complements previous efforts that looked at trends over time in specific countries30 or at regional and global levels47,48.
The Spectrum estimates indicate that many countries are making progress in reducing the prevalence of syphilis in adult women. The estimated prevalence in 2016 was lower than in 2012 in 54 of the 132 countries with sufficient data to meet our study entry criteria, and in 5 countries this decrease was substantive from a public health perspective. In the 78 countries where the prevalence was higher in 2016 than in 2012 the increase was substantive in 10. At the current rate of declines only 12 of the 132 countries are on course, assuming this decline continues at the same rate, to meet the WHO target of a 90% reduction in the incidence of syphilis in adults from 2018 to 20301.
Seventy-four countries had insufficient data to meet the criteria for generating a Spectrum estimate. For 34 of these, by expanding the study entry criteria to include blood donors, we were able to generate a trend estimate; this subset of countries had a much lower prevalence of infection. Of the remaining countries, 20 had at least one data point and these had observed prevalences lower than countries in the default and blood donor-based estimates (SI4). For another 20 countries we were not able to identify any data post-1999 from a study in an ANC or general population; these were primarily high-income countries in the European and Eastern Mediterranean regions where ANC women are screened for syphilis but the data are either not collated for surveillance purposes, or not reported through international mechanisms like the Global AIDS Monitoring system (see SI4 and SI5).
Restricting the analysis to those studies where individuals were tested with both treponemal and non-treponemal tests (n = 85) resulted in an increasing proportion of countries with declining rates from 41% to 53%. This different result is in part due to a selective, higher-prevalence set of countries (with the countries with higher prevalence at 2012 more often showing a subsequent decline, Fig. 3b), and it may in part reflect a more rigid data set less affected by uncertainties and potential mis-classifications in diagnostics tests and their adjustment factors (which were based on a meta-analysis of studies pre-201428). The larger declines estimated for the higher-prevalence countries also illustrate that at regional and global levels syphilis may still be declining, even if equal number of countries (but more often, less populous and lower-prevalence countries) showed increasing trends.
At a country level the Spectrum-STI syphilis estimates concur with recent historic trend estimates, for those few countries with such an independent estimate available30. At regional level, a recent meta-regression of the Spectrum database (in a year’s older, less complete version), with data aggregated across countries, estimated average proportional annual decline rates over 1990 to 2016 of around 5% for the African Region, 8% for the Region of the Americas, 16% for the Eastern Mediterranean Region, 6% for the European Region, 10% for the South-East Asian Region and 3% for the Western Pacific Region29, which are consistent with our Spectrum-based estimates for countries in these regions. These prior syphilis trend analyses29,30 have generally highlighted a declining trend over longer time horizons, reflecting behavioral risk reductions (e.g. increased use of condoms and fewer partners)49,50, improvements in the coverage of ANC-based syphilis screening and treatment as well as treatment of partners48, and increased use of antibiotics such as oral penicillin, tetracyclines and macrolides, commonly prescribed for skin, respiratory, and other non STI-infections, which have activity against syphilis as well51. This includes over 741 million doses of azithromycin administered as part of mass trachoma treatment campaigns since 1999 in trachoma-endemic countries52.
Our analysis shows that in many other, mainly lower-prevalence countries, syphilis prevalence tended to increase from 2012 to 2016. Possible reasons for recent increases in these countries may include: saturation of prior declines associated with roll-out of ANC-based services; fatigue with safer sex and behaviour-based HIV prevention, possibly in response to the rapid expansion of access to HIV treatment drugs; shifts from condom usage to longer-term hormonal contraception53; and the recent global shortage of benzathine-penicillin used as first-line treatment for syphilis54. Furthermore, measuring prevalence using RPR/TPHA dual testing or RPR-based testing may obscure recent declines owing to the time lag for a person to test RPR negative after treatment. For example, if the median time for RPR to sero-revert after treatment is 1 to 2 years55–57 real prevalence reductions will only become apparent after a 1 to 2 year delay.
Limitations
The quality of statistical trend estimates reflects the quantity, quality and representativeness of the input data. The Spectrum syphilis database draws on data from a variety of sources including the Global AIDS Monitoring System and is the most global comprehensive database on syphilis now. Experience from country workshops22,25–27, however, suggests that there are additional data available within countries. Country outreach can also improve data quality, for example, by providing information on the specific diagnostic test types.
The Spectrum estimates were based on data primarily from ANC women (79% of data points, 63% of tests). The focus on data from ANC women means we may be over-estimating the prevalence in the general female population, as ANC women are sexually active and younger. Also recent syphilis declines may be larger in ANC populations than the overall adult population, as multiparous pregnant woman may have been diagnosed and treated in an earlier pregnancy. The HIV field has noted that ANC sentinel surveys and ANC-based HIV screening historically over-estimate HIV prevalence compared to general population surveys, as sentinel sites and routine screening were usually first established in urban, higher-risk areas58,59; however, syphilis prevalence has not consistently been higher in urban areas, and may in fact be higher in underserved rural populations, as a function of access to health care and exposure to antibiotics.
Our analysis included a small number of data points from community-based studies in men, and assumed a male-to-female ratio of 1 to 1, based on a recent global meta-analysis29. Removing these data points had almost no impact on the results (sensitivity analysis, Scenario D). The lack of male data, however, is a considerable challenge when generating national syphilis burden estimates. The few countries with data highlight that there is considerable variation in the male-to-female ratio between countries and within countries.
The Spectrum-STI model is a work in progress and, like the Spectrum HIV models, is being refined as more data become available. The current version of the model is focused on modeling trends over time in the general population—data from populations at higher risk of infection are not incorporated. A new variant of Spectrum-STI is under development that will include sub-group estimates such as for FSW and MSM, incorporating data from other sources like integrated bio-behavioural surveillance surveys (see26 for an example of sub-groups estimation). This will make it possible to remove the 10% adjustment factor for high-risk populations from the current version of Spectrum-STI. For Morocco, Mongolia and Colombia, comparative prevalences of syphilis in FSW relative to Spectrum-STI estimates for women, combined with national estimates of numbers of FSWs22,25,27, suggested that a 10% uplift in prevalence was a reasonable adjustment for them. However, contributions of higher-risk populations vary across countries and may be higher, in countries with low STI prevalences26. Several higher-income countries60,61 as well as China62 recently recorded rising syphilis prevalence in MSM, which may be driving reversals from past prevalence declines among men and in the general adult population63. Other refinements that could be considered for future Spectrum versions include sub-national disaggregation and looking in more detail at age. For large countries like India, Indonesia, China, Nigeria and the Democratic Republic of the Congo, national aggregation is a limitation62,64.
Conclusion
Spectrum-STI provides a useful tool for countries to monitor trends over time in the prevalence of syphilis. The quality and usefulness of these estimates reflects both the quality and quantity of the data available. As countries improve their syphilis surveillance, estimations should also improve and countries then should be in a better position to set targets for syphilis control and elimination, and to mobilize funding to expand access to existing services and launch and intensify complementary services, such as screening and treatment for key populations. The trend analysis presented in this paper suggests that whilst the prevalence of syphilis fell in a number of countries between 2012 and 2016, the prevalence increased in more countries than it fell. This highlights the need to intensify efforts to improve syphilis surveillance and control if countries are to meet the WHO’s syphilis reduction targets for 2030.
Electronic supplementary material
Supplementary Information files 2, 3 and 4
Acknowledgements
The views expressed in this paper are those of the authors and do not necessarily represent the official position of Avenir Health, the World Health Organization, the United States of America Centers for Disease Control and Prevention, or other affiliated organization. Estimates presented in the Results section do not present official WHO estimates. We thank Lori Newman and Mary Kamb (USA Centers for Disease Control and Prevention), Saman Wijesooriya (independent consultant) and Nicolas Kassebaum (Institute for Health Metrics and Evaluation, University of Washington) for inputs, advice and links to data relevant to the analysis, Alex Smolak (Weill Cornell Medical College Qatar) for supporting literature review of prevalence studies in Northern Africa and Eastern Mediterranean countries, Sarwat Mahmud (Weill Cornell Medical College Qatar) for support in visualizing results, and John Stover and Robert Glaubius (Avenir Health) for advice on methods and analyses. The project was funded by the World Health Organization, Department of Reproductive Health and Research, STI programme. LJA acknowledges the support of Qatar National Research Fund (NPRP 9-040-3-008).
Author Contributions
E.L.K. and J.R. conceived the study; J.R., R.W., R.M.C., E.L.K., M.T. and L.J.A. collated data; G.M., N.N., C.P. and E.L.K. designed the estimation approach and performed estimations; J.R., R.M.C. and L.J.A. provided biomedical modelling assumptions; G.M. and C.P. programmed the estimation method; E.L.K. and J.R. wrote the article; all authors analyzed final results and contributed to the final article.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29805-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global health sector strategy on sexually transmitted infections 2016–2021. Towards ending STIs. Report No. WHO/RHR/16.09, (Geneva, 2016).
- 2.World Health Organization. The global elimination of congenital syphilis: rationale and strategy for action. 48 (Geneva, 2007).
- 3.Korenromp EL, Wi T, Resch S, Stover J, Broutet N. Costing of National STI Program Implementation for the Global STI Control Strategy for the Health Sector, 2016–2021. PLoS One. 2017;12:e0170773. doi: 10.1371/journal.pone.0170773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman L, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassebaum NJ, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korenromp, E. L. et al. Estimating prevalence trends in adult gonorrhoea and syphilis prevalence in low- and middle-income countries with the Spectrum-STI model: results for Zimbabwe and Morocco from 1995 to 2016. Sex Transm Infectsextrans-2016-052953, doi:10.1136/sextrans-2016-052953 (2017). [DOI] [PMC free article] [PubMed]
- 7.Rowley, J. & Korenromp, E. L. Spectrum STI module–Manual, https://spectrummodel.zendesk.com/hc/en-us/articles/115001964191-Spectrum-STI-Module-Overview-Manual (2017).
- 8.Avenir Health & World Health Organization Reproductive Health and Research department. Spectrum-STI model estimating national STI rates: country surveillance data inputs/Guide to Country data needed to inform a national STI estimation. (Glastonbury (CT), 2017). https://spectrummodel.zendesk.com/hc/en-us/articles/115008306167-Application-of-the-Spectrum-STI-estimation-model-estimating-STI-prevalence-and-time-trends-Collating-Country-Data-.
- 9.Park SH. Experimental Designs for Fitting Segmented Polynomial Regression Models. Technometrics. 1978;20:151–154. doi: 10.1080/00401706.1978.10489639. [DOI] [Google Scholar]
- 10.Mahiane SG, Laeyendecker O. Segmented polynomials for incidence rate estimation from prevalence data. Stat Med. 2017;36:334–344. doi: 10.1002/sim.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahiané, S. G., Korenromp, E., Pretorius, C. & Stover, J. Second Order Segmented Polynomials for Syphilis and Gonorrhea Prevalence and Incidence Trends Estimation: Application to Spectrum’s Guinea-Bissau and South Africa Data InternationalJournal of Biostatistics (accepted, June 2018). [DOI] [PubMed]
- 12.Chico RM, et al. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA. 2012;307:2079–2086. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 13.R Core Team. R: A language and environment for statistical computing, https://www.R-project.org/ (2017).
- 14.World Health Organization. Global health observatory indicator data: Antenatal care (ANC) attendees tested for syphilis at first ANC visit. (World Health Organization, Geneva, 2017). http://apps.who.int/gho/data/node.imr.PerctestedANC?lang=en.
- 15.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections–2008. (Geneva, 2012).
- 16.World Health Organization. Prevalence and incidence of selected sexually transmitted infections–Chlamydia trachomatis, Neisseria gonorrheae, syphilis and Trichomonas vaginalis. Methods and results used by WHO to generate2005 estimates. (Geneva, 2011).
- 17.Institute for Health Metrics and Evaluation. Global Health Data Exchange (GHDx) database. (Seattle, 2017). http://ghdx.healthdata.org/.
- 18.Abu-Raddad LJ, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS. 2010;24(Suppl 2):S5–23. doi: 10.1097/01.aids.0000386729.56683.33. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Raddad, L. J. et al. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: Time for Strategic Action. (The World Bank Press, 2010).
- 20.Joseph Davey DL, et al. Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015: A Systematic Review. Sex Transm Dis. 2016;43:450–458. doi: 10.1097/OLQ.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams, R. et al. The Sustainable Development Goals and curable sexually transmitted infections among pregnant women: A systematic review and meta-analysis. (manuscript in preparation).
- 22.Bennani A, et al. The prevalence and incidence of active syphilis in Morocco, 1995–2016: model-based estimation and implications for STI surveillance. PLoS One. 2017;12:e0181498. doi: 10.1371/journal.pone.0181498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Kettani A, et al. Trends in adult chlamydia and gonorrhea prevalence, incidence and urethral discharge case reporting in Morocco over 1995 to 2015 – estimates using the Spectrum-Sexually Transmitted Infection model. Sex Transm Dis. 2017;44:557–564. doi: 10.1097/OLQ.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badrakh, J. et al. Trends in adult chlamydia and gonorrhea prevalence, incidence and urethral discharge case reporting in Mongolia over 1995–2016 – estimates using the Spectrum-STI model. Western Pac Surveill Response J8, 10.5365/wpsar.2017.8.2.007 (2017). [DOI] [PMC free article] [PubMed]
- 25.Enkhbat E, et al. Estimating adult female syphilis prevalence, Congenital Syphilis case incidence and adverse birth outcomes due to Congenital Syphilis using the Spectrum Sexually Transmitted Infection surveillance tool, Mongolia 2000–2016. Infectious Disease Modelling. 2018;3:13–22. doi: 10.1016/j.idm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowley, J. & Korenromp, E. L. A pilot application of the Spectrum-STI model in a low-prevalence setting: Estimation of STI prevalence and incidence trends in Georgia. Technical Report, based on a workshop in Tbilisi, Georgia, August 23–24th 2017. (London, 2017). https://spectrummodel.zendesk.com/hc/en-us/articles/115003492452-Georgia-Spectrum-STI-estimation-2017-final-report & https://spectrummodel.zendesk.com/hc/en-us/articles/115003469011-Georgia-Spectrum-STI-estimation-2017-annex-to-final-report.
- 27.Korenromp, E. L. et al. Adult syphilis, chlamydia and gonorrhea prevalence and incidence, and congenital syphilis incidence in Colombia, 1995-2016 – estimates using the Spectrum-STI model. Pan-American journal of Public Health in press (2018). [DOI] [PMC free article] [PubMed]
- 28.Ham DC, Lin C, Newman L, Wijesooriya NS, Kamb M. Improving global estimates of syphilis in pregnancy by diagnostic test type: A systematic review and meta-analysis. Int J Gynaecol Obstet. 2015;130(Suppl 1):S10–14. doi: 10.1016/j.ijgo.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smolak, A. et al. Trends and predictors of syphilis prevalence in the general population: Global pooled analyses of 1103 prevalence measures including 136 million syphilis tests. Clin Infect Dis Nov 9 10.1093/cid/cix975 (2017). [DOI] [PMC free article] [PubMed]
- 30.Kenyon CR, Osbak K, Tsoumanis A. The Global Epidemiology of Syphilis in the Past Century - A Systematic Review Based on Antenatal Syphilis Prevalence. PLoS Negl Trop Dis. 2016;10:e0004711. doi: 10.1371/journal.pntd.0004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ICAP project at Columbia University, Centers for Disease Control USA, Zimbabwe National AIDS Council (NAC), Zimbabwe National Statistics Agency (ZIMSTAT) & Zimbabwe Biomedical Research and Training Institute (BRTI). Zimbabwe population-based HIV impact assessment ZIMPHIA 2015-2016. Fact sheet. (Washington DC, 2016).
- 32.ICAP project at Columbia University, Centers for Disease Control USA & Westat. Uganda population-based HIV impact assessment UPHIA 2016-2017. Summary sheet: preliminary findings. (Washington DC, 2017).
- 33.Zambia Ministry of Communicable Diseases Maternal and Child Health et al. Zambia Population-based HIV impact assessment (ZAMPHIA) 2016. First report. (Lusaka, 2017).
- 34.Soedarmono, Y. Blood safety status in Indonesia, https://www.ipfa.nl/UserFiles/File/WS%202017/Yogyakarta%202017/Proceedings%20publicly%20published%20Yogyakarta%202017/7_3%20SOEDARMONO%20Presentation%20YSM%20IPFA%2003032017.pdf (2016).
- 35.Pasaribu, L. R. et al. RTI prevalence among pregnant women in several cities in Indonesia. (Indonesia Ministry of Health & NIHRD, Jakarta, 2016).
- 36.Pasaribu, L. R. et al. Study testing services and RTI & HIV prevalence, and antiretroviral therapy service for pregnant women in Kupang city. (Indonesia Ministry of Health & NIHRD, Jakarta, 2016).
- 37.Mohammadali, F. & Pourfathollah, A. A. Changes in frequency of HBV, HCV, HIV and syphilis infections among blood donors in Tehran province 2005–2011. Arch Iran Med17, 613–620, 0141709/AIM.006 (2014). [PubMed]
- 38.Attaullah S, Khan S, Khan J. Trend of transfusion transmitted infections frequency in blood donors: provide a road map for its prevention and control. J Transl Med. 2012;10:20. doi: 10.1186/1479-5876-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qadeer MI, Hasnain S, Yasmeen H. Sero-Prevalence of Sexually Transmitted Disease (Hiv, Syphilis, Hepatitis-B and Hepatitis-C) in Volunteer Donors of Gaol Inmates and Student Community in Punjab Province of Pakistan. Sex Transm Infect. 2013;89(P3):367. [Google Scholar]
- 40.Waheed U, et al. Prevalence of transfusion transmitted infections among blood donors of a teaching hospital in Islamabad. Ann Pak Inst Med Sci. 2012;8:236–239. [Google Scholar]
- 41.Nazir S, et al. Prevalence of syphilis in Pakistani blood donors. Advancements in Life Sciences. 2013;1:27–30. [Google Scholar]
- 42.Arshad A, et al. Prevalence of transfusion transmissible infections in blood donors of Pakistan. BMC Hematol. 2016;16:27. doi: 10.1186/s12878-016-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demirel Y, Toktamis A, Duran B, Cetin M. Seroprevalence of syphilis, hepatitis B and C, and human immunodeficiency virus infections among women. Saudi Medical Journal. 2004;25:2037–2038. [PubMed] [Google Scholar]
- 44.Ensari T, et al. An eight-year retrospective analysis of antenatal screening results for syphilis: is it still cost effective? J Infect Dev Ctries. 2015;9:1011–1015. doi: 10.3855/jidc.6064. [DOI] [PubMed] [Google Scholar]
- 45.Dayan S, et al. HBsAg, anti-HCV, anti-HIV 1/2 and syphilis seroprevalence in healthy volunteer blood donors in southeastern Anatolia. J Infect Dev Ctries. 2013;7:665–669. doi: 10.3855/jidc.2835. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. The2016 global Status Report on blood safety and availability. (Geneva, 2017).
- 47.GBD 2015 Disease and Injury Incidence and Prevention Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet388, 1545–1602, doi:S0140-6736(16)31678-6 (2016). [DOI] [PMC free article] [PubMed]
- 48.Wijesooriya NS, et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Health. 2016;4:e525–533. doi: 10.1016/S2214-109X(16)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korenromp EL, et al. The effect of HIV, behavioural change, and STD syndromic management on STD epidemiology in sub-Saharan Africa: simulations of Uganda. Sex Transm Infect. 2002;78(Suppl 1):i55–63. doi: 10.1136/sti.78.suppl_1.i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awad SF, Abu-Raddad LJ. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s? Epidemics. 2014;8:9–17. doi: 10.1016/j.epidem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 51.South Africa Ministry of Health. National HIV and Syphilis Sero-Prevalence Survey of women attending Public Antenatal Clinics in South Africa 2000. (Johannesburg, 2000).
- 52.International Trachoma Initiative. Progress in Our Global Program; ITI performance metrics in 2017, 2018).
- 53.McNicholas, C. P., Klugman, J. B., Zhao, Q. & Peipert, J. F. Condom use and incident sexually transmitted infection after initiation of long-acting reversible contraception. Am J Obstet Gynecol217, 672 e671-672 e676, S0002-9378(17)31115-8. [DOI] [PMC free article] [PubMed]
- 54.Nurse-Findlay, S. et al. Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews. PLoS Med14, e1002473, 10.1371/journal.pmed.1002473. [DOI] [PMC free article] [PubMed]
- 55.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005–1009. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 56.Anderson J, Mindel A, Tovey SJ, Williams P. Primary and secondary syphilis, 20 years’ experience. 3: Diagnosis, treatment, and follow up. Genitourin Med. 1989;65:239–243. doi: 10.1136/sti.65.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeter AL, Lucas JB, Price EV, Falcone VH. Treatment for early syphilis and reactivity of serologic tests. JAMA. 1972;221:471–476. doi: 10.1001/jama.1972.03200180015003. [DOI] [PubMed] [Google Scholar]
- 58.Montana LS, Mishra V, Hong R. Comparison of HIV prevalence estimates from antenatal care surveillance and population-based surveys in sub-Saharan Africa. Sex Transm Infect. 2008;84(Suppl 1):i78–i84. doi: 10.1136/sti.2008.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabiani M, et al. Adjusting HIV prevalence data from a program for the prevention of mother-to-child transmission for surveillance purposes in Uganda. J Acquir Immune Defic Syndr. 2007;46:328–331. doi: 10.1097/QAI.0b013e31815724e7. [DOI] [PubMed] [Google Scholar]
- 60.Mohammed H, et al. Increase in Sexually Transmitted Infections among Men Who Have Sex with Men, England, 2014. Emerg Infect Dis. 2016;22:88–91. doi: 10.3201/eid2201.151331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.United States of America Centers for Disease Control. 2016 Sexually Transmitted Diseases Surveillance. (Atlanta, 2017).
- 62.Tucker JD, Cohen MS. China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50–55. doi: 10.1097/QCO.0b013e32834204bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, et al. Monetary incentives for provision of syphilis screening, Yunnan, China. Bull World Health Organ. 2017;95:657–662. doi: 10.2471/BLT.17.191635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahy M, et al. Redefining the HIV epidemic in Nigeria: from national to state level. AIDS. 2014;28(Suppl 4):S461–467. doi: 10.1097/QAD.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information files 2, 3 and 4