Abstract
The role of melatonin in the regulation of fruit ripening and the mechanism involved remain largely unknown. In “Moldova” grape berries, melatonin accumulated rapidly from onset of veraison, reached the maximum at 94 days after bloom (DAB) and then exhibited low levels at late stages of berry ripening. By contrast, abscisic acid (ABA) and hydrogen peroxide (H2O2) exhibited different accumulation patterns, and ethylene was primarily produced immediately before veraison. Further experiments demonstrated that 10 and particularly 100 µM melatonin treatments increased the levels of ABA, H2O2, and ethylene production and promoted berry ripening compared with the control treatment, whereas 0.1 and 1.0 µM melatonin did not lead to clear effects. Additionally, the application of inhibitors indicated that ABA, H2O2, and ethylene participated in the regulation of berry ripening induced by melatonin, and the suppression of ethylene biosynthesis produced the greatest inhibitory effects on melatonin-induced berry ripening compared with those of ABA and H2O2. Melatonin also promoted ethylene production via ABA. In summary, 10 and particularly 100 µM melatonin treatments promoted berry ripening, which was accomplished, at least partially, via the other signaling molecules of ABA, H2O2, and particularly ethylene. This research provides insight into melatonin signaling during berry ripening and may advance the application of melatonin to accelerate berry ripening.
Ripening: waking the grape
Melatonin, the same hormone that regulates sleep and wakefulness in animals, promotes ripening of grapes by triggering the production of other ripening hormones. Melatonin was previously known to play a part in fruit ripening, but the mechanism remains poorly understood. Yu-Xin Yao and co-workers at Shandong Agricultural University in China tested how applying various quantities of melatonin affected ripening of grapes, and also investigated the mechanism of action by measuring levels of other hormones related to ripening, such as ethylene. They found that adding melatonin made grapes ripen sooner, and increasing the quantity increased the effect. Applying substances that inhibited the other ripening hormones repressed ripening, indicating that melatonin cannot act alone. These results illuminate how melatonin triggers ripening, and may help in developing methods to induce ripening in various plants.
Introduction
Grapevine is one of the most important fruit crops and is planted worldwide. The high economic and nutritional value of grapevine has encouraged many researchers to study the physiological and molecular basis of berry development and particularly berry quality formation. Berry development involves two growth periods; the first period is characterized by rapid cell division and growth and the accumulation of organic acids, and the second period is characterized by the decline in organic acids and the accumulation of sugar, anthocyanin, and flavor compounds. Veraison is a transition phase from the first to the second period1.
Grapes are classified as non-climacteric fleshy fruits and might be considered a model species to study the ripening of non-climacteric species2. Although the mechanism involved in the ripening of non-climacteric fruits remains largely unclear, several signaling molecules participate in the control of ripening in grape berry3,4. Based on the substantial accumulation during fruit maturation in non-climacteric fruits, abscisic acid (ABA) plays an important role in accelerating fruit ripening, and based on RNA-Seq analysis, is a primary regulator of grape berry ripening onset5,6. Additionally, one of the best-known roles of ABA is the ability to upregulate anthocyanin production of grape berries7. By contrast, a few recent studies show that grape berry tissues have a fully functional pathway for ethylene biosynthesis that is activated immediately before veraison; furthermore, ethylene perception is critical for some grape berry ripening8,9. Exogenous ethylene also positively affects anthocyanin production in grape berry8,10. Additionally, reactive oxygen species (ROS) are involved in regulating fruit ripening and senescence11, and H2O2 can regulate the process of ripening by regulating ripening-related genes12. Particularly, the accumulation of ROS is a characteristic of grape berry ripening13, and H2O2 promotes the early ripening of “Kyoho” berry4.
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine that is synthesized from l-tryptophan metabolism via serotonin. Some recent studies report that melatonin participates in the regulation of fruit ripening and senescence. Melatonin promotes ripening and improves quality of climacteric tomato fruit during postharvest life14, and further proteomics analysis provides insights into the physiological and molecular mechanisms underlying melatonin-mediated tomato fruit ripening15. In banana, another typical climacteric fruit, melatonin is also an indicator for fruit ripening in various varieties16. By contrast, exogenous application of melatonin delays postharvest senescence in banana, strawberry, and peach fruits16–18. Therefore, melatonin treatment is proposed to positively regulate fruit ripening but negatively regulate fruit senescence, although the two processes are intrinsically linked15,16. However, melatonin also regulates fruit metabolism or abiotic resistance via interaction with other signaling molecules. Melatonin treatment increases ethylene production and accelerates the climacteric phase of tomato fruits14, but exogenous application of melatonin reduces ethylene production of banana through regulation of the expression of ACO1 and ACS116. Additionally, melatonin treatment can alter ABA levels, e.g., melatonin treatment results in a rapid decrease in ABA levels during seed germination of cucumber under salt stress and in apple leaves in drought conditions through regulation of ABA biosynthesis and catabolic enzymes19,20. By contrast, the application of melatonin results in increased ABA content in drought-primed plants when exposed to cold stress21. Moreover, melatonin delays senescence of peach fruit partly via decreasing the levels of superoxide anion and H2O217.
The objectives of this study were to investigate the changes in melatonin and the other signaling molecules, including ABA, H2O2, and ethylene during berry ripening, to elucidate the effects of exogenous melatonin application on berry ripening and the accumulations of the three signaling molecules, and to reveal whether melatonin can regulate berry ripening through the other signaling molecules. The underlying mechanism of melatonin in the regulation of berry ripening was further revealed in this study, and these results may help promote the application of melatonin in regulating berry ripening.
Results
Changes in total soluble solids, titratable acid and anthocyanin during berry ripening
The onset of ripening (veraison) is indicated by increases in sugar content, and in red and black grapes, anthocyanin biosynthesis and by decline in acid content. In 2016, titratable acid continued to decrease and total soluble solids (TSS) continued to increase from 60 days after bloom (DAB), anthocyanin began to accumulate at 78 DAB, and coloring began at 94 DAB (Fig. 1a). In 2017, particularly with more sampling time points, the dramatic decrease in titratable acid and TSS beginning to continuously and prominently accumulate from 60 DAB were more clear than in 2016; and anthocyanin was detectable at 70 DAB, followed by the rapid increase (Fig. 1b). Titratable acid reached the minimum, and the TSS and anthocyanin contents reached their maximums in the ripened berries (124 DAB) (Fig. 1a, b). Therefore, the onset of veraison occurred approximately at 70 DAB, which varied slightly between the two years.
Fig. 1. Berry coloration and changes in berry TSS and anthocyanin and titratable acid.
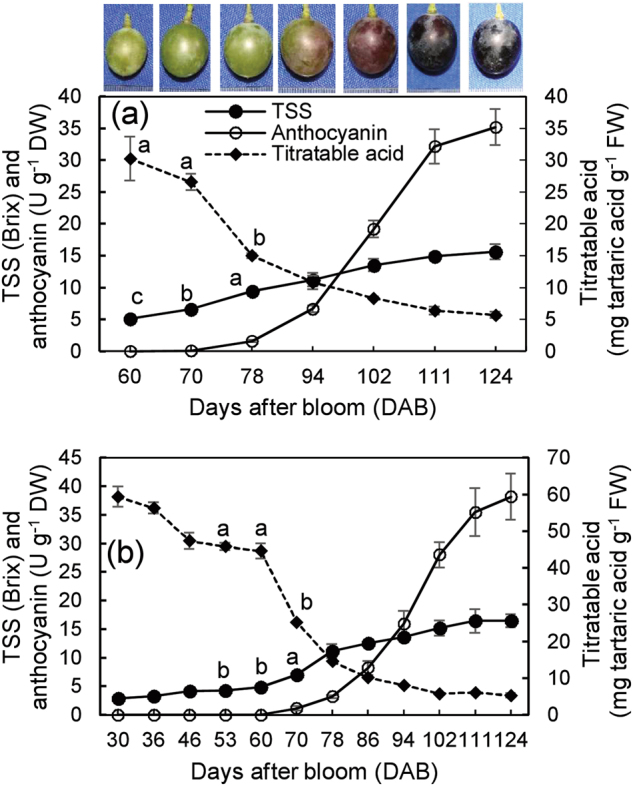
The data in panels a and b were determined in 2016 and 2017, respectively. DW dry weight, FW fresh weight. Values represent the mean ± SD of three replicates, and each replication contained 240 berries, which were randomly sampled from the middle of the clusters of 10 vines. The differences are not significant at the 5% significance level among the values with the same small letter at the different time points
Changes in 5-hydroxyserotonin, melatonin and 2-hydroxymelatonin, and the other signaling molecules during berry ripening
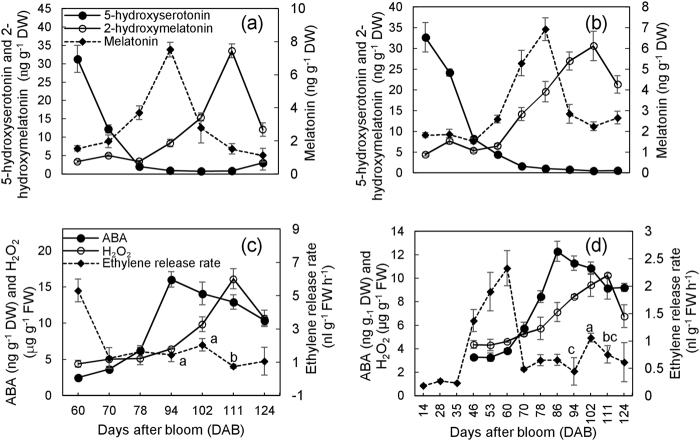
When veraison occurred, melatonin increased rapidly and peaked at 94 DAB with a 3.89- and 3.54-fold increase compared with melatonin at 70 DAB in 2016 and 2017, respectively (Fig. 2a, b). Thereafter, melatonin declined sharply at 102 DAB, but different changes were found at 111 and 124 DAB between the two years (Fig. 2a, b), suggesting the influence of environmental factors. By contrast, 2-hydroxymelatonin also promptly accumulated during berry ripening but reached the maximum at 111 DAB. Additionally, 5-hydroxyserotonin, a precursor of melatonin biosynthesis, decreased sharply from the first sampling time point and remained at relatively low levels in the late stages (Fig. 2a, b), indicating the transformation of 5-hydroxyserotonin into melatonin.
Fig. 2. Accumulations of melatonin and its precursor (5-hydroxyserotonin) and metabolite (2-hydroxymelatonin) and the other signaling molecules during berry ripening.
The data in (a) and (b) were determined in 2016, and those in (c) and (d) were from 2017. In (d), ethylene release rate was detected from 14 DAB, and ABA and H2O2 were detected from 46 DAB. DW dry weight, FW fresh weight. Values represent the mean ± SD of three replicates, and each replication contained 240 berries, which were randomly sampled from the middle of the clusters of 10 vines. The differences are not significant at the 5% significance level among the values with the same small letter in (c) and (d)
ABA continuously accumulated from 60 DAB and reached the peak at 94 DAB, which was 2.82-fold higher than that at 60 DAB in 2016 (Fig. 2c). By contrast, the determinations in a small time interval showed that the peak of ABA occurred at 86 DAB in 2017 (Fig. 2d). Thereafter, ABA content declined slowly but remained at a high level (Fig. 2c, d). By contrast, the peak of H2O2 was at 111 DAB in both years, and the concentration of H2O2 at the peak was 1.42- and 1.21-fold higher than the value at 60 DAB in 2016 and 2017, respectively (Fig. 2c, d). In 2016, high ethylene release rates were detected at 60 DAB, with relatively low values found at the other time points, suggesting an ethylene peak at 60 DAB or earlier time point (Fig. 2c). Further determinations in 2017 indicated that the peak of ethylene release also occurred at 60 DAB, which was 3.80-fold higher than that at 70 DAB (Fig. 2d). Additionally, a small peak of ethylene release was observed at 102 DAB in 2016 and particularly in 2017 (Fig. 2c, d)
Collectively, the peak of ethylene release occurred before veraison, and the peaks of ABA, melatonin, and 2-hydroxymelatonin/H2O2 occurred in chronological order after onset of veraison.
Exogenous melatonin treatments increased the levels of melatonin and the other signaling molecules and promoted berry ripening
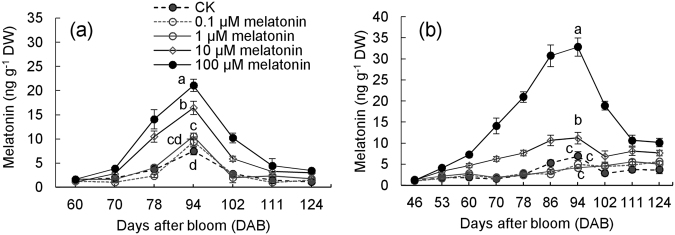
The role of melatonin in promoting berry ripening was evaluated by applying exogenous melatonin treatment. First, the influences of exogenous melatonin concentration on the content of berry melatonin were determined (Fig. 3). In 2016, the content of melatonin in berries treated once with 10- and 100-µM melatonin at 60 DAB largely increased, and the increments increased with the increase in concentration of applied melatonin. Increments of 1.18- and 1.80-fold were generated at 94 DAB in the 10- and 100-µM melatonin-treated berries, respectively, compared with the control check (CK) berries. By contrast, 0.1- and 1.0-µM melatonin treatments did not lead to clear changes in melatonin content of berries (Fig. 3a). To further increase melatonin levels before the occurrence of the ethylene release peak, melatonin treatments at two times, i.e., at 46 and 53 DAB, were performed in 2017 (Fig. 3b). Two-time treatment with 10- and 100-µM melatonin largely increased melatonin levels of grape berries and led to 3.89- and 2.51-fold increments, respectively, at 60 DAB compared with the level in the CK treatment. The increase reached 1.61- and 4.74-fold at 94 DAB under 10- and 100-µM melatonin treatments, respectively, compared with the CK (Fig. 3b). By contrast, 0.1- and 1.0-µM melatonin treatment did not clearly alter melatonin levels, similar to results in 2016 (Fig. 3a, b).
Fig. 3.
Changes in melatonin content of grape berries under the treatments of different concentrations of exogenous melatonin. The berries were treated once at 60 DAB in 2016 (a) and were treated two times at 46 and 53 DAB in 2017 (b). DW dry weight. Values represent the mean ± SD of three replicates, and each replication contained 240 berries, which were randomly sampled from the middle of the clusters of 10 vines. The differences are not significant at the 5% significance level among the values with the same small letter at the same DAB
Second, the effects of the 10- and 100-µM melatonin treatments on ABA, H2O2, and ethylene release were determined in 2017. Treated with 10-µM melatonin, berries accumulated 30.0% more ABA than that of the control berries from 60 to 124 DAB, and treatment with 100-µM melatonin caused greater increases in ABA content than did 10-µM melatonin (Fig. 4a). Similarly, exogenous melatonin treatments promoted the accumulation of H2O2 at the same time points, and 100-µM melatonin caused a greater effect on H2O2 than that of 10-µM melatonin. Moreover, with melatonin treatments, the peak of H2O2 occurred in advance (Fig. 4b). By contrast, melatonin treatments not only largely increased ethylene release rate of berries but also altered accumulation patterns (Fig. 4c). In the CK berries, the ethylene release rate decreased from 70 DAB and then remained at very low levels. By contrast, two peaks of ethylene release were produced at 78 and 102 DAB in the melatonin-treated berries. At 78 DAB, 7.28- and 6.06-fold increments in melatonin content were observed in the 100- and 10-µM-treated berries, respectively, compared with the control berries, and more than 11-fold increments were produced under the 100- and 10-µM treatments at 102 DAB (Fig. 4c).
Fig. 4. Changes of the signaling molecules and the ripening-related parameters under melatonin treatments.
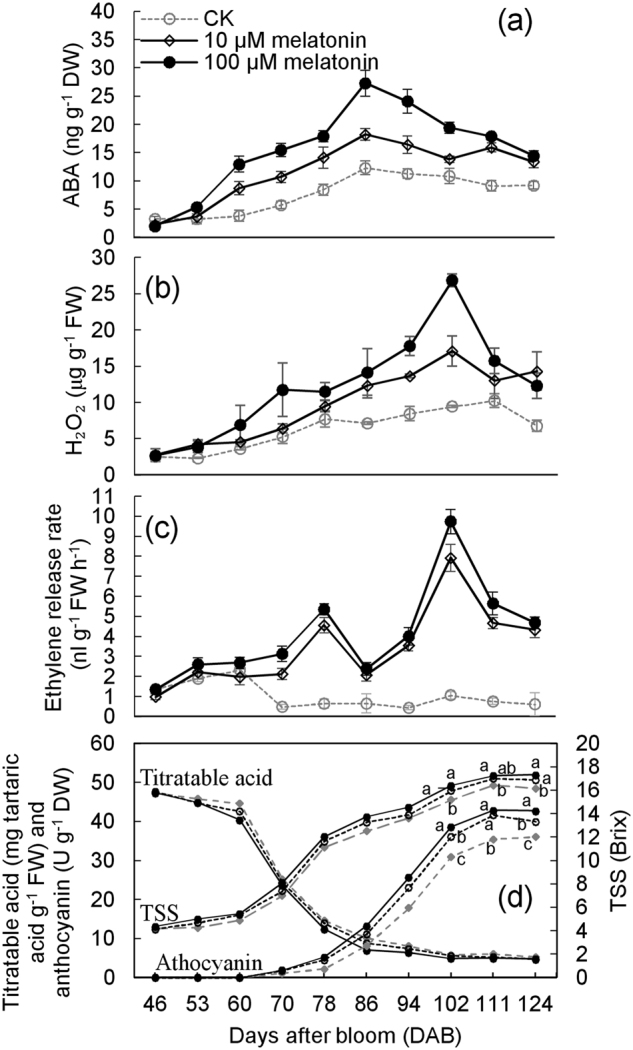
ABA (a), H2O2 (b), ethylene release rate (c) and the ripening-related parameters (d) were determined in 2017. The berries were treated two times at 46 and 53 DAB. The ripening-related parameters included TSS, anthocyanin, and titratable acid (d). FW fresh weight, DW dry weight. Values represent the mean ± SD of three replicates, and each replication contained 240 berries, which were randomly sampled from the middle of the clusters of 10 vines. The differences are not significant at the 5% significance level among the values with the same small letter at the same DAB in (d)
Last, the effects of 10- and 100-µM melatonin treatments on berry ripening were determined in 2017 (Fig. 4d). The berries treated with melatonin at 10 and particularly 100 µM accumulated high TSS and anthocyanin and low titratable acid compared with those of the CK berries at the same time points. Additionally, contents of TSS and anthocyanin in the melatonin-treated berries at 102 DAB surpassed the values in the ripened CK berries, and the melatonin-treated berries at 102 DAB accumulated lower titratable acid than that in the ripened CK berries. Therefore, 10- and 100-µM melatonin treatments promoted berry ripening.
In summary, exogenous melatonin treatments increased the levels of melatonin, ABA, H2O2, and ethylene production in the berries and promoted berry ripening, suggesting that melatonin might promote berry ripening via these three signaling molecules.
Exogenous melatonin treatments promoted berry ripening via the signaling molecules ABA, ethylene, and H2O2
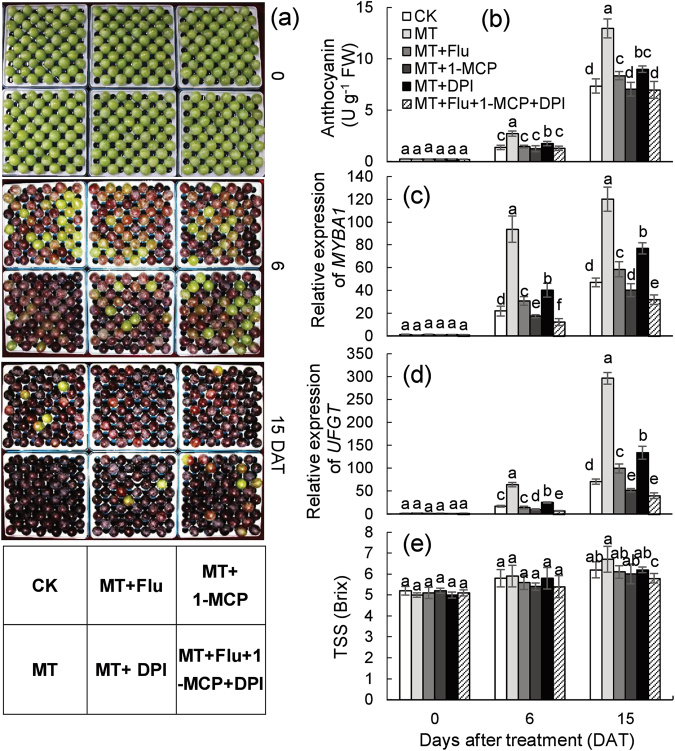
To further investigate whether melatonin promoted berry ripening via the other signaling molecules, melatonin, fluridone (Flu; an inhibitor of ABA biosynthesis), diphenylene iodonium (DPI; an inhibitor of H2O2 biosynthesis), and 1-methylcyclopropene (1-MCP; an inhibitor of ethylene response at the receptor level) were used to treat the berries in vitro at 60 DAB in 2017 (Fig. 5). When the berries were treated with melatonin at 100 µM, most accumulated much anthocyanin and exhibited red purple color at 6 days after treatment (DAT), in contrast to only approximately 65% of the CK berries (Fig. 5a). A 1.9- and 1.76-fold increase of anthocyanin in melatonin-treated berries was also detected at 6 and 15 DAT, respectively, compared with the CK berries (Fig. 5b). By contrast, the treatment of melatonin plus DPI, Flu, or 1-MCP significantly reduced the anthocyanin content at 6 and 15 DAT compared with the treatment of melatonin alone. The application of 1-MCP caused the greatest inhibitory effects on melatonin-induced anthocyanin accumulation, and the berries under melatonin plus 1-MCP treatment accumulated similar levels of anthocyanin to those of the CK berries. By contrast, the anthocyanin content in melatonin plus Flu or DPI was significantly higher than that in the CK berries. The treatment of melatonin plus all three inhibitors produced similar effects on anthocyanin to those of the treatment of melatonin plus 1-MCP (Fig. 5a, b). Additionally, the gene expression of MYBA1 and UFGT, two marker genes in the regulation of anthocyanin biosynthesis, indicated the same results as those of anthocyanin assays (Fig. 5c, d). For TSS, the patterns of change were similar to those of anthocyanin under the different treatments at 15 DAT, although the differences were small, and the treatment of melatonin plus the three inhibitors significantly reduced the melatonin-induced increase in TSS (Fig. 5e). Therefore, exogenous melatonin treatments promoted berry ripening, at least partially, via ethylene, ABA, and H2O2, and ethylene produced the greatest effect on ripening compared with that of ABA and H2O2 under the applied conditions.
Fig. 5.
Changes in the ripening-related parameters and expression of MYBA1 and UFGT in the berries with different treatments. The berries at 60 DAB were used for different treatments. Half of each berry cluster was immersed in the treatment solution, which was refreshed every 3 days. The photos (a) were taken and the corresponding anthocyanin content (b), gene expression (c, d) and TSS accumulation were determined at different DAT. MT melatonin, Flu fluridone, DPI diphenylene iodonium, 1-MCP 1-methylcyclopropene. Values represent the mean ± SD of three replicates, and each replication contained 50 berries, which were randomly sampled from the same vine. The differences are not significant at the 5% significance level among the values with the same small letter at the same DAT in (b–e)
Melatonin promoted ethylene release partially via ABA
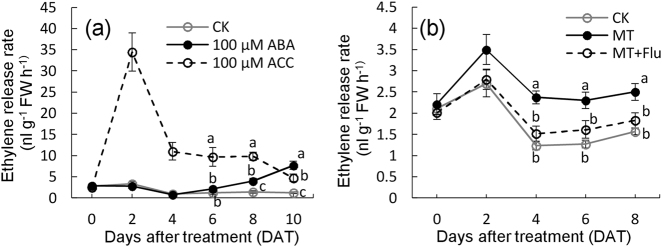
In a previous study, ABA treatment promoted ethylene release in grape pulp22, and therefore, we determined whether melatonin could promote ethylene release by increasing levels of ABA (Fig. 6). Under CK conditions, ethylene release in berries declined after a small increase and then remained at a low level. Ethylene release rate largely increased with 1-aminocyclopropane-1-carboxylate (ACC) treatment (as a positive control) at 2 DAT and thereafter decreased gradually. By contrast, ABA treatment promoted ethylene production from 6 DAT and led to a higher level of ethylene production than that with ACC at 10 DAT (Fig. 6a). Additionally, ethylene release rate was determined under the treatments of MT and MT + Flu (Fig. 6b). The results showed that melatonin promoted ethylene production of the in vitro berries, but the application of Flu largely reduced the increase in ethylene production caused by melatonin treatment. For example, melatonin treatment produced a 1.59-fold increase in ethylene release rate compared with that of the CK, whereas the increment declined to 1.17-fold under the treatment of MT + Flu at 8 DAT. Collectively, these results indicated that melatonin promoted ethylene biosynthesis partially via ABA.
Fig. 6. Modifications in ethylene release rate in the berries with different treatments.
The berries at 60 DAB were used in the in vitro treatment, i.e., half of each berry cluster was immersed in the treatment solution, which was refreshed every 3 days. The berries at different DAT were used for the determinations of ethylene release rate. The solution of 100 µM 1-aminocyclopropane-1-carboxylate (ACC) and water (CK) were used as positive and negative controls, respectively. MT melatonin, Flu fluridone, FW fresh weight. Values represent the mean ± SD of three replicates, and each replication contained 50 berries, which were randomly sampled from the same vine. The differences are not significant at the 5% significance level among the values with the same small letter at the same DAT
Discussion
Melatonin plays different roles in the regulation of berry ripening and senescence
The accelerated accumulations of TSS and anthocyanins and the declines in titratable acid were indications of the role of melatonin in promoting berry ripening. Additionally, the role of melatonin in the regulation of ripening was dose-dependent (Fig. 4d). Similar increases in TSS, glucose, and pigment accumulations are also observed in melatonin-treated tomato fruits14. However, melatonin treatment decreases total soluble sugars or solids and delays postharvest senescence of peach and banana fruits, and a concentration-dependent effect is observed in banana fruits16,17. In particular, exogenous application of melatonin positively regulates fruit ripening; whereas, fruit senescence is negatively regulated15. Therefore, melatonin may play different roles in regulating berry ripening and senescence, and the different roles might correspond to two functions of melatonin. First, melatonin can act as an endogenous elicitor and signaling molecule23. One of the first roles proposed for melatonin in plants was possible action as a growth regulator because of the structural similarity to indole-3-acetic acid (IAA);23 some studies also indicate that melatonin can promote plant growth with a considerable auxinic effect24. Additionally, melatonin promotes seed germination in cucumber19, promotes tomato ripening14, and increases the size and synchronicity of grape berries25. Moreover, the multiple changes in gene expression caused by melatonin are an indication of a role as a multiregulatory molecule capable of coordinating many aspects of plant development23. Therefore, melatonin may promote fruit ripening as a signaling molecule. Second, melatonin, as a “suicide” antioxidant, can directly scavenge radicals and radical products23. Fruit senescence is related to reactive oxygen metabolism and membrane lipid peroxidation, which can directly lead to tissue death26. Melatonin decreases the levels of superoxide anion and H2O2 and delays fruit senescence of peach17 and also delays physiological deterioration of cassava roots postharvest by lowering H2O2 accumulation27. Therefore, melatonin might delay fruit senescence via scavenging ROS as an antioxidant.
Roles of melatonin, ABA, H2O2, and ethylene in triggering or promoting berry ripening
Many studies indicate that the patterns of melatonin accumulation during ripening are different among fruit species, and that the mechanism of melatonin to regulate fruit ripening is complicated. Melatonin levels continue to decrease with fruit ripening in mulberry;28 however, the highest melatonin content is detected in the ripest tomato fruit and sweet cherries29,30. In the “Moldova” berry, melatonin exhibited a peak in the berries at 94 DAB in late veraison (Fig. 2a, b); similarly, melatonin content at veraison is higher than that at preveraison in seed and flesh of “Merlot” grape31. Additionally, the continuous decline in 5-hydroxyserotonin indicated transformation into melatonin at veraison (Fig. 2a, b). Melatonin can be directly transformed to 2-hydroxymelatonin by the catalysis of melatonin 2-hydroxylase32. Comparison of the changing patterns in melatonin and 2-hydroxymelatonin suggested the transformation of melatonin into 2-hydroxymelatonin after veraison under the condition of low supply of 5-hydroxyserotonin (Fig. 2a, b). Therefore, melatonin might be primarily accumulated at veraison in grape. Comparisons of melatonin level and the parameters involved in ripening showed that the abrupt increase in melatonin content coincided with the increases in TSS and total anthocyanin content at veraison, but anthocyanin and TSS continued to increase throughout the ripening period, whereas simultaneously, melatonin levels started to decrease (Figs. 1a, b and 2a, b)33. All these results suggest that melatonin is a regulator of berry ripening onset.
Numerous studies of ABA determination, transcriptomics, and proteomics show that ABA plays a primary role in controlling the berry ripening process3,6. Although sugar accumulation correlates with an increase in ABA content of the berry33, ABA treatment does not affect sugar content, and ABA treatment at 3 weeks before veraison even decreases the sugar content of the berry33,34. Additionally, ABA did not necessarily sustain the accumulation of anthocyanin and TSS, because anthocyanins and TSS continued to increase throughout the ripening period, whereas ABA started to decrease from 94 DAB (Figs. 1a, b and 2c, d)33. Therefore, ABA might also be a regulator of berry ripening.
Although grape berry is a non-climacteric fruit, an oxidative burst occurs with a characteristic rapid accumulation of H2O213, and the H2O2 likely acts as a signaling molecule to accelerate berry ripening of grape rather than as a toxic by-product in the ripening of the berry4. Strong evidence also indicates the involvement of H2O2 in tomato ripening12. In this study, H2O2 rapidly accumulated from 86 DAB and peaked at 111 DAB (Fig. 2c, d), suggesting that the role of H2O2 in regulating berry ripening occurred after the initiation of berry ripening. H2O2 has been reported as a potential signaling molecule that regulates fruit ripening in the middle stage of fruit development but may also function as a primary toxic molecule in the late stage of peach fruit35. These studies show that the role of H2O2 as a signal or toxic molecule depends on the fruit developmental stage.
During grape ripening, the typical respiration peak does not occur. Nevertheless, the peak of ethylene release occurred immediately before veraison in “Moldova” grape (Fig. 2c, d); similar results are also reported in the grape cultivar “Muscat Hamburg”22 and Cabernet Sauvignon8. Additionally, the peak of ACC oxidase transcript accumulation also occurs immediately before veraison or in green berries in other grapevine cultivars36,37. These results all provide support that the peak of ethylene release occurs before veraison. Therefore, ethylene might be involved in the trigger of berry ripening. Additionally, the application of 2-chloroethylphosphonic acid (2-CEPA), an ethylene-releasing compound, on berries at veraison promotes anthocyanin accumulation in Cabernet Sauvignon berries;38 however, 2-CEPA application delayed ripening when applied early in berry development39, which might involve an increase in production of IAA caused by ethylene40. From the above discussion, the inference is that ethylene might regulate berry ripening via interplay with other regulators, whereas the effects of exogenous ethylene on berry ripening depend on the developmental stage of the berries at the time of application.
Collectively, melatonin, ABA, and H2O2 can promote berry ripening; however, their exact role as either a primary promoter or simply a participant in controlling berry ripening requires further research. Ethylene is a potential signaling molecule that can trigger berry ripening, but more decisive evidence is required.
Melatonin promotes berry ripening via other signaling molecules
Grape berry development and ripening is a complex process, and berry ripening involves the integration of multiple hormonal signals, with some hormones acting as promoters and others as repressors. The mechanisms involved in perceiving and signaling melatonin during berry ripening remain poorly understood. In order to reveal the interaction of melatonin with other signaling molecules in berry ripening, exogenous melatonin treatment was performed and the biosynthesis of ABA and H2O2 and ethylene signaling were inhibited by applying their specific inhibitors, Flu, DPI, and 1-MCP22,41.
In this study, melatonin treatment increased the ABA levels of grape berries, and ABA mediated the melatonin-induced berry ripening (Figs. 4a and 5). Some published data indicate that ABA might act as a downstream signal of melatonin in a stress response, because neither ABA nor fluridone changes endogenous melatonin42. Additionally, some studies indicate a role for melatonin in regulating ABA levels, but different effects are observed. For example, melatonin treatment increases ABA accumulation and alleviates cold-induced oxidative damage in leaves of Elymus nutans42, but melatonin decreases ABA levels during seed germination under salt stress and in heat-induced senescence19,43. Therefore, melatonin can regulate ABA metabolism and signaling but causes varying effects under different conditions or in different tissues.
The effects of melatonin behavior on ethylene are complicated. Results indicated that ethylene mediated melatonin-induced berry ripening (Fig. 5); similarly, melatonin treatment promotes ethylene biosynthesis in tomato postharvest ripening14. However, in banana postharvest ripening, melatonin treatment reduces ethylene production through regulation of the expression of ACO1 and ACS116. These opposite effects of melatonin on ethylene suggest that the regulation of melatonin on ethylene might involve the other regulators of ethylene biosynthesis. This hypothesis is supported by a study in which melatonin treatment inhibits ethylene production in banana leaves, whereas combined treatments of melatonin and Fusarium wilt cause induction of ethylene levels44. Additionally, several studies reveal that ABA treatment can induce ethylene evolution in fruit22,45. In particular, this study determined that ABA induced ethylene production and mediated melatonin-induced ethylene release (Fig. 6). Therefore, melatonin promoted ethylene production at least partially via ABA in berry ripening. This conclusion could also explain the peak in ethylene production at 102 DAB, which might be attributed to the high level of ABA under melatonin treatment.
This study revealed that melatonin increased the levels of H2O2 during berry ripening, and that H2O2 participated in melatonin-induced berry ripening (Figs. 4b and 5). Melatonin and H2O2 as antioxidant and ROS, respectively, did not exhibit an adversarial relationship, suggesting that melatonin and H2O2 act predominantly as signaling molecules during berry ripening. By contrast, melatonin scavenges ROS as an antioxidant in fruit senescence of banana and peach16,17. The interplay between melatonin and H2O2 remains largely unknown and awaits further studies.
Conclusions
Melatonin and ABA prominently accumulated at veraison, and thereafter, although melatonin sharply declined, ABA remained at relatively high levels; the large accumulation of H2O2 was late compared with that of melatonin and ABA during berry ripening. By contrast, the strongest ethylene production occurred before veraison. The different accumulation patterns indicated their different roles in the regulation of berry ripening. Additionally, 10- and 100-µM melatonin treatments increased the levels of ABA, H2O2, and ethylene production, and promoted berry ripening in a concentration-dependent manner by increasing endogenous melatonin content. Further experiments determined that ABA, H2O2, and particularly ethylene participated in melatonin-induced berry ripening. Moreover, melatonin promotion of ethylene production was partially dependent on ABA. In summary, melatonin promoted berry ripening at least partially through ABA, H2O2, and particularly ethylene.
Materials and methods
Plant materials and experimental treatments
The present experiment was undertaken at an experimental vineyard in Tai-An City (36°.17′N, 117°.16′E), Shandong Province, China. Daily average temperature in the experimental site ranged between 7.5 and 31.0 °C from April to August (Fig. S1). The grapevines used in this study were “Moldova” (Vitis vinifera × labrusca). The vines, each of which had 15 vertical fruiting shoots on the horizontal cordon, were planted at a row × vine spacing of 2.5 × 2.0 m. Each fruiting shoot was controlled to produce two clusters. In 2016, the berries at 60 DAB were treated with melatonin. Each grape cluster on a vine was soaked for 5 s in melatonin solution at a concentration of 0.1, 1.0, 10.0, or 100 µM plus 0.05% (v/v) Triton X-100 in a 2000-mL plastic beaker. CK berries were soaked in 0.05% (v/v) Triton X-100. In 2017, melatonin treatments were applied twice at 46 and 53 DAB using the same methods. Additionally, the berries at 60 DAB were collected for in vitro treatment in 2017. The berries were soaked in solutions of 100-µM melatonin plus 50-µM Flu, 200-µM DPI, or 5 µL L−1 1-MCP under a 14-/10-h (light/dark) photoperiod at approximately 600 mmol m−2 s−1 at 25 °C for 15 days. The berries at different DAB or DAT were collected, rinsed, frozen in liquid nitrogen, and stored at −70 °C for the determinations of ripening-related parameters, melatonin, and other hormones. Each treatment was conducted with three replications, and each replication contained 10 vines and 50 berries for in vivo and in vitro treatments, respectively.
Determination of relative anthocyanin content, TSS, and titratable acid
Relative anthocyanin content was determined according to the method described by Neff and Chory46 with minor modifications. Ten milliliters of methanol in a 1% (v/v) HCl solution was added to 0.5 g ground lyophilized berry skins and 1.5 g fresh berry skins from in vivo and in vitro treatments, respectively. Sonicated for 3 min. After 24 h of incubation in the dark, the extraction was centrifuged for 10 min at 10 000 rpm, and the aqueous phase was subjected to spectrophotometric quantification at 530 and 657 nm. The relative unit was calculated by the formula OD = A530 − 0.25 × A657. The relative anthocyanin content is expressed as U mg−1 dry weight or fresh weight (FW).
Fresh berry pulp was mixed, homogenized, and filtered. The filtrate was used for the determination of TSS and titratable acid. TSS was measured using a manual, digital-displayed sugar meter (PAL-1; Atago, Tokyo, Japan), and the results are expressed as Brix. Titratable acid was determined by the titration of the filtrate with 0.1 M NaOH to an end point at pH 8.3. The results are expressed as mg tartaric acid per g FW.
Extraction and determination of 5-hydroxyserotonin, melatonin, and 2-hydroxymelatonin
Melatonin was extracted as previously reported14 with some modifications. The extraction was conducted entirely under dim green light. One gram of ground lyophilized grape (skin and pulp) was extracted three times in 8 mL of methanol via an ultrasonic bath for 15 min each time. After centrifuging at 5000 rpm for 15 min, the supernatant was filtered on a filter paper, and the filtrate was evaporated to dryness at 30 °C in a rotary evaporator. The residue was dissolved in 5 mL of chromatographic grade methanol and transferred to a C18 solid phase extraction cartridge (ProElutTM; DIKMA, China) for the purification of melatonin.
The samples were separated on an Acquity UHPLC system (Waters, Milford, MA, USA). The separation was performed by injecting 10 µL of sample onto a BEH C18 column (Waters, 2.1 mm internal diameter × 50 mm length, and 1.7 µm particle size). Mass spectrometry (MS) analyses were performed using a QTof-Micro mass spectrometer (Waters, Milford, MA, USA). The parameters and conditions of ultra-high performance liquid chromatography (UHPLC)-MS analysis were set according to our previous study47. The mobile phases consisted of water with 0.05% (v/v) acetic acid (A) and methanol (B) delivered at 0.3 mL min−1. The elution started at a composition of 80% A and 20% B, held for 0.8 min; a 1.4 min linear gradient to 40% A, held for 3 min; return to the initial ratio of A and B by a 0.1 min gradient, held for 2.9 min. Detection conditions were the following: a column temperature of 25 °C; a capillary temperature of 300 °C; a spray voltage of 3000 V; an auxiliary pressure of 15 V; and a sheath pressure of 35 V.
ABA extraction and determination
Extraction and determination of ABA were performed as described by Ré et al.48 with some modifications. One-half gram of ground lyophilized berries was extracted three times in 5 mL of cold 80% methanol (v/v) mixed with 30 μg mL−1 sodium diethyldithiocarbamate. The extracts were centrifuged at 8000 × g for 10 min, and the supernatant was concentrated to dryness under vacuum. The residue was dissolved in 4 ml of 0.4 M phosphate buffer (pH 8.0). After centrifuging, the aqueous phase was collected and supplemented with polyvinylpyrrolidone to remove the phenolics and then centrifuged at 8000 × g for 10 min. The supernatant was extracted with 4 mL of ethyl acetate (pH 3.0) twice. The upper phase was collected and concentrated to dryness under vacuum. The residue was dissolved in 1 mL of 0.5% (v/v) acetic acid/methanol (55:45, v/v) and finally filtered through a 0.45 μm filter.
ABA was quantified using an LC-electrospray ionization (ESI)-MS instrument. Ten microliters of the sample was injected into a Thermo Scientific Hypersil Gold column (50 × 2.1 mm, 1.9 μm) in a Scientific Ultimate 3000 HPLC system (Thermo, San Jose, CA, USA). The HPLC solvents were as follows: A, 0.04% acetate acid in water; and B, methanol (0.4 ml min−1). The two mobile phases were used in the gradient mode under the following time/concentration (min/%) of B: 0.0/20, 0.5/20, 2.5/90, 3.5/90, 3.6/20, and 5.0/20. Detection and quantification were performed using a TSQ Quantum Access MAX system (Thermo, San Jose, CA, USA). ABA was detected in the ESI negative mode and selected reaction monitoring with the following parameters: parent mass by charge (m/z) of 263.1; daughter mass by charge (m/z) of 153.0; collision energy of 14 eV.
Determination of ethylene production rate
The ethylene production rate was measured using a GC-9A gas chromatograph (Shimadzu, Japan) equipped with a GDX-502 column and a flame ionization detector. The detached berries collected from different vines or the in vitro-treated berries were enclosed in a 2500-mL jar and incubated for 3 h. Five milliliters of the headspace gas was withdrawn from each jar through the septum stopper using a gas-tight syringe and was assayed.
H2O2 extraction and determination
H2O2 was extracted and determined according to the methods of Patterson49 and Macnevin and Urone50. One gram of fresh berries was homogenized with 2.5 mL of chilled acetone and then centrifuged at 10 000 rpm for 15 min at 4 °C. One hundred microliters of the supernatant was incubated with 100 µL of Ti(SO4)2 (5%) and 200 µL of NH3·H2O. Centrifuged at 3000 rpm for 10 min, the precipitate was dissolved with 500 µL of 2 mol L−1 H2SO4, and the extract was immediately photometrically determined at 415 nm.
Real-time quantitative reverse transcription-PCR
Total RNA was extracted using the hot borate method as described previously51. Two micrograms of total RNA was used to synthesize first-strand cDNA. The quantitative reverse transcription-PCR assays were conducted using a BIO-RAD iQ5 instrument (USA). All reactions were conducted in a 20-µL system containing 2 µL of Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, USA), 3 µL of diluted cDNA, and 0.5 µM each primer. Fluorescence was measured at the end of each annealing step. The specificity of the amplification was determined with a dissociation curve analysis. Expression values were normalized against the VvUbiquitin gene. All primers are listed in Supplementary Table S1.
Statistical analysis
The statistical analysis was performed by the SPSS (V19.0) statistical software package. A one-way analysis of variance followed by Duncan’s multiple range test was employed.
Data availability
Data supporting the results can be found in this paper.
Electronic supplementary material
Table S1. Sequences of the primers used in this paper
Fig. S1 Daily average temperature in the experimental site (36º.17'N, 117º.16'E) in 2016 and 2017
Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (ZR2018MC021), Funds of Shandong “Double Tops” Program (SYL2017YSTD10), China’s Agricultural Research System (CARS-29), and the Changjiang Scholars and lnnovative Research Team in University (IRT15R42).
Authors’ contributions
Y.Y. conceived and designed the experiments. L.X., Q.Y., and G.X. carried out the experiments. L.X., Y.Y., and F.B. performed data analysis. Y.Y. wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41438-018-0045-y).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coombe BG, Mccarthy MG. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2010;6:131–135. doi: 10.1111/j.1755-0238.2000.tb00171.x. [DOI] [Google Scholar]
- 2.Kuhn N, et al. Berry ripening: recently heard through the grapevine. J. Exp. Bot. 2014;65:4543–4559. doi: 10.1093/jxb/ert395. [DOI] [PubMed] [Google Scholar]
- 3.Fortes AM, Teixeira RT, Agudelo-Romero P. Complex interplay of hormonal signals during grape berry ripening. Molecules. 2015;20:9326–9343. doi: 10.3390/molecules20059326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi FF, et al. Comparison of reactive oxygen species metabolism during grape berry development between ‘Kyoho’ and its early ripening bud mutant ‘Fengzao’. Plant Physiol. Biochem. 2017;118:634–642. doi: 10.1016/j.plaphy.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Leng P, Zhang G, Li X. Cloning and functioning analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J. Plant Physiol. 2009;166:1241–1252. doi: 10.1016/j.jplph.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Pilati S, et al. Abscisic acid is a major regulator of grape berry ripening onset: new insights into ABA signaling network. Front. Plant Sci. 2017;8:1093–1108. doi: 10.3389/fpls.2017.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagné S, Cluzet S, Mérillon JM, Gény L. ABA initiates anthocyanin production in grape cell cultures. J. Plant Growth Regul. 2011;30:1–10. doi: 10.1007/s00344-010-9165-9. [DOI] [Google Scholar]
- 8.Chervin C, et al. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004;167:1301–1305. doi: 10.1016/j.plantsci.2004.06.026. [DOI] [Google Scholar]
- 9.Chervin C, et al. Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol. Plant. 2008;134:534–546. doi: 10.1111/j.1399-3054.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu MY, et al. The effects of light and ethylene and their interaction on the regulation of proanthocyanidin and anthocyanin synthesis in the skins of Vitis vinifera berries. Plant Growth Regul. 2016;79:377–390. doi: 10.1007/s10725-015-0141-z. [DOI] [Google Scholar]
- 11.Tian S, Qin G, Li B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013;82:593–602. doi: 10.1007/s11103-013-0035-2. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, et al. Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma. 2016;253:581–594. doi: 10.1007/s00709-015-0836-z. [DOI] [PubMed] [Google Scholar]
- 13.Pilati S, et al. The onset of grapevine berry ripening is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 2014;14:87–101. doi: 10.1186/1471-2229-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun QQ, et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015;66:657–668. doi: 10.1093/jxb/eru332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun QQ, et al. A label-free differential proteomics analysis reveals the effect of melatonin in promoting fruit ripening and anthocyanin accumulation upon post-harvest in tomatoes. J. Pineal Res. 2016;61:138–153. doi: 10.1111/jpi.12315. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017;65:9987–9994. doi: 10.1021/acs.jafc.7b03354. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, et al. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016;118:103–110. doi: 10.1016/j.postharvbio.2016.03.006. [DOI] [Google Scholar]
- 18.Aghdam MS, Fard JR. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017;221:1650–1657. doi: 10.1016/j.foodchem.2016.10.123. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014;56:39–50. doi: 10.1111/jpi.12095. [DOI] [PubMed] [Google Scholar]
- 20.Li C, et al. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behavior in two Malus species under drought stress. J. Exp. Bot. 2015;66:669–680. doi: 10.1093/jxb/eru476. [DOI] [PubMed] [Google Scholar]
- 21.Li C, et al. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016;61:218–229. doi: 10.1111/jpi.12342. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, et al. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010;10:257–267. doi: 10.1186/1471-2229-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnao MB, Hernández-Ruiz J. Functions of melatonin in plants: a review. J. Pineal Res. 2015;59:133–150. doi: 10.1111/jpi.12253. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Ruiz J, Cano A, Arnao MB. Melatonin: growth-stimulating compound present in lupin tissues. Planta. 2004;220:140–144. doi: 10.1007/s00425-004-1317-3. [DOI] [PubMed] [Google Scholar]
- 25.Meng JF, et al. Melatonin treatment of pre-veraison grape berries to increase size and synchronicity of berries and modify wine aroma components. Food Chem. 2015;185:127–134. doi: 10.1016/j.foodchem.2015.03.140. [DOI] [PubMed] [Google Scholar]
- 26.Watada AE, Herner RC, Kader AA, Romani RJ, Staby GL. Terminology for the description of developmental stages of horticultural crops. Hort. Sci. 1984;62:217–254. [Google Scholar]
- 27.Ma Q, Zhang T, Zhang P, Wang ZY. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016;60:424–434. doi: 10.1111/jpi.12325. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, et al. Effect of cultivar, temperature, and environmental conditions on the dynamic change of melatonin in mulberry fruit development and wine fermentation. J. Food Sci. 2016;81:958–967. doi: 10.1111/1750-3841.13263. [DOI] [PubMed] [Google Scholar]
- 29.Van Tassel DL, Roberts N, Lewy A, O’Neill SD. Melatonin in plant organs. J. Pineal Res. 2001;31:8–15. doi: 10.1034/j.1600-079X.2001.310102.x. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Gomez D, et al. Detection and quantification of melatonin and serotonin in eight sweet cherry cultivars (Prunus avium L.) Eur. Food Res. Technol. 2009;229:223–229. doi: 10.1007/s00217-009-1042-z. [DOI] [Google Scholar]
- 31.Vitalini S, et al. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011;51:331–337. doi: 10.1111/j.1600-079X.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 32.Byeon Y, et al. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015;58:470–478. doi: 10.1111/jpi.12232. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler S, Loveys B, Ford C, Davies C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009;15:195–204. doi: 10.1111/j.1755-0238.2008.00045.x. [DOI] [Google Scholar]
- 34.Berli FJ, Fanzone Mn, Piccoli P, Bottini R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011;59:4874–4884. doi: 10.1021/jf200040z. [DOI] [PubMed] [Google Scholar]
- 35.Huan C, et al. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016;104:294–303. doi: 10.1016/j.plaphy.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Deluc LG, et al. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics. 2007;8:429–470. doi: 10.1186/1471-2164-8-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilati S, et al. Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics. 2007;8:428–449. doi: 10.1186/1471-2164-8-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Kereamy A, et al. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003;119:175–182. doi: 10.1034/j.1399-3054.2003.00165.x. [DOI] [Google Scholar]
- 39.Böttcher C, Harvey KE, Boss PK, Davies C. Ripening of grape berries can be advanced or delayed by reagents that either reduce or increase ethylene levels. Funct. Plant Biol. 2013;40:566–581. doi: 10.1071/FP12347. [DOI] [PubMed] [Google Scholar]
- 40.Böttcher C, Burbidge CA, Boss PK, Davies C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013;13:222–235. doi: 10.1186/1471-2229-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong XQ, Luo Z, Dong HZ, Eneji AE, Li WJ. H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J. Exp. Bot. 2016;67:2247–2261. doi: 10.1093/jxb/erw026. [DOI] [PubMed] [Google Scholar]
- 42.Fu J, et al. Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci. Rep. 2017;7:39865–39875. doi: 10.1038/srep39865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, et al. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.) Environ. Exp. Bot. 2017;138:36–45. doi: 10.1016/j.envexpbot.2017.02.012. [DOI] [Google Scholar]
- 44.Wei Y, et al. Identification, transcriptional and functional analysis of heat-shock protein 90s in banana (Musa acuminate L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J. Pineal Res. 2017;62:1–12. doi: 10.1111/jpi.12367. [DOI] [PubMed] [Google Scholar]
- 45.Zaharah SS, Singh Z, Symons GM, Reid JB. Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol. Technol. 2013;75:37–44. doi: 10.1016/j.postharvbio.2012.07.009. [DOI] [Google Scholar]
- 46.Neff M, Chory J. Genetic interactions between phytochrome A, phytochrome B, and Cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian FE, et al. [Effect of root-applied melatonin on endogenous melatonin and chlorophyll fluorescence characteristics in grapevine under NaCl stress] Sci. Agric. Sinica. 2018;51:952–963. [Google Scholar]
- 48.Ré DA, Raud B, Chan RL, Baldwin IT, Bonaventure G. RNAi-mediated silencing of the HD-Zip gene HD20 in Nicotiana attenuate affects benzyl acetone emission from corollas via ABA levels and the expression of metabolic genes. BMC Plant Biol. 2012;12:60–74. doi: 10.1186/1471-2229-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson BD, Macrae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium (IV) Anal. Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- 50.Macnevin WM, Urone PF. Separation of hydrogen peroxide from organic hydroperoxides. Anal. Chem. 1953;25:1760–1761. doi: 10.1021/ac60083a052. [DOI] [Google Scholar]
- 51.Yao YX, Li M, Liu Z, Hao YJ, Zhai H. A novel gene, screened by cDNA-AFLP approach, contributes to lowering the acidity of fruit in apple. Plant Physiol. Biochem. 2007;45:139–145. doi: 10.1016/j.plaphy.2007.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sequences of the primers used in this paper
Fig. S1 Daily average temperature in the experimental site (36º.17'N, 117º.16'E) in 2016 and 2017
Data Availability Statement
Data supporting the results can be found in this paper.