Abstract
It is now established that adverse conditions during pregnancy can trigger a fetal origin of cardiovascular dysfunction and/or increase the risk of heart disease in later life. Suboptimal environmental conditions during early life that may promote the development of cardiovascular dysfunction in the offspring include alterations in fetal oxygenation and nutrition as well as fetal exposure to stress hormones, such as glucocorticoids. There has been growing interest in identifying the partial contributions of each of these stressors to programming of cardiovascular dysfunction. However, in humans and in many animal models this is difficult, as the challenges cannot be disentangled. By using the chicken embryo as an animal model, science has been able to circumvent a number of problems. In contrast to mammals, in the chicken embryo the effects on the developing cardiovascular system of changes in oxygenation, nutrition or stress hormones can be isolated and determined directly, independent of changes in the maternal or placental physiology. In this review, we summarise studies that have exploited the chicken embryo model to determine the effects on prenatal growth, cardiovascular development and pituitary–adrenal function of isolated chronic developmental hypoxia.
Keywords: fetus, hypoxia, IUGR, programming, cardiovascular disease
Introduction
Laws of nature predict that the younger we are the greater the impact the environment has upon us. This is unsurprising because our physiology is much more plastic in fetal than in adult life. Therefore, we now understand that adverse environmental conditions during early life, such as pre‐conceptually and during pregnancy, may be just as if not more important than postnatal lifestyle in interacting with genes to set a risk of cardiovascular disease. Since this concept of developmental origins of health and disease was introduced (Barker et al. 1993; Gluckman et al. 2008), it has been understood that intrauterine growth restriction (IUGR) is a surrogate marker for adverse intrauterine conditions and there has been a keen interest in identifying which and how sub‐optimal environmental conditions may promote IUGR and programme an increased cardiovascular risk in the offspring.
Data from human epidemiological studies and from experimental animal models of IUGR pregnancy have consistently associated chronic fetal hypoxia with the slowed fetal growth and an increased cardiovascular risk in later life. However, the contribution of chronic fetal hypoxia in promoting IUGR and programmed cardiovascular risk has been difficult to isolate for a number of reasons. For instance, it is established that high altitude pregnancy leads to IUGR (Moore, 1990; Giussani et al. 2001; Moore et al. 2011; Soria et al. 2013). However, most high altitude populations are impoverished with significant maternal malnutrition (Giussani et al. 2001; Niermeyer et al. 2001; Julian et al. 2009). Therefore, the contribution of chronic fetal hypoxia versus chronic fetal undernutrition in slowing fetal growth and in setting future cardiovascular risk under these conditions is uncertain. The same applies to sea level pregnancy complicated by preeclampsia, placental insufficiency, gestational diabetes and even maternal obesity. All these conditions are associated with an increase in placental vascular resistance (Macfarlane & Tsakalakos, 1985; Okamura et al. 1990; Gagnon, 2003; Kendall & Peebles, 2005; Hayes et al. 2012; Giussani, 2016), which will decrease oxygen as well as nutrient delivery to the growing fetus. Similarly, several studies including our own in mammalian experimental animal models have shown that maternal chronic hypoxia during pregnancy can lead to IUGR and programme increased cardiovascular risk in the offspring (Thompson & Weiner, 1999; Camm et al. 2010; Thompson et al. 2011; Giussani et al. 2012). However, because experimental induction of chronic hypoxia in rodents can reduce maternal food intake and/or alter the quality of the maternal milk (Williams et al. 2005b; Wlodek et al. 2005; O'Dowd et al. 2008; Camm et al. 2010), the contribution of chronic fetal hypoxia versus chronic fetal and/or neonatal under‐nutrition under these conditions, again, remains uncertain. By using the chicken embryo as an animal model, science has been able to circumvent a number of these problems because, in contrast to mammals, with the exception of monotremes, in the chicken the effects of changes in oxygenation on the embryo can be isolated and determined directly, independent of changes in the maternal physiology.
The development of the chicken embryo is supported by three gas exchange organ systems: the yolk sac, the chorioallantoic membrane (CAM) and the lungs (Wangensteen & Rahn, 1971; Druyan et al. 2007). The vascular portion of the yolk sac is the principal gas exchange organ during early development (Baumann & Meuer, 1992). In addition to its role as an early gas exchange organ, the yolk sac provides the chicken embryo with essential nutrients for its growth. Protein, fat, carbohydrates and minerals are stored in the yolk of the egg for future utilisation by the embryo (Speake et al. 1998; Yadgary et al. 2013). At approximately embryonic day 8, the allantoic sac develops and fuses with the chorion to create the CAM (Wangensteen & Rahn, 1971). This highly vascular structure, in conjunction with the porosity of the eggshell, permits diffusion of oxygen and carbon dioxide between the environment and the blood, thereby replacing the yolk sac as the primary source of oxygen uptake (Wangensteen & Rahn, 1971; Druyan et al. 2007; Mohammed et al. 2015). Finally, transition to ex ovo life in chickens is initiated around incubation day 19–20, when the embryo pips internally through to the air cell of the egg with its beak and begins air breathing for the first time. From this point on, the embryo relies on both the CAM and the lungs for gas exchange (Dzialowski et al. 2012). Therefore, during the second half of incubation, chicken embryos are endowed with a gas‐exchange organ, the CAM, and a choriovitelline nutritional organ, the yolk sac (Wangensteen & Rahn, 1971; Druyan et al. 2007; Dzialowski et al. 2012; Mohammed et al. 2015). Interestingly, these two extra‐embryonic organs are perfused by two separate vascular systems that show important differences in their responses to vasoactive agents (Mohammed et al. 2015). It is also known that incubation of fertilised eggs under varying degrees of oxygenation will induce different effects depending on the activity of the major gas exchange organ system at that time (Chan & Burggren, 2005). For instance, exposure to hypoxia (15% O2) during days 1–6 of incubation induces accelerated yolk consumption (Chan & Burggren, 2005). In contrast, exposure to hypoxia during mid‐incubation accelerates yolk consumption (Chan & Burggren, 2005) and increases CAM vascularity (Strick et al. 1991; Verhoelst et al. 2011). The latter promotes an increased functional surface area for gas exchange (Chan & Burggren, 2005).
Chronic hypoxia and fetal growth: studies in the chicken embryo
We have previously reported that incubation of fertilised eggs laid by sea level hens at high altitude (3600 m) reduced fetal , increased haematocrit and reduced embryonic weight by 45% at the end of the incubation period (Fig. 1 A; Giussani et al. 2007; Salinas et al. 2010). Importantly, the reduction in embryonic weight persisted when it was expressed relative to the egg mass at the start of the incubation period, to account for very real differences in size of eggs laid by sea level hens compared to high altitude hens (Fig. 1 B). Incubation of eggs laid by high altitude hens at high altitude also led to significant fetal growth restriction, although the change was smaller (by 22.2%) compared to the effect of high altitude on chicken embryos of sea level hens. This protection of prenatal growth by prolonged residence at high altitude has also been reported for human Andean populations (Giussani et al. 2001; Moore et al. 2004; Soria et al. 2013), and the effect has been termed the Andean curse on the conquistadors (Giussani, 2007). Further data revealed that high altitude‐induced growth restriction of fertilised eggs laid by sea level hens was prevented by supplementing the incubator with oxygen at pressures to equate with sea level conditions (Giussani et al. 2007). Importantly, chicken embryos incubated at high altitude were asymmetrically growth restricted (Fig. 1 C), with a marked increase in the brain weight and head diameter relative to the embryonic body mass (Giussani et al. 2007; Salinas et al. 2010). It is well established that the asymmetric growth restriction is a consequence of fetal blood flow redistribution in response to chronic hypoxia, commonly referred to as the fetal brain sparing effect (Giussani & Davidge, 2013; Allison et al. 2016; Giussani, 2016). This fetal phenotypic response to chronic fetal hypoxia is well conserved across species including the human, sheep and rat (Mulder et al. 1998; Lang et al. 2000; Ruijtenbeek et al. 2000; Giussani et al. 2001, 2007; Fowden et al. 2006; Camm et al. 2010), thereby supporting the chicken embryo as an appropriate and comparable animal model to study the consequences of chronic developmental hypoxia on asymmetric growth restriction prior to hatching.
Figure 1. Fetal growth in the chicken embryo at day 19–20 of incubation.
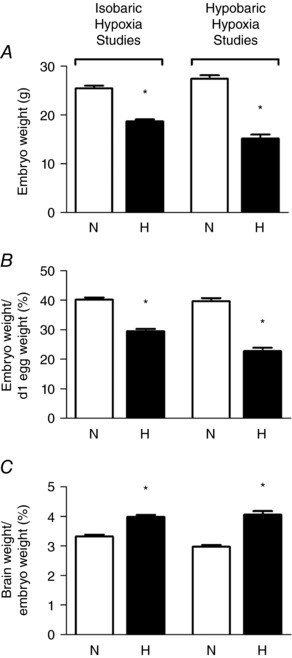
Values are mean ± SEM at day 19–20 of absolute embryo weight (A), relative embryo weight (B), and brain weight relative to body weight (C) of chicken embryos incubated in either normoxia (N, n = 21) or hypoxia (H, n = 20) for isobaric hypoxia studies, and normoxia at sea level (N, n = 31) or hypoxia at high altitude (H, n = 16) for hypobaric hypoxia studies. *Significantly (P < 0.05) different from corresponding control. Data adapted from Giussani et al. (2007), Salinas et al. (2010) and Itani et al. (2016, 2017).
The significant induction of embryonic growth restriction by developmental hypoxia has also been reported by us and others using incubation of chicken embryos under isobaric (sea level atmospheric pressure) hypoxic conditions (Fig. 1 and Table 1; Ruijtenbeek et al. 2000; Dzialowski et al. 2002; Miller et al. 2002; Rouwet et al. 2002; Villamor et al. 2004; Sharma et al. 2006; Giussani et al. 2007; Wei et al. 2007; Lindgren & Altimiras, 2009; Van der Sterren et al. 2009; Zoer et al. 2009; Salinas et al. 2010; Zoer et al. 2010; Lindgren & Altimiras, 2011; Lindgren et al. 2011; Sahan et al. 2011; Moonen et al. 2012; Itani et al. 2016; Itani et al. 2017). Varying magnitudes of the reduction in embryo weight in these studies may reflect differences in the humidity, temperature, the length and timing of hypoxia as well as the breed of chicken used. However, taken together, past studies describing the significant effect of incubation of fertilised eggs under hypobaric (lower than normal atmospheric pressure) or isobaric hypoxic conditions in slowing embryonic growth add robust evidence to the literature to strongly support a role for isolated fetal hypoxia in mediating fetal growth restriction.
Table 1.
Studies showing a reduction in chicken embryo weight following exposure to chronic hypoxia
| Oxygen (%) | Body weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breed | Study design | Temp (°C) | Humidity (%) | Control | Hypoxia | Day body weight was measured | Control | Hypoxia | Difference (%) | Reference |
| Black Leghorn | Hypobaric hypoxia | 38 | 60 | 20 | 13.7 | 20 | 27.4 ± 0.8 | 15.2 ± 0.8 | 44.6 | Giussani et al. (2007) |
| Black Leghorn | Hypobaric hypoxia | 38 | 60 | 20 | 13.7 | 20 | 28 ± 1 | 15 ± 1 | 46.4 | Salinas et al. (2010) |
| Broiler Ross 308 | Hypobaric hypoxia | 37.5 | 55 | 20.7 | 18.5 | 18 | 48.1 ± 0.7 | 46.0 ± 0.6 | 4 | Sahan et al. (2011) |
| Bovans Brown | Isobaric hypoxia | 37.9 | 45 | 21 | 14 | 19 | 25.2 ± 0.9 | 19.4 ± 0.5 | 23 | Itani et al. (2016) |
| White Leghorn | Isobaric hypoxia from day 6 | 38 | 60 | 21 | 15 | 19 | 25.4 ± 0.6 | 21.9 ± 0.4 | 13.8 | Ruijtenbeek et al. (2000) |
| White Leghorn | Isobaric hypoxia from day 6 | 37.8 | 45 | 21 | 15 | 19 | 29.9 ± 5.6 | 25.3 ± 5.5 SD | 15.4 | Van der Sterren et al. (2009) |
| White Leghorn | Isobaric hypoxia from day 6 | 38 | 60 | 21 | 15 | 19 | 26.6 ± 0.7 | 22.4 ± 0.5 | 15.8 | Villamor et al. (2004) |
| White Leghorn | Isobaric hypoxia | 37.8 | 60 | 21 | 15 | 19 | 28.0 ± 2.9 SD | 24.8 ± 1.9 SD | 11.4 | Moonen et al. (2012) |
| White Leghorn | Isobaric hypoxia | 37.8 | 45 | 21 | 15 | 19 | 28.3 ± 0.4 SD | 23.7 ± 0.6 SD | 16.3 | Zoer et al. (2010) |
| White Leghorn | Isobaric hypoxia | 37 | 60 | 21 | 15 | 19 | 24.7 ± 0.4 | 21.9 ± 0.5 | 11.3 | Rouwet et al. (2002) |
| White Leghorn | Isobaric hypoxia | 38 | 60‐70 | 21 | 15 | 3.5 | 0.038 ± 0.002 | 0.033 ± 0.001 | 13.2 | Sharma et al. (2006) |
| White Leghorn | Isobaric hypoxia | 38.5 | 50‐60 | 21 | 14 | 15 | 12.2 ± 1.7 SD | 9.1 ± 1.2 SD | 25.5 | Miller et al. (2002) |
| White Leghorn | Isobaric hypoxia from day 10 | 38.5 | 50‐60 | 21 | 14 | 15 | 12.2 ± 1.7 SD | 10.8 ± 1.6 SD | 10.8 | Miller et al. (2002) |
| White Leghorn | Isobaric hypoxia between day 6–12 | 37.5 | 75‐95 | 21 | 15 | 18 | 23.4 ± 0.5 | 20.7 ± 0.5 | 11.5 | Dzialowski et al. (2002) |
| White Leghorn | Isobaric hypoxia between day 12–18 | 37.5 | 75‐95 | 21 | 15 | 18 | 23.4 ± 0.5 | 19.7 ± 0.5 | 15.8 | Dzialowski et al. (2002) |
| White Leghorn | Isobaric hypoxia from day 6 | 37.8 | 45 | 21 | 15 | 19 | 29.2 ± 0.3 SD | 25.9 ± 0.4 SD | 11.3 | Zoer et al. (2009) |
| Broiler | Isobaric hypoxia from day 6 | 37.8 | 45 | 21 | 15 | 19 | 31.4 ± 0.6 SD | 25.6 ± 0.5 SD | 18.5 | Zoer et al. (2009) |
| Broiler Ross 308 | Isobaric hypoxia | 37.8 | 45 | 20.95 | 14 | 19 | 31.7 ± 3.2 SD | 21.5 ± 2.8 SD | 32.2 | Lindgren & Altimiras, (2009) |
| Broiler Ross 308 | Isobaric hypoxia | 37.8 | 45 | 21 | 14 | 19 | 34.8 ± 0.5 | 25.7 ± 0.6 | 26.1 | Lindgren et al. (2011) |
Body weight data are mean ± SEM unless otherwise stated. SD, standard deviation.
Chronic hypoxia and the fetal HPA axis: studies in the chicken embryo
It is well established that the hypothalamo–pituitary–adrenal (HPA) axis is functional long before hatching in the chicken embryo (Woods et al. 1971; Wise & Frye, 1973; Kalliecharan & Hall, 1974; Jenkins & Porter, 2004). Consequently, possible effects of hypoxia on the developing HPA axis can be isolated using this model (Salinas et al. 2011). In one of the few studies addressing HPA function in the chronically hypoxic chicken embryo, Salinas et al. (2011) reported that incubation at high altitude of fertilised eggs laid by sea level hens significantly increased plasma adrenocorticotropic hormone (ACTH) concentrations by embryonic day 20, while circulating levels of corticosterone in these embryos were markedly reduced compared to chicken embryos of sea level hens incubated at sea level. In addition, incubation at high altitude of eggs laid by high altitude hens produced similar changes in the levels of fetal plasma ACTH and corticosterone. Moreover, supplementing sea level eggs with oxygen during incubation at high altitude completely prevented the observed changes in ACTH and corticosterone production, thereby supporting hypoxia rather than hypobaria in inducing the adrenocortical blunting. Correlation analysis revealed that the levels of plasma ACTH and corticosterone were positively related in sea level embryos incubated at sea level or at high altitude with oxygen supplementation. However, this correlation was no longer apparent in all other groups. Therefore, data in this study support previous human and mammalian studies reporting dissociated HPA activities in development complicated by chronic fetal hypoxia (Challis et al. 1989; Hooper et al. 1990; Harvey et al. 1993; Murotsuki et al. 1996; Stratford & Hooper, 1997). Importantly, the chicken embryo data showed that the plasma corticosterone/ACTH ratio was positively correlated to embryonic body weight and to chorioallantoic blood , providing further evidence for a direct relationship between changes in HPA activity and changes in oxygenation during embryonic development (Salinas et al. 2011).
In line with the findings of Salinas et al. (2011) in the chicken embryo, a study using the sheep fetus demonstrated that development at high altitude promoted the enhanced processing of the precursor proopiomelanocortin into ACTH (Myers et al. 2005a). In addition, ACTH receptor expression and enzymatic capacity to synthesise cortisol were both reduced in the fetal sheep adrenal glands in the same experimental model (Myers et al. 2005b). In subsequent studies, Myers and Ducsay proposed that nitric oxide (NO) and leptin could be potential mediators of the inhibition of cortisol synthesis in the adrenal glands of fetal sheep exposed to long‐term high altitude hypoxia (Monau et al. 2009; Myers & Ducsay, 2012). The authors suggested that leptin and NO limit the capacity of elevated fetal plasma ACTH levels to stimulate cortisol production in the chronically hypoxic fetus. In sheep, this prevents the premature induction of labour while allowing for the maintenance of the normal prepartum surge in fetal plasma cortisol that occurs close to term, which is essential for appropriate fetal maturation.
In addition to adrenocortical blunting, high altitude incubation of chicken embryos from hens native to sea level or to high altitude induced a significant increase in adrenal concentrations of both adrenaline and noradrenaline, and oxygen supplementation during high altitude incubation prevented this (Salinas et al. 2011). Chronic hypoxia‐induced sensitisation of the sympathoadrenal medullary system has been reported in a range of animal models including the sheep, llama, rat and chicken (Roigas et al. 1996; Simonetta et al. 1997; Ruijtenbeek et al. 2000; Gardner et al. 2002; Llanos et al. 2003; Lindgren & Altimiras, 2013). For instance, in the chicken embryo, chronic hypoxia sensitises cardiac β‐adrenergic receptors (Lindgren & Altimiras, 2009, 2013). In addition, chronically hypoxic chicken embryos have increased noradrenaline levels and enhanced sympathetic innervation in the peripheral vasculature (Ruijtenbeek et al. 2000). Importantly, there was a strong negative correlation between arterial and adrenal catecholamine content in chicken embryos incubated at sea level and high altitude (Salinas et al. 2011). These data therefore not only support a similar relationship between plasma noradrenaline levels and reported in fetal sheep (Simonetta et al. 1997), but provide further evidence for a direct effect of isolated developmental hypoxia on the reactivity of the sympathoadrenomedullary system.
Blunting of fetal basal adrenal cortical but not medullary function may be an appropriate homeostatic response to prolonged periods of fetal hypoxia to protect sensitive tissues from sustained elevations in plasma glucocorticoid levels (Ducsay, 1998; Myers et al. 2005b). Conversely, sensitisation of the sympathoadrenal system may maintain appropriate glucogenic capacity during fetal development under conditions of chronic fetal hypoxia. The biological trade‐offs of the divergent adaptations in adrenal responses may yield newborns with adrenocortical suppression and adrenergic hyper‐reactivity. Both will have clear consequences for the programming of cardio‐metabolic and endocrine dysfunction in later life (Reynolds et al. 2001; Peyronnet et al. 2002; Kajantie et al. 2003; Watterberg et al. 2004) and this warrants further investigation.
Chronic fetal hypoxia and programmed cardiovascular risk: studies in the chicken embryo and in the adult bird
It is widely established that adverse conditions during development not only promote IUGR and alterations in the HPA axis, but they also induce early origins of cardiovascular remodelling and dysfunction (see Gluckman et al. 2008; Giussani & Davidge, 2013). In humans, IUGR pregnancy is associated with ventricular hypertrophy, and increased aortic stiffness and thickness in the offspring before and after birth (Veille et al. 1993; Skilton et al. 2005; Akira & Yoshiyuki, 2006; Cosmi et al. 2009). Studies in rodent animal models have provided evidence to support that the human IUGR‐induced programming of cardiovascular remodelling is at least partly due to suspected chronic fetal hypoxia. For instance, hypoxic pregnancy induces similar thickening of the aortic wall and aortic stiffening in the rodent as well as ovine fetus (Williams et al. 2005a; Camm et al. 2010; Thompson et al. 2011; Giussani et al. 2012). Studies by Zhang and colleagues have also reported that hypoxic pregnancy in rodents leads to cardiac remodelling in the offspring (Bae et al. 2003; Tong & Zhang, 2012). Aortic hypertrophy often precedes the clinical manifestation of hypertension, atherosclerosis and coronary heart disease (Arnett et al. 1994). Consequently, the remodelling of the heart and major vessels early in life due to suspected chronic fetal hypoxia may increase the risk of developing cardiovascular disease in adulthood (Williams et al. 2005b; Crispi et al. 2010; Giussani et al. 2012; Giussani & Davidge, 2013).
Again, studies using the chicken embryo have shown that hypoxic incubation can recapitulate the adverse cardiovascular phenotype described either in studies using mammalian animal models of chronic hypoxia or in human clinical studies of IUGR pregnancies, supporting that chronic fetal hypoxia is an important mechanism. The relatively large size of the chicken embryo at term (>25 g on day 19, term is 21 days; Table 1) compared to the rodent fetus (<4 g on day 20, term is 21 days; see Camm et al. 2010) means that the chicken embryo model has the added advantage of facilitating study of cardiovascular function in ovo or in isolated organs, for instance using the Langendorff preparation (Itani et al. 2016) or the myograph (Ruijtenbeek et al. 2003b; Villamor et al. 2004; Van der Sterren et al. 2009; Moonen et al. 2012; Itani et al. 2016, 2017). Interestingly, and perhaps surprisingly, the chronology of cardiac development in the chicken is much more comparable to the human than is the rodent, in which cardiac development continues into the postnatal period (Sissman, 1970; Monie, 1976; Marcela et al. 2012; see Fig. 2). Combined, these advantages have convinced many investigators to use the chicken embryo as a useful animal model to isolate the effects of chronic hypoxia on cardiovascular development (Table 2). Studies have reported that chicken embryos of both sea level and high altitude hens following incubation at high altitude have increased relative heart weight as well as relative left (LV) and right (RV) ventricular area and wall thickness (Salinas et al. 2010). These embryos also had a higher aortic wall:lumen area ratio, indicating that developmental exposure to hypobaric hypoxia induced aortic hypertrophy prior to hatching. This morphometric analysis of the cardiovascular system of the high altitude chicken embryo has been expanded recently to include analysis on cardiovascular morphology of chicken embryos exposed to isobaric hypoxia throughout the incubation period (Itani et al. 2016). In contrast to high altitude incubation, chronic isobaric hypoxia induced cardiac dilatation with reduced LV wall while enhancing LV lumen volume, yielding a marked reduction, rather than an increase, in the LV wall:lumen area ratio (Itani et al. 2016). Similarly, both dilatation and hypertrophy of the aorta in chronically hypoxic chicken embryos have been reported by independent groups (Figs 2 and 3; Rouwet et al. 2002; Salinas et al. 2010; Itani et al. 2016). The reasons underlying the differential effects of chronic hypobaric versus isobaric hypoxia of similar magnitude and duration on cardiovascular remodelling in the developing embryo are not clear at present.
Figure 2. Comparison of the key stages of cardiac development in different animal models.
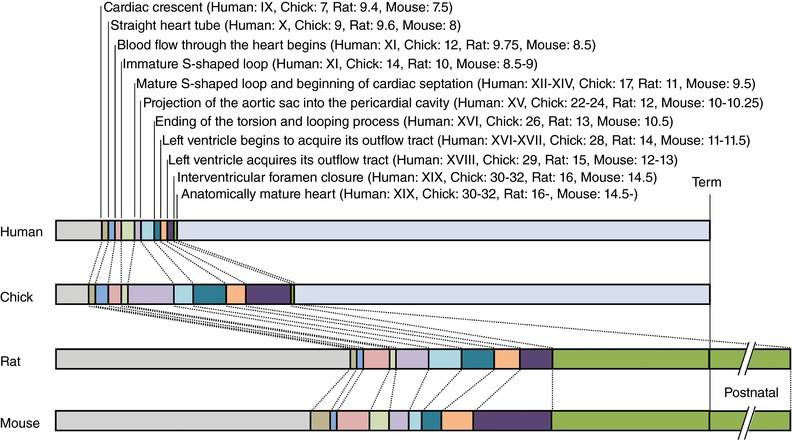
Bars represent the proportion (%) of development. Roman and Arabic numerals refer to human Carnegie stage (Streeter horizons), chick Hamburger and Hamilton stage, rat and mouse embryonic days. Term: human, 38 weeks; chick, 21 days; rat, 21 days; mouse, 19 days. While the main anatomical development of the heart is complete in the rat and mouse by day 16 and 14.5, respectively, the acquisition of mature septum, valves and tendinea cords is not yet complete until after birth (Marcela et al. 2012). Drawn from data provided in Sissman (1970) and Marcela et al. (2012).
Table 2.
Studies showing effects of chronic developmental hypoxia on the cardiovascular system in chicken embryos and/or adult birds
| Breed | Study design | Temp (°C), humidity (%) | Oxygen (%) N, H | Effects on cardiovascular function | Morphological and/or histochemical effects on the heart | Morphological and/or histochemical effects on vessels | Reference |
|---|---|---|---|---|---|---|---|
| Black Leghorn | Hypobaric hypoxia | 38, 60 | 20, 13.7 | Increased heart weight, LV and RV area and wall thicknesses relative to body weight | Increased aortic wall:lumen area ratio on embryonic day 20 | Salinas et al. (2010) | |
| Black Leghorn | Hypobaric hypoxia | 20, 13.7 | Significantly lower systolic, diastolic and mean arterial pressure at 6 months of age. Increased HR and lower pulse interval in the males but not females | Herrera et al. (2013) | |||
| Black Leghorn | Hypobaric hypoxia | 38, 60 | 20, 13.7 | Increased tricuspid pressure gradient. Reduced mitral valve pressure gradient in males | Thicker right ventricular wall | Increased pulmonary artery diameter in males | Salinas et al. (2014) |
| Bovans Brown | Isobaric hypoxia | 37.9, 45 | 21, 14 | Reduced systolic pressure and higher diastolic pressure, impaired peripheral endothelial vasodilatation | LV dilatation | Aortic dilatation | Itani et al. (2016, 2017) |
| White Leghorn | Isobaric hypoxia from day 6 | 38, 60 | 21, 15 | Reduced sensitivity of femoral artery to noradrenaline | Increased noradrenaline content | Higher perivascular sympathetic innervation in femoral artery | Ruijtenbeek et al. (2000) |
| White Leghorn | Isobaric hypoxia from day 6 | 38, 60–70 | 21, 15 | Impaired NO‐dependent endothelial relaxation to ACh. Enhanced contractile response of the femoral artery to electrical stimulation 14–15 weeks post‐hatching | Perivascular hyperinnervation no longer present 14–15 weeks after hatching | Ruijtenbeek et al. (2003a) | |
| White Leghorn | Isobaric hypoxia from day 6 | 38, 60 | 21, 15 | Impaired NO‐dependent vasodilatation of the femoral artery on day 19 | Ruijtenbeek et al. (2003b) | ||
| White Leghorn | Isobaric hypoxia from day 6 | 38, 60 | 21, 15 | Impaired contractile function of the pulmonary artery. Impaired femoral arterial relaxation to ACh | Increased heart mass and LV and RV wall thickness and areas relative to body weight | Villamor et al. (2004) | |
| White Leghorn | Isobaric hypoxia from day 6 | 37.8, 45 | 21, 15 | Shortened ductus arteriosus. Enhanced ductus arteriosus contractile response to α‐adrenergic agonists and impaired endothelium‐dependent, ‐independent and β‐AR agonist‐induced relaxation | Van der Sterren et al. (2009) | ||
| White Leghorn | Isobaric hypoxia from day 6 | 37.8, 45 | 21, 15 | Endothelium‐dependent relaxation of intrapulmonary arteries are not affected | Zoer et al. (2009) | ||
| Broiler | Isobaric hypoxia from day 6 | 37.8, 45 | 21, 15 | Endothelium‐dependent relaxation of intrapulmonary arteries are not affected | Zoer et al. (2009) | ||
| White Leghorn | Isobaric hypoxia | 37.8, 45 | 21, 15 | Hypoxia did not affect the Rho‐kinase inhibitor hydroxyfasudil‐induced relaxation | Zoer et al. (2010) | ||
| White Leghorn | Isobaric hypoxia | 37.8, 60 | 21, 15 | Enhanced vascular response to noradrenaline on day 19. Enhanced response to endothelin‐1 on day 15 and postnatal day 1. Diminished response to ACh on day 15. | Moonen et al. (2012) | ||
| White Leghorn | Isobaric hypoxia | 37, 60 | 21, 15 | Reduced systolic pressure, enhanced peripheral arterial tone | Heart weight increased relative to body weight, reduced septum thickness | Hypertrophic growth of the aorta | Rouwet et al. (2002) |
| White Leghorn | Isobaric hypoxia | 37, 60 | 21, 15 | LV systolic and diastolic dysfunction at day 20 and 8 months post‐hatching | Dilated left ventricle with enhanced fibrosis, cardiomyocyte degeneration and disorganisation | Tintu et al. (2009) | |
| White Leghorn | Isobaric hypoxia | 38, 60–70 | 21, 15 | Reduced peak systolic pressure, systolic volume and cardiac output | Sharma et al. (2006) | ||
| Broiler strain Ross 308 | Isobaric hypoxia | 37.8, 45 | 20.95, 14 | Decreased density and enhanced sensitivity of β‐AR at day 19, followed by decreased sensitivity to β‐AR stimulation by day 35 post‐hatching | Increased relative heart mass in the embryo and in 14‐day‐old hatchlings, but the effect disappears by day 35 post‐hatching | Lindgren & Altimiras (2009) | |
| Broiler strain Ross 308 | Isobaric hypoxia | 37.8, 45 | 21, 14 | Hypotension and enhanced β‐AR sensitivity | Lindgren et al. (2011) | ||
| Broiler strain Ross 308 | Isobaric hypoxia | 37.8, 45 | 21, 14 | Increased β1‐AR activity and in vivo systolic dysfunction | Increased relative heart mass and increased systolic lumen diameter | Lindgren & Altimiras (2013) | |
| White Leghorn | Isobaric hypoxia | 38, 60 | 21, 15 | Blunted in vivo cardiovascular response to superimposed acute hypoxia and NO stimulation | Iversen et al. (2014) | ||
| White Leghorn | Isobaric hypoxia | 38, 60 | 21, 15 | Reduced stroke volume and cardiac output, and impaired left ventricular contractility and relaxability | Increased relative heart and LV mass. Reduced expression of the genes involved in excitation–contraction coupling | Jonker et al. (2015) | |
| Broiler strain Ross 308 | Isobaric hypoxia | 37.8, 45 | 21, 14 | Increased relative heart mass. No effect on cardiomyocyte density or size, indicating a reduction in the cell number by day 19 | Österman et al. (2015) |
β‐AR, β‐adrenergic receptor; ACh, acetylcholine; LV, left ventricle; RV, right ventricle.
Figure 3. Aortic morphology in the chicken embryo at day 19–20 of incubation.
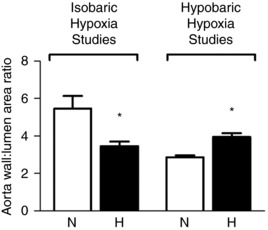
Values are mean ± SEM at day 19–20 of wall:lumen area ratio of chicken embryos incubated in either normoxia (N, n = 10) or hypoxia (H, n = 10) for isobaric hypoxia studies, and normoxia at sea level (N, n = 8) or hypoxia at high altitude (H, n = 7) for hypobaric hypoxia studies. *Significantly (P < 0.05) different from corresponding control. Data adapted from Salinas et al. (2010) and Itani et al. (2016).
In addition to morphological remodelling of the developing heart, a number of studies in humans and mammalian animal models have reported that chronic fetal hypoxia has pronounced adverse effects on cardiac and vascular function in the offspring. In humans, IUGR pregnancy is associated with impaired cardiac contractility and ventricular filling, together with reduced ventricular ejection force in the offspring before and after birth (Rizzo et al. 1995; Gardiner et al. 2001). Human epidemiological studies have also reported endothelial dysfunction in children and adults with low birth weight (Goodfellow et al. 1998; Martin et al. 2000; Leeson et al. 2001). In line with observations in human IUGR offspring, there are now elegant non‐human primate data derived from cardiac magnetic resonance imaging that reports IUGR also being associated with cardiac remodelling in young adult baboons (Kuo et al. 2017a,b). Studies in rodent mammalian animal models have also reported that chronic fetal hypoxia can programme cardiac and vascular dysfunction in later life (Thompson & Weiner, 1999; Kim et al. 2005; Williams et al. 2005a; Giussani et al. 2012; Giussani & Davidge, 2013; Kane et al. 2013).
Collectively, studies in the chicken embryo suggest that cardiovascular dysfunction in children and in mammalian animal models of IUGR pregnancy may again be attributable to chronic fetal hypoxia. Thus, incubation of chicken embryos under hypoxic conditions was also associated with reduced left ventricular ejection fraction and contractility, and diminished left ventricular developed pressure, all indicative of significant systolic dysfunction (Rouwet et al. 2002; Sharma et al. 2006; Tintu et al. 2009; Jonker et al. 2015; Itani et al. 2016). Several candidate pathways may contribute to the hypoxia‐induced cardiovascular dysfunction, including those involving vascular endothelial growth factor (VEGF) (Tintu et al. 2009; Moonen et al. 2012; Itani et al. 2016) and Rho‐kinase (Zoer et al. 2010). Exposure to chronic hypoxia increased both gene and protein expressions of VEGF in the embryonic heart, and systemic administration of recombinant VEGF mimicked the hypoxia‐induced cardiac dilatation (Tintu et al. 2009; Itani et al. 2016). Itani and colleagues have also reported that hypoxic incubation increases indices of oxidative stress, reduces the expression and activity of endogenous antioxidant enzymes and impairs levels of nitric oxide species in the chick embryo heart by the end of the incubation period (Itani et al. 2016, 2017).
Incubation of chicken embryos under hypoxic conditions can also recapitulate impaired vasodilatation and/or enhanced contractile responses in pulmonary and systemic arteries in response to pharmacological or electrical stimulation (Table 2; Ruijtenbeek et al. 2003b; Villamor et al. 2004; Van der Sterren et al. 2009; Moonen et al. 2012; Itani et al. 2016, 2017). Endothelial function has been mainly studied by the use of acetylcholine (ACh). In the chicken embryo, ACh induced an endothelium‐dependent and, at least partially, NO‐mediated relaxation of pulmonary (Villamor et al. 2002), femoral (le Noble et al. 2000; Villamor et al. 2002), mesenteric (Moonen & Villamor, 2011) and carotid arteries (le Noble et al. 2000), as well as in the ductus arteriosus (Agren et al. 2008). Interestingly, chronic isobaric hypoxia led to impairment of ACh‐induced relaxation in the systemic (Ruijtenbeek et al. 2003a,b; Villamor et al. 2004; Van der Sterren et al. 2009; Moonen et al. 2012) but not in the pulmonary arteries (Villamor et al. 2004). In contrast, chronic hypobaric hypoxia impaired endothelium‐independent relaxation in the ductus arteriosus (Van der Sterren et al. 2009), did not affect it in femoral and pulmonary arteries (Ruijtenbeek et al. 2003a,b; Villamor et al. 2004) and increased it in mesenteric arteries (Moonen et al. 2012). Altogether, this suggests that the effects of hypoxia in endothelium‐dependent and ‐independent relaxation in chicken embryo vessels are strongly vascular bed dependent.
Fewer studies have investigated the consequence of hypoxic incubation on the cardiovascular system of the adult bird (Table 2). Chronically instrumented adult chickens raised from eggs incubated at high altitude were significantly hypotensive with lower systolic and diastolic arterial pressures (Herrera et al. 2013). Adult chickens raised from eggs incubated at high altitude also showed echocardiographic indices of pulmonary hypertension and right heart dysfunction, relative to birds raised from eggs incubated at sea level (Salinas et al. 2014). One important consideration for studies of cardiovascular function at high altitude in humans and experimental animals at adulthood is that the effects on cardiovascular dysfunction triggered by post‐natal versus pre‐natal hypoxia cannot be disentangled. However, studies from independent laboratories have reported that adult birds raised in a normoxic environment but incubated under hypoxic conditions do indeed show altered contractile and relaxant responses in the peripheral vasculature (Ruijtenbeek et al. 2003a,b), systolic and diastolic dysfunction and marked increases in indices of myocardial fibrosis (Tintu et al. 2009; Lindgren & Altimiras, 2013). These studies therefore support an effect of hypoxia during the incubation period rather than after hatching as the relevant stimulus in programing future cardiovascular risk at adulthood.
There is growing evidence for the importance of addressing sex differences in the programming of cardiovascular disease by adverse developmental conditions (Gilbert & Nijland, 2008; Aiken & Ozanne, 2013). The chicken embryo model offers a cost‐effective experimental means to address some of these questions. Indeed, incubation of eggs at high altitude has been reported to have differential effects on cardiovascular function in male and female adult chickens (Herrera et al. 2013). Relative to sea level controls, hens that were incubated and raised at high altitude displayed an increased cardiac baroreflex gain, while this was significantly impaired in cockerels which were incubated and raised at high altitude (Herrera et al. 2013). Pre‐ and post‐hatching development of chickens at high altitude is also associated with echocardiographic indices of pulmonary hypertension at adulthood in a highly sex‐dependent manner (Salinas et al. 2014). Male but not female chickens incubated and reared at high altitude had significantly greater right ventricular wall thickness during systole and diastole than their same sex sea level counterparts. Further, the tricuspid pressure gradient was greatly enhanced in highland male and female chickens relative to sea level controls. However, the increment in the tricuspid pressure gradient and the pulmonary artery diameter was significantly greater in highland cockerels than in highland hens. In fact, pre‐ and post‐hatching development at high altitude did not affect the pulmonary artery diameter significantly in female chickens (Salinas et al. 2014).
Summary and perspectives
In contrast to observations in humans and experiments in mammalian animal models, the direct effects of prenatal hypoxia on the individual can be isolated using the chicken embryo. Importantly, these effects on the embryonic and adult bird are independent of effects of hypoxic exposure on the maternal physiology, on the release of placental hormones and on the quality of the milk for lactation, as in mammalian species. The chicken compared to the rat or the mouse is a precocial rather than altricial species. Therefore, the temporal developmental trajectory of cardiovascular structure and function is also much more similar between chickens and humans. Data generated from chicken embryo incubations under hypoxic conditions are now beginning to reveal cellular and molecular mechanisms through which chronic hypoxia directly affects growth and the HPA axis during prenatal development and the setting of a future risk of cardiovascular, metabolic or endocrine disease. An additional advantage of the chicken embryo model is that it is high throughput and cost‐effective. Therefore, interventional strategies to protect against growth restriction and the developmental programming of cardiovascular disease by chronic hypoxia can be tested in parallel, in different doses and at varying times of administration within one experimental design. Recent studies have reported that treatment of hypoxic incubations with antioxidants or agents that increase NO bioavailability, such as with melatonin or sildenafil (Itani et al. 2016, 2017), can not only protect but also rescue the cardiovascular phenotype of the offspring even when therapy is started long after the induction of chronic developmental hypoxia. The latter is an important advance for human translational therapy, as in the clinical setting, IUGR resulting from chronic fetal hypoxia in adverse pregnancy needs to be diagnosed before it can be treated.
Additional information
Competing interests
None declared.
Author contributions
N.I., C.E.S., C.E.B., M.V., S.K.L., C.B., E.V., C.E.B. and D.A.G. were all involved in the design and experimentation of relevant studies summarised and the writing of the manuscript.
Funding
D.G. is supported by the British Heart Foundation, The Lister Institute, The Biotechnology and Biological Sciences Research Council, The Royal Society, The Wellcome Trust, Action Medical Research and the Isaac Newton Trust.
Acknowledgements
We thank Dr Diva Bellido, Dr Wilma Tellez, Mr Armando Rodriguez, Mrs Martha Aguilar, Mrs Loyola Riveros, Mr Wilmar Velasquez and Mr Didi Maquera at IBBA, La Paz, Dr Ginella, Dr Nioshi and Dr Roca at CENETROP, Santa Cruz, and Ms Lilian Kessels at Maastricht University for their invaluable help with the original chicken embryo studies arising from work in Bolivia. We also thank Mr Sage Ford and the Barcroft Centre technical staff for their assistance with the avian studies at Cambridge.
Biographies
Nozomi Itani is a Postdoctoral Research Associate at the Department of Physiology, Development and Neuroscience at the University of Cambridge. She was the recipient of the Thomas McDonald Foundation Award for outstanding research by an Early Career Investigator at the 2017 meeting of the Society for Reproductive Investigation.

Carlos Salinas is a cardiologist and Director of the Bolivian Institute for High Altitude Biology (IBBA) in La Paz, Bolivia. His research interests are the effects of high altitude hypoxia on cardiopulmonary function across all life stages.
Dino Giussani is Professor of Cardiovascular Physiology & Medicine at the Department of Physiology Development & Neuroscience at the University of Cambridge. He is the current President of the Fetal & Neonatal Physiological Society. His current programmes of research use an integrative approach at the whole animal, isolated organ, cellular and molecular levels to determine the role of fetal oxygenation and reactive oxygen species in cardiovascular development, and in setting an increased risk of cardiovascular disease in later life.
Edited by: Ole H. Petersen & Laura Bennet
N. Itani and C. E. Salinas contributed equally.
References
- Agren P, van der Sterren S, Cogolludo AL, Frazziano G, de Mey JG, Blanco CE & Villamor E (2008). Developmental changes in endothelium‐dependent relaxation of the chicken ductus arteriosus. J Physiol Pharmacol 59, 55–76. [PubMed] [Google Scholar]
- Aiken CE & Ozanne SE (2013). Sex differences in developmental programming models. Reproduction 145, R1–R13. [DOI] [PubMed] [Google Scholar]
- Akira M & Yoshiyuki S (2006). Placental circulation, fetal growth, and stiffness of the abdominal aorta in newborn infants. J Pediatr 148, 49–53. [DOI] [PubMed] [Google Scholar]
- Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, Botting KJ, Cross CM, Itani N, Skeffington KL, Beck C & Giussani DA (2016). Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 594, 1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Evans GW & Riley WA (1994). Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 140, 669–682. [DOI] [PubMed] [Google Scholar]
- Bae S, Xiao Y, Li G, Casiano CA & Zhang L (2003). Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285, H983–H990. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA & Robinson JS (1993). Fetal nutrition and cardiovascular disease in adult life. Lancet 341, 938–941. [DOI] [PubMed] [Google Scholar]
- Baumann R & Meuer H‐J (1992). Blood oxygen transport in the early avian embryo. Physiol Rev 72, 941–965. [DOI] [PubMed] [Google Scholar]
- Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW & Giussani DA (2010). Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol 203, 495.e424–495.e434. [DOI] [PubMed] [Google Scholar]
- Challis JR, Fraher L, Oosterhuis J, White SE & Bocking AD (1989). Fetal and maternal endocrine responses to prolonged reductions in uterine blood flow in pregnant sheep. Am J Obstet Gynecol 160, 926–932. [DOI] [PubMed] [Google Scholar]
- Chan T & Burggren W (2005). Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus). Respir Physiol Neurobiol 145, 251–263. [DOI] [PubMed] [Google Scholar]
- Cosmi E, Visentin S, Fanelli T, Mautone AJ & Zanardo V (2009). Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet Gynecol 114, 1109–1114. [DOI] [PubMed] [Google Scholar]
- Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A & Gratacos E (2010). Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 121, 2427–2436. [DOI] [PubMed] [Google Scholar]
- Druyan S, Cahaner A & Ashwell C (2007). The expression patterns of hypoxia‐inducing factor subunit α‐1, heme oxygenase, hypoxia upregulated protein 1, and cardiac troponin T during development of the chicken heart. Poult Sci 86, 2384–2389. [DOI] [PubMed] [Google Scholar]
- Ducsay CA (1998). Fetal and maternal adaptations to chronic hypoxia: prevention of premature labor in response to chronic stress. Comp Biochem Physiol A Mol Integr Physiol 119, 675–681. [DOI] [PubMed] [Google Scholar]
- Dzialowski EM, Sirsat T, van der Sterren S & Villamor E (2012). Prenatal cardiovascular shunts in amniotic vertebrates. Respir Physiol Neurobiol 178, 66–74. [DOI] [PubMed] [Google Scholar]
- Dzialowski EM, von Plettenberg D, Elmonoufy NA & Burggren WW (2002). Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 131, 713–724. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA & Forhead AJ (2006). Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37. [DOI] [PubMed] [Google Scholar]
- Gagnon R (2003). Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 110(Suppl 1), S99–S107. [DOI] [PubMed] [Google Scholar]
- Gardiner H, Brodszki J & Marsal K (2001). Ventriculovascular physiology of the growth‐restricted fetus. Ultrasound Obstet Gynecol 18, 47–53. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJ, Bloomfield MR, Fowden AL & Giussani DA (2002). Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol 540, 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS & Nijland MJ (2008). Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol 295, R1941–R1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA (2007). Hypoxia, fetal growth and early origins of disease: the Andean curse on the Conquistadors. J Physiol 582, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA (2016). The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7, e31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S & Barker DJ (2001). Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49, 490–494. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Salinas CE, Villena M & Blanco CE (2007). The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol 585, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C & Thornburg KL (2008). Effect of in utero and early‐life conditions on adult health and disease. N Engl J Med 359, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, Davies DP & Henderson AH (1998). Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res 40, 600–606. [DOI] [PubMed] [Google Scholar]
- Harvey LM, Gilbert RD, Longo LD & Ducsay CA (1993). Changes in ovine fetal adrenocortical responsiveness after long‐term hypoxemia. Am J Physiol Endocrinol Metab 264, E741–E747. [DOI] [PubMed] [Google Scholar]
- Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez‐Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC & Raha S (2012). Adverse fetal and neonatal outcomes associated with a life‐long high fat diet: role of altered development of the placental vasculature. PLoS One 7, e33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Salinas CE, Blanco CE, Villena M & Giussani DA (2013). High altitude hypoxia and blood pressure dysregulation in adult chickens. J Dev Orig Health Dis 4, 69–76. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Coulter CL, Deayton JM, Harding R & Thorburn GD (1990). Fetal endocrine responses to prolonged hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 259, R703–R708. [DOI] [PubMed] [Google Scholar]
- Itani N, Skeffington KL, Beck C & Giussani DA (2017). Sildenafil therapy for fetal cardiovascular dysfunction during hypoxic development: studies in the chick embryo. J Physiol 595, 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani N, Skeffington KL, Beck C, Niu Y & Giussani DA (2016). Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J Pineal Res 60, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen NK, Wang T, Baatrup E & Crossley DA 2nd (2014). The role of nitric oxide in the cardiovascular response to chronic and acute hypoxia in White Leghorn chicken (Gallus domesticus). Acta Physiol (Oxf) 211, 346–357. [DOI] [PubMed] [Google Scholar]
- Jenkins SA & Porter TE (2004). Ontogeny of the hypothalamo‐pituitary‐adrenocortical axis in the chicken embryo: a review. Domest Anim Endocrinol 26, 267–275. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Giraud GD, Espinoza HM, Davis EN & Crossley DA 2nd (2015). Effects of chronic hypoxia on cardiac function measured by pressure‐volume catheter in fetal chickens. Am J Physiol Regul Integr Comp Physiol 308, R680–R689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ & Moore LG (2009). Evolutionary adaptation to high altitude: a view from in utero. Am J Human Biol 21, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Dunkel L, Turpeinen U, Stenman UH, Wood PJ, Nuutila M & Andersson S (2003). Placental 11β‐hydroxysteroid dehydrogenase‐2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab 88, 493–500. [DOI] [PubMed] [Google Scholar]
- Kalliecharan R & Hall BK (1974). A developmental study of the levels of progesterone, corticosterone, cortisol, and cortisone circulating in plasma of chick embryos. Gen Comp Endocrinol 24, 364–372. [DOI] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Camm EJ & Giussani DA (2013). Vitamin C prevents intrauterine programming of in vivo cardiovascular dysfunction in the rat. Circ J 77, 2604–2611. [DOI] [PubMed] [Google Scholar]
- Kendall G & Peebles D (2005). Acute fetal hypoxia: the modulating effect of infection. Early Hum Dev 81, 27–34. [DOI] [PubMed] [Google Scholar]
- Kim YH, Veille JC, Cho MK, Kang MS, Kim CH, Song TB & Figueroa JP (2005). Chronic hypoxia alters vasoconstrictive responses of femoral artery in the fetal sheep. J Korean Med Sci 20, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW & Clarke GD (2017a). Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. J Physiol 595, 4245–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW & Clarke GD (2017b). Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol 595, 1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang U, Baker RS, Khoury J & Clark KE (2000). Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol Regul Integr Comp Physiol 279, R53–R59. [DOI] [PubMed] [Google Scholar]
- Leeson CP, Kattenhorn M, Morley R, Lucas A & Deanfield JE (2001). Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103, 1264–1268. [DOI] [PubMed] [Google Scholar]
- le Noble FA, Ruijtenbeek K, Gommers S, de Mey JG & Blanco CE (2000). Contractile and relaxing reactivity in carotid and femoral arteries of chicken embryos. Am J Physiol Heart Circ Physiol 278, H1261–H1268. [DOI] [PubMed] [Google Scholar]
- Lindgren I & Altimiras J (2009). Chronic prenatal hypoxia sensitizes β‐adrenoceptors in the embryonic heart but causes postnatal desensitization. Am J Physiol Regul Integr Comp Physiol 297, R258–R264. [DOI] [PubMed] [Google Scholar]
- Lindgren I & Altimiras J (2011). Sensitivity of organ growth to chronically low oxygen levels during incubation in Red Junglefowl and domesticated chicken breeds. Poult Sci 90, 126–135. [DOI] [PubMed] [Google Scholar]
- Lindgren I & Altimiras J (2013). Prenatal hypoxia programs changes in β‐adrenergic signaling and postnatal cardiac contractile dysfunction. Am J Physiol Regul Integr Comp Physiol 305, R1093–R1101. [DOI] [PubMed] [Google Scholar]
- Lindgren I, Crossley D 2nd, Villamor E & Altimiras J (2011). Hypotension in the chronically hypoxic chicken embryo is related to the β‐adrenergic response of chorioallantoic and femoral arteries and not to bradycardia. Am J Physiol Regul Integr Comp Physiol 301, R1161–R1168. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Riquelme RA, Sanhueza EM, Hanson MA, Blanco CE, Parer JT, Herrera EA, Pulgar VM, Reyes RV, Cabello G & Giussani DA (2003). The fetal llama versus the fetal sheep: different strategies to withstand hypoxia. High Alt Med Biol 4, 193–202. [DOI] [PubMed] [Google Scholar]
- Macfarlane CM & Tsakalakos N (1985). Evidence of hyperinsulinaemia and hypoxaemia in the cord blood of neonates born to mothers with gestational diabetes. S Afr Med J 67, 81–84. [PubMed] [Google Scholar]
- Marcela SG, Cristina RM, Angel PG, Manuel AM, Sofia DC, Patricia de LR, Bladimir RR & Concepcion SG (2012). Chronological and morphological study of heart development in the rat. Anat Rec (Hoboken) 295, 1267–1290. [DOI] [PubMed] [Google Scholar]
- Martin H, Hu J, Gennser G & Norman M (2000). Impaired endothelial function and increased carotid stiffness in 9‐year‐old children with low birthweight. Circulation 102, 2739–2744. [DOI] [PubMed] [Google Scholar]
- Miller SL, Green LR, Peebles DM, Hanson MA & Blanco CE (2002). Effects of chronic hypoxia and protein malnutrition on growth in the developing chick. Am J Obstet Gynecol 186, 261–267. [DOI] [PubMed] [Google Scholar]
- Mohammed R, Cavallaro G, Kessels CG & Villamor E (2015). Functional differences between the arteries perfusing gas exchange and nutritional membranes in the late chicken embryo. J Comp Physiol B 185, 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monau TR, Vargas VE, King N, Yellon SM, Myers DA & Ducsay CA (2009). Long‐term hypoxia increases endothelial nitric oxide synthase expression in the ovine fetal adrenal. Reprod Sci 16, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monie IW (1976). Comparative development of the nervous, respiratory, and cardiovascular systems. Environ Health Perspect 18, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen RM, Kessels CG, Zimmermann LJ & Villamor E (2012). Mesenteric artery reactivity and small intestine morphology in a chicken model of hypoxia‐induced fetal growth restriction. J Physiol Pharmacol 63, 601–612. [PubMed] [Google Scholar]
- Moonen RM & Villamor E (2011). Developmental changes in mesenteric artery reactivity in embryonic and newly hatched chicks. J Comp Physiol B 181, 1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LG (1990). Maternal O2 transport and fetal growth in Colorado, Peru, and Tibet high‐altitude residents. Am J Human Biol 2, 627–637. [DOI] [PubMed] [Google Scholar]
- Moore LG, Charles SM & Julian CG (2011). Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol 178, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E & Vargas E (2004). Maternal adaptation to high‐altitude pregnancy: an experiment of nature—a review. Placenta 25(Suppl A), S60–S71. [DOI] [PubMed] [Google Scholar]
- Mulder AL, van Golde JC, Prinzen FW & Blanco CE (1998). Cardiac output distribution in response to hypoxia in the chick embryo in the second half of the incubation time. J Physiol 508, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsuki J, Gagnon R, Matthews SG & Challis JR (1996). Effects of long‐term hypoxemia on pituitary‐adrenal function in fetal sheep. Am J Physiol Endocrinol Metab 271, E678–E685. [DOI] [PubMed] [Google Scholar]
- Myers DA, Bell PA, Hyatt K, Mlynarczyk M & Ducsay CA (2005a). Long‐term hypoxia enhances proopiomelanocortin processing in the near‐term ovine fetus. Am J Physiol Regul Integr Comp Physiol 288, R1178–R1184. [DOI] [PubMed] [Google Scholar]
- Myers DA & Ducsay CA (2012). Adrenocortical and adipose responses to high‐altitude‐induced, long‐term hypoxia in the ovine fetus. J Pregnancy 2012, 681306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DA, Hyatt K, Mlynarczyk M, Bird IM & Ducsay CA (2005b). Long‐term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near‐term ovine fetus. Am J Physiol Regul Integr Comp Physiol 289, R1707–R1714. [DOI] [PubMed] [Google Scholar]
- Niermeyer S, Zamudio S & Moore LG (2001). The People In High Altitude: An Exploration of Human Adaptation. Marcel Dekker, New York. [Google Scholar]
- O'Dowd R, Kent JC, Moseley JM & Wlodek ME (2008). Effects of uteroplacental insufficiency and reducing litter size on maternal mammary function and postnatal offspring growth. Am J Physiol Regul Integr Comp Physiol 294, R539–R548. [DOI] [PubMed] [Google Scholar]
- Okamura K, Watanabe T, Tanigawara S, Shintaku Y, Endo H, Iwamoto M, Murotsuki J & Yajima A (1990). Biochemical evaluation of fetus with hypoxia caused by severe preeclampsia using cordocentesis. J Perinat Med 18, 441–447. [DOI] [PubMed] [Google Scholar]
- Österman H, Lindgren I, Lindström T & Altimiras J (2015). Chronic hypoxia during development does not trigger pathologic remodeling of the chicken embryonic heart but reduces cardiomyocyte number. Am J Physiol Regul Integr Comp Physiol 309, R1204–1214. [DOI] [PubMed] [Google Scholar]
- Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP & Lagercrantz H (2002). Long‐lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch 443, 858–865. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB & Phillips DI (2001). Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab 86, 245–250. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Capponi A, Rinaldo D, Arduini D & Romanini C (1995). Ventricular ejection force in growth‐retarded fetuses. Ultrasound Obstet Gynecol 5, 247–255. [DOI] [PubMed] [Google Scholar]
- Roigas J, Roigas C, Heydeck D & Papies B (1996). Prenatal hypoxia alters the postnatal development of β‐adrenoceptors in the rat myocardium. Biol Neonate 69, 383–388. [DOI] [PubMed] [Google Scholar]
- Rouwet EV, Tintu AN, Schellings MW, van Bilsen M, Lutgens E, Hofstra L, Slaaf DW, Ramsay G & Le Noble FA (2002). Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 105, 2791–2796. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, Kessels LC, De Mey JG & Blanco CE (2003b). Chronic moderate hypoxia and protein malnutrition both induce growth retardation, but have distinct effects on arterial endothelium‐dependent reactivity in the chicken embryo. Pediatr Res 53, 573–579. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, Kessels CG, Janssen BJ, Bitsch NJ, Fazzi GE, Janssen GM, De Mey J & Blanco CE (2003a). Chronic moderate hypoxia during in ovo development alters arterial reactivity in chickens. Pflugers Arch 447, 158–167. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE & De Mey JG (2000). Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation 102, 2892–2897. [DOI] [PubMed] [Google Scholar]
- Sahan U, Ipek A, Yilmaz‐Dikmen B, Aydin C & Kederli E (2011). Effect of oxygen supplementation in the hatcher at high altitude on the incubation results of broiler eggs laid at low altitude. Br Poult Sci 52, 388–394. [DOI] [PubMed] [Google Scholar]
- Salinas CE, Blanco CE, Villena M, Camm EJ, Tuckett JD, Weerakkody RA, Kane AD, Shelley AM, Wooding FB, Quy M & Giussani DA (2010). Cardiac and vascular disease prior to hatching in chick embryos incubated at high altitude. J Dev Orig Health Dis 1, 60–66. [DOI] [PubMed] [Google Scholar]
- Salinas CE, Blanco CE, Villena M & Giussani DA (2014). High‐altitude hypoxia and echocardiographic indices of pulmonary hypertension in male and female chickens at adulthood. Circ J 78, 1459–1464. [DOI] [PubMed] [Google Scholar]
- Salinas CE, Villena M, Blanco CE & Giussani DA (2011). Adrenocortical suppression in highland chick embryos is restored during incubation at sea level. High Alt Med Biol 12, 79–87. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K & Keller BB (2006). Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res 59, 116–120. [DOI] [PubMed] [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS & McMillen IC (1997). Impact of placental restriction on the development of the sympathoadrenal system. Pediatr Res 42, 805–811. [DOI] [PubMed] [Google Scholar]
- Sissman NJ (1970). Developmental landmarks in cardiac morphogenesis: comparative chronology. Am J Cardiol 25, 141–148. [DOI] [PubMed] [Google Scholar]
- Skilton MR, Evans N, Griffiths KA, Harmer JA & Celermajer DS (2005). Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 365, 1484–1486. [DOI] [PubMed] [Google Scholar]
- Soria R, Julian CG, Vargas E, Moore LG & Giussani DA (2013). Graduated effects of high‐altitude hypoxia and highland ancestry on birth size. Pediatr Res 74, 633–638. [DOI] [PubMed] [Google Scholar]
- Speake BK, Murray AM & Noble RC (1998). Transport and transformations of yolk lipids during development of the avian embryo. Prog Lipid Res 37, 1–32. [DOI] [PubMed] [Google Scholar]
- Stratford LL & Hooper SB (1997). Effect of hypoxemia on tissue glycogen content and glycolytic enzyme activities in fetal sheep. Am J Physiol Regul Integr Comp Physiol 272, R103–R110. [DOI] [PubMed] [Google Scholar]
- Strick DM, Waycaster RL, Montani J‐P, Gay WJ & Adair TH (1991). Morphometric measurements of chorioallantoic membrane vascularity: effects of hypoxia and hyperoxia. Am J Physiol Heart Circ Physiol 260, H1385–H1389. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Richardson BS, Gagnon R & Regnault TR (2011). Chronic intrauterine hypoxia interferes with aortic development in the late gestation ovine fetus. J Physiol 589, 3319–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP & Weiner CP (1999). Effects of acute and chronic hypoxia on nitric oxide‐mediated relaxation of fetal guinea pig arteries. Am J Obstet Gynecol 181, 105–111. [DOI] [PubMed] [Google Scholar]
- Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, van Bilsen M, Carmeliet P, Staff AC, Tjwa M, Cetin I, Gratacos E, Hernandez‐Andrade E, Hofstra L, Jacobs M, Lamers WH, Morano I, Safak E, Ahmed A & le Noble F (2009). Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long‐term consequences. PLoS One 4, e5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W & Zhang L (2012). Fetal hypoxia and programming of matrix metalloproteinases. Drug Discov Today 17, 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sterren S, Agren P, Zoer B, Kessels L, Blanco CE & Villamor E (2009). Morphological and functional alterations of the ductus arteriosus in a chicken model of hypoxia‐induced fetal growth retardation. Pediatr Res 65, 279–284. [DOI] [PubMed] [Google Scholar]
- Veille JC, Hanson R, Sivakoff M, Hoen H & Ben‐Ami M (1993). Fetal cardiac size in normal, intrauterine growth retarded, and diabetic pregnancies. Am J Perinatol 10, 275–279. [DOI] [PubMed] [Google Scholar]
- Verhoelst E, De Ketelaere B, Bruggeman V, Villamor E, Decuypere E & De Baerdemaeker J (2011). Development of a fast, objective, quantitative methodology to monitor angiogenesis in the chicken chorioallantoic membrane during development. Int J Dev Biol 55, 85–92. [DOI] [PubMed] [Google Scholar]
- Villamor E, Kessels CG, Ruijtenbeek K, van Suylen RJ, Belik J, de Mey JG & Blanco CE (2004). Chronic in ovo hypoxia decreases pulmonary arterial contractile reactivity and induces biventricular cardiac enlargement in the chicken embryo. Am J Physiol Regul Integr Comp Physiol 287, R642–651. [DOI] [PubMed] [Google Scholar]
- Villamor E, Ruijtenbeek K, Pulgar V, De Mey JG & Blanco CE (2002). Vascular reactivity in intrapulmonary arteries of chicken embryos during transition to ex ovo life. Am J Physiol Regul Integr Comp Physiol 282, R917–R927. [DOI] [PubMed] [Google Scholar]
- Wangensteen OD & Rahn H (1971). Respiratory gas exchange by the avian embryo. Respir Physiol 11, 31–45. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, Couser RJ, Garland JS, Rozycki HJ, Leach CL, Backstrom C & Shaffer ML (2004). Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 114, 1649–1657. [DOI] [PubMed] [Google Scholar]
- Wei ZH, Zhang H, Jia CL, Ling Y, Gou X, Deng XM & Wu CX (2007). Blood gas, hemoglobin, and growth of Tibetan chicken embryos incubated at high altitude. Poult Sci 86, 904–908. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC & Davidge ST (2005a). Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288, R360–R367. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Hemmings DG, Mitchell JM, McMillen IC & Davidge ST (2005b). Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 565, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM & Frye BE (1973). Functional development of the hypothalamo‐hypophyseal‐adrenal cortex axis in the chick embryo, Gallus domesticus . J Exp Zool 185, 277–292. [DOI] [PubMed] [Google Scholar]
- Wlodek ME, Westcott KT, O'Dowd R, Serruto A, Wassef L, Moritz KM & Moseley JM (2005). Uteroplacental restriction in the rat impairs fetal growth in association with alterations in placental growth factors including PTHrP. Am J Physiol Regul Integr Comp Physiol 288, R1620–R1627. [DOI] [PubMed] [Google Scholar]
- Woods JE, De Vries GW & Thommes RC (1971). Ontogenesis of the pituitary‐adrenal axis in the chick embryo. Gen Comp Endocrinol 17, 407–415. [DOI] [PubMed] [Google Scholar]
- Yadgary L, Kedar O, Adepeju O & Uni Z (2013). Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult Sci 92, 1634–1640. [DOI] [PubMed] [Google Scholar]
- Zoer B, Blanco CE & Villamor E (2010). Role of Rho‐kinase in mediating contraction of chicken embryo femoral arteries. J Comp Physiol B 180, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoer B, Kessels L, Vereijken A, De Mey JG, Bruggeman V, Decuypere E, Blanco CE & Villamor E (2009). Effects of prenatal hypoxia on pulmonary vascular reactivity in chickens prone to pulmonary hypertension. J Physiol Pharmacol 60, 119–130. [PubMed] [Google Scholar]


