Naïve CD4+CD25− T cells activated in the presence of naïve splenic B cells may differentiate into CD4+CD25+Foxp3− regulatory T cells, which were named Treg-of-B cells by our group (Table 1). In addition, the oral delivery of antigen-activated naive Peyer’s patch B cells induced Treg-of-B(P) cells with an increased suppressive function.3 Our recent article reported that peritoneal B-1a cells produced more IL-10; moreover, B-1a cells promoted the induction of Treg-of-B1a cells in an IL-10-independent manner.1 In our studies, splenic B2-cell-induced Treg-of-B cells, Peyer’s patch B-cell-induced Treg-of-B (P) and peritoneal B-1a-cell-induced Treg-of-B1a cells shared similar phenotypes and functions. Our group subsequently reported that adoptive transfer these Treg-of-B cells protected mice from several disease models, including allergic asthma, rheumatoid arthritis and inflammatory bowel disease. Here, we summarize the suppression and induction mechanism of Treg-of-B cells (Figure 1).
Table 1.
Recent studies on B-cell-induced CD4+Foxp3− regulatory T cells
| Generation | Phenotype | Application | Implication | Reference |
|---|---|---|---|---|
| Splenic B2 and peritoneal B1a cell/monoclonal antibodies/CD4+CD25− T cell | Treg-of-B2 and Treg-of-B1a, CD4+CD25+Foxp3− | In vitro suppression assay | Treg-of-B cells were generated in a cell–cell contact-dependent manner and had cell–cell contact-dependent inhibition | 1 |
| Splenic B cell/OVA peptide/DO11.10 CD4+CD25− T cell | Treg-of-B, CD4+CD25+Foxp3− ICOS+CTLA4+PD1+LAG3+ | Allergic asthma | Treg-of-B cells may be involved in oral tolerance and had non-antigen-specific inhibition | 2 |
| Peyer’s patch B cell/OVA peptide/DO11.10 CD4+CD25− T cell | Treg-of-B(P), CD4+CD25+Foxp3− | Allergic asthma | Treg-of-B(P) cells may be involved in oral tolerance | 3 |
| Peyer’s patch B cell/OVA peptide/DO11.10 CD4+CD25− T cell | Treg-of-B(P), CD4+CD25+Foxp3−LAG3+ | Allergic asthma | LAG3 and IL-10 play roles in the suppression of Treg-of-B(P) cells | 4 |
| Splenic B cell/monoclonal antibodies/CD4+CD25− T cell | Treg-of-B, CD4+CD25+Foxp3−LAG3+ | Rheumatoid arthritis | LAG3 and IL-10 play roles in the suppression of Treg-of-B cells | 5 |
| Splenic B cell/OVA peptide/DO11.10 CD4+CD25− T cell/long culture period | Long-term Treg-of-B, CD4+CD25+Foxp3−cMaf+ICOS+CTLA4+LAG3+PD1+ | In vitro suppression assay | CTLA4 and IL-10 play roles in the suppression of long-term Treg-of-B cells but not short-term Treg-of-B cells | 6 |
| Splenic B cell/monoclonal antibodies/CD4+CD25− T cell | Treg-of-B, CD4+CD25+Foxp3−ICOS+PD1+LAG3+ | Inflammatory bowel disease | IL-10 is not essential for the suppressive ability of Treg-of-B cells | 7 |
| Splenic B cell/OVA peptide/DO11.10 CD4+CD25− T cell | T-of-B, CD4+CD25+ Foxp3−CD62L+ | Graft versus host disease | B-T form an immunological synapse during induction | 8 |
| Splenic B cell/OVA peptide/DO11.10 CD4+CD25− T cell | T-of-B, CD4+CD25+Foxp3−CD62L+ | In vitro suppression assay | Additional CD28 signals decrease the suppressive ability of B-cell-induced T cells | 9 |
Figure 1.
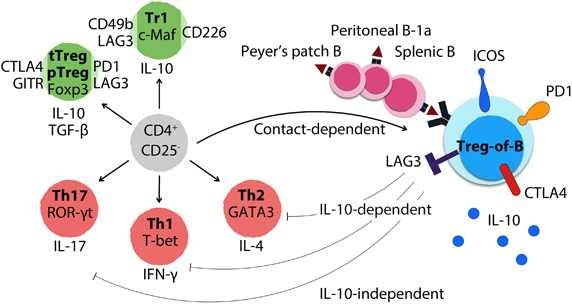
B-cell-induced CD4+Foxp3− regulatory T cells. When exposed to B cells from the spleen and Peyer’s patch, as well as B-1a cells from the peritoneal cavity, CD4+CD25− T cells are triggered to differentiate into CD4+CD25+Foxp3− regulatory T cells dependent on cell–cell contact. These B-cell-induced Treg cells are named Treg-of-B cells, which express both regulatory T-cell-related molecules, such as ICOS, PD1, OX40, LAG3 and CTLA4, and inhibitory cytokines, such as IL-10 and TGF-β. Treg-of-B cells express lower or no master transcription factors of thymus-derived Treg, Tr1, Th1, Th2 and Th17 cells. Treg-of-B cells exert antigen-specific and non-antigen-specific suppressive functions in IL-10-dependent, IL-10-independent and cell contact-dependent manners. Notably, prophylactic transfer of Treg-of-B cells prevents Th2-dominant allergic asthma, Th1/Th17-mediated inflammatory bowel disease and rheumatoid arthritis in mice.
We and other groups have demonstrated that antigen-presenting B cells may play a critical role in tolerance. Oral delivery of antigen-activated Peyer’s patch B cells induced Treg-of-B(P) cells with enhanced suppressive ability.3 Furthermore, oral delivery antigen-activated CD4+CD25+ T cells, similar to B-cell-induced Treg-of-B cells, increased the levels of CTLA4, ICOS and PD1 and improved their suppressive functions. Both the oral delivery of antigen and adoptive transfer of single antigen-specific Treg-of-B cells protected mice from allergic asthma in antigen-specific and non-antigen-specific fashions.2 Consistent with these findings, lentiviral-enforced collagen peptide-presenting B cells protected mice from collagen-induced arthritis, which may, in part, involve the induction of CD4+ regulatory T cells.10 These studies indicated that B cells possess an ability to promote the generation of CD4+ regulatory T cells as part of the sophisticated tolerance mechanism.
Our results indicated that Treg-of-B cells were induced by monoclonal antibodies and specific peptides expressed as regulatory T-related markers, including inducible co-stimulator (ICOS and CD278), lymphocyte-activation gene 3 (LAG3), programmed cell death protein 1 (PD1 and CD279) and tumor necrosis factor receptor superfamily member 4 (TNFRSF4, OX40 and CD134). Treg-of-B cells and long-term cultured Treg-of-B cells expressed low or no master transcription factors of Th1, Th2, Th17 or thymus-derived regulatory T cells, including T-bet, GATA3, ROR-γt, Foxp3 and Helios. Both short-term and long-term Treg-of-B cells produced IL-10 and exhibited an IL-10-mediated inhibitory function. Moreover, Treg-of-B cells exerted IL-10-independent suppressive abilities and differed from Tr1 cells that mainly rely on IL-10-mediated inhibition.6,7 These findings indicated that Treg-of-B cells have a regulatory T-related phenotype and new characteristics from the well-established Foxp3+ regulatory T cells and IL-10-dominant Tr1 cells.
Recently, our group investigated the complex suppressive function of Treg-of-B cells. Treg-of-B cells produced higher levels of IL-10 and TGF-β and lower levels of IFN-γ and IL-2 than naïve CD4+CD25− T cells.2 Treg-of-B1a cells exhibited a soluble-mediated suppression, whereas Treg-of-B cells appeared to rely more on cell–cell contact-dependent suppression.1 In the following studies, we reported that LAG3 was expressed on the Treg-of-B-cell surface and cooperated with IL-10 in the inhibitory mechanism.4,5 However, genetic IL-10-deficient Treg-of-B cells did not diminish their suppression in vitro or in vivo, which indicates that Treg-of-B cells possess an IL-10-independent suppressive ability.7 We further determined that long-term Treg-of-B cells increased the expression levels of IL-10, IL-4, musculoaponeurotic fibrosarcoma oncogene homolog (c-Maf), cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and ICOS. IL-10 and CTLA4 play roles in the suppressive activity of long-term Treg-of-B cells but not short-term Treg-of-B cells.6 In contrast to IL-10- and CTLA4-mediated suppression of long-term Treg-of-B cells, short-term Treg-of-B cells exert IL-10-independent and cell–cell contact-dependent inhibitory abilities. In addition to monoclonal antibody-induced Treg-of-B cells, our group investigated the antigen specificity of Treg-of-B cells induced by a synthetic peptide and transgenic T cells bearing antigen-specific TCR. OVA323–339 peptide-presenting naïve B-cell-induced DO11.10 naive T cells (in which transgenic TCR recognizes OVA323–339 presented in H-2d) differentiated into Treg-of-B cells, and single antigen-specific Treg-of-B cells exhibited inhibition in non-antigen-specific fashions in vitro.2 We have demonstrated that the prophylactic transfer of Treg-of-B cells prevented the development of Th2-mediated allergic asthma,2,3,4 Th1/Th17-mediated inflammatory bowel disease7 and collagen-induced arthritis.5 These findings demonstrated that Treg-of-B cells could exert suppressive functions both in vitro and in vivo.
It has been demonstrated that naïve B-T cells form a mature immunologic synapse during the generation of Treg-of-B cells.8 Our group has shown that cell–cell contact rather than IL-10 is important for the generation of Treg-of-B cells. However, the IL-10 level was higher in a peritoneal B1a-T co-culture than that of a splenic B2-T co-culture.1 The levels of IL-10, IL-4, IL-27 and TGF-β increased in long-term Treg-of-B-cell cultures, and IL-10 and IL-27 have been reported to promote the induction of Tr1 cells. Nevertheless, IL-10 and IL-27 blockade did not alter the generation of long-term Treg-of-B cells. The results of a transwell co-culture suggested that cell contact between B-T cells is required for the suppressive ability and phenotype of long-term Treg-of-B cells.6 Another group reported that additional CD28 signaling broke the development of B-cell-induced regulatory T cells, which suggests the lack of or a low CD28 costimulation signal was essential.9 These findings indicate that naïve B cells are able to promote CD4+Foxp3− regulatory T cells without supplemental cytokines or chemicals in a cell–cell contact-dependent manner, and a fine-tuning costimulation signal is important for the generation.
In conclusion, we have induced and identified a particular subset of regulatory T cells stimulated by B cells. In addition, we have successfully applied these Treg-of-B cells to the treatment of allergic airway inflammation, a murine model of collagen-induced arthritis and inflammatory bowel disease. We have also initially shown that the LAG3 molecule may play a critical role in the functions of Treg-of-B cells. Furthermore, the results demonstrated that Foxp3 and IL-10 were not necessary for the development and functions of Treg-of-B cells. These results suggested that Treg-of-B cells are different from the thymus-derived regulatory T cells and Tr1 cells. The regulatory T cells described in our proposal may compose a novel subset of regulatory T cells, which may provide new approaches for the pathway of immune regulation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hsu LH, Li KP, Chu KH, Chiang BL. A B-1a cell subset induces Foxp3(−) T cells with regulatory activity through an IL-10-independent pathway. Cell Mol Immunol. 2015;12:354–365. doi: 10.1038/cmi.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien CH, Yu HH, Chiang BL. Single allergen-induced oral tolerance inhibits airway inflammation in conjugated allergen immunized mice. J Allergy Clin Immunol. 2015;136:1110–1113.e1114. doi: 10.1016/j.jaci.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Chu KH, Chiang BL. Regulatory T cells induced by mucosal B cells alleviate allergic airway hypersensitivity. Am J Respir Cell Mol Biol. 2012;46:651–659. doi: 10.1165/rcmb.2011-0246OC. [DOI] [PubMed] [Google Scholar]
- 4.Chu KH, Chiang BL. Characterization and functional studies of forkhead box protein 3(-) lymphocyte activation gene 3(+) CD4(+) regulatory T cells induced by mucosal B cells. Clin Exp Immunol. 2015;180:316–328. doi: 10.1111/cei.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SY, Hsu WT, Chen YL, Chien CH, Chiang BL. Lymphocyte-activation gene 3(+) (LAG3(+)) forkhead box protein 3(−) (FOXP3(−)) regulatory T cells induced by B cells alleviates joint inflammation in collagen-induced arthritis. J Autoimmun. 2016;68:75–85. doi: 10.1016/j.jaut.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Chien CH, Yu HC, Chen SY, Chiang BL. Characterization of c-Maf+Foxp3- regulatory T cells induced by repeated stimulation of antigen-presenting B cells. Sci Rep. 2017;7:46348. doi: 10.1038/srep46348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao TY, Hsu LH, Chien CH, Chiang BL. Novel Foxp3(−) IL-10(−) regulatory T-cells induced by B-cells alleviate intestinal inflammation in vivo. Sci Rep. 2016;6:32415. doi: 10.1038/srep32415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 9.Etemire E, Krull M, Hasenberg M, Reichardt P, Gunzer M. Transiently reduced PI3K/Akt activity drives the development of regulatory function in antigen-stimulated naive T-cells. PLoS One. 2013;8:e68378. doi: 10.1371/journal.pone.0068378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson SE, Eneljung T, Tengvall S, Jirholt P, Stern A, Henningsson L, et al. Collagen epitope expression on B cells is sufficient to confer tolerance to collagen-induced arthritis. Arthritis Res Ther. 2016;18:140. doi: 10.1186/s13075-016-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


