Abstract
The stomatal complex is critical for gas and water exchange between plants and the atmosphere. Originating over 400 million years ago, the structure of the stomata has evolved to facilitate the adaptation of plants to various environments. Although the molecular mechanism of stomatal development in Arabidopsis has been widely studied, the evolution of stomatal structure and its molecular regulators in different species remains to be answered. In this study, we examined stomatal development and the orthologues of Arabidopsis stomatal genes in a basal angiosperm plant, Nymphaea colorata, and a member of the eudicot CAM family, Kalanchoe laxiflora, which represent the adaptation to aquatic and drought environments, respectively. Our results showed that despite the conservation of core stomatal regulators, a number of critical genes were lost in the N. colorata genome, including EPF2, MPK6, and AP2C3 and the polarity regulators BASL and POLAR. Interestingly, this is coincident with the loss of asymmetric divisions during the stomatal development of N. colorata. In addition, we found that the guard cell in K. laxiflora is surrounded by three or four small subsidiary cells in adaxial leaf surfaces. This type of stomatal complex is formed via repeated asymmetric cell divisions and cell state transitions. This may result from the doubled or quadrupled key genes controlling stomatal development in K. laxiflora. Our results show that loss or duplication of key regulatory genes is associated with environmental adaptation of the stomatal complex.
How plants breathe in different environments
Researchers in China have shown how plants adapted to different environments by altering the structure of pores used for gas and water exchange, known as stomata. Shang Wu of Fujian Agriculture and Forestry University searched for stomatal development genes in the genomes of a range of plants, including a drought-tolerant species and a water lily, which diverged from other flowering plants early in their evolution. While the function of most stomatal genes has been conserved, some genes were absent in the water lily and some were doubled in the drought-resistant species. Analysis of the morphology of developing stomata in the two species revealed changes in the number and organization of cell divisions. Overall, the findings improve our understanding of how the evolution of stomata and may help guide the breeding or engineering improved varieties.
Introduction
Stomata are a pore-like structure in multiple organs, including leaves and stems, which facilitates gas and water exchange. When environmental conditions are unfavourable, plants can regulate water evapotranspiration and reduce CO2 uptake by opening and closing the stomata. For instance, Crassulacean acid metabolism (CAM) plants are adapted to arid conditions1. The stomata in CAM plants remain closed during the day to reduce evapotranspiration while staying open at night to absorb CO2. These physiological traits make CAM plants resistant to diverse stresses, including strong irradiance and drought2.
Stomatal structure is highly conserved across land plants. The basic core structure with two guard cells surrounding the stomatal pore has remained unchanged during evolution3. However, the patterning of the mature stomatal structure differs among plant groups and can be generally summarized by three classes: anomocytic, stephanocytic, and paracytic4. The widely used model plant Arabidopsis thaliana exhibits anomocytic stomata. However, there are a few species (for example, CAM families) among the eudicots with paracytic stomata5. Most grass species have paracytic mature stomata6. Amborella trichopoda in ANITA possesses stephanocytic stomata7. The diverse architecture of mature stomatal structures may suggest the evolution of their different developmental regulations and their adaption to different environments.
In A. thaliana, meristem mother cells (MMCs) undergo up to three asymmetrical divisions to form guard mother cells (GMCs). In grasses, meristemoids divide asymmetrically to form GMCs, and the lateral neighbouring axial cell lineage surrounding the GMC undergoes asymmetric division to give rise to lateral subsidiary cells (LSCs)8. In A. trichopoda, however, protodermal cells can directly become GMCs or divide asymmetrically to produce a GMC9. Hence, the regulation of stomatal development is highly diverse in different groups of land plants.
In the past, A. thaliana and Oryza sativa were often used as model systems to study stomatal patterning and development. Based on those studies, we now have a good understanding of the basic molecular network behind stomatal development. In A. thaliana, a complex signalling cascade of several genes has been identified to promote stomatal development. The secreted peptides of the EPIDERMAL PATTERNING FACTOR (EPF)/EPF-LIKE (EPFL) family act with a mitogen-activated protein kinase (MAPK) cascade to regulate the activity of basic-helix-loop-helix (bHLH) transcription factors10. EPF1 and EPF2 specifically bind to leucine-rich repeat receptor (LRR) kinase complexes that include members of TOO MANY MOUTHS receptor-like protein (TMM) and the ERECTA family (ER). EPF1 is expressed in late-stage meristemoids, GMCs and young guard cells, whereas EPF2 is expressed in early-stage protodermal cells11,12. In the downstream pathway, a number of mitogen-activated protein (MAP) kinases, including MAPKKK YODA, MPKK4/5, MPKK7/9, and MAPK MPK3/6, were found to transduce the signalling for stomatal development13. Five bHLH transcription factors positively regulate the stomatal–lineage transition and differentiation. For example, SPEECHLESS (SPCH), MUTE, and FAMA act sequentially to promote the cellular transition in a stage-specific manner. SPCH regulates asymmetric divisions in MMC and MUTE involved in GMC differentiation14,15. FAMA promotes the last step to form GCs16. Two additional bHLH proteins, SCREAM/ICE1 and SCREAM2, act redundantly to heterodimerize SPCH, MUTE, and FAMA to coordinate the regulation17.
Polarity information is critical in stomatal development and directs asymmetric cell division and possibly cell fate determination. In A. thaliana, two unique polarity proteins, POLAR LOCALIZATION DURING ASYMMETRIC DIVISION AND REDISTRIBUTION (POLAR) and BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL), show mostly overlapping localization during asymmetric stomatal divisions18,19. In the grass, the asymmetric division taking place in the lateral neighbouring cell to produce the subsidiary cell relies on two LRR receptor-like kinases, PANGLOSS1 (PAN1) and PAN220,21. PAN proteins are located at the poles in SMCs at the site of contact with GMCs, which precedes the polar accumulation of small GTPases (ROPs) and F-actin22. Interestingly, recent observations in Brachypodium distachyon found that BdMUTE regulates subsidiary cells through cell-to-cell movement23. In contrast, the MUTE homologue in A. thaliana is immobile23,24.
Although stomata morphologies across land plants have been widely examined, questions on the early evolution of angiosperms and the adaptation of stomata to diverse environments remain to be answered. It is not clear how molecular regulation of stomatal development evolved and how that relates to the diverse stomata morphologies among the land plants. Immediately above the root node of angiosperm evolution is the ANITA grade (basal angiosperms), which includes Amborella, Nymphaeales, Illiciaceae, Trimeniaceae and Austrobaileyaceae7. In this study, we took advantage of the newly sequenced genome of Nymphaea colorata (not released yet), a typical base angiosperm, to examine stomata regulation in early angiosperm evolution.
The structure and function of stomata are important for environmental adaptation. In some species, stomata underwent radical modifications to facilitate habituation to a particular environment. A recent study indicated that Z. marina lost all the genes involved in stomatal differentiation, which is coincident with its marine habituation. Nymphaea colorata is also an aquatic plant, so it is interesting to know if its stomata-related genes also changed during evolution. By contrast, Kalanchoe laxiflora, a CAM species, has adapted to drought conditions and has evolved specialized stomata functions. To understand how the evolution of the molecular regulation of stomatal development is associated with environmental adaptation, we analysed stomatal morphologies and related regulatory cascades in both Nymphaea colorata and K. laxiflora. Our analysis showed that although generally conserved, loss or duplication of key genes could be associated with structural and physiological renovations required for individual adaptation of plants to local environments.
Materials and methods
Plant materials and growth condition
A. thaliana Columbia seeds were germinated and grown on 1/2 MS medium with 1% agar, 1% sucrose and 0.05% (wt/vol) morpholinoethansulfonic acid monohydrate (pH 5.7) under a 16/8-h light/dark cycle at 23 °C. Plants were imaged 3–4 days after planting. O. sativa and K. laxiflora were grown at 28 °C with a 16/8-h light/dark photoperiod. N. colorata were cultivated in water at 23 °C in the greenhouse. Leaves of Spirodela polyrhiza were collected in winter 2017 at the Fujian Agriculture and Forestry University.
Methods
Microscopy and image processing
For Differential Interference Contrast (DIC) imaging, the protocol was modified slightly according to Raissig et al.23,25. Samples from the mid-regions of leaves were cut into small squares and cleared using a solution (ethanol: acetic acid glacial, in proportions 4:1 by volume) to remove chlorophyll; then, samples were subjected to a basic solution (a mixture of 7% NaOH in 60% ethanol). Finally, samples were washed briefly with 40% ethanol and mounted in water for visualization and microscopy analysis. Samples were examined using a Nikon ECLIPSE Ni-U microscope fitted with a Nikon DS-Ri 2 digital camera. Images were processed using ImageJ.
Phylogenetic analysis
We surveyed a number of genomes, such as A. thaliana, K. laxiflora, Sorghum bicolor, O. sativa, Zea mays, Ananas comosus, S. polyrhiza, and A. trichopoda, from Phytozome v12. Nelumbo nucifera and Phalaenopsis equestris were retrieved from ftp://ftp.ncbi.nih.gov/genomes/. Ginkgo biloba was found from GigaDB (http://gigadb.org/). N. colorata was recently sequenced by Liangsheng Zhang’s Lab in Fujian Agriculture and Forestry University, and sequences were available in the water lily genome database (eplant.org). To obtain probable orthologous genes, we performed BLASTp (protein query–proteins database) and tBLASTn (protein query–nucleic acid database) searches to selectively look for similar protein sequences from these genomes26. A MAFFT (Multiple Sequence Alignment program) was chosen to produce an alignment of all amino-acid sequences with a BLAST score of at least 60 against A. thaliana27. The phylogenetic tree was reconstructed using the maximum likelihood (ML) method in FastTree228.
Protein domains were identified using the National Center for Biotechnology Information conserved domain search tool. PEST domains were identified using emboss.bioinformatics.nl/cgi-bin/emboss/epestfind.
Results
Loss of stomatal development genes in N. colorata
It was reported that different stomatal development patterns occur in plants of the ANITA grade. A. trichopoda possesses mostly perigenous and mesoperigenous stomata9. In this species, protodermal cells can directly become GMCs or divide asymmetrically to produce GMCs and stomatal lineage ground cells9. However, in Nymphaea, protodermal cells seemed to skip asymmetric divisions and directly gave rise to GMCs7,9. It is still to be determined whether asymmetric division is an ancestral stomata-forming step during evolution.
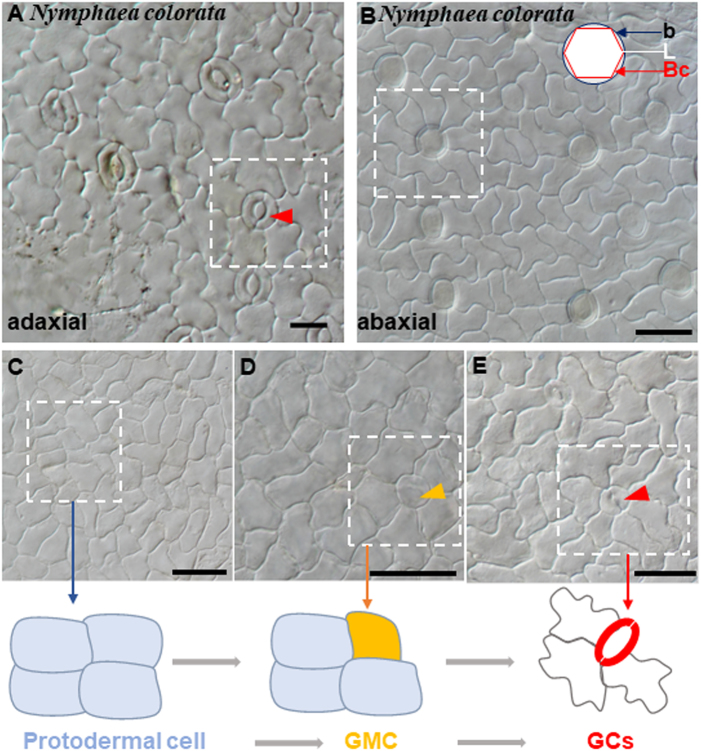
To gain a deeper understanding of the ancestral development of stomatal structure, we performed anatomic observation of the stomatal structure in N. colorata. We found that N. colorata stomata are only present on the adaxial surface of the floating leaf, with each stoma surrounded by 4–8 neighbouring cells (Fig. 1a). On the abaxial surface of N. colorata, we only found hydropote complexes with lens-shaped cells and bowl-shaped cells, which appeared to be surrounded by specialized rosettes of epidermal cells (Fig. 1b). It was hypothesized that the hydropote in Nymphaea colorata is homologous to stomatal complexes, and its functions and morphologies are highly associated with aquatic habitats29. Similarly, another floating plant, S. polyrhiza, has lost stomata on the abaxial surface (Figure S1). These results reveal that floating plants tend to lose stomata or create special stomata-like structures to adapt to the aquatic environment. It can also be exemplified by seagrass, Zostera marina, in which no stomata are present on leaves, and coincidently, entire stomatal genes are lost to adapt to the marine lifestyle30. Although anatomical descriptions of stomatal development have been reported for many taxa, little is known about the evolution of the molecular machine of stomatal formation across land plants.
Fig. 1. Stomatal structures and development process in Nymphaea colorata.
a The upper epidermis of N. colorata with anomocytic stomata. b Abaxial hydropote complex structures of N. colorata with base (b) formed by anticlinal contact cell walls, the lens-shaped cell (L), and the bowl-shaped cell (Bc). c-e Micrograph of stomata at different developmental stages in adaxial leaf surfaces. c Squared patterning, a protodermal cell. d Large round cells are putative GMCs (orange arrow). e Stage with maturing stomata (red arrow). Schematic diagram of stomatal development. A protodermal cell (pale blue) that differentiated directly into a guard mother cell (orange); then, the GMC divided into GCs (red)
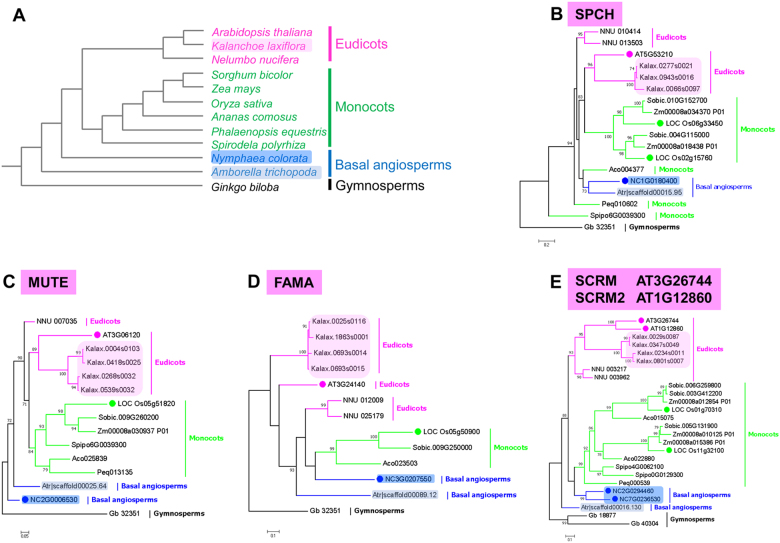
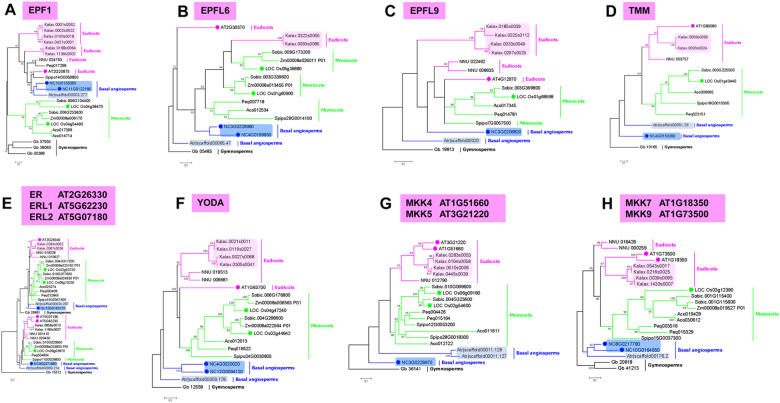
One way to understand the evolution of these essential regulators of stomatal development is to analyse their phylogenies. This is currently feasible based on the genome sequences for many species, including the eudicots A. thaliana and K. laxiflora; the monocot plants O. sativa and Z. mays. To facilitate our understanding of the early evolution of these regulators, we included basal angiosperms A. trichopoda, and we recently sequenced the genome of an early-divergent angiosperm N. colorata (see Materials and methods for information on genome data) (Fig. 2a). To understand some special features of stomata formation in N. colorata, we analysed the potential orthologues of A. thaliana genes involved in stomatal formation using the unique unpublished genome data of water lily. In line with A. thaliana, we found high conservation of the core genes required for stomatal formation in N. colorata, including an orthologue of an SPCH-like gene, NcSPCH (Fig. 2b); orthologue of a MUTE-like gene, NcMUTE (Fig. 2c); orthologue of a FAMA-like gene, NcFAMA (Fig. 2d), and two orthologues of an ICE/SCRM-like gene, NcICE1 and NcSCRM2 (Fig. 2e). We further analysed the conservation of the homologous domain of these proteins and found a high degree of domain conservation (Fig. 3). However, we also found a number of genes missing from the N. colorata genome, including the peptide ligands EPF2, MPK6, and AP2C3 and the polarity controllers BASL and POLAR (Fig. 4). Interestingly, the function of lost genes seems to be highly specific to the asymmetric stomatal development stages.
Fig. 2. Phylogenetic trees of stomatal bHLH genes in representative species.
a The molecular tree summarizes the phylogenetic relationships of representative species, including gymnosperms (e.g., Ginkgo biloba), basal angiosperms (e.g., Amborella trichopoda and Nymphaea colorata), monocots (e.g., Oryza sativa and Spirodela polyrhiza), and eudicots (e.g., Arabidopsis thaliana and Kalanchoe laxiflora). b-e Gene trees of master regulatory bHLH transcription factors SPCH (b), MUTE (c), FAMA (d) and ICE1/2 (e) in stomatal development. Amino-acid sequences from G. biloba (Gb), A. trichopoda (Atr, grey shade), N. colorata (Nc, blue shade), S. polyrhiza (Spipo), Phalaenopsis equestris (Peq), Zea mays (Zm), O. sativa (Loc_Os, green circle), Nelumbo nucifera (NNU), K. laxiflora (Kalax, peachy shade) and A. thaliana (AT, peachy circle) were used to generate trees
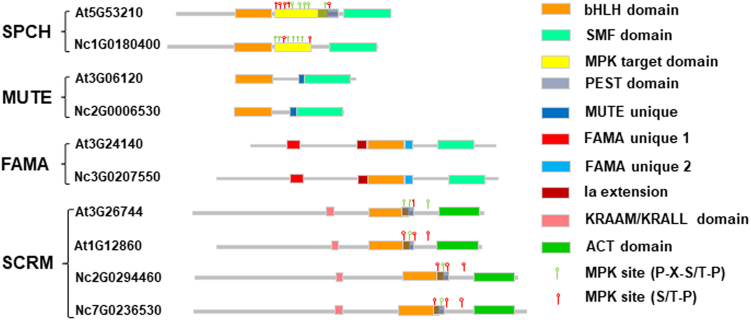
Fig. 3. Schematics of the domain architecture of SPCH, MUTE, FAMA, and ICE-like sequences from N. colorata (Nc) and A. thaliana (At).
NcSPCH shares the bHLH domain (orange) and C-terminal SMF domain (light blue) with AtSPCH but has no protein degradation-associated PEST domain (grey) and has a shorter MAPK target domain (yellow). Both NcMUTE and AtMUTE genes have a unique conserved region (MUTE unique, dark blue) and lack some residues preceding the bHLH domain that are present in all the other bHLH Ia members with various lengths. Both NcFAMA and AtFAMA genes have high AA sequence similarity and harbour three unique domains (FAMA unique 1, red; FAMA unique 2, blue; Ia extension, brown). Both NcICE-like and AtICE1/2 have highly conserved bHLH domains, potential PEST domains and ACT domains (green)
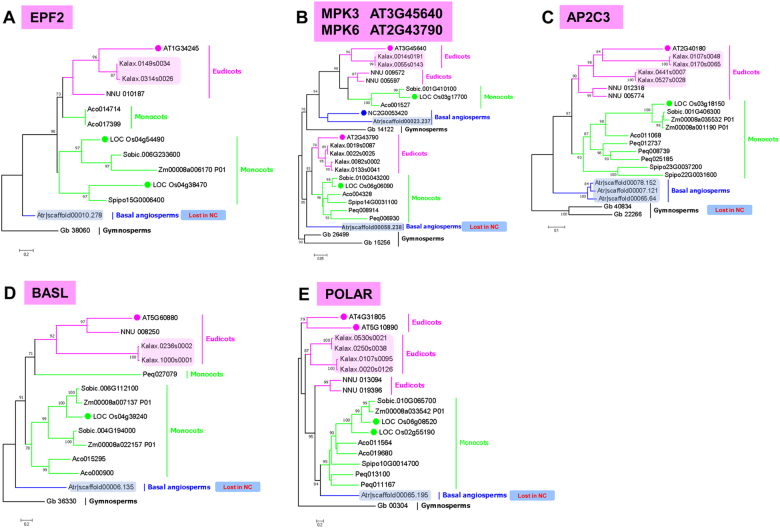
Fig. 4. Phylogenetic analysis of genes lost in N. colorata.
Phylogenetic trees constructed using amino-acid sequences of selected A. thaliana EPF2 (a), MPK3/MPK6 (b), AP2C3 (c), BASL (d) and POLAR (e) gene family members. Amino-acid sequences from G. biloba (Gb), A. trichopoda (Atr, grey shade), N. colorata (Nc, blue shade), S. polyrhiza (Spipo), P. equestris (Peq), Z. mays (Zm), O. sativa (Os, green circle), N. nucifera (NNU), K. laxiflora (Kalax, peachy shade) and A. thaliana (AT, peachy circle) were used to generate trees
Stomatal development gene duplications in K. laxiflora
Whole-genome duplications (WGDs) are a common phenomenon during evolution, and the resulting gene duplications (GDs) provide redundant functions or specified novel functions31–34. WGDs are the source of functional diversity or novelty in the genome for adaption to environmental changes35. It has been suggested that two distinct WGDs occur in the K. laxiflora lineage and generate four gene copies across the genome36.
To understand the evolution of CAM stomata-related genes, we performed genome phylogenetic analysis in K. laxiflora. Intercellular signalling networks, such as peptide ligands, transmembrane receptors TMM/ER, MAPK modules, and bHLH transcription factors, are important for stomatal patterning11–17. In the EPF/TMM/ER module, our phylogenetic analysis shows that EPF2, EPFL6, TMM and ER/ERL have two copies, whereas EPF1 and EPFL9 have six and four orthologous genes, respectively, in K. laxiflora (Figs. 4a, 5). Furthermore we found each YODA, MKK4/MKK5, MKK7/MKK9, MAPKs MPK3/MPK6, and AP2C3 gene has only one copy in A. thaliana while expanded to four homologous genes in K. laxiflora (Figs. 4b, c, 5). Similarly, the group of bHLH transcription factors in K. laxiflora has also expanded to four orthologous (Fig. 2). In addition, the copy of the cell fate determining regulators, HDG2 and FLP/MYB88 also became quadrupled in K. laxiflora (Figures S2A, B). To understand if the asymmetric division is also associated with polarity in K. laxiflora, we analysed polar genes in K. laxiflora. Our analysis indicates that K. laxiflora genome contains homologous genes for PAN1, PAN2, POLAR, BASL, and ROP (Figures S2C, S2D, S2E; Fig. 4d, e). Together, these findings suggest that four copies of stomatal orthologous genes in K. laxiflora possibly derived from maximally two rounds of genome duplication (Table 1).
Fig. 5. Phylogenetic analysis of stomatal regulators.
a-e Phylogenetic analysis of ligand-receptor EPF/TMM/ER models. Phylogenetic trees constructed using amino-acid sequences of selected A. thaliana EPF1 (a), EPFL6 (b), EPFL9 (c), TMM (d) and ER/ERL (e) gene family members. f-h Phylogenetic analysis of the MAPK modules. Phylogenetic trees constructed using amino acid sequences of selected A. thaliana YODA (f), MKK4/5 (g), and MKK7/9 (h) gene family members. Amino-acid sequences from G. biloba (Gb), A. trichopoda (Atr, grey shade), N. colorata (Nc, blue shade), S. polyrhiza (Spipo), P. equestris (Peq), Z. mays (Zm), O. sativa (Os, green circle), N. nucifera (NNU), K. laxiflora (Kalax, peachy shade) and A. thaliana (AT, peachy circle) were used to generate trees
Table 1.
Gene involved in stomata development in N. colorata compared with other representative plant
| Gene name | Symbol | A. thaliana | K. laxiflora | N. nucifera | O. sativa | S. polyrhiza | N. colorata | A. trichopoda | G. biloba |
|---|---|---|---|---|---|---|---|---|---|
| Differentiation genes | |||||||||
| SPEECHLESS | SPCH | AT5G53210 | Kalax.0066s0097 Kalax.0943s0016 Kalax.0277s0021 |
NNU 010414 NNU 013503 |
LOC Os06g33450 LOC Os02g15760 |
Spipo6G0039300 | NC1G0180400 | Atr|scaffold00015.95 | Gb 32351 |
| MUTE | MUTE | AT3G06120 | Kalax.0004s0103 Kalax.0418s0025 Kalax.0268s0032 Kalax.0539s0032 |
NNU 007035 | LOC Os05g51820 | NC2G0006530 | Atr|scaffold00025.64 | ||
| FAMA | FAMA | AT3G24140 | Kalax.0693s0014 Kalax.1863s0001 Kalax.0693s0015 Kalax.0693s0014 |
NNU 012009 NNU 025179 |
LOC Os05g50900 | NF | NC3G0207550 | Atr|scaffold00089.12 | |
| SCREAM/ICE1 | SCRM | AT3G26744 | Kalax.0347s0049 Kalax.0029s0087 Kalax.0801s0007 Kalax.0234s0011 |
NNU 003962 NNU 003217 |
LOC Os11g32100 LOC Os01g70310 |
Spipo4G0062100 Spipo0G0129300 |
NC2G0294460 NC7G0236530 |
Atr|scaffold00016.130 | Gb 18877Gb 40304 |
| SCREAM2 | SCRM2 | AT1G12860 | |||||||
| FOUR LIPS | FLP | AT1G14350 | Kalax.0757s0004 Kalax.0556s0006 Kalax.0031s0030 Kalax.0089s0020 |
NNU 022886 NNU 000781 |
LOC Os07g43420 | Spipo0G0157900 | NC2G0034590 | Atr|scaffold00010.370 | Gb 06045 |
| MYB88 | MYB88 | AT2G02820 | |||||||
| HOMEODOMAIN GLABROUS2 | HDG2 | AT1G05230 | Kalax.0393s0043 Kalax.0069s0102 Kalax.1016s0007 Kalax.1527s0001 |
NNU 019425 NNU 014296 |
LOC Os04g53540 LOC Os08g08820 LOC Os08g04190 |
Spipo7G0015400 | NC1G0306950 | Atr|scaffold00004.265 | Gb 18862Gb 16030 |
| Spacing and patterning genes | |||||||||
| EPIDERMAL PATTERNING FACTOR1 | EPF1 | AT2G20875 | Kalax.0168s0064 Kalax.1136s0002 Kalax.0140s0018 Kalax.0421s0001 Kalax.0001s0052 Kalax.0002s0022 |
NNU 024753 | LOC Os04g54490 LOC Os04g38470 |
Spipo14G0058800 | NC1G0135060 NC11G0122180 |
Atr|scaffold00003.277 | Gb 37555Gb 00388Gb 38060 |
| EPIDERMAL PATTERNING FACTOR2 | EPF2 | AT1G34245 | Kalax.0149s0034 Kalax.0314s0026 |
NNU 010187 | Spipo15G0006400 | NF | Atr|scaffold00010.278 | ||
| STOMAGEN/EPF-LIKE9 | EPFL9 | AT4G12970 | Kalax.0185s0039 Kalax.0025s0112 Kalax.0033s0049 Kalax.0297s0025 |
NNU 022462 NNU 008635 |
LOC Os01g68598 | Spipo7G0057500 | NC3G0208820 | Atr|scaffold00020 | Gb 18813 |
| CHALLAH/EPF-LIKE6 | EPFL6 | AT2G30370 | Kalax.0093s0085 Kalax.0322s0055 |
NF | LOC Os01g60900 LOC Os05g39880 |
Spipo29G0014100 | NC3G0226990 NC4G0199650 |
Atr|scaffold00065.47 | Gb 05485 |
| ERECTA | ER | AT2G26330 | Kalax.0387s0036 Kalax.0284s0052 |
NNU 018228 NNU 010627 | LOC Os06g10230 LOC Os02g53720 |
Spipo15G0047400 | NC10G0163170 | Atr|scaffold00024.267 | Gb 26881 |
| ERECTA-LIKE1 | ERL1 | AT5G62230 | Kalax.0858s0013 Kalax.1180s0007 |
NNU 001410 | LOC Os06g03970 | Spipo11G0029800 | NC9G0271680 | Atr|scaffold00069.214 | Gb 15512 |
| ERECTA-LIKE2 | ERL2 | AT5G07180 | NNU 020430 | ||||||
| TOO MANY MOUTHS | TMM | AT1G80080 | Kalax.0093s0024 Kalax.0058s0095 |
NNU 003757 | LOC Os01g43440 | Spipo18G0010300 | NC4G0153390 | Atr|scaffold00051.26 | Gb 10165 |
| STOMATAL DENSITY AND DISTRIBUTION1 | SDD1 | AT1G04110 | Kalax.0525s0015 Kalax.0155s0004 |
NNU 010999 | LOC Os03g04950 | Spipo1G0013100 | NC4G0239300 | Atr|scaffold00039.113 | Gb 35657 |
| CO2 RESPONSE SECRETED PROTEASE | CRSP | AT1G20160 | NF | NNU 013210 | LOC Os09g30458 | Spipo3G0019800 | NC2G037260 | Atr|scaffold00152.21 | Gb 39463 |
| YODA | YDA | AT1G63700 | Kalax.0027s0088 Kalax.0305s0041 Kalax.0021s0011 Kalax.0119s0027 |
NNU 019513 NNU 006681 |
LOC Os02g44642 LOC Os04g47240 |
Spipo24G0030800 | NC4G0020020 NC12G0094120 |
Atr|scaffold00009.178 | Gb 12558 |
| MPK3 | MPK3 | AT3G45640 | Kalax.0014s0191 Kalax.0055s0143 |
NNU 009572 NNU 005597 |
LOC Os03g17700 | NF | NC2G0053420 | Atr|scaffold00023.237 | Gb 14122 |
| MPK6 | MPK6 | AT2G43790 | Kalax.0019s0087 Kalax.0022s0025 Kalax.0082s0002 Kalax.0133s0041 |
NF | LOC Os06g06090 | Spipo14G0031100 | NF | Atr|scaffold00058.238 | Gb 26499Gb 15256 |
| MKK4 | MKK4 | AT1G51660 | Kalax.0510s0006 Kalax.0445s0039 Kalax.0283s0053 Kalax.0104s0058 |
NNU 012790 | LOC Os02g54600 LOC Os06g09180 |
Spipo12G0053200 Spipo28G0018300 |
NC3G0229970 | Atr|scaffold00011.127 Atr|scaffold00011.128 |
Gb 36141 |
| MKK5 | MKK5 | AT3G21220 | |||||||
| MKK7 | MKK7 | AT1G18350 | Kalax.0543s0017 Kalax.0216s0025 Kalax.0039s0095 Kalax.1433s0007 |
NNU 016426 NNU 000259 |
LOC Os03g12390 | Spipo15G0037300 | NC8G0217780 NC10G0164550 |
Atr|scaffold00176.2 | Gb 41213Gb 20818 |
| MKK9 | MKK9 | AT1G73500 | |||||||
| ARABIDOPSIS PROTEIN PHOPHATASE 2C | AP2C3 | AT2G40180 | Kalax.0107s0048 Kalax.0170s0065 Kalax.0441s0007 Kalax.0527s0028 |
NNU 012318 NNU 005774 |
LOC Os03g18150 | Spipo22G0031600 Spipo23G0037200 |
NF | Atr|scaffold00065.64 Atr|scaffold00078.152 Atr|scaffold00007.121 |
Gb 40834Gb 22266 |
| Polarity and division asymmetry genes | |||||||||
| PANGLOSS1 | PAN1 | AT2G42290, AT3G57830 | Kalax.0222s0039 Kalax.0637s0020 |
NNU 012890 | LOC Os08g39590 | Spipo12G0035200 | NC1G0088630 | Atr|scaffold00022.305 | Gb 28844 |
| PANGLOSS2 | PAN2 | AT4G20940 | Kalax.0016s0247 Kalax.0114s0005 |
NNU 026348 | LOC Os07g05190 | Spipo32G0009300 Spipo0G0142000 |
NC14G0281210 | Atr|scaffold00175.33 | Gb 30406Gb 18587 |
| RHO-RELATED PROTEIN FROM PLANTS 9 | ROP9 | AT4G28950 | Kalax.0192s0051 Kalax.0015s0042 Kalax.1214s0006 |
NNU 005916 NNU 003451 |
LOC Os05g43820 | Spipo26G0003200 | NC6G0252910 | Atr|scaffold00002.129 | Gb 09833 |
| BREAKING OF ASYMMETRY IN THE STOMATAL LINEGAE | BASL | At5g60880 | Kalax.0236s0002 Kalax.1000s0001 |
NNU 008250 | LOC Os04g39240 | NF | NF | Atr|scaffold00006.135 | Gb 36330 |
| POLAR LOCALIZATION DURING ASYMMETRIC DIVISION AND REDISTRIBUTION | POLAR | AT4G31805 | Kalax.0020s0126 Kalax.0107s0095 Kalax.0250s0038 Kalax.0530s0021 |
NNU 019396NNU 013094 | LOC Os06g08520 LOC Os02g55190 |
Spipo10G0014700 | NF | Atr|scaffold00065.195 | Gb 00304 |
| Mitosis and cytokinesis genes | |||||||||
| STOMATAL CYTOKINESIS DEFECTIVE 1 | SCD1 | AT1G49040 | Kalax.0061s0068 Kalax.0190s0062 |
NNU 012674 | LOC Os01g39380 | Spipo21G0025200 | NC3G0202830 | Atr|scaffold00104.16 | Gb 36258 |
| Hormone and environmental signalling genes | |||||||||
| CRYPTOCHROME | CRY1 | AT4G08920 | Kalax.0428s0010 Kalax.1365s0004 Kalax.0290s0014 Kalax.0239s0053 |
NNU 001876 NNU 015266 |
LOC Os04g37920 LOC Os02g36380 |
Spipo15G0011900 | NC8G0218290 | Atr|scaffold00038.124 | Gb 13122 |
| CRY2 | AT1G04400 | Kalax.0094s0015 Kalax.0075s0050 |
NNU 010890 NNU 018834 |
LOC Os02g41550 | Spipo1G0003600 | NC12G0249420 | Atr|scaffold00148.69 | Gb 13122 | |
| PHYTOCHROME | PHYA | AT1G09570 | Kalax.0106s0002 Kalax.0005s0079 Kalax.0038s0184 Kalax.0172s0035 |
NNU 026354 | LOC Os03g51030 | Spipo6G0014200 | NC10G0166490 | Atr|scaffold00045.165 | Gb 21967 |
| PHYB | AT2G18790 | Kalax.0613s0014 Kalax.0391s0019 Kalax.0996s0003 |
NNU 014452 | LOC Os03g19590 | Spipo6G0031800 | NC5G0160900 | Atr|scaffold00003.45 | Gb 17897 | |
| PYTOCHROME-INTERACTING FACTOR 4 | PIF4 | AT2G43010 | Kalax.0495s0020 Kalax.0759s0011 |
NNU 026428 | LOC Os03g43810 LOC Os07g05010 LOC Os03g56950 |
Spipo13G0048400 | NC10G0166270 | Atr|scaffold00039.9 | Gb 07156 |
| CONSTITUTIVE PHOTOMORPHOGENIC 1 | COP1 | AT2G32950 | Kalax.0049s0041 Kalax.0049s0041 |
NNU 005078 NNU 015709 |
LOC Os02g53140 | Spipo31G0000500 | NC1G0178350 | Atr|scaffold00074.24 | Gb 15627 |
| CONSTITUTIVE PHOTOMORPHOGENIC 10 | COP10 | AT3G13550 | Kalax.0340s0003 Kalax.0021s0072 |
NNU 019762 | LOC Os07g38940 | Spipo2G0063200 | NC1G0193740 | Atr|scaffold00061.43 | Gb 07763 |
| HIGH CARBON DIOXIDE | HIC1 | AT2G46720 | Kalax.0018s0006 Kalax.0090s0007 Kalax.1015s0012 Kalax.0013s0142 Kalax.1015s0014 |
NNU 006085 NNU 003630 |
LOC Os05g49900 LOC Os02g11070 LOC Os06g39750 |
Spipo14G0001700 Spipo21G0006400 |
NC6G0254440 NC1G0129310 |
Atr|scaffold00052.41 | Gb 23820 |
| BRI SUPPRESSOR1 | BSU1 | AT1G03445 | Kalax.0084s0077 Kalax.1286s0001 Kalax.0045s0074 Kalax.0289s0053 |
NNU 001649 NNU 024344 |
LOC Os05g05240 | Spipo6G0007500 | NC1G0193170 | Atr|scaffold00004.204 | Gb 36990 |
| BRASSINOSTEROID INSENSTIVIE 2 | BIN2 | AT4G18710 | Kalax.0092s0006 Kalax.0164s0037 Kalax.1441s0002 Kalax.0375s0036 Kalax.0283s0042 Kalax.0104s0069 |
NNU 025519 | LOC Os01g10840 LOC Os05g11730 LOC Os02g14130 LOC Os06g35530 |
Spipo18G0019800 Spipo14G0030500 |
NC9G0114290 NC13G0028550 |
Atr|scaffold00170.9 | Gb 21469 |
NF not found
Novel formation of subsidiary cells in K. laxiflora
CAM increases water-use efficiency and drought resistance in plants, which is characterized by nocturnal opening and diurnal closing of the stomata36. Therefore, stomatal control in the leaves is particularly important for this type of plant to reduce evapotranspiration in the daytime and increase carbon dioxide (CO2) collection at night2,36. The physiological traits probably improve the resistance of CAM plants to diverse environmental stresses, including drought1,2.
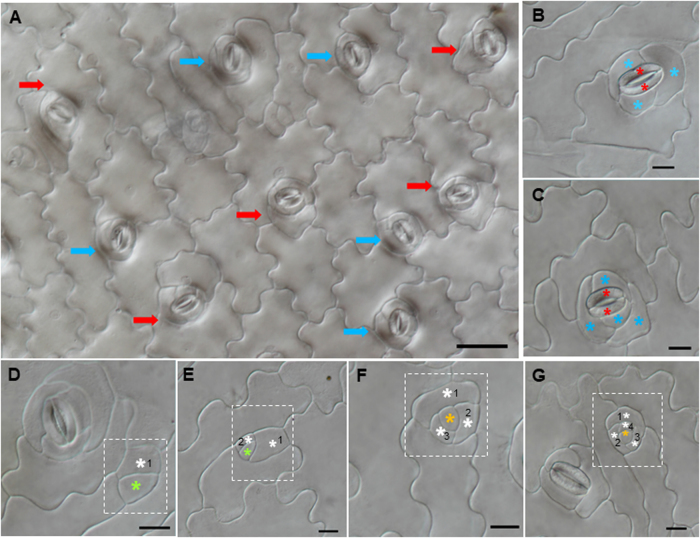
To gain a better understanding of the stomatal complex in CAM plants, we performed anatomical observation of K. laxiflora, a member of the eudicot CAM family. In K. laxiflora, stomata are surrounded by three to four small subsidiary cells in adaxial leaf surfaces (Fig. 6a). Similarly, we found that the stomata of Phalaenopsis equestris, another CAM monocot species, is also surrounded by approximately four subsidiary cells (Figure S3). This innovation of stomatal architecture could derive from differential regulation of stomatal formation. We found that in K. laxiflora, stomata formed via a series of asymmetric cell divisions and cell state transitions: protodermal cells entered the stomatal lineage and took on a MMC identity; the MMC underwent three or four asymmetrical divisions to form GMC and Stomatal lineage ground cell (SLGC) (Fig. 6d-g). The GMC underwent a symmetric division to form a pair of guard cells, and SLGCs eventually became subsidiary cells surrounding the guard cell (Fig. 6b, c).
Fig. 6. Stomatal development of Kalanchoe laxiflora on adaxial leaf epidermis.
a There are two types of mature stomata equably distributed on adaxial leaf surfaces; the guard cells are surrounded by three (blue arrow) or four subsidiary cells (red arrow). b A stoma with three subsidiary cells. c A stoma with four subsidiary cells. d-g DIC of different stages with asymmetric division finally form two mature stomatal types. Meristemoid (green star), surrounding cells (white star), guard mother cell (orange star), guard cells (red star), and subsidiary cells (blue star)
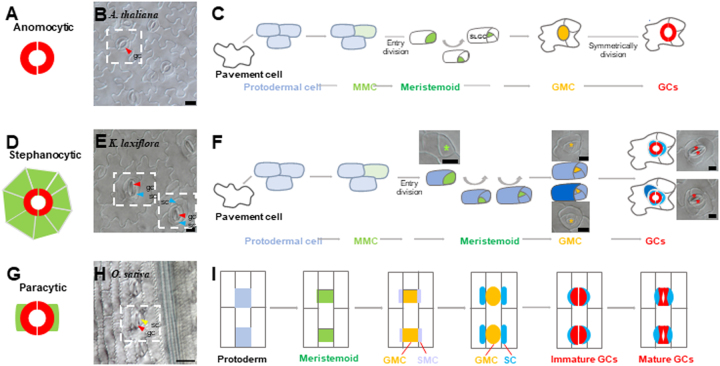
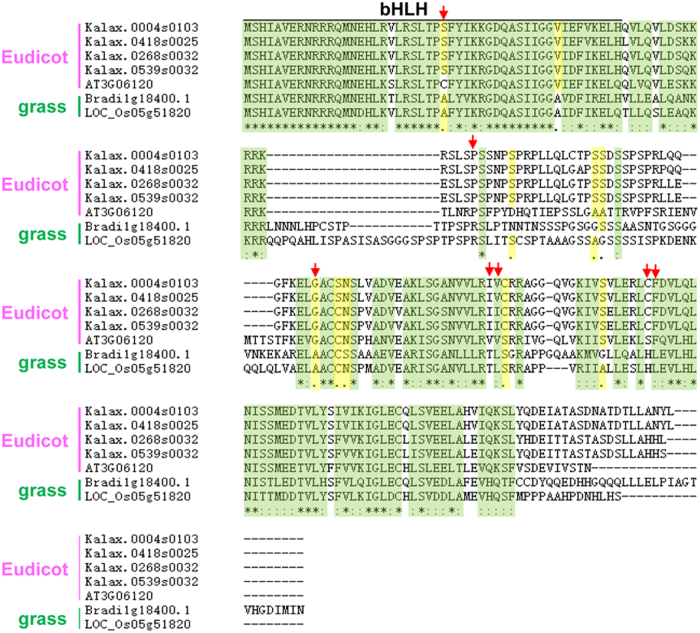
It is widely accepted that different stomatal patternings reflect the asymmetric division of precursor cells and lateral divisions of neighbouring cells37. For example, in anomocytic stomata occurring in the eudicot A. thaliana (Fig. 7a, b), the MMC underwent three asymmetric divisions to give rise to a GMC and SLGCs, which was followed by a transition from SLGCs to pavement cells (Fig. 7c). Although both A. thaliana and K. laxiflora are eudicots, K. laxiflora possesses stephanocytic stomata (Fig. 7d, e). Developmentally, there is a similarity between these two types of stomata: meristemoids undergo a series of asymmetric divisions to produce SLGCs surrounding guard cells (Fig. 7f), and different cell fate choices of SLGCs finally give rise to different stomatal complexes (Figure S4). In monocot species such as O. sativa, the type of mature stomata is named the paracytic type, in which the guard cell is surrounded by two subsidiary cells (Fig. 7g, h). In this type, the stomatal meristemoid divides asymmetrically to form a larger SLGC and a smaller meristemoid that directly forms the GMC. Before the GMC divides, it induces neighbouring cell files to adopt an SMC identity, which subsequently forms SCs via asymmetric divisions. The GMC then undergoes symmetric mitosis to eventually form guard cells (Fig. 7i). Therefore, subsidiary cells can develop through different ways: one is through asymmetric division in O. sativa, and the other is through SLGC differentiation in K. laxiflora. In K. laxiflora, subsidiary cells are noticeably visible, but little is known about the factors defining subsidiary cell identity. In Brachypodium distachyon, subsidiary cells are formed through asymmetric divisions. BdMUTE is an orthologue of A. thaliana MUTE that has been identified as sufficient for SC formation based on its acquisition of cell-to-cell mobility23. In A. thaliana, AtMUTE, which is associated with GMC identity, is nonmobile. The question is whether the KalaxMUTE could also specify SC identity by being mobile. To address this, we compared MUTE orthologues of the representative species with B. distachyon, A. thaliana and K. laxiflora to test potential mobility motifs in K. laxiflora (Fig. 8). Our results show high conservation in the bHLH functional domain. The differences in potential mobility residues of KalaxMUTE from its homologue in B. distachyon are similar to those in A. thaliana. Thus, the subsidiary cells in K. laxiflora may not be specified by KalaxMUTE mobility.
Fig. 7. Mature stomatal types and development in diverse species.
a, d, g Mature stomatal types. Diagrams show the guard cell pair (red) and subsidiary cells (green). a Anomocytic stomata lack subsidiary cells. d Stephanocytic stomata possess a ring of subsidiary cells. g Paracytic stomata possess one pair of lateral subsidiary cells oriented parallel to the guard cells. b, c Example of eudicot stomata in A. thaliana. b The upper epidermis of A. thaliana with anomocytic stomata. c Schematic diagram of stomatal development transitions. A subset of protodermal cells (pale blue) enter the stomatal lineage and take on an MMC identity; the MMC (pale green) undergoes asymmetric cell division producing a smaller meristemoid (green) and larger SLGCs (white). Then, the meristemoid differentiates into a GMC (orange), and the GMC undergoes a symmetric division to form a pair of guard cells (red). e, f Example of eudicot stomata in K. laxiflora. e The upper epidermis of K. laxiflora with stephanocytic stomata. f Schematic diagram of stomatal development. Protodermal cells (pale blue) take on an MMC identity. The MMC (pale green) divides through three or four asymmetric divisions to give rise to a GMC (orange), and a round of neighbouring cells (dark blue) eventually become subsidiary cells (blue) surrounding the guard cells (red). h, i Example of monocot stomata in O. sativa. h The upper epidermis of O. sativa with linear cell files and paracytic stomata. i Diagrams illustrating stomatal development for the stomatal complex. Cell protoderm files (pale blue) asymmetrically divide to create a meristemoid (green), and the meristemoid differentiates into a GMC (orange). Then, neighbouring cell files (SMC, pale purple) divide asymmetrically to form SCs (blue). Finally, the GMC divides once symmetrically to form GCs (red), and the GCs and SCs terminally differentiate and form mature dumbbell-shaped stomata. Key: protodermal cell that will give rise to the stomatal lineage, pale blue; MMC (meristemoid mother cell), pale green; meristemoid, green; SLGCs (stomatal-lineage ground cell), white; GMC (guard mother cell), orange; GCs (guard cells), red; SMC (subsidiary mother cell), pale purple; SCs (subsidiary cells), blue
Fig. 8. Alignment of grass and eudicot MUTE orthologues to identify potential mobility residues.
MUTE orthologues of the representative grass species Brachypodium (BdMUTE—Bradi1g18400) and rice (OsMUTE—LOC_Os05g51820) were aligned with the MUTE orthologues of the representative eudicot species Arabidopsis (AtMUTE—AT3G06120) and Kalax.0004s0103/Kalax.0418s0025/Kalax.0268s0032/Kalax.0539s0032 using ClustalW (http://www.genome.jp/tools-bin/clustalw). The bHLH domain spans the first 50 amino acids and is indicated. Green shaded amino acids represent high similarity, whereas yellow shaded amino acids represent intermediate similarity. Candidate amino acids that are either consistently different between grasses and eudicots or are conserved among grasses but not in eudicots, or vice versa, are marked with a red asterisk and represent potential mobility motifs
Discussion
Stomatal patterning is diverse among different land plants. In Physcomitrella patens, stomata exhibit partial or complete division to form a single GC or paired GCs, respectively38. Moss does not have genes encoding MUTE or SPCH and uses genes encoding two bHLH proteins, PpSMF1 and PpSCRM1, to promote stomatal formation39. In A. thaliana, the stomata are surrounded by two kidney-shaped guard cells, and polar localization of BASL is required for a series of asymmetric divisions to form the stomatal structure40. In O. sativa, polar localization of PAN protein is responsible for subsidiary cell asymmetry in the stomatal complex10. In B. distachyon, BdMUTE is necessary and sufficient for SC formation. However, AtMUTE in A. thaliana defines GC precursor fate23. Overall, it appears that the function of most genes is conserved during stomatal formation across plant evolution, but there are novel genes recruited to regulate unique aspects of stomatal patterning in some species.
The regulatory machine of stomata development appeared to be flexible and adaptable during evolution. The adaptation pressure could quickly change the division and differentiation pattern during stomata formation. For example, all the genes involved in stomatal differentiation are lost in seagrass Zostera to enhance its adaptation to marine lifestyle30. Plants of the ANITA grade form specialized structures in the epidermal cells to adapt to its habitat29. Similarly, N. colorata has lost genes, which could be associated with its unique stomatal development. However, further molecular and genetic manipulations are needed for functional verification.
Compared with our understanding of stomatal development in model systems, little is known about the molecular evolution of stomatal morphology, particularly in basal angiosperms. Alongside the completion of the genome, we are beginning to find the comparative molecular basis of the evolution of stomatal development and identify orthologues of stomatal regulator genes in a selected range of phylogenetic taxa. However, it is still technically difficult to analyse the function of orthologues. In the N. colorata genome, we found that a number of the genes that are highly specific to the stomatal asymmetric division were missing. Taken together, these results suggest that most core regulators of stomata formation remain conserved during evolution, whereas some gene loss events can occur to modify stomata formation processes, such as asymmetric division. These changes at the genetic and morphological levels of individual species may result from adaptation to inhabitant environments rather than evolutionary changes.
Recent studies have indicated that WGD events are ubiquitous in the evolution of angiosperms, and WGDs tend to retain multiple family duplications to increase the frequency of multiplication and the function of genes41. Thus, WGDs are widely thought to provide genomic novelties and complexities to promote plant adaptation to environments42. Large-scale GDs involved in stomata development through WGDs in K. laxiflora have been identified36.
Analysis of the genes involved in stomata formation showed that the protein sequences of the core genes required to instigate and pattern stomata are conserved in K. laxiflora (Table 1). It is unclear whether the expression or protein modification of these regulators is different in K. laxiflora compared with that in A. thaliana. Indeed, the duplication of stomata regulator genes appears to be a common theme in K. laxiflora, but the extent to which this represents a divergence in gene function requires further studies.
It seemed that genes encoding critical developmental regulators were more likely to be retained during evolution43,44. For stomatal development, subsidiary cells can occur from an adjacent cell file or the same cell as the guard cells. Based on sequence conservation, the mobility of KalaxMUTE could be similar to its homologue in Arabidopsis. Thus, it is less likely that the modification of KalaxMUTE leads to featured stomatal subsidiary cells in K. laxiflora. Further work is needed to investigate whether the gene gains in K. laxiflora are associated with subsidiary cell establishment.
Electronic supplementary material
Acknowledgements
This study was supported by the 1000-Talents Plan from China for young researchers (grant KXR15012A), the National Key Research and Development Program of China (2016YFD0100700) and a grant from the National Natural Science Foundation of China (31722006) to S.W.
Author contributions
S.W. and L.Z. designed the research; M.X. and S.Q. performed the experiments; M.X., S.Q., F.C., and S.W. analysed the data; M.Z. and S.W. wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Luttge U. Ability of crassulacean acid metabolism plants to overcome interacting stresses in tropical environments. AoB Plants. 2010;2010:plq005. doi: 10.1093/aobpla/plq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borland AM, et al. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant. Sci. 2014;19:327–338. doi: 10.1016/j.tplants.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vatén A, Bergmann DC. Mechanisms of stomatal development: an evolutionary view. EvoDevo. 2012;3:11. doi: 10.1186/2041-9139-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson KM, Rychel AL, Torii KU. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. Plant Cell. 2010;22:296–306. doi: 10.1105/tpc.109.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Males J, Griffiths H. Stomatal biology of CAM plants. Plant Physiol. 2017;174:550–560. doi: 10.1104/pp.17.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, et al. Molecular evolution of grass stomata. Trends Plant. Sci. 2017;22:124–139. doi: 10.1016/j.tplants.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter KJ. Stomatal architecture and evolution in basal angiosperms. Am. J. Bot. 2005;92:1595–1615. doi: 10.3732/ajb.92.10.1595. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher K, Smith LG. Roles for polarity and nuclear determinants in specifying daughter cell fates after an asymmetric cell division in the maize leaf. Curr. Biol. 2000;10:1229–1232. doi: 10.1016/S0960-9822(00)00730-2. [DOI] [PubMed] [Google Scholar]
- 9.Rudall PJ, Knowles EVW. Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Ann. Bot. 2013;112:1031–1043. doi: 10.1093/aob/mct169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillitteri LJ, Torii KU. Mechanisms of stomatal development. Annu. Rev. Plant. Biol. 2012;63:591–614. doi: 10.1146/annurev-arplant-042811-105451. [DOI] [PubMed] [Google Scholar]
- 11.Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt L, Gray JE. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr. Biol. 2009;19:864–869. doi: 10.1016/j.cub.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- 15.Pillitteri LJ, Bogenschutz NL, Torii KU. The bHLH protein, MUTE, controls differentiation of stomata and the hydathode pore in Arabidopsis. Plant Cell Physiol. 2008;49:934–943. doi: 10.1093/pcp/pcn067. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi-Ito K, Bergmann DC. Arabidopsis FAMA controls the final proliferation/ differentiation switch during stomatal development. Plant Cell. 2006;18:2493–2505. doi: 10.1105/tpc.106.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaoka MM, et al. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong J, MacAlister CA, Bergmann DC. BASL controls asymmetric cell division in Arabidopsis. Cell. 2009;137:1320–1330. doi: 10.1016/j.cell.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell. 2011;23:3260–3275. doi: 10.1105/tpc.111.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright HN, Humphries JA, Smith LG. PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science. 2009;323:649–651. doi: 10.1126/science.1161686. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Identification of PAN2 by quantitative proteomics as a leucine-rich repeat-receptor-like kinase acting upstream of PAN1 to polarize cell division in maize. Plant Cell. 2012;24:4577–4589. doi: 10.1105/tpc.112.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphries JA, et al. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell. 2012;23:2273–2284. doi: 10.1105/tpc.111.085597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raissig MT, et al. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science. 2017;355:1215–1218. doi: 10.1126/science.aal3254. [DOI] [PubMed] [Google Scholar]
- 24.Cai S, Papanatsiou M, Blatt MR, Chen ZH. Speedy grass stomata: emerging molecular and evolutionary features. Mol. Plant. 2017;10:912–914. doi: 10.1016/j.molp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Raissig MT, Abrash E, Bettadapur A, Vogel JP, Bergmann DC. Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc. Natl. Acad. Sci. USA. 2016;113:8326–8331. doi: 10.1073/pnas.1606728113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter KJ. Specialized structures in the leaf epidermis of basal angiosperms: morphology, distribution, and homology. Am. J. Bot. 2006;93:665–681. doi: 10.3732/ajb.93.5.665. [DOI] [PubMed] [Google Scholar]
- 30.Olsen JL, et al. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature. 2016;530:331–335. doi: 10.1038/nature16548. [DOI] [PubMed] [Google Scholar]
- 31.Pontes O, et al. Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc. Natl. Acad. Sci. USA. 2004;101:18240–18245. doi: 10.1073/pnas.0407258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell-Olds T, Schmitt J. Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature. 2006;441:947–952. doi: 10.1038/nature04878. [DOI] [PubMed] [Google Scholar]
- 33.Madlung A, et al. Genomic changes in synthetic Arabidopsis polyploids. Plant J. 2005;41:221–230. doi: 10.1111/j.1365-313X.2004.02297.x. [DOI] [PubMed] [Google Scholar]
- 34.Long M, Betrán E, Thornton K, Wang W. The origin of new genes: glimpses from the young and old. Nat. Rev. Genet. 2003;4:865–875. doi: 10.1038/nrg1204. [DOI] [PubMed] [Google Scholar]
- 35.Jiao Y, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, et al. The Kalanchoë genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nat. Commun. 2017;8:1899. doi: 10.1038/s41467-017-01491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrash EB, Bergmann DC. Asymmetric cell divisions: a view from plant development. Dev. Cell. 2009;16:783–796. doi: 10.1016/j.devcel.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Merced A, Renzaglia KS. Patterning of stomata in the moss Funaria: a simple way to space guard cells. Ann. Bot. 2016;117:985–994. doi: 10.1093/aob/mcw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chater CC, et al. Origin and function of stomata in the moss Physcomitrella patens. Nat. Plants. 2016;2:16179. doi: 10.1038/nplants.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao W, Dong J. Polarity in plant asymmetric cell division: division orientation and cell fate differentiation. Dev. Biol. 2016;419:121–131. doi: 10.1016/j.ydbio.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren R, et al. Wide-spread whole genome duplications contribute to genome complexity and species diversity in angiosperms. Mol. Plant. 2018;18:30022–30024. doi: 10.1016/j.molp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Curr. Biol. 2008;18:R435–R444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 43.McGrath CL, Gout J, Johri P, Doak TG, Lynch M. Differential retention and divergent resolution of duplicate genes following whole-genome duplication. Genome Res. 2014;24:1665–1675. doi: 10.1101/gr.173740.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aury JM, et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature. 2006;444:171–178. doi: 10.1038/nature05230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.